The Role of Vitekang Soil Conditioner and Arbuscular Mycorrhizae Fungi in Mitigating Cadmium Stress in Solanum lycopersicum Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Soil
2.3. AMF Propagation with Clover Culture
2.4. Experimental Design
2.4.1. Experiment 1
2.4.2. Experiment 2
2.5. Sampling and Growth Index Measurement
2.6. Determination of Cadmium Content
2.7. Methods for Physiological Measurements
2.8. RNA Extraction and QRT–PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of Different Soil Conditioner Concentrations on Cadmium Content and Agronomic Biofortification in Solanum lycopersicumes (Experiment 1)
3.1.1. Cadmium Content in Roots and Leaves
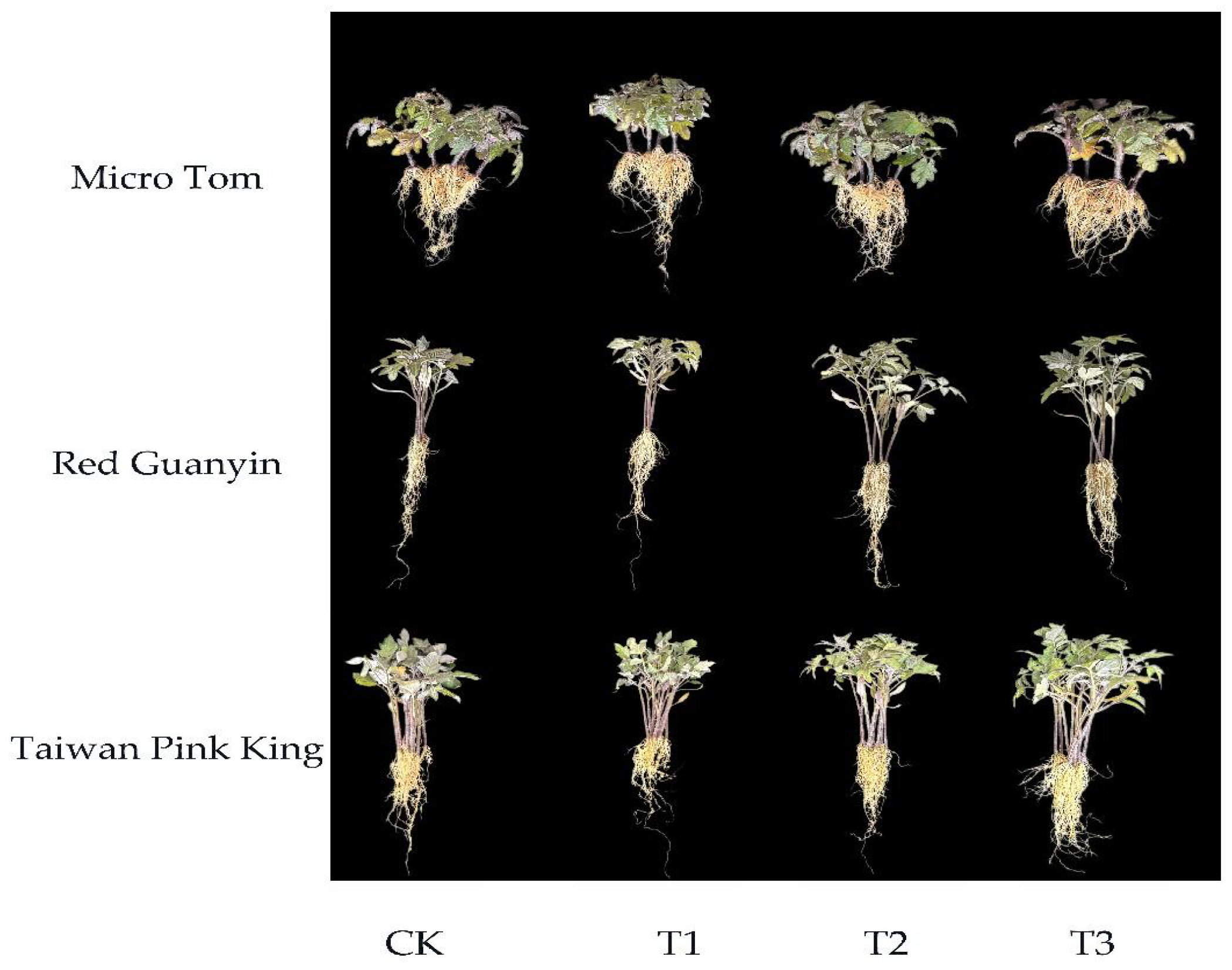
3.1.2. Agronomic Traits
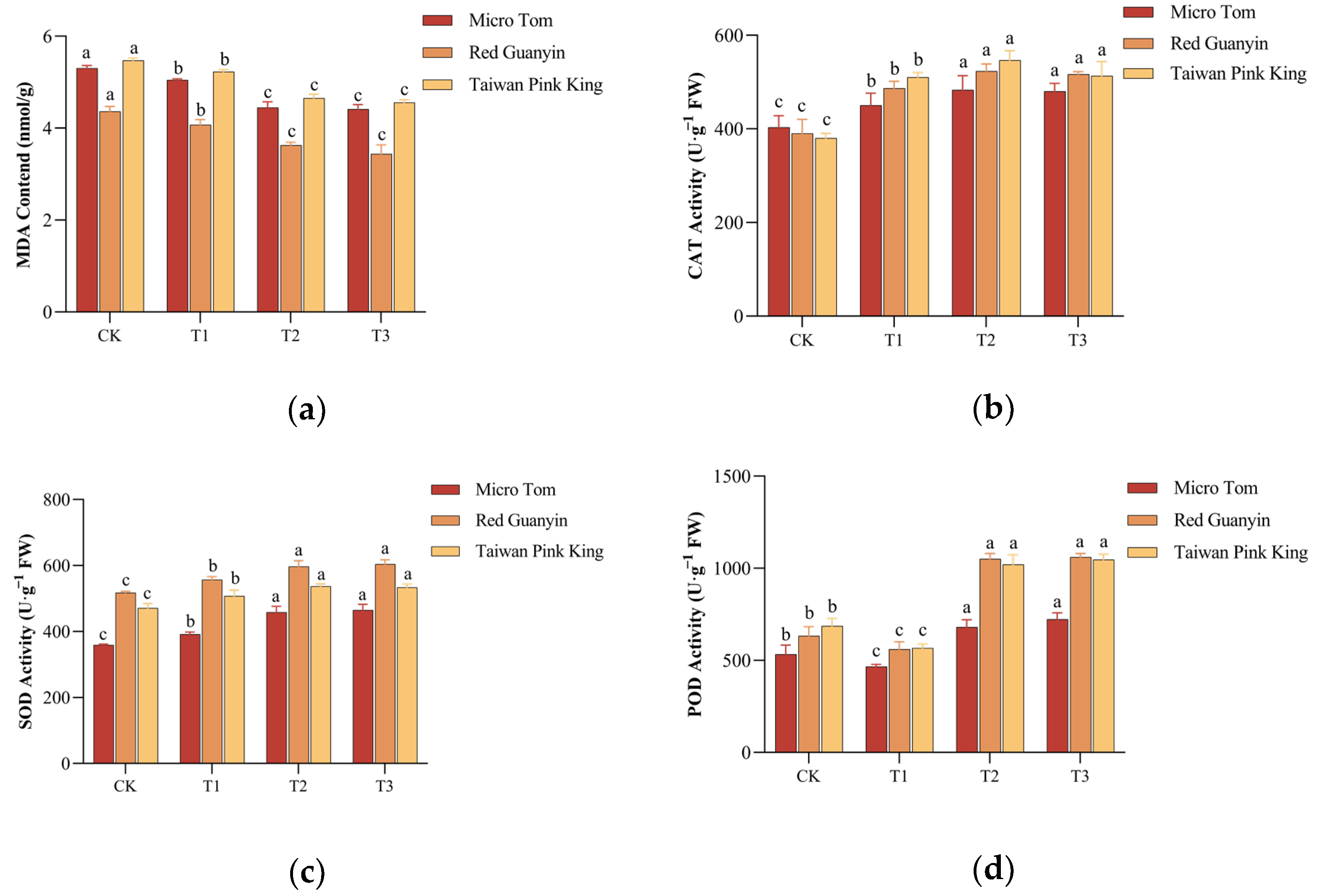
3.1.3. MDA Content and Antioxidant Enzyme Activities
3.1.4. DAB and NBT Staining
3.1.5. Effects of the Expression of NRAMP6 and HMA3 Genes
3.2. Effects of VT and AMF Treatments Alone and in Combination on Cadmium Content and Quality of Solanum lycopersicum (Experiment 2)
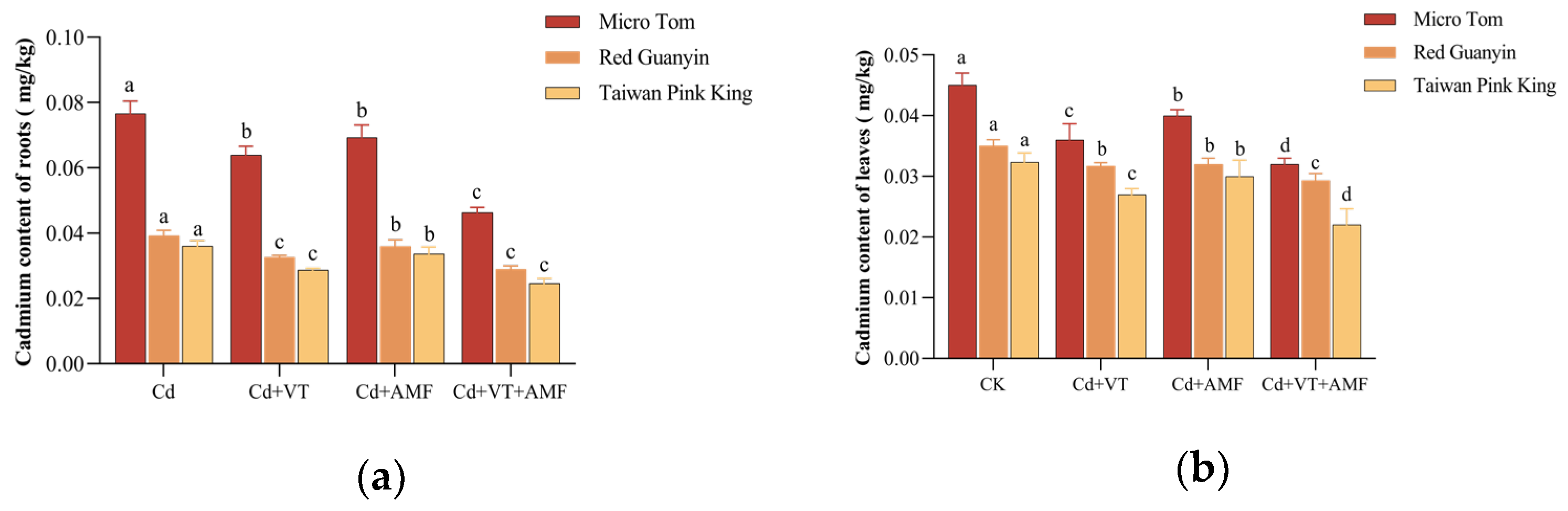
3.2.1. Cadmium Content in Roots and Leaves
3.2.2. Agronomic Traits
3.2.3. MDA Content and Antioxidant Enzyme Activities
4. Discussion
4.1. Effects of Soil Conditioner Concentration on Solanum lycopersicum Under Cadmium Stress
4.2. Effects of AMF Inoculation on Solanum lycopersicum Under Cadmium Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, N.C.; Zheng, Y.J.; He, X.F.; Li, X.F.; Zhang, X.X. Analysis of the Bulletin on the Nationwide Survey of Soil Pollution. J. Agro-Environ. Sci. 2017, 36, 1689–1692. [Google Scholar]
- Cao, L.; Ren, W.; Liu, L.; Zheng, J.; Tao, C.; Zhu, W.; Xiang, M.; Wang, L.; Liu, Y.; Zheng, P. CDR1, a DUF946 domain containing protein, positively regulates cadmium tolerance in Arabidopsis thaliana by maintaining the stability of OPT3 protein. J. Hazard. Mater. 2024, 477, 135313. [Google Scholar] [CrossRef]
- Dong, P.F.; Liu, T.B.; Chen, K.; Li, D.; Li, Y.; Lian, C.Y.; Wang, Z.Y.; Wang, L. Cadmium targeting transcription factor EB to inhibit autophagy-lysosome function contributes to acute kidney injury. J. Adv. Res. 2024, in press. [CrossRef] [PubMed]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competi-tion with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Zhao, H.K.; Zhang, X.Y.; Zeng, H.Y.; Deng, J.; Chen, X.; Song, L. Effects of Vitekang Soil Conditioner on soil physical and properties and physiological characteristics of non-heading Chinese cabbage. Acta Agron. Boreali-Sin. 2021, 3, 312–319. [Google Scholar]
- Wang, D.; Zhou, W.; Zhang, Y.Y.; Wang, M.Y. Effects of Vitekang Soil Conditioner on the improvement of severely cadmium-polluted and the accumulation of cadmium in crops. Anhui Agric. Sci. 2024, 52, 7376. [Google Scholar]
- Zhang, Q.; Zhang, L.; Liu, T.; Liu, B.; Huang, D.; Zhu, Q.; Xu, C. The influence of liming on cadmium accumulation in rice grains via iron-reducing bacteria. Sci. Total Environ. 2018, 645, 109–118. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Yin, J.; Qi, L.; Feng, Y. Combined amendments of nano-hydroxyapa-tite immobilized cadmium in contaminated soil-potato (Solanum tuberosum L.) system. Bull. Environ. Contam. Toxicol. 2018, 100, 581–587. [Google Scholar] [CrossRef]
- Moscowskiy Bio-Organic Catalyst, Inc. The Program and the Results of Pilot Study on the Use of Bio-Organic Catalyst Phyto-C3 TM for Treatment of Agricultural Plants on the Basis of JSC Agricultural Complex; Contentree: Reno, NV, USA, 2022. [Google Scholar]
- Wang, H.; Hao, Z.; Zhang, X.; Xie, W.; Chen, B. Arbuscular Mycorrhizal Fungi Induced Plant Resistance against Fusarium Wilt in Jasmonate Biosynthesis Defective Mutant and Wild Type of Solanum lycopersicum. J. Fungi 2022, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Feng, G. Arbuscular mycorrhizal fungi alleviate the negative effects of increases in phosphorus (P) resource diversity on plant community structure by improving P resource utilization. Plant Soil 2021, 461, 295–307. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Zhao, J.; Zhang, L.; Li, Y.; Wang, H.; Teng, H.; Yuan, Z.; Yuan, Z. Physio-biochemical and transcriptomic features of arbuscular mycorrhizal fungi relieving cadmium stress in wheat. Antioxidants 2022, 11, 2390. [Google Scholar] [CrossRef]
- Chen, L.; Wang, F.; Zhang, Z.; Chao, H.; He, H.; Hu, W.; Zeng, Y.; Duan, C.; Liu, J.; Fang, L. Influences of arbuscular mycorrhizal fungi on crop growth and potentially toxic element accumulation in contaminated soils: A meta-analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1795–1816. [Google Scholar] [CrossRef]
- Wu, Z.X.; Liu, J.J.; Ma, W.D. Research Progress on Enhanced Phytoremediation of Heavy Metal-Contaminated Soils by the Combined Application of Biochar and Arbuscular Mycorrhizal Fungi. Environ. Pollut. Control. 2024, 46, 1040–1046. [Google Scholar]
- Cui, Q.; Beiyuan, J.; Chen, Y.; Li, M.; Qiu, T.; Zhao, S.; Zhu, X.; Chen, H.; Fang, L. Synergistic enhancement of plant growth and cadmium stress defense by Azospirillum brasilense and plant heme: Modulating the growth–defense relationship. Sci. Total Environ. 2024, 946, 174503. [Google Scholar] [CrossRef]
- Xin, J. Enhancing soil Health to minimize Cadmium accumulation in agro-products: The role of microorganisms, organic amendments, and nutrients. Environ. Pollut. 2024, 348, 123890. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality: Risk Management Standard for Soil Contamination of Agricultural Land (Trial). Ministry of Ecology and Environment of the People’s Republic of China; General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2018.
- Wang, Y.S.; Wang, X.Y.; Sun, H.; Zhang, S.B.; Xing, L.J. Methods for Collection, Cultivation, and Preservation of Arbuscular Mycorrhizal Fungi Species Resources. Bio Protoc. 2022, 101, e2104422. [Google Scholar]
- Liu, J.; Zhang, N.M.; Yuan, Q.H. Study on the effects of different passivators on the passivation lead-cadmium co-contaminated soil and influencing factors. Ecol. Environ. Sci. 2021, 30, 732–741. [Google Scholar]
- Zheng, H.; Wang, H.F. Research Progress in Soil Moisture Measurement Technologies. Metrol. Sci. Technol. 2022, 66, 31–36+40. [Google Scholar]
- Zhang, J.; Zhao, R.; Li, X.; Zhang, J. Potential of arbuscular mycorrhizal fungi for soil health. Pedosphere 2024, 34, 279–288. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Abid, M.; Mehmood, K.; Aziz, A.; Sarwar, A.; Akhtar, N.; Mehran, M. Ionic Homeostasis and Growth Characteristics of Solanum lycopersicum (Solanum lycopersicum L.) Grown with Municipal Wastewater by Supplying Silicon, Farmyard Manure and Plant Growth Promoting Rhizobacteria. Silicon 2022, 14, 12855–12867. [Google Scholar] [CrossRef]
- Zhu, H.; He, Y.Y. Effects of different Vitekang Soil Conditioners on soil properties, yield, and fruit quality of continuously Solanum lycopersicum. China Veg. 2023, 36, 104–108. [Google Scholar]
- Qu, M.; Qin, L.N.; Liu, Y.J.; Fan, H.C.; Zhu, S.; Wang, J.F. Comparison of two methods for detecting SOD enzyme activity. J. Food Saf. Qual. Test. 2014, 5, 3318–3323. [Google Scholar]
- Yan, Q.; Li, X.; Xiao, X.; Chen, J.; Liu, J.; Lin, C.; Guan, R.; Wang, D. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Cinnamomum migao by enhancing physio-biochemical responses. Ecol. Evol. 2022, 12, e9091. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, F.; Li, B.; Guo, C.; Hu, T.; Zhang, M.; Liang, Y.; Zhu, J.; Zhan, X. A bHLH transcription factor, SlbHLH96, promotes drought tolerance in tomato. Hortic. Res. 2022, 9, uhac198. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.J.; Yao, D.H.; Wei, Z.Q.; Wu, J.F. A study on the effect of chemical fertilizer combined with Vitekang Soil Conditioner on acidified red paddy soil. Acta Agric. Univ. Jiangxiensis 2020, 42, 1277–1284. [Google Scholar]
- Liang, J.; Wang, Z.; Ren, Y.; Jiang, Z.; Chen, H.; Hu, W.; Tang, M. The alleviation mechanisms of cadmium toxicity in Broussonetia papyrifera by arbuscular mycorrhizal symbiosis varied with different levels of cadmium stress. J. Hazard. Mater. 2023, 459, 132076. [Google Scholar] [CrossRef]
- Hou, S.S.; Pu, Z.T.; Zhang, C. Advances in research on alleviation of soil excessive trace elements and metals toxicity to plants by arbuscular mycorrhizal fungi. Soil Bull. 2023, 54, 39–749. [Google Scholar]
- Adedayo, A.A.; Babalola, O.O.; Prigent-Combaret, C.; Cruz, C.; Stefan, M.; Kutu, F.; Glick, B.R. The application of plant growth-promoting rhizobacteria in Solanum lycopersicum production in the agricultural system: A review. PeerJ 2022, 10, e13405. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, J.; Wang, S.; Lin, Q.; Ruan, D.; Chi, H.; Zheng, M.; Chao, Y.; Qiu, R.; Yang, Y. Effects of cadmium-resistant plant growth-promoting rhizobacteria and Funneliformis mosseae on the cadmium tolerance of Solanum lycopersicum (Lycopersicon esculentum L.). Int. J. Phytoremediat. 2020, 22, 451–458. [Google Scholar] [CrossRef] [PubMed]
- de Leon, V.; Orr, K.; Stelinski, L.L.; Mandadi, K.; Ibanez-Carrasco, F. Inoculation of Solanum lycopersicum with Plant Growth Promoting Rhizobacteria Affects the Solanum lycopersicum-Potato Psyllid-Candidatus Liberibacter Solanacearum Interactions. J. Econ. Entomol. 2023, 116, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Guo, W.L.; Chen, B.H.; Niu, L.; Ji, S. Effects of biological fertilizer and Vitekang Soil Conditioner on growth, fruit setting, and diseases of spring Solanum lycopersicum in plastic greenhouses. Hubei Agric. Sci. 2018, 57, 37–45. [Google Scholar]



| Treatment | Experimental Scheme | |||
|---|---|---|---|---|
| Micro Tom | A-CK (0 g/kg) | A-T1 (1.2 g/kg) | A-T2 (2.4 g/kg) | A-T3 (4.8 g/kg) |
| Red Guanyin | B-CK (0 g/kg) | B-T1 (1.2 g/kg) | B-T2 (2.4 g/kg) | B-T3 (4.8 g/kg) |
| Taiwan Pink King | C-CK (0 g/kg) | C-T1 (1.2 g/kg) | C-T2 (2.4 g/kg) | C-T3 (4.8 g/kg) |
| Variety | Micro Tom | Red Guanyin | Taiwan Pink King | |||
|---|---|---|---|---|---|---|
| Treatment | Cd in the Roots (mg/kg) | Cd in the Leaves (mg/kg) | Cd in the Roots (mg/kg) | Cd in the Leaves (mg/kg) | Cd in the Roots (mg/kg) | Cd in the Leaves (mg/kg) |
| CK | 0.0731 ± 0.0053 c | 0.0443 ± 0.0032 c | 0.0392 ± 0.0025 c | 0.0346 ± 0.0021 c | 0.0375 ± 0.0022 c | 0.0321 ± 0.0024 c |
| T1 | 0.0686 ± 0.0047 b | 0.0416 ± 0.0028 b | 0.0352 ± 0.0017 b | 0.0292 ± 0.0018 b | 0.0326 ± 0.0023 b | 0.0293 ± 0.0016 b |
| T2 | 0.0562 ± 0.0029 a | 0.0352 ± 0.0031 a | 0.0319 ± 0.0012 a | 0.0279 ± 0.0013 a | 0.0284 ± 0.0016 a | 0.0255 ± 0.0012 a |
| T3 | 0.0559 ± 0.0035 a | 0.0348 ± 0.0023 a | 0.0318 ± 0.0014 a | 0.0277 ± 0.0011 a | 0.0282 ± 0.0014 a | 0.0256 ± 0.0014 a |
| Treatment | Plant Height | Stem Diameter | SPAD | Above Ground -FW | Under Ground -FW | Above Ground -DW | Under Ground -DW |
|---|---|---|---|---|---|---|---|
| A-CK | 5.28 ± 0.117 b | 1.978 ± 0.053 c | 35.65 ± 0.708 c | 2.305 ± 0.081 c | 0.669 ± 0.041 b | 0.353 ± 0.013 c | 0.158 ± 0.005 b |
| A-T1 | 5.733 ± 0.063 a | 2.098 ± 0.039 bc | 37.05 ± 0.299 bc | 2.438 ± 0.036 bc | 0.658 ± 0.013 b | 0.398 ± 0.011 b | 0.16 ± 0.004 b |
| A-T2 | 5.75 ± 0.778 a | 2.398 ± 0.056 a | 39.95 ± 0.601 a | 3.358 ± 0.051 a | 0.773 ± 0.030 a | 0.485 ± 0.010 a | 0.18 ± 0.004 a |
| A-T3 | 5.243 ± 0.028 b | 2.17 ± 0.051 b | 38.55 ± 0.348 ab | 2.573 ± 0.109 b | 0.673 ± 0.023 b | 0.393 ± 0.005 b | 0.163 ± 0.003 b |
| B-CK | 8.495 ± 0.181 c | 1.69 ± 0.022 c | 33.828 ± 0.624 b | 1.899 ± 0.025 b | 0.236 ± 0.009 b | 0.313 ± 0.005 b | 0.053 ± 0.005 b |
| B-T1 | 9.573 ± 0.204 b | 1.968 ± 0.021 b | 32.86 ± 1.115 b | 1.95 ± 0.172 ab | 0.249 ± 0.051 b | 0.323 ± 0.032 b | 0.045 ± 0.006 b |
| B-T2 | 12.113 ± 0.194 a | 2.073 ± 0.037 a | 37.135 ± 0.780 a | 3.287 ± 0.899 a | 0.445 ± 0.049 a | 0.485 ± 0.051 a | 0.088 ± 0.006 a |
| B-T3 | 11.755 ± 0.321 a | 2.053 ± 0.037 ab | 37.515 ± 0.602 a | 2.555 ± 0.343 ab | 0.443 ± 0.058 a | 0.438 ± 0.053 ab | 0.083 ± 0.005 a |
| C-CK | 9.925 ± 0.890 a | 1.593 ± 0.019 c | 32.325 ± 1.222 b | 1.658 ± 0.029 b | 0.278 ± 0.019 b | 0.263 ± 0.020 b | 0.056 ± 0.005 a |
| C-T1 | 10.52 ± 0.944 a | 1.68 ± 0.0147 b | 34.775 ± 0.969 ab | 1.875 ± 0.057 b | 0.338 ± 0.009 a | 0.315 ± 0.006 ab | 0.068 ± 0.005 a |
| C-T2 | 11.253 ± 1.148 a | 1.758 ± 0.035 a | 36.235 ± 1.139 a | 2.198 ± 0.080 a | 0.353 ± 0.009 a | 0.335 ± 0.013 a | 0.061 ± 0.004 a |
| C-T3 | 10.585 ± 1.237 a | 1.7 ± 0.004 ab | 35.36 ± 1.261 ab | 2.333 ± 0.127 a | 0.36 ± 0.008 a | 0.308 ± 0.029 ab | 0.068 ± 0.009 a |
| Treatment | Plant Height | Stem Diameter | SPAD | AG-FW | UG-FW | AG-DW | UG-DW | |
|---|---|---|---|---|---|---|---|---|
| Micro Tom | -Cd | 15.633 ± 1.501 a | 8.030 ± 0.227 a | 46.400 ± 1.966 b | 10.890 ± 0.032 a | 1.223 ± 0.009 b | 1.795 ± 0.009 a | 0.249 ± 0.002 b |
| CK | 9.033 ± 0.273 d | 4.940 ± 0.290 d | 40.000 ± 0.961 d | 5.710 ± 0.015 d | 0.750 ± 0.006 e | 0.893 ± 0.022 d | 0.194 ± 0.003 e | |
| Cd+VT | 11.567 ± 0.176 c | 6.043 ± 0.249 c | 48.867 ± 0.961 ab | 7.503 ± 0.242 c | 1.220 ± 0.006 d | 1.127 ± 0.060 c | 0.293 ± 0.005 d | |
| Cd+AMF | 13.300 ± 0.625 b | 6.270 ± 0.202 b | 47.800 ± 0.961 b | 7.877 ± 0.018 b | 1.417 ± 0.015 c | 1.187 ± 0.019 bc | 0.344 ± 0.004 c | |
| Cd+VT+AMF | 16.100 ± 0.896 a | 7.117 ± 0.163 a | 52.300 ± 0.961 a | 10.280 ± 0.081 a | 1.673 ± 0.009 a | 1.525 ± 0.013 a | 0.420 ± 0.004 a | |
| Red Guanyin | -Cd | 55.880 ± 2.517 a | 7.967 ± 0.342 a | 40.567 ± 0.961 d | 8.323 ± 0.109 b | 1.740 ± 0.046 b | 1.277 ± 0.016 b | 0.422 ± 0.010 b |
| CK | 38.033 ± 1.50 d | 4.030 ± 0.227 c | 38.400 ± 1.966 b | 10.890 ± 0.0322 c | 1.223 ± 0.009 e | 1.795 ± 0.009 d | 0.249 ± 0.002 e | |
| Cd+VT | 48.367 ± 2.673 d | 5.593 ± 0.259 b | 49.233 ± 1.966 a | 13.330 ± 0.071 c | 1.340 ± 0.015 d | 2.024 ± 0.004 c | 0.273 ± 0.004 | |
| Cd+AMF | 48.733 ± 0.681 d | 5.553 ± 0.188 b | 50.600 ± 1.967 a | 13.687 ± 0.175 c | 1.603 ± 0.012 c | 1.930 ± 0.056 cd | 0.320 ± 0.003 c | |
| Cd+VT+AMF | 53.400 ± 2.498 b | 6.250 ± 0.320 ab | 53.467 ± 1.157 a | 17.930 ± 0.829 b | 1.807 ± 0.028 b | 2.738 ± 0.192 b | 0.361 ± 0.006 b | |
| Taiwan Pink King | -Cd | 37.267 ± 0.410 a | 6.637 ± 0.122 a | 41.000 ± 1.097 b | 23.333 ± 0.622 a | 2.950 ± 0.046 a | 3.454 ± 0.143 a | 0.578 ± 0.005 a |
| CK | 30.633 ± 2.413 c | 3.057 ± 0.310 c | 34.733 ± 3.037 c | 11.860 ± 0.0589 e | 1.253 ± 0.017 c | 1.857 ± 0.004 e | 0.252 ± 0.003 c | |
| Cd+VT | 32.433 ± 1.593 b | 4.107 ± 0.363 b | 45.200 ± 1.332 ab | 13.460 ± 0.068 d | 1.370 ± 0.032 b | 2.091 ± 0.011 d | 0.276 ± 0.006 b | |
| Cd+AMF | 34.033 ± 0.884 ab | 4.240 ± 0.387 ab | 44.700 ± 1.572 ab | 15.500 ± 0.281 c | 1.693 ± 0.009 a | 2.419 ± 0.040 c | 0.341 ± 0.002 a | |
| Cd+VT+AMF | 38.400 ± 0.520 a | 4.490 ± 0.215 ab | 47.600 ± 2.050 a | 18.277 ± 0.105 a | 1.753 ± 0.009 a | 2.866 ± 0.016 a | 0.353 ± 0.002 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, Y.; Chen, G.; Liu, X.; Tanveer, M.; Guo, Y.; Zeng, P.; Huang, L. The Role of Vitekang Soil Conditioner and Arbuscular Mycorrhizae Fungi in Mitigating Cadmium Stress in Solanum lycopersicum Plants. Horticulturae 2025, 11, 179. https://doi.org/10.3390/horticulturae11020179
Wang Q, Liu Y, Chen G, Liu X, Tanveer M, Guo Y, Zeng P, Huang L. The Role of Vitekang Soil Conditioner and Arbuscular Mycorrhizae Fungi in Mitigating Cadmium Stress in Solanum lycopersicum Plants. Horticulturae. 2025; 11(2):179. https://doi.org/10.3390/horticulturae11020179
Chicago/Turabian StyleWang, Qianqian, Yue Liu, Guangxin Chen, Xing Liu, Mohsin Tanveer, Yongjun Guo, Peng Zeng, and Liping Huang. 2025. "The Role of Vitekang Soil Conditioner and Arbuscular Mycorrhizae Fungi in Mitigating Cadmium Stress in Solanum lycopersicum Plants" Horticulturae 11, no. 2: 179. https://doi.org/10.3390/horticulturae11020179
APA StyleWang, Q., Liu, Y., Chen, G., Liu, X., Tanveer, M., Guo, Y., Zeng, P., & Huang, L. (2025). The Role of Vitekang Soil Conditioner and Arbuscular Mycorrhizae Fungi in Mitigating Cadmium Stress in Solanum lycopersicum Plants. Horticulturae, 11(2), 179. https://doi.org/10.3390/horticulturae11020179







