Glutathione Reductase Affects Hyphal Growth and Fruiting Body Development by Regulating Intracellular ROS Levels in Hypsizygus marmoreus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Culture Conditions
2.2. Gene Cloning and RNAi Transformants
2.3. Mycelial Growth Rate and ROS Measurement
2.4. Observations of Mitochondrial Morphology and Measurement of Mitochondrial Complex Activity Levels
2.5. Determination of Antioxidant Enzyme Activity Levels
2.6. Analysis of Redox-Active Compounds
2.7. Determination of the Activity Level of Lignocelluloses
2.8. Gene Expression Analysis by qRT-PCR
2.9. Statistical Analysis
3. Results
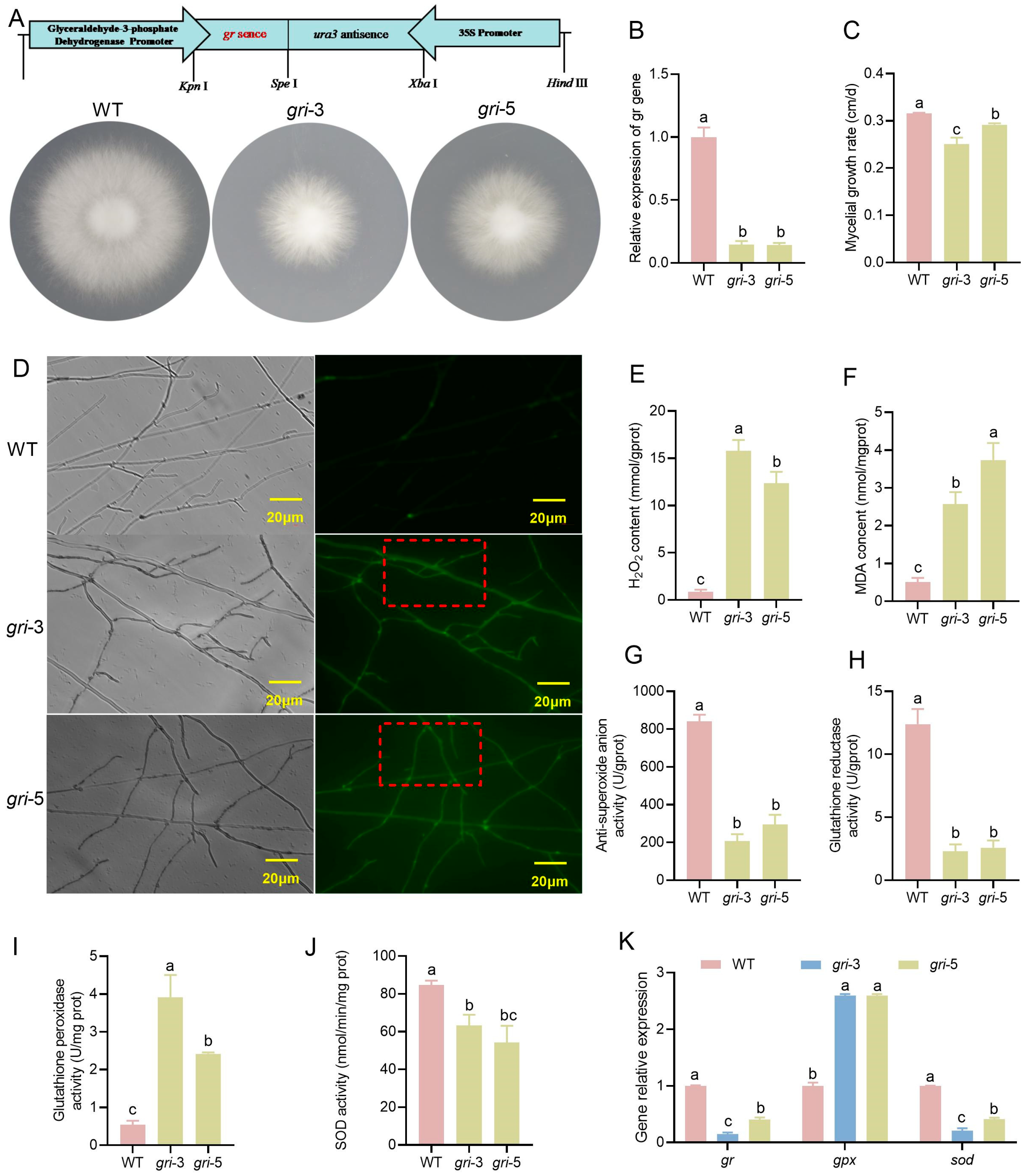
3.1. The gr Gene Impaired Mycelial Growth and Induced Oxidative Stress in H. marmoreus
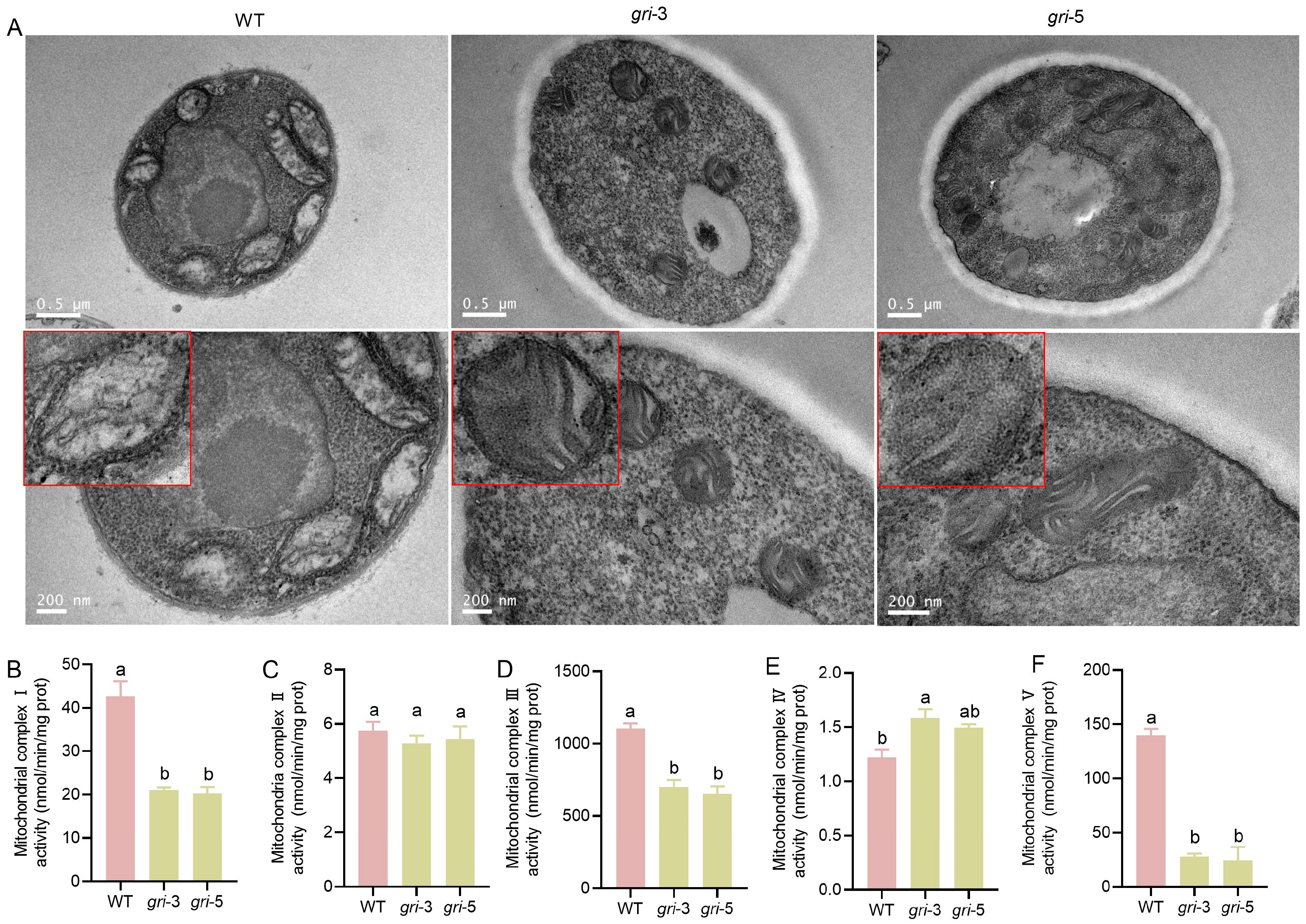
3.2. The gr Gene Disrupts Mitochondrial Structure and Suppresses the Activity of Key Respiratory Complexes in H. marmoreus
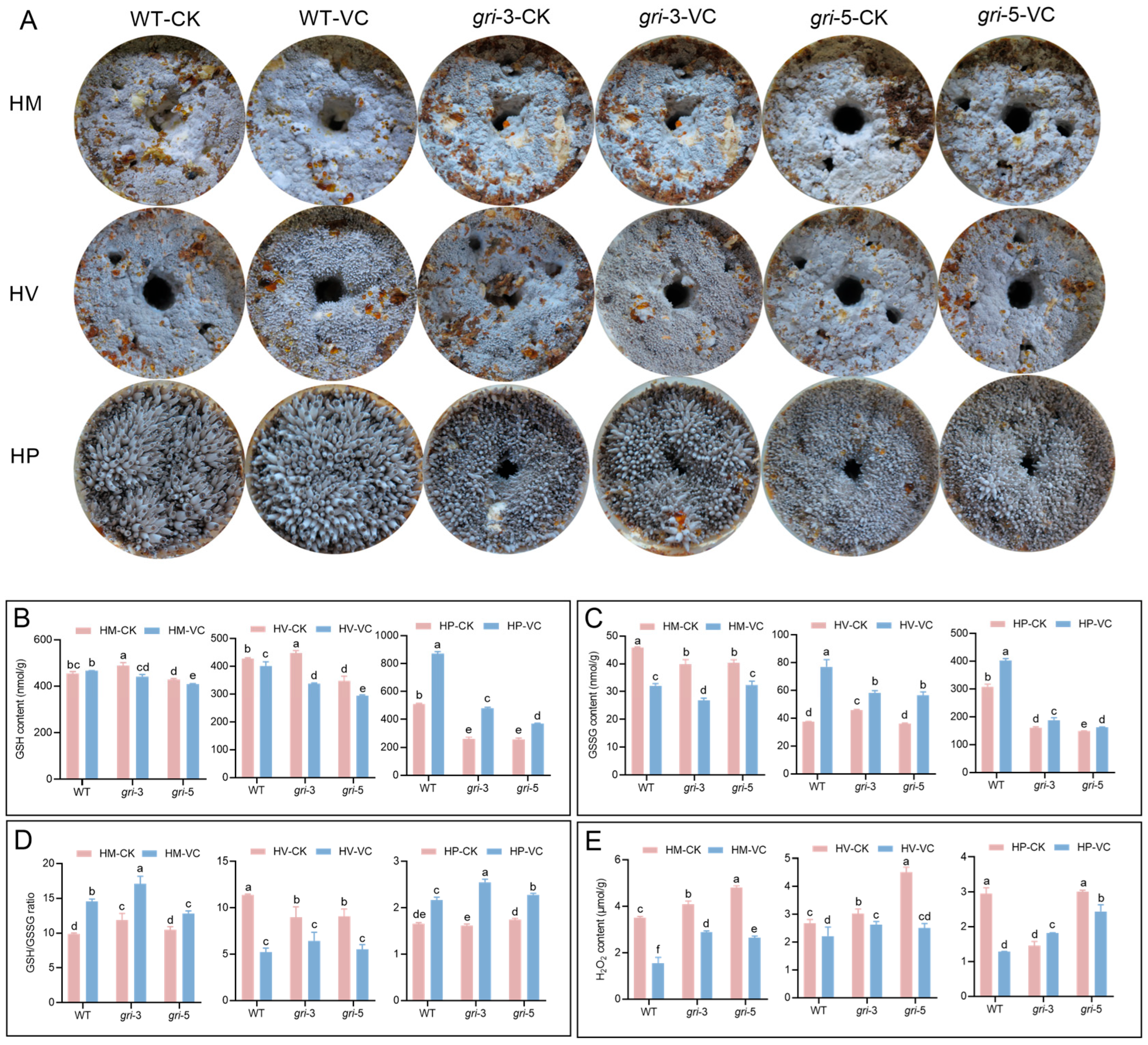
3.3. The gr Gene Regulates Fruiting Body Development and Mediates Redox Substance Accumulation in H. marmoreus
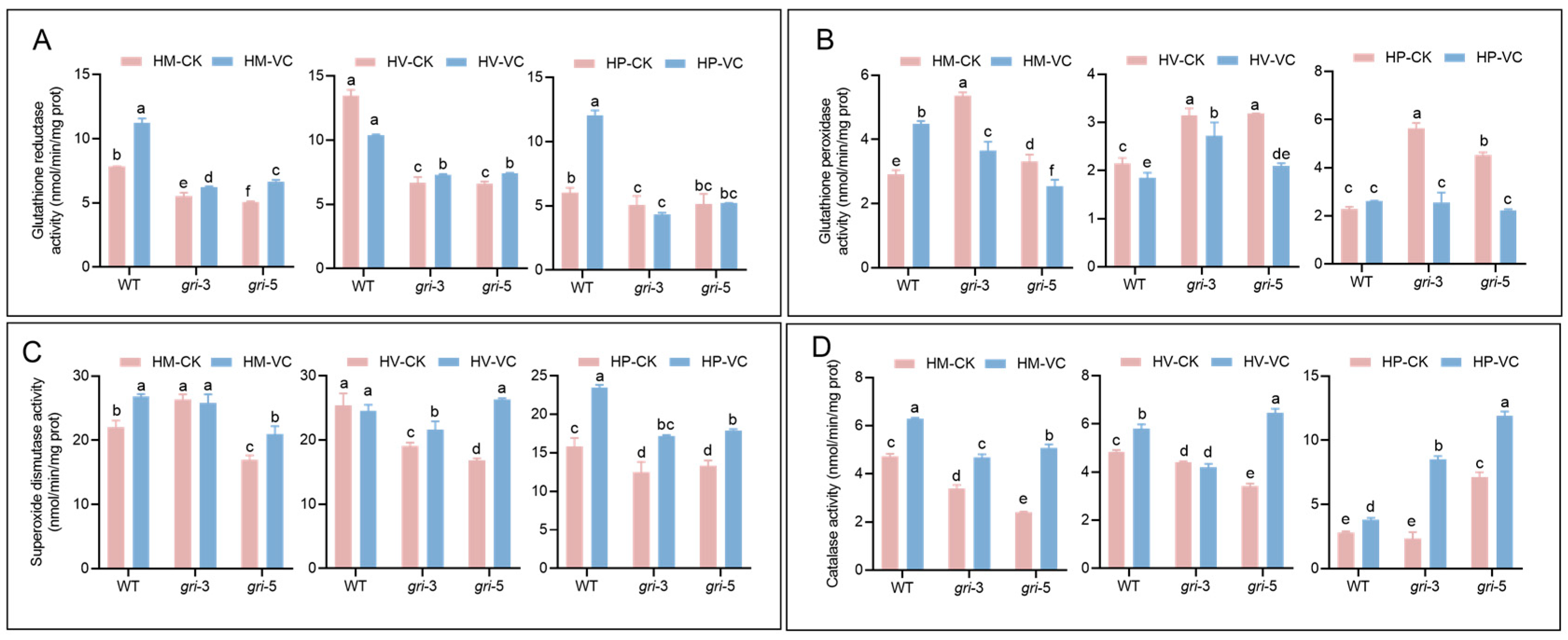
3.4. The gr Gene Influences Antioxidant Enzyme Activity During the Development of H. marmoreus
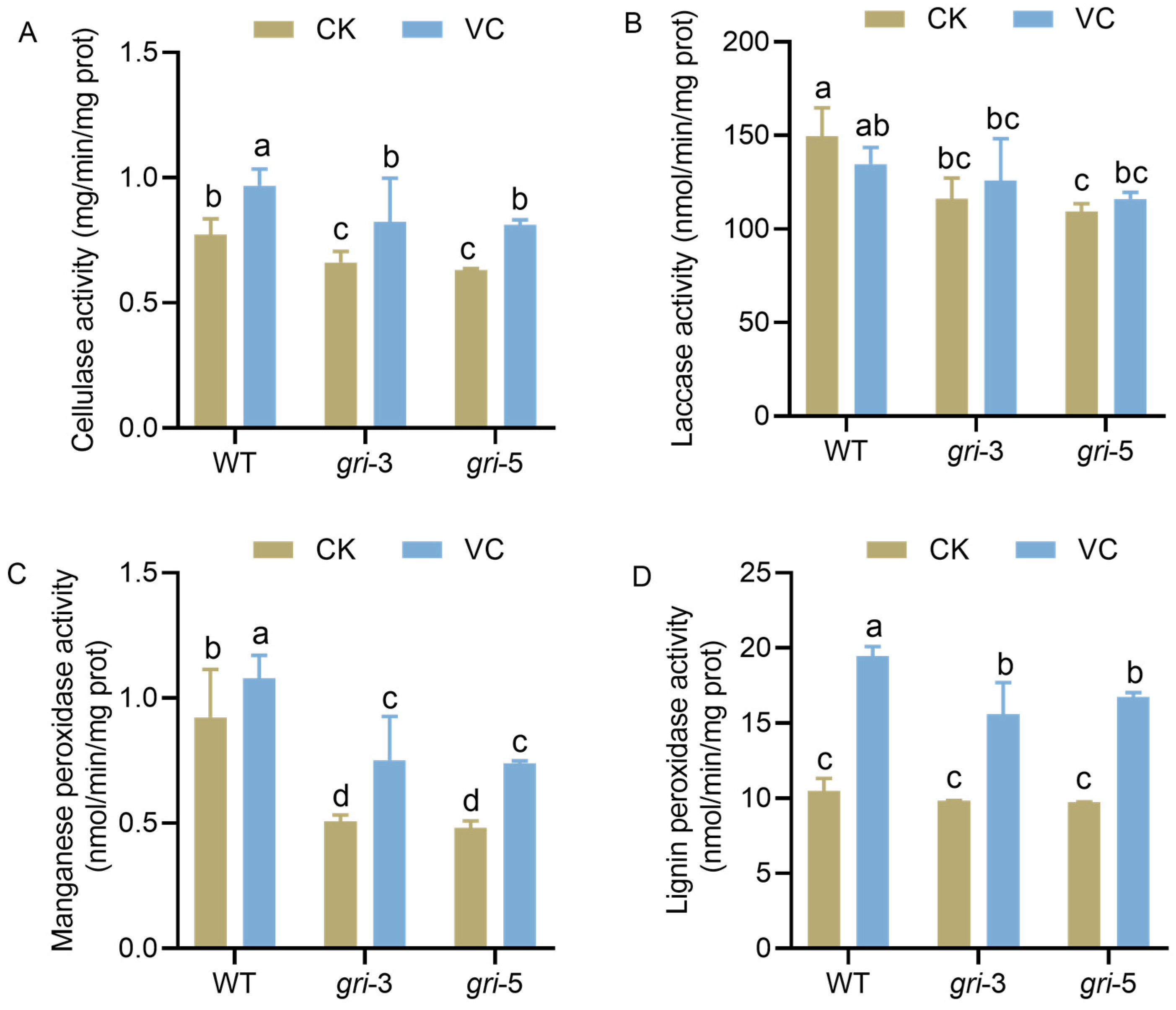
3.5. Vc Supplementation Restores Lignocellulose-Degrading Enzyme Activity in H. marmoreus gri Strains
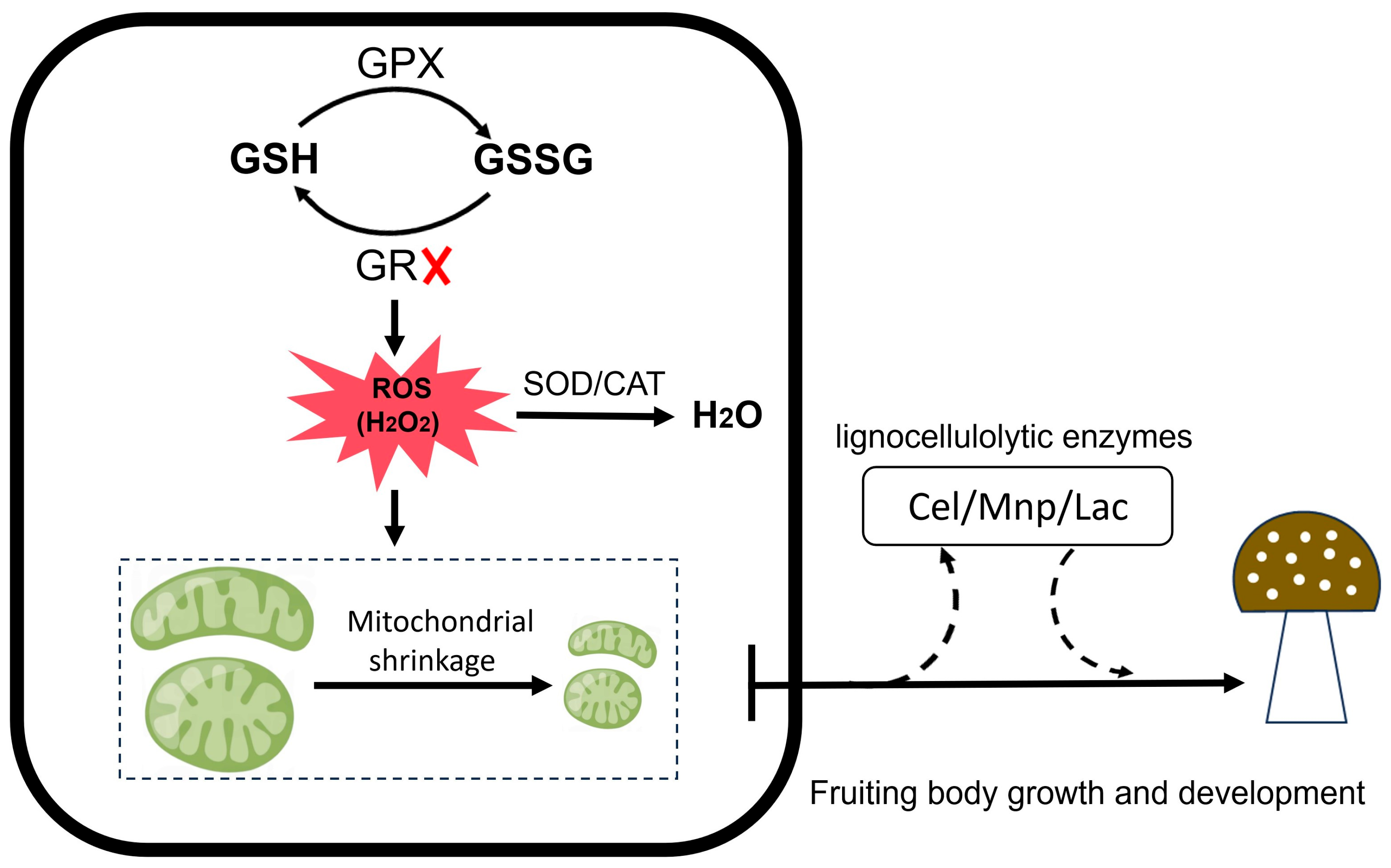
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Son, S.Y.; Park, Y.J.; Jung, E.S.; Singh, D.; Lee, Y.W.; Kim, J.-G.; Lee, C.H. Integrated metabolomics and transcriptomics unravel the metabolic pathway variations for different sized beech mushrooms. Int. J. Mol. Sci. 2019, 20, 6007. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, J.; Yan, J.; Zhang, C.; Wang, T.; Gan, B. Evaluation of the nutritional value, umami taste, and volatile organic compounds of Hypsizygus marmoreus by simulated salivary digestion in vitro. Curr. Res. Food Sci. 2023, 7, 100591. [Google Scholar] [CrossRef]
- Kala, K.; Lazur, J.; Karnafał, J.; Pająk, W.; Sulkowska-Ziaja, K.; Muszynska, B. Beech mushroom (Hypsizygus marmoreus, Agaricomycetes) cultivation and outstanding health-promoting properties: A Review. Int. J. Med. Mushrooms 2024, 26, 15–26. [Google Scholar] [CrossRef]
- Bhatia, J.N.; Yadav, A.N. A comprehensive review on multifunctional bioactive properties of elm oyster mushroom Hypsizygus ulmarius (Bull.) Redhead (Agaricomycetes): Current research, challenges and future trends. Heliyon 2025, 11, 2. [Google Scholar]
- Xu, L.L.; Guo, L.Z.; Yu, H. Label-Free Comparative proteomics analysis revealed heat stress responsive mechanism in Hypsizygus marmoreus. Front. Microbiol. 2021, 11, 541967. [Google Scholar] [CrossRef]
- Kała, K.; Pająk, W.; Sułkowska-Ziaja, K.; Krakowska, A.; Lazur, J.; Fidurski, M.; Marzec, K.; Zięba, P.; Fijałkowska, A.; Szewczyk, A.; et al. Hypsizygus marmoreus as a source of indole compounds and other bioactive substances with health-promoting activities. Molecules 2022, 27, 8917. [Google Scholar] [CrossRef]
- Xiang, Q.; Arshad, M.; Li, Y.; Zhang, H.; Gu, Y.; Yu, X.; Zhao, K.; Ma, M.; Zhang, L.; He, M.; et al. Transcriptomic profiling revealed important roles of amino acid metabolism in fruiting body formation at different ripening times in Hypsizygus marmoreus. Front. Microbiol. 2023, 14, 1169881. [Google Scholar] [CrossRef]
- Tudzynski, P.; Heller, J.; Siegmund, U. Reactive oxygen species generation in fungal development and pathogenesis. Curr. Opin. Microbiol. 2012, 15, 653–659. [Google Scholar] [CrossRef]
- Chen, H.; Hai, H.; Wang, H.; Wang, Q.; Chen, M.; Feng, Z.; Ye, M.; Zhang, J. Hydrogen-rich water mediates redox regulation of the antioxidant system, mycelial regeneration and fruiting body development in Hypsizygus marmoreus. Fungal Biol. 2018, 122, 310–321. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, H.; Wu, X.; Wang, Q.; Chen, M.; Feng, Z.; Chen, H. The functions of glutathione peroxidase in ROS homeostasis and fruiting body development in Hypsizygus marmoreus. Appl. Microbiol. Biotechnol. 2020, 104, 10555–10570. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, L.; Chen, D.; Ren, A.; Gao, T.; Zhao, M. Functional analysis of the role of glutathione peroxidase (GPx) in the ROS signaling pathway, hyphal branching and the regulation of ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2015, 82, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Cano-Dominguez, N.; Alvarez-Delfin, K.; Hansberg, W.; Aguirre, J. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot. Cell 2008, 7, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The key role of GSH in keeping the redox balance in mammalian cells: Mechanisms and significance of GSH in detoxification via formation of conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Tang, G.; Li, N.; Liu, Y.; Yu, L.; Yan, J.; Luo, L. Sinorhizobium meliloti glutathione reductase is required for both redox homeostasis and symbiosis. Appl. Environ. Microbiol. 2018, 84, e01937-17. [Google Scholar] [CrossRef]
- Sato, I.; Shimizu, M.; Hoshino, T.; Takaya, N. The glutathione system of Aspergillus nidulans involves a fungus-specific glutathione s-transferase. J. Biol. Chem. 2009, 284, 8042–8053. [Google Scholar] [CrossRef]
- Wangsanut, T.; Pongpom, M. The role of the glutathione system in stress adaptation, morphogenesis and virulence of pathogenic fungi. Int. J. Mol. Sci. 2022, 23, 10645. [Google Scholar] [CrossRef] [PubMed]
- Hamad, D.; El-Sayed, H.; Ahmed, W.; Sonbol, H.; Ramadan, M.A.H. GC-MS analysis of potentially volatile compounds of Pleurotus ostreatus polar extract: In vitro antimicrobial, cytotoxic, immunomodulatory, and antioxidant activities. Front. Microbiol. 2022, 13, 834525. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Nakade, K.; Sato, S.; Yoshida, K.; Miyazaki, K.; Natsume, S.; Konno, N. Lentinula edodes genome survey and postharvest transcriptome analysis. Appl. Environ. Microbiol. 2017, 83, e02990-16. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Wang, L.; Song, S.; Zhu, J.; Liu, R.; Shi, L.; Ren, A.; Zhao, M. GCN4 regulates secondary metabolism through activation of antioxidant gene expression under nitrogen limitation conditions in Ganoderma lucidum. Appl. Environ. Microbiol. 2021, 87, e00156-21. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Chen, M.; Wang, H.; Wang, Q.; Song, X.; Hao, H.; Feng, Z. Kojic acid-mediated damage responses induce mycelial regeneration in the basidiomycete Hypsizygus marmoreus. PLoS ONE 2017, 12, e0187351. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Chen, M.; Wang, H.; Song, X.; Feng, Z. Construction and application of a gene silencing system using a dual promoter silencing vector in Hypsizygus marmoreus. J. Basic Microbiol. 2017, 57, 78–86. [Google Scholar] [CrossRef]
- Piotr, K.; Marcin, G.; Beata, P.; Anna, S.; Katarzyna, W.; Jan, F.; Kurlandzka, A. Newly identified protein Imi1 affects mitochondrial integrity and glutathione homeostasis in Saccharomyces cerevisiae. FEMS Yeast Res. 2015, 15, fov048. [Google Scholar] [CrossRef]
- Chen, H.; Hao, H.; Han, C.; Wang, H.; Wang, Q.; Chen, M.; Juan, J.; Feng, Z.; Zhang, J. Exogenous l-ascorbic acid regulates the antioxidant system to increase the regeneration of damaged mycelia and induce the development of fruiting bodies in Hypsizygus marmoreus. Fungal Biol. 2020, 124, 551–561. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, G.S. Effect of diethyl phthalate on biochemical indicators of carp liver tissue. Toxin Rev. 2015, 34, 21–27. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Hao, H.; Feng, Z.; Chen, M.; Wang, H.; Ye, M. Hydrogen-rich water increases postharvest quality by enhancing antioxidant capacity in Hypsizygus marmoreus. AMB Express 2017, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, H.; Chen, M.; Wang, H.; Feng, Z.; Chen, H. Hydrogen-rich water alleviates the toxicities of different stresses to mycelial growth in Hypsizygus marmoreus. AMB Express 2017, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- You, B.J.; Chang, W.T.; Chung, K.R.; Kuo, Y.H.; Yang, C.S.; Tien, N.; Hsieh, H.-C.; Lai, C.-C.; Lee, H.-Z. Effect of solid-medium coupled with reactive oxygen species on ganoderic acid biosynthesis and MAP kinase phosphorylation in Ganoderma lucidum. Food Res. Int. 2012, 49, 634–640. [Google Scholar] [CrossRef]
- Tong, X.; Wang, F.; Zhang, H.; Bai, J.; Dong, Q.; Yue, P.; Jiang, X.; Li, X.; Wang, L.; Guo, J. iTRAQ-based comparative proteome analyses of different growth stages revealing the regulatory role of reactive oxygen species in the fruiting body development of Ophiocordyceps sinensis. Peer J. 2021, 9, e10940. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- James, M.R.; Doss, K.E.; Cramer, R.A. New developments in Aspergillus fumigatus and host reactive oxygen species responses. Curr. Opin. Microbiol. 2024, 80, 102521. [Google Scholar] [CrossRef]
- Mattila, H.K.; Österman-Udd, J.; Mali, T.L.E.; Lundell, T. Basidiomycota fungi and ROS: Genomic perspective on key enzymes involved in generation and mitigation of reactive oxygen species. Front. Fungal Biol. 2022, 3, 837605. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Hongsanan, S.A.; Nwankwegu, U.P.; Xie, N. Exploring the critical environmental optima and biotechnological prospects of fungal fruiting bodies. Microb. Biotechnol. 2025, 18, e70210. [Google Scholar] [CrossRef]
- Korge, P.; Calmettes, G.; Weiss, J.N. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta. 2015, 1847, 514–525. [Google Scholar] [CrossRef]
- Wu, T.M.; Lin, W.R.; Kao, C.H.; Hong, C.Y. Gene knockout of glutathione reductase 3 results in increased sensitivity to salt stress in rice. Plant Mol. Biol. 2015, 87, 555–564. [Google Scholar] [CrossRef]
- Ren, A.; Liu, R.; Miao, Z.; Zhang, X.; Cao, P.; Chen, T.; Li, C.; Shi, L.; Jiang, A.; Zhao, M. Hydrogen-rich water regulates effects of ROS balance on morphology, growth and secondary metabolism via glutathione peroxidase in Ganoderma lucidum. Environ. Microbiol. 2017, 19, 566–583. [Google Scholar] [CrossRef]
- Gostimskaya, I.; Grant, C.M. Yeast mitochondrial glutathione is an essential antioxidant with mitochondrial thioredoxin providing a back-up system. Free Radic. Biol. Med. 2016, 94, 55–65. [Google Scholar] [CrossRef]
- Ježek, P.; Jabůrek, M.; Holendová, B.; Engstová, H.; Dlasková, A. Mitochondrial cristae morphology reflecting metabolism, superoxide formation, redox homeostasis, and pathology. Antioxid. Redox Signal. 2023, 39, 635–683. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, H.C.; Chang, C.J.; Lee, S.Y. Mitochondrial glutathione in cellular redox homeostasis and disease manifestation. Int. J. Mol. Sci. 2024, 25, 1314. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Zhang, S.B.; Zhai, H.C.; Lv, Y.Y.; Hu, Y.S.; Cai, J.P. Hexanal induces early apoptosis of Aspergillus flavus conidia by disrupting mitochondrial function and expression of key genes. Appl. Microbiol. Biotechnol. 2021, 105, 6871–6886. [Google Scholar] [CrossRef]
- Liu, R.; Cao, P.; Ren, A.; Wang, S.; Yang, T.; Zhu, T.; Shi, L.; Zhu, J.; Jiang, A.-L.; Zhao, M.-W. SA inhibits complex III activity to generate reactive oxygen species and thereby induces GA overproduction in Ganoderma lucidum. Redox Biol. 2018, 16, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, R.; Maqsood, M.F.; Shahbaz, M.; Naz, N.; Zulfiqar, U.; Ali, M.F.; Jamil, M.; Khalid, F.; Ali, Q.; Sabir, M.A.; et al. Exogenous ascorbic acid as a potent regulator of antioxidants, osmo-protectants, and lipid peroxidation in pea under salt stress. BMC Plant Biol. 2024, 24, 247. [Google Scholar] [CrossRef]
- Galic, M.; Stajic, M.; Simonić, J. Hypsizygus Marmoreus—A novel potent degrader of lignocellulose residues. Cellul. Chem. Technol. 2020, 54, 977–982. [Google Scholar] [CrossRef]
- Li, A.; Wang, W.; Guo, S.; Li, C.; Wang, X.; Fei, Q. Insight into the role of antioxidant in microbial lignin degradation: Ascorbic acid as a fortifier of lignin-degrading enzymes. Biotechnol. Biofuels Bioprod. 2025, 18, 16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Zhang, Y.; Wang, Q.; Xiao, T.; Yue, Y.; Zhang, J.; Chen, H. Glutathione Reductase Affects Hyphal Growth and Fruiting Body Development by Regulating Intracellular ROS Levels in Hypsizygus marmoreus. Horticulturae 2025, 11, 1441. https://doi.org/10.3390/horticulturae11121441
Hao H, Zhang Y, Wang Q, Xiao T, Yue Y, Zhang J, Chen H. Glutathione Reductase Affects Hyphal Growth and Fruiting Body Development by Regulating Intracellular ROS Levels in Hypsizygus marmoreus. Horticulturae. 2025; 11(12):1441. https://doi.org/10.3390/horticulturae11121441
Chicago/Turabian StyleHao, Haibo, Yuchen Zhang, Qian Wang, Tingting Xiao, Yihong Yue, Jinjing Zhang, and Hui Chen. 2025. "Glutathione Reductase Affects Hyphal Growth and Fruiting Body Development by Regulating Intracellular ROS Levels in Hypsizygus marmoreus" Horticulturae 11, no. 12: 1441. https://doi.org/10.3390/horticulturae11121441
APA StyleHao, H., Zhang, Y., Wang, Q., Xiao, T., Yue, Y., Zhang, J., & Chen, H. (2025). Glutathione Reductase Affects Hyphal Growth and Fruiting Body Development by Regulating Intracellular ROS Levels in Hypsizygus marmoreus. Horticulturae, 11(12), 1441. https://doi.org/10.3390/horticulturae11121441





