Boesenbergia Kuntze (Zingiberaceae) in Cambodia: Four New Records with Notes on Their Potential Horticultural Significance, Cultivation Guidelines, and Lectotypification of B. xiphostachya (Gagnep.) Loes.
Abstract
1. Introduction
2. Materials and Methods
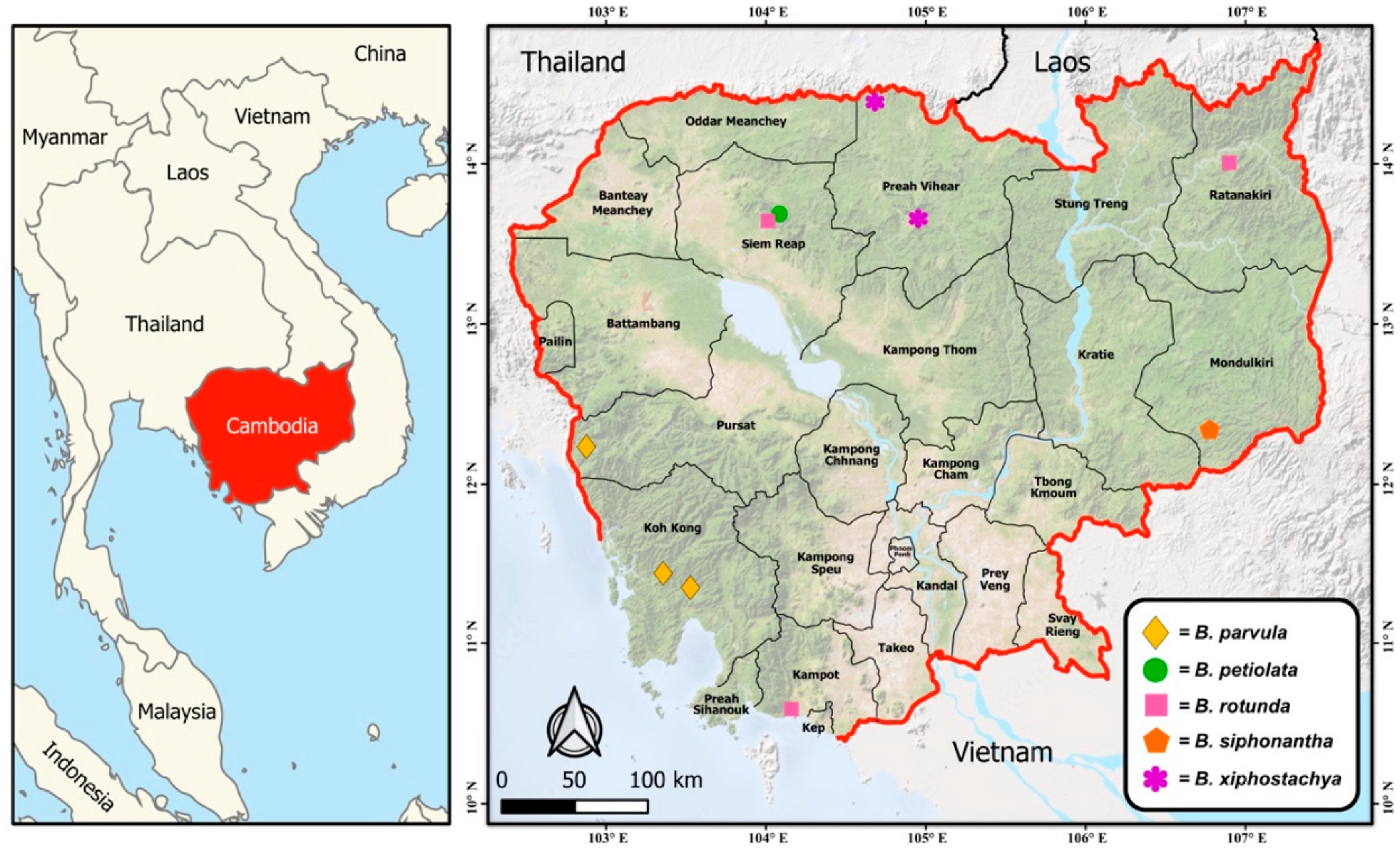
2.1. Plant Materials and Study Area
2.2. Procedures
3. Results
3.1. Taxonomic Treatment
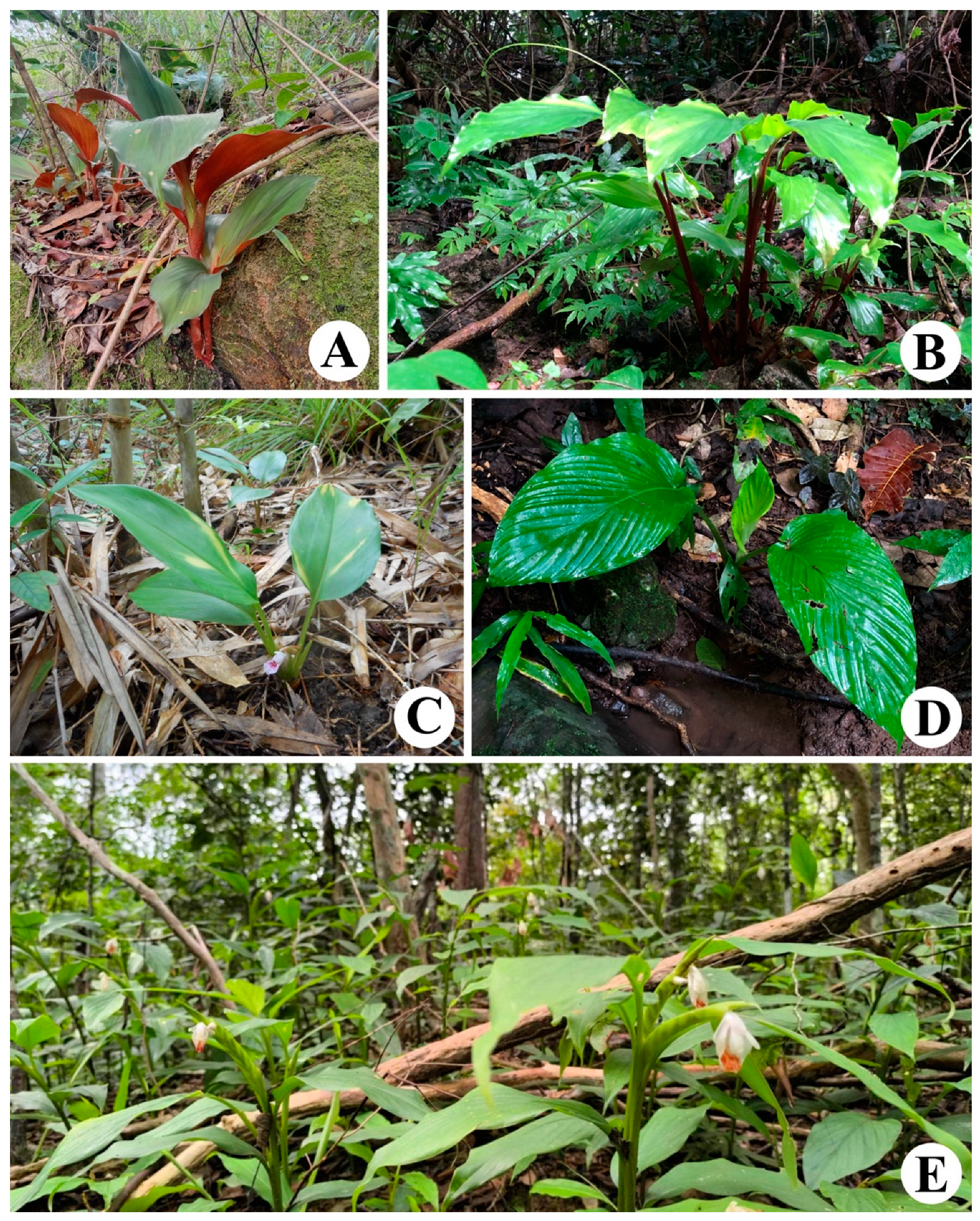
3.1.1. Boesenbergia parvula (Wall. ex Baker) Kuntze var. parvula [2,20]
3.1.2. Boesenbergia petiolata Sirirugsa [11]
3.1.3. Boesenbergia rotunda (L.) Mansf. [2]
3.1.4. Boesenbergia siphonantha (King ex Baker) M.Sabu, Prasanthk., & Škorničk [14]
3.1.5. Boesenbergia xiphostachya (Gagnep.) Loes. [2,12]
3.2. Proposal for Conservation Status of Five Boesenbergia Species in Cambodia
3.3. Potential Horticultural Significance
3.3.1. Ornamental and Aesthetic Value
3.3.2. Medicinal and Culinary Significance
3.3.3. Functional Roles in Landscaping and Gardening
3.3.4. Low Maintenance and Adaptability
3.3.5. Economic Opportunities in Cultivation
3.3.6. Planting Between Rows in Fruit Orchards
3.3.7. Future Prospects for Research and Development
3.4. Cultivation Guidelines for Boesenbergia Species
3.4.1. Site Preferences and Growth Conditions
3.4.2. Effective Propagation Methods
3.4.3. Watering and Nutrient Management
3.4.4. Maintenance and Pest Control
3.4.5. Applications in Landscaping and Agriculture
3.4.6. Potential for Cultivar Development
3.4.7. Rhizome Storage and Dormancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Amer. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Plant of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: www.plantsoftheworldonline.org/ (accessed on 30 November 2024).
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N.; et al. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef] [PubMed]
- Boonma, T.; Saensouk, S.; Saensouk, P. Diversity and traditional utilization of the Zingiberaceae plants in Nakhon Nayok Province, Central Thailand. Diversity 2023, 15, 904. [Google Scholar] [CrossRef]
- Boonma, T.; Saensouk, S.; Saensouk, P. Biogeography, conservation Status, and traditional uses of Zingiberaceae in Saraburi Province, Thailand, with Kaempferia chaveerachiae sp. nov. Horticulturae 2024, 10, 934. [Google Scholar] [CrossRef]
- Picheansoonthon, C.; Chaiyoot, A.; Sukrong, S. Jirawongsea, a new genus of the family Zingiberaceae. Folia Malays. 2008, 9, 1–16. [Google Scholar]
- Mood, J.D.; Veldkamp, J.F.; Dey, S.; Prince, L.M. Nomenclatural changes in Zingiberaceae: Caulokaempferia is a superfluous name for Monolophus and Jirawongsea is reduced to Boesenbergia. Bull. Sing. 2014, 66, 215–231. [Google Scholar]
- Mood, J.D.; Ardiyani, M.; Veldkamp, J.F.; Mandáková, T.; Prince, L.M.; de Boer, H.J. Nomenclatural changes in Zingiberaceae: Haplochorema is reduced to Boesenbergia. Bull. Sing. 2020, 72, 77–95. [Google Scholar] [CrossRef]
- Holttum, R.E. Boesenbergia. The Zingiberaceae of the Malay Peninsula. Bull. Sing. 1950, 13, 106–117. [Google Scholar]
- Tong, S.Q. A new species of Boesenbergia from Yunnan. Acta Phytotax Sin. 1986, 24, 323. [Google Scholar]
- Sirirugsa, P. Three new species and one new combination in Boesenbergia (Zingiberaceae) from Thailand. Nord. J. Bot. 1987, 7, 421–425. [Google Scholar] [CrossRef]
- Sirirugsa, P. A revision of the genus Boesenbergia Kuntze (Zingiberaceae) in Thailand. Nat. Hist. Bull. Siam Soc. 1992, 40, 67–90. [Google Scholar]
- Saensouk, S.; Larsen, K. Boesenbergia baimaii, a new species of Zingiberaceae from Thailand. Nordic J. Bot. 2002, 21, 595–597. [Google Scholar] [CrossRef]
- Sabu, M.; Prasanthkumar, M.G.; Škorničková, J.; Jayasree, S. Transfer of Kaempferia siphonantha Baker to Boesenbergia Kuntze (Zingiberaceae). Rheedea 2004, 14, 55–59. [Google Scholar]
- Larsen, K.; Larsen, S.S. Ginger of Thailand. Queen Sirikit Botanical Garden: Chiang Mai, Thailand, 2006. [Google Scholar]
- Mood, J.D.; Prince, L.M.; Veldkamp, J.F.; Dey, S. The history and identity of Boesenbergia longiflora (Zingiberaceae) and descriptions of five related new taxa. Bull. Sing. 2013, 65, 47–95. [Google Scholar]
- Veldkamp, J.F. Nomenclatural notes on Boesenbergia Kuntze (Zingiberaceae). Philippine J. Sci. 2013, 142, 215–221. [Google Scholar]
- Mood, J.D.; Tanaka, N.; Aung, M.M.; Murata, J. The genus Boesenbergia (Zingiberaceae) in Myanmar with two new records. Bull. Sing. 2016, 68, 299–318. [Google Scholar] [CrossRef]
- Mood, J.D.; Trần, H.Đ.; Prince, L.M.; Veldkamp, J.F. Boesenbergia siphonantha (Zingiberaceae)—A new record for Thailand and Vietnam. Bull. Sing. 2016, 68, 125–137. [Google Scholar]
- Mood, J.D.; Trần, H.Đ.; Veldkamp, J.F.; Prince, L.M. Taxonomy of Boesenbergia parvula (Zingiberaceae) with new synonymy. Thai For. Bull. Bot. 2018, 46, 10–24. [Google Scholar] [CrossRef]
- Aishwarya, K.; Sabu, M. Boesenbergia pulcherrima and B. tiliifolia (Zingiberaceae) in India: Notes on the identity, variability and typification. Rheedea 2015, 25, 59–68. [Google Scholar]
- Aishwarya, K.; Sabu, M. On the IUCN status of Boesenbergia albolutea and B. rubrolutea (Zingiberaceae) and typification of B. rubrolutea. J. Threatened Taxa 2021, 13, 20133–20135. [Google Scholar] [CrossRef]
- Aishwarya, K.; Prabhu Kumar, K.M.; Sabu, M. Boesenbergia kingii (Zingiberaceae): A new record for South India. Webbia 2015, 70, 319–322. [Google Scholar] [CrossRef]
- Aishwarya, K.; Vinitha, M.R.; Thomas, G.; Sabu, M. A new species of Boesenbergia and rediscovery of B. rotunda (Zingiberaceae) from India. Phytotaxa 2015, 197, 186–196. [Google Scholar] [CrossRef]
- Lý, N.S. Boesenbergia quangngaiensis (Zingiberaceae), a new species from Central Vietnam. Phytotaxa 2017, 324, 83–88. [Google Scholar] [CrossRef]
- Tanaka, N.; Tagane, S.; Naiki, A.; Aung, M.M.; Tanaka, N.; Dey, S.; Mood, J.; Murata, J. Contributions to the Flora of Myanmar I: Nine taxa of monocots newly recorded from Myanmar. Bull. Natl. Mus. Nat. Sci. Ser. B 2018, 44, 31–39. [Google Scholar]
- Chen, J.; Xia, N.H. A taxonomic revision of Chinese Boesenbergia (Zingiberaceae), with a new record. Phytotaxa 2019, 424, 217–231. [Google Scholar] [CrossRef]
- Saensouk, S.; Saensouk, P. Boesenbergia isanensis (Zingiberaceae), a new species from Thailand. J. Jpn. Bot. 2020, 95, 65–68. [Google Scholar]
- Lam, N.F.; Ibrahim, H.; Sam, Y.Y.; Zakaria, R.M.; Poulsen, A.D. Two new species of Boesenbergia (Zingiberaceae) from Sabah, Malaysia. Phytokeys 2022, 211, 81–92. [Google Scholar] [CrossRef]
- Lam, N.F.; Ibrahim, H.; Sam, Y.Y.; Zakaria, R.M.; Poulsen, A.D. Three new species of Boesenbergia (Zingiberaceae) from Sabah, Malaysia. Phytokeys 2024, 247, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Yang, H.J.; Ye, Y.S.; Kang, Y.; Zhang, W.; Jiang, X.L.; Pan, Y.X.; Zheng, X.L. Boesenbergia quangngaiensis N.S.Lý, a newly recorded species of Zingiberaceae from China. J. Trop. Subtrop. Bot. 2020, 28, 241–244. [Google Scholar] [CrossRef]
- Saravanan, T.S.; Kaliamoorthy, S. Boesenbergia kalakadensis (Zingiberaceae), a new species from southern Western Ghats, India. Nord. J. Bot. 2024, 2024, e04445. [Google Scholar]
- Leong-Škorničková, J.; Newman, M.F. Gingers of Cambodia, Laos and Vietnam, 1st ed.; Oxford Graphic Printers Pte Ltd: Singapore, 2015; pp. 170–175. [Google Scholar]
- Chaveerach, A.; Mokkamul, P.; Sudmoon, R.; Tanee, T. A new species of Alpinia Roxb. (Zingiberaceae) from Northeastern Thailand. Taiwania 2008, 53, 1–5. [Google Scholar]
- Chaveerach, A.; Mokkamul, P.; Sudmoon, R.; Tanee, T.; Garcia, V.F. A new species of Stahlianthus (Zingiberaceae) from Northeastern Thailand. Taiwania 2007, 52, 315–319. [Google Scholar]
- Bongcheewin, B.; Darbyshire, I.; Satitpatipan, V.; Kongsawadworakul, P. Taxonomic revision of Clinacanthus (Acanthaceae) in Thailand. Phytotaxa 2019, 391, 253–263. [Google Scholar] [CrossRef]
- Bongcheewin, B.; Pramali, K.; Traiperm, P.; Chantaranothai, P.; Paton, A. Pogostemon nudus sp. nov. (Lamiaceae) from Thailand. Nord. J. Bot. 2017, 35, 289–299. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 24 October 2024).
- Jing, L.J.; Mohamed, M.; Rahmat, A.; Bakar, M.F.A. Phytochemicals, antioxidant properties and anticancer investigations of the different parts of several gingers species (Boesenbergia rotunda, Boesenbergia pulchella var. attenuata and Boesenbergia armeniaca). J. Med. Plants Res. 2010, 4, 27–32. [Google Scholar]
- Mohan, S.; Hobani, Y.H.; Shaheen, E.; Abou-Elhamd, A.S.; Abdelhaleem, A.; Alhazmi, H.A.; Abdelwahab, S.I. Ameliorative effect of Boesenbergin A, a chalcone isolated from Boesenbergia rotunda (Fingerroot) on oxidative stress and inflammation in ethanol-induced gastric ulcer in vivo. J. Ethnopharmacol. 2020, 261, 113104. [Google Scholar] [CrossRef]
- Janni, M.; Maestri, E.; Gulli, M.; Marmiroli, M.; Marmiroli, N. Plant responses to climate change, how global warming may impact on food security: A critical review. Plant Sci. 2024, 14, 1297569. [Google Scholar] [CrossRef] [PubMed]


| 1a. | Staminodes pinkish; androecial cup absent | B. rotunda |
| 1b. | Staminodes white to pale yellow; androecial cup present | 2 |
| 2a. | Leaves base cordate | 3 |
| 2b. | Leaves base attenuate | 4 |
| 3a. | Floral tube 2–3 cm long; only terminal inflorescence | B. petiolata |
| 3b. | Floral tube 7–9 cm long; both terminal and radical inflorescence | B. siphonantha |
| 4a. | Spike fusiform, 4–5 cm long | B. parvula |
| 4b. | Spike gladiate, 12–17 cm long | B. xiphostachya |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saensouk, P.; Saensouk, S.; Boonma, T.; Song, D.; Maknoi, C.; Setyawan, A.D. Boesenbergia Kuntze (Zingiberaceae) in Cambodia: Four New Records with Notes on Their Potential Horticultural Significance, Cultivation Guidelines, and Lectotypification of B. xiphostachya (Gagnep.) Loes. Horticulturae 2025, 11, 178. https://doi.org/10.3390/horticulturae11020178
Saensouk P, Saensouk S, Boonma T, Song D, Maknoi C, Setyawan AD. Boesenbergia Kuntze (Zingiberaceae) in Cambodia: Four New Records with Notes on Their Potential Horticultural Significance, Cultivation Guidelines, and Lectotypification of B. xiphostachya (Gagnep.) Loes. Horticulturae. 2025; 11(2):178. https://doi.org/10.3390/horticulturae11020178
Chicago/Turabian StyleSaensouk, Piyaporn, Surapon Saensouk, Thawatphong Boonma, Det Song, Charun Maknoi, and Ahmad Dwi Setyawan. 2025. "Boesenbergia Kuntze (Zingiberaceae) in Cambodia: Four New Records with Notes on Their Potential Horticultural Significance, Cultivation Guidelines, and Lectotypification of B. xiphostachya (Gagnep.) Loes." Horticulturae 11, no. 2: 178. https://doi.org/10.3390/horticulturae11020178
APA StyleSaensouk, P., Saensouk, S., Boonma, T., Song, D., Maknoi, C., & Setyawan, A. D. (2025). Boesenbergia Kuntze (Zingiberaceae) in Cambodia: Four New Records with Notes on Their Potential Horticultural Significance, Cultivation Guidelines, and Lectotypification of B. xiphostachya (Gagnep.) Loes. Horticulturae, 11(2), 178. https://doi.org/10.3390/horticulturae11020178








