Evaluation of Pollination Potential in ‘Jinfeng’ Kiwifruit Seedling Male Plants Based on Floral Traits and Pollen Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Collection
2.3. Floral Trait Recording and Measurement
2.4. Pollen Viability Measurement
2.5. Scanning Electron Microscopy (SEM) Observation and Morphological Analysis of Pollen
2.6. Data Analysis
3. Results
3.1. Analysis of Floral Traits in ‘Jinfeng’ Seedling Male Plants
3.2. Pollen Viability Evaluation of ‘Jinfeng’ Seedling Male Plants
3.3. Scanning Electron Microscopy of Pollen from ‘Jinfeng’ Seedling Male Plants
3.4. Correlation Analysis
3.5. Principal Component Analysis of All Male Floral Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karakaya, E.; Uzundumlu, S.A. Kiwi production forecasts for the leading countries in the period 1983–2027. Appl. Fruit Sci. 2025, 67, 66. [Google Scholar] [CrossRef]
- Kumawat, P.; Rathore, R.S.; Singh, R.P.; Kumar, S. Study the effect of different pollination techniques on date palm production. Sugar Tech. 2022, 24, 1887–1893. [Google Scholar] [CrossRef]
- Bocianowski, J.; Leśniewska-Bocianowska, A. Towards the identification of candidate genes for pollen morphological traits in Rubus L. using association mapping. Forests 2025, 16, 1395. [Google Scholar] [CrossRef]
- Negi, S.; Rawat, R.; Tiwari, P. Pollen morphology of Lauraceae members from Garhwal Himalaya, (Uttarakhand, India). Microsc. Res. Tech. 2025, 88, 3009–3016. [Google Scholar] [CrossRef]
- Nazish, M.; Kamal, A.; Khan, M.A.; Rahman, K.U.; Elshikh, M.S.; Razak, A.S.; Bibi, F.; Alkahtani, J.; Zaman, W. Integration of multivariate indices to correlate pollen morphology and evolutionary adaptations in halophytes. Genet. Resour. Crop Evol. 2025, 72, 8259–8280. [Google Scholar] [CrossRef]
- Gu, X.B.; Lu, L.H.; Fan, F.; Gao, J.; Song, G.H.; Zhang, H.Q. Analysis on the genetic characteristics of male flower traits in the hybrid progeny of kiwifruit Jinli and Moshan No.4. Acta Agric. Nucl. Sin. 2025, 39, 2388–2397. [Google Scholar]
- Liang, Y.P.; Mo, S.; Chen, Y.; Li, K.M. Morphological characteristics of male Actinidia chinensis floral organ. Guizhou Agric. Sci. 2023, 51, 126–132. [Google Scholar]
- Zhong, M.; Liao, G.L.; Li, Z.Y.; Zou, L.F.; Huang, Q.; Chen, L.; Huang, C.H.; Tao, J.J.; Zhu, B.; Xu, X.B. Genetic diversity of wild male kiwifruit (Actinidia eriantha Benth.) germplasms based on SSR and morphological markers. J. Fruit Sci. 2018, 35, 658–667. [Google Scholar] [CrossRef]
- EI Hanafi, S.; Cherkaoui, S.; Kehel, Z.; Sanchez-Garcia, M.; Sarazin, J.B.; Baenziger, S.; Tadesse, W. Hybrid seed set in relation with male floral traits, estimation of heterosis and combining abilities for yield and its components in wheat (Triticum aestivum L.). Plants 2022, 11, 508. [Google Scholar] [CrossRef]
- Xue, D.H.; Wu, Y.X.; Xu, S.Q.; Chen, J.B.; Wang, Y.J.; Zhang, Y.J.; Song, W.X.; He, T.M.; Zhang, F. Study on genetics of several traits of floral organs in hybrid offspring of ‘Korla fragrant pear’ and ‘Cuiguan’. J. Fruit Res. 2025, 6, 1–6+32. [Google Scholar] [CrossRef]
- Xin, Y.; Yu, X.K.; Li, X.B.; Yu, H. Study on the variation of floral organ, pollen and SSR genetic diversity of different strawberry cultivars. Acta Agric. Nucl. Sin. 2024, 38, 1468–1475. [Google Scholar]
- Kim, J.; Jeong, H.; Kim, S.; Chae, W. Pollen traits significantly associated with fruit yield traits under heat stress among large fruit but not cherry fruit tomatoes. Hortic. Environ. Biotechnol. 2025, 66, 1121–1132. [Google Scholar] [CrossRef]
- Karimi, H.R.; Zeraatkar, H. Effects of artificial pollination using pollen suspension spray on nut and kernel quality of pistachio cultivar Owhadi. Int. J. Fruit Sci. 2016, 16, 171–181. [Google Scholar] [CrossRef]
- Machado, C.D.A.; Moura, C.R.F.; Lemos, E.E.P.D.; Ramos, S.R.R.; Ribeiro, F.E.; Lédo, A.D.S. Pollen grain viability of coconut accessions at low temperatures. Acta Sci. 2014, 36, 227–232. [Google Scholar] [CrossRef]
- Rakonjac, V.; Nikolić, D.; Čolić, S.; Glišić, I.; Đorđević, M.; Popovska, M.; Radičević, S. Investigation of pollen morphology and viability of sweet and sour cherry genotypes by multivariate analysis. Microsc. Res. Tech. 2024, 88, 42–52. [Google Scholar] [CrossRef]
- Meng, M.; Liu, Z.Y.; Xu, Z.S.; Zhang, H.L.; She, H.B.; Qian, W. Observation of nine types of spinach pollen morphology by scanning electron microscopy. Horticulturae 2024, 10, 1358. [Google Scholar] [CrossRef]
- Patil, A.S.; Nimbalkar, S.M.; Pagariya, C.M.; Kulkarni, A.J.; Jadhav, P.R.; Mane, M.P.; Magdum, A.B.; Saha, T.N.; Shinde, K.V.; Prasad, K.V.; et al. Pollen morphology and variability among Indian cultivars of Chrysanthemum morifolium and comparative analysis with genera of the Asteraceae family. Genet. Resour. Crop Evol. 2024, 72, 2227–2247. [Google Scholar] [CrossRef]
- Wang, W.C. Scanning electron microscopy analysis of pollen morphology in wild Pyrus germplasm resources. J. Fruit Res. 2025, 6, 1–7. [Google Scholar] [CrossRef]
- He, L.X.; Wang, L.H.; Zhuang, Q.G.; Zhang, Y.; Wang, F.Q.; Zhang, Q. Observation and analysis of pollen morphology of 41 germplasm of 9 species of kiwifruit. South China Fruits 2024, 53, 132–139. [Google Scholar] [CrossRef]
- Qi, X.J.; Wang, R.; Lan, Y.P.; Chen, J.Y.; Gu, H.; Fang, J.B. Morphologic study of pollens of three cultivated Actinidia species by scanning electron microscopy. J. Fruit Sci. 2017, 34, 1365–1373. [Google Scholar] [CrossRef]
- Tu, G.Q.; Liao, G.L.; Liu, Q.; Li, B.M.; Huang, C.H.; Jia, D.F.; Zhao, S.G.; Xu, X.B. Biological characteristics and main cultivation techniques of a new Actinidia chinensis yellow-fleshed cultivar ‘Fenghuang No. 1’. South China Fruits 2020, 49, 153–156. [Google Scholar] [CrossRef]
- GB/T 19557.11-2022; Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability—Actinidia (Actinidia L.). State Administration for Market Regulation (Standardization Administration of China): Beijing, China, 2022.
- Shen, G.H.; Wang, X.Q.; Luo, J.; Zhang, S.L.; Qian, P.H.; Jin, F.L. Effects of greenhouse culture on pear pollen quantity per anther and pollen viability. Acta Agric. Shanghai 2008, 3, 54–57. [Google Scholar]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain. Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kristen, U.; Kappler, R. The pollen tube growth test. Methods Mol. Biol. 1995, 43, 189–198. [Google Scholar] [CrossRef]
- Kang, N.; Wang, S.M.; Huang, R.H.; Wu, X.W. Study on the pollen morphology of 9 Actinidia genotypes. Plant Sci. J. 1993, 2, 111–116. [Google Scholar]
- Zhu, C.Z.; Xu, G.J.; Xu, L.S.; Li, P. Study on the pollen morphology of 12 Actinidia genotypes. J. China Pharm. Univ. 1995, 3, 139–143. [Google Scholar]
- Jiang, Z.W.; Wang, S.M.; Zhang, Z.H.; Huang, H.W. Pollen morphology of Actinidia and its systematic significance. J. Syst. Evol. 2004, 43, 245–260. [Google Scholar]
- Wang, B.Q. A tentative study on the morphological character of the pollen of Actinidia in the north east area. J. Jilin Eng. Norm. Univ. 2008, 24, 92–94. [Google Scholar]
- Sharma, A.; Bala, N.; Sharma, M.; Katnoria, J.K.; Bahel, S. Study on evaluation of effects of electromagnetic radiation on pollen viability in some commonly occurring plant species following different staining methods. Protoplasma 2025, 1–16. [Google Scholar] [CrossRef]
- Zhong, M.; Xie, M.; Zhang, W.B.; Tao, J.J.; Huang, C.H.; Zhu, B.; Xu, X.B. Observation on the pollen morphology of male genotypes in wild Actinidia eriantha population. J. Fruit Sci. 2016, 33, 1251–1258. [Google Scholar] [CrossRef]
- Habibi, M.; Attar, F.; Falahati Anbaran, M. Taxonomic importance of pollen micromorphology in Prunus L. subgenus cerasus pers (Rosaceae) from Iran. Iran. J. Bot. 2022, 28, 96–112. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C.C. Studies on pollen morphology of rosaceae. Acta Bot. Gall. 1994, 141, 183–193. [Google Scholar] [CrossRef]
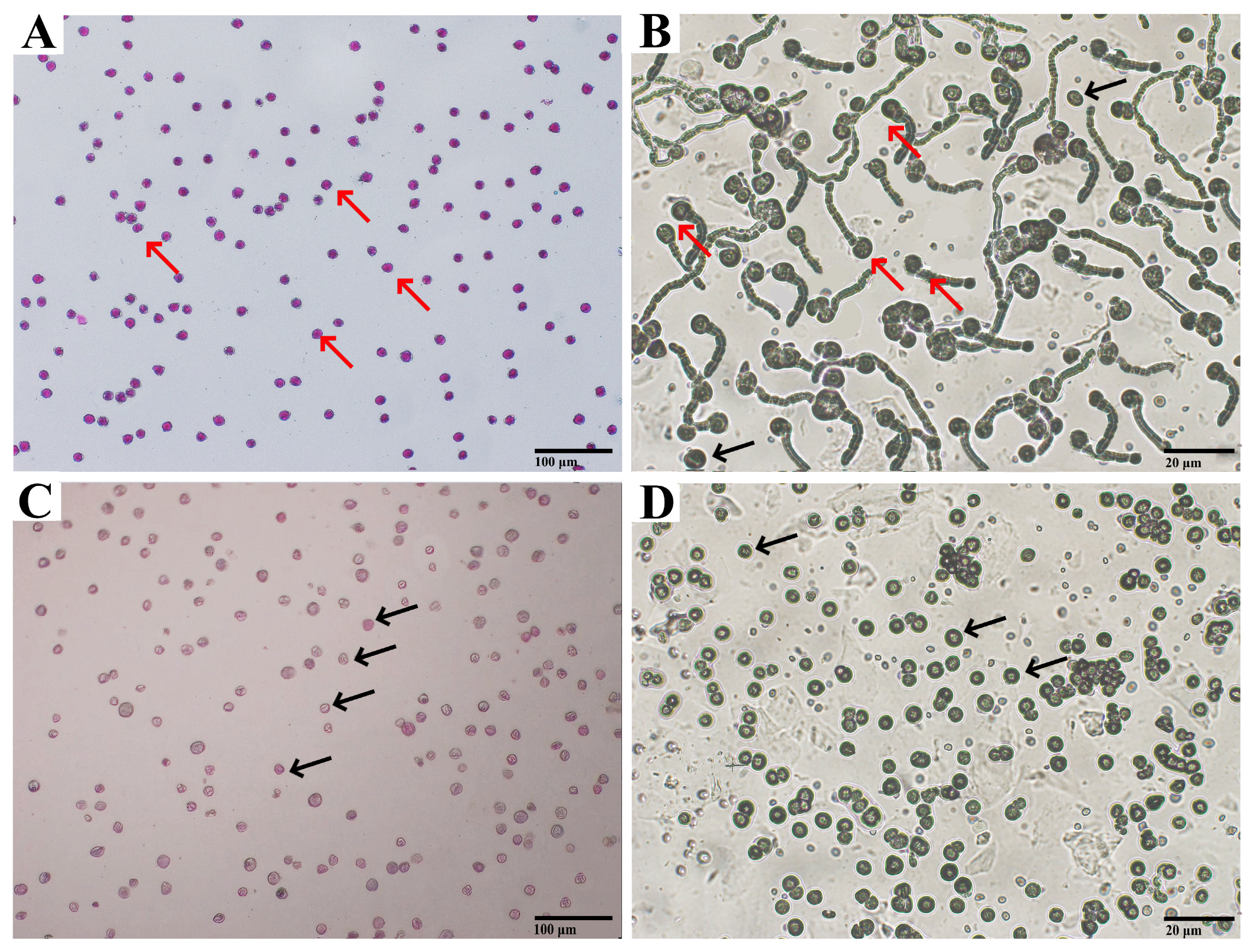
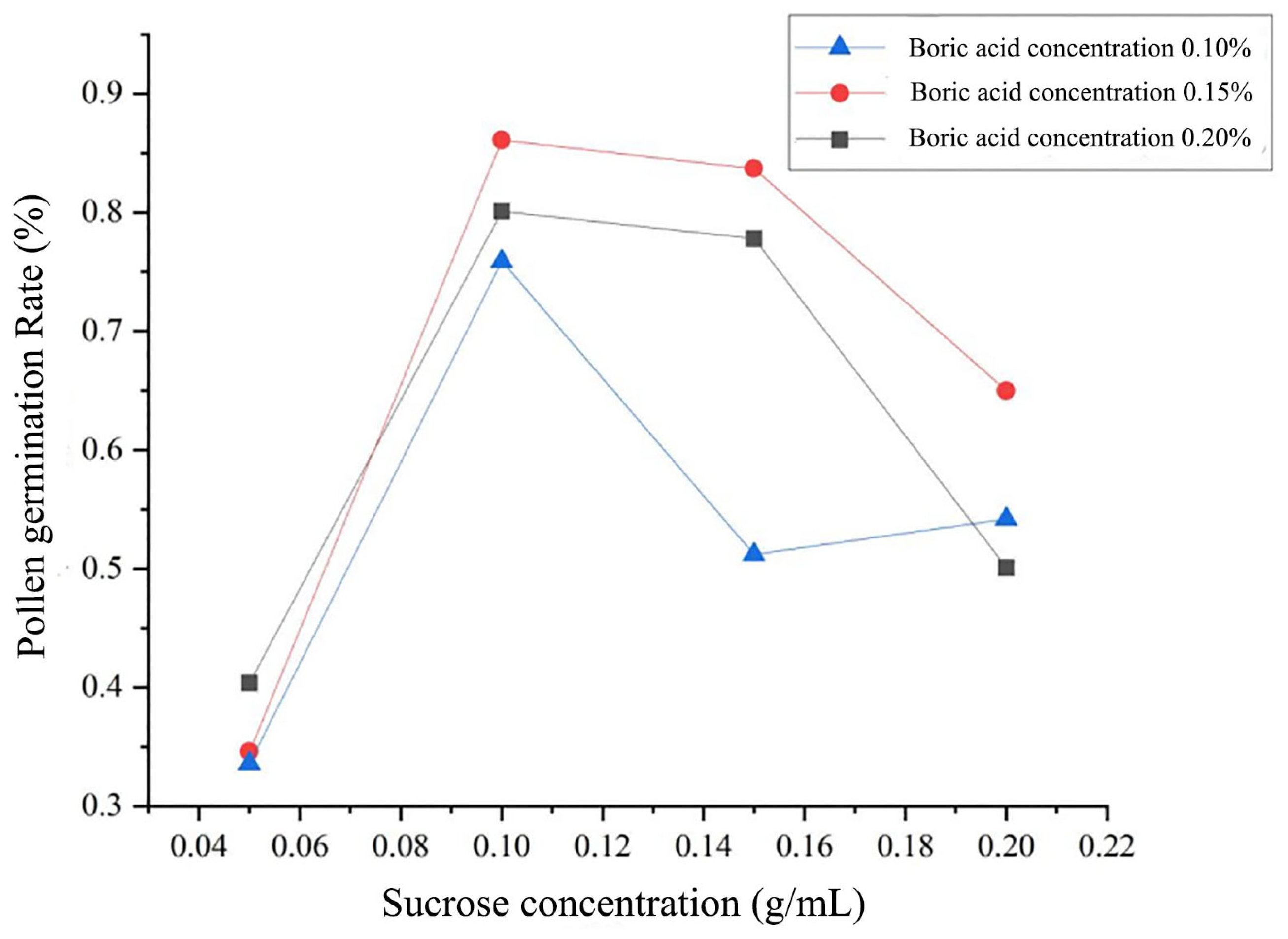
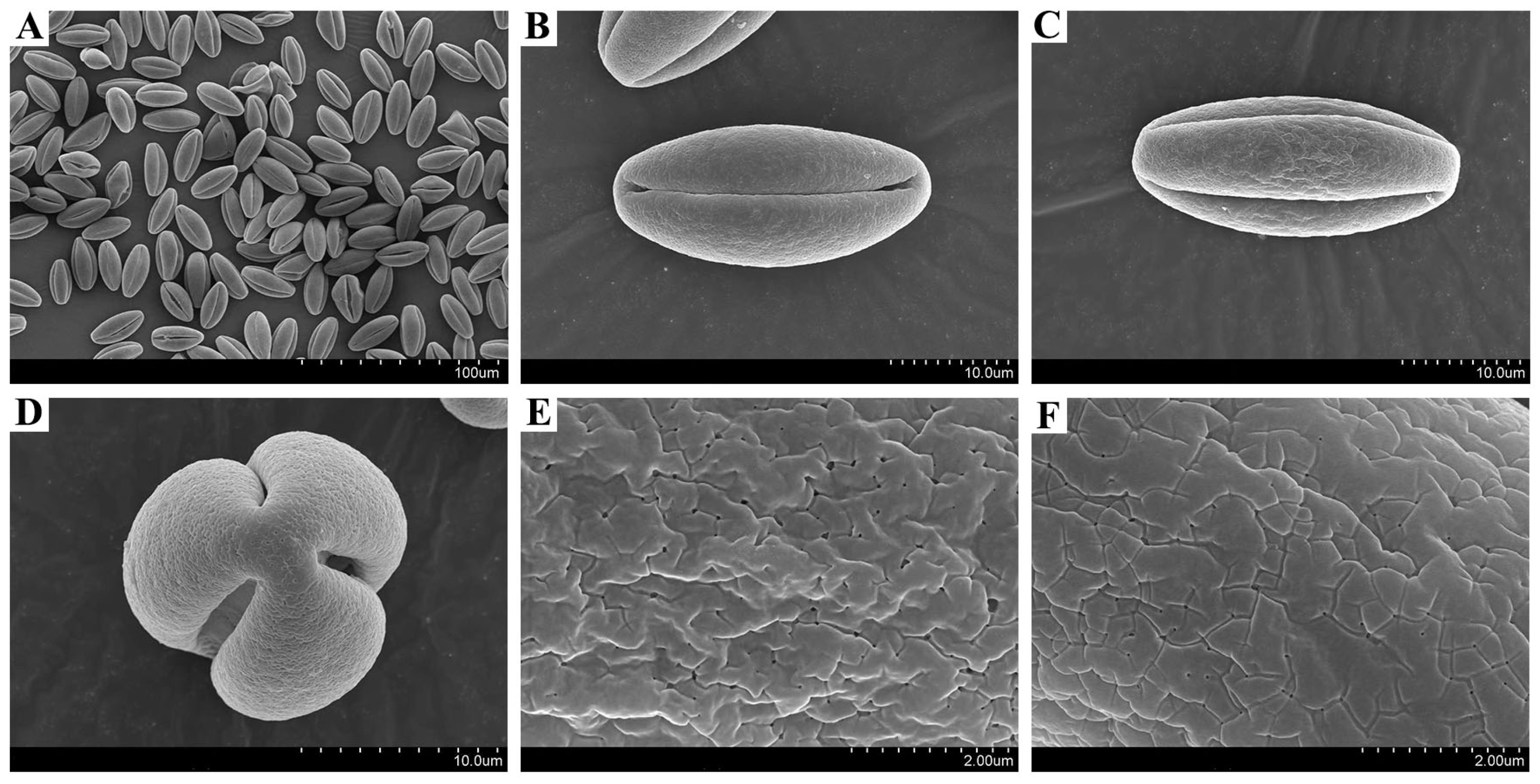
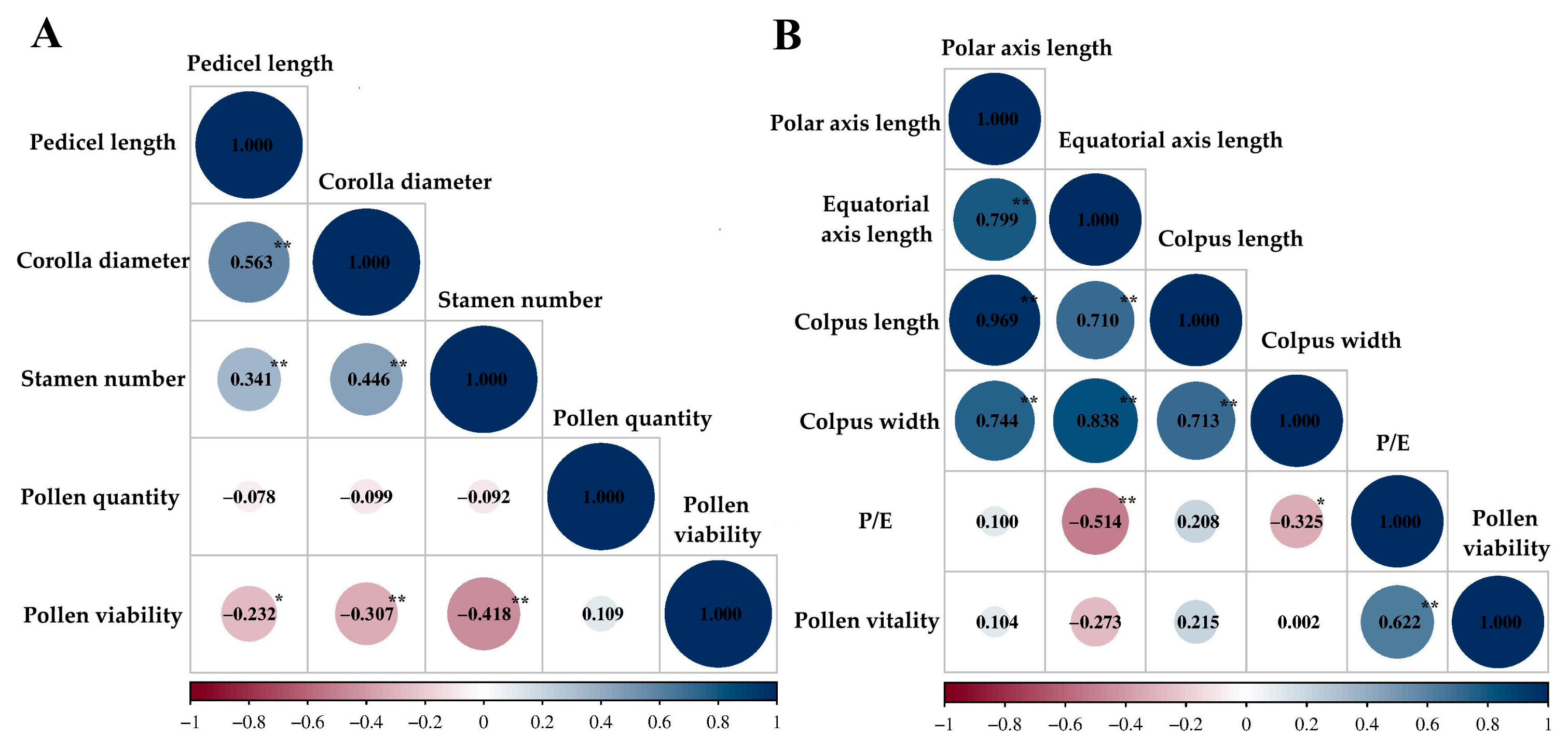
| Index | Pedicel Length/cm | Corolla Diameter/cm | Stamen Number | Pollen Quantity | Pollen Viability/% |
|---|---|---|---|---|---|
| Average | 3.73 | 3.83 | 96.03 | 9654.48 | 72.78 |
| SD | 0.91 | 0.52 | 27.77 | 4381.56 | 31.60 |
| Median | 3.58 | 3.78 | 90.00 | 8273.50 | 90.09 |
| Mode | 2.89 | 3.07 | 79.00 | 6906.00 | 97.06 |
| Min. | 1.87 | 2.85 | 55.00 | 3398.00 | 0.33 |
| Max. | 6.18 | 5.11 | 178.00 | 25,117.00 | 100.00 |
| CV/% | 24.36 | 13.57 | 28.92 | 45.38 | 43.41 |
| Range | 4.31 | 2.26 | 123.00 | 21,719.00 | 99.67 |
| Number | Staining Method | Pollen Germination Method | Number | Staining Method | Pollen Germination Method |
|---|---|---|---|---|---|
| 7-601-4 | 1.000 ± 0.000 | 0.759 ± 0.049 | 1-54 | 0.155 ± 0.043 | 0.000 ± 0.000 |
| 5-327 | 1.000 ± 0.000 | 0.841 ± 0.062 | 2-96 | 0.131 ± 0.039 | 0.007 ± 0.012 |
| 7-601 | 0.997 ± 0.004 | 0.774 ± 0.102 | 5-321 | 0.116 ± 0.028 | 0.000 ± 0.000 |
| 3-156 | 0.995 ± 0.007 | 0.720 ± 0.116 | 7-600-1 | 0.105 ± 0.103 | 0.000 ± 0.000 |
| 11-510 | 0.994 ± 0.006 | 0.678 ± 0.060 | 1-51 | 0.068 ± 0.019 | 0.000 ± 0.000 |
| 4-290-1 | 0.993 ± 0.003 | 0.716 ± 0.079 | 11-512 | 0.050 ± 0.030 | 0.000 ± 0.000 |
| 5-311 | 0.992 ± 0.008 | 0.610 ± 0.096 | 1-81 | 0.037 ± 0.038 | 0.000 ± 0.000 |
| 2-132-1 | 0.992 ± 0.006 | 0.744 ± 0.103 | 1-8 | 0.009 ± 0.001 | 0.000 ± 0.000 |
| 1-56 | 0.991 ± 0.008 | 0.529 ± 0.130 | 1-74 | 0.005 ± 0.004 | 0.000 ± 0.000 |
| 2-136 | 0.991 ± 0.016 | 0.614 ± 0.029 | 2-120 | 0.003 ± 0.006 | 0.000 ± 0.000 |
| Index | Polar Axis Length/μm | Equatorial Axis Length/μm | Colpus Length/μm | Colpus Width/μm | P/E | Floral Vitality/% |
|---|---|---|---|---|---|---|
| Average | 28.45 | 13.51 | 24.34 | 7.94 | 2.11 | 71.45 |
| SD | 2.12 | 1.14 | 2.25 | 0.73 | 0.10 | 33.60 |
| Median | 27.64 | 13.29 | 23.74 | 7.89 | 2.11 | 90.63 |
| Mode | 28.14 | 12.34 | 22.75 | 7.48 | 2.14 | 100.00 |
| Min. | 25.34 | 11.72 | 20.86 | 6.56 | 1.71 | 3.72 |
| Max. | 34.62 | 16.17 | 30.58 | 9.76 | 2.29 | 100.00 |
| CV/% | 0.07 | 0.08 | 0.09 | 0.09 | 0.05 | 0.47 |
| Range | 9.28 | 4.45 | 9.72 | 3.20 | 0.58 | 96.28 |
| Item | Principal Component | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Pedicel length | −0.06 | 0.48 | 0.17 |
| Corolla diameter | 0.04 | 0.40 | 0.07 |
| Stamen number | −0.04 | 0.27 | −0.10 |
| Polar axis length | 0.28 | 0.06 | 0.14 |
| Equatorial axis length | 0.25 | −0.04 | −0.20 |
| Colpus length | 0.27 | 0.05 | 0.20 |
| Colpus width | 0.27 | −0.15 | −0.15 |
| P/E | −0.02 | 0.15 | 0.53 |
| Pollen vitality | 0.07 | −0.12 | 0.35 |
| Eigenvalues | 3.69 | 2.49 | 1.39 |
| Contribution rates/% | 38.39 | 24.12 | 21.79 |
| Cumulative contribution rates/% | 38.39 | 62.50 | 84.29 |
| Number | Comprehensive Evaluation Score | Pollen Vitality/% |
|---|---|---|
| 5-327 | 1.20 | 100 |
| 1-13 | 1.19 | 78.62 |
| 5-322 | 1.18 | 27.4 |
| 5-163-1 | 1.05 | 34.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Qian, S.; Dai, J.; Shi, J.; Tu, H.; Le, C.; Tao, J.; Huang, C. Evaluation of Pollination Potential in ‘Jinfeng’ Kiwifruit Seedling Male Plants Based on Floral Traits and Pollen Viability. Horticulturae 2025, 11, 1432. https://doi.org/10.3390/horticulturae11121432
Chen Y, Qian S, Dai J, Shi J, Tu H, Le C, Tao J, Huang C. Evaluation of Pollination Potential in ‘Jinfeng’ Kiwifruit Seedling Male Plants Based on Floral Traits and Pollen Viability. Horticulturae. 2025; 11(12):1432. https://doi.org/10.3390/horticulturae11121432
Chicago/Turabian StyleChen, Yanyan, Shilong Qian, Jiliang Dai, Jikang Shi, Hui Tu, Chenxi Le, Junjie Tao, and Chunhui Huang. 2025. "Evaluation of Pollination Potential in ‘Jinfeng’ Kiwifruit Seedling Male Plants Based on Floral Traits and Pollen Viability" Horticulturae 11, no. 12: 1432. https://doi.org/10.3390/horticulturae11121432
APA StyleChen, Y., Qian, S., Dai, J., Shi, J., Tu, H., Le, C., Tao, J., & Huang, C. (2025). Evaluation of Pollination Potential in ‘Jinfeng’ Kiwifruit Seedling Male Plants Based on Floral Traits and Pollen Viability. Horticulturae, 11(12), 1432. https://doi.org/10.3390/horticulturae11121432







