Resistance Phenotyping and WGCNA Identify Oxidative-Defense Hub Regulators in Strawberry Challenged by Colletotrichum siamense
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Isolation of Strawberry Anthracnose Isolate Cs.4J
2.3. Sequence Amplification of Cs.4J rDNA
2.4. Pathogenicity Assay of Cs.4J
2.5. Statistical Disease Index and Incidence
2.6. Morphological and Histological Observations
2.7. Measurements of Antioxidant Enzyme Activities and Oxidative Stress Biomarkers in Pathogen-Infected Plant Leaves
2.8. Transcriptome Sequencing of Resistant and Susceptible Varieties
2.9. WGCNA
2.10. Real-Time Quantitative PCR
3. Results
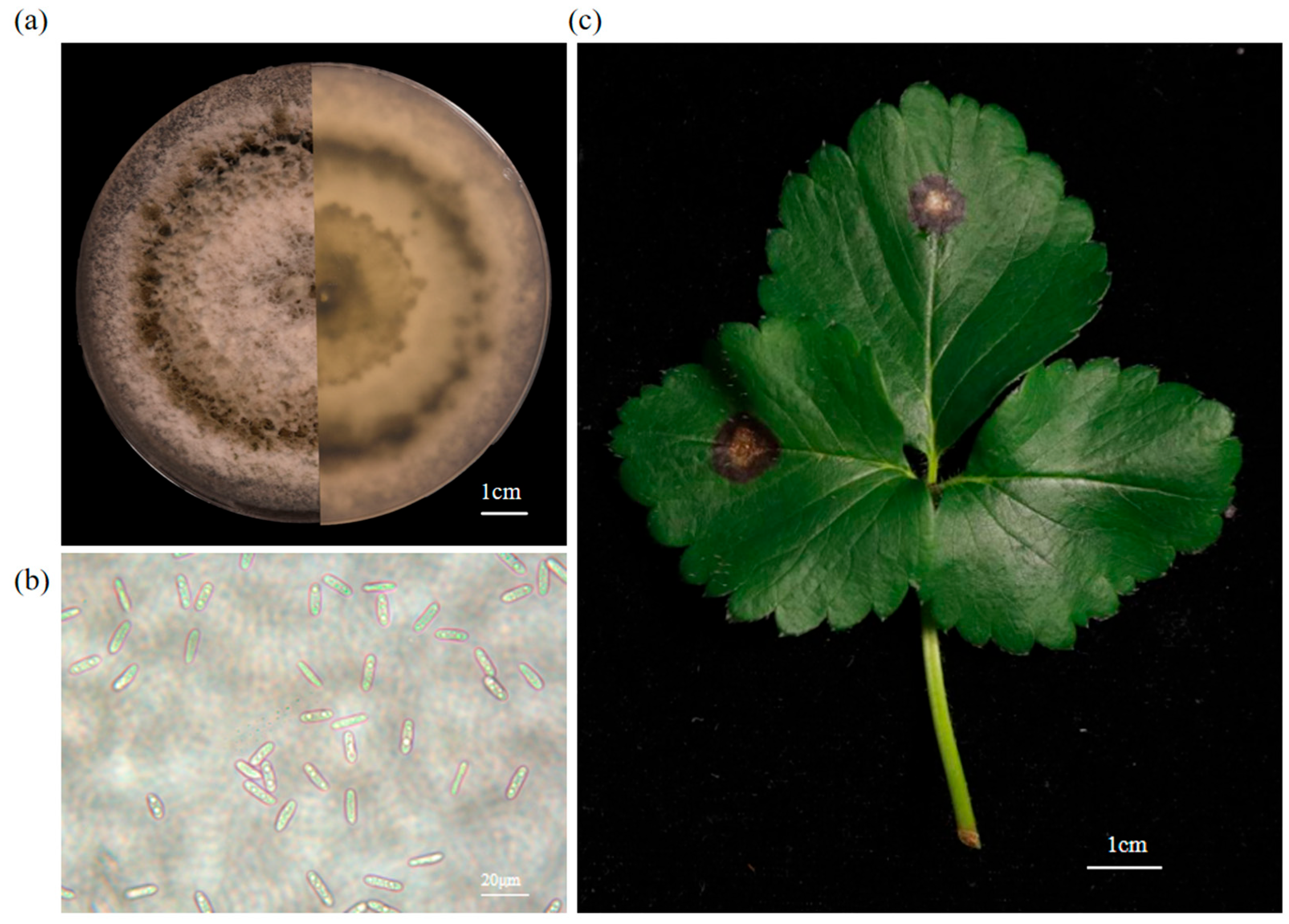
3.1. Classification Status of Pathogenic Fungi
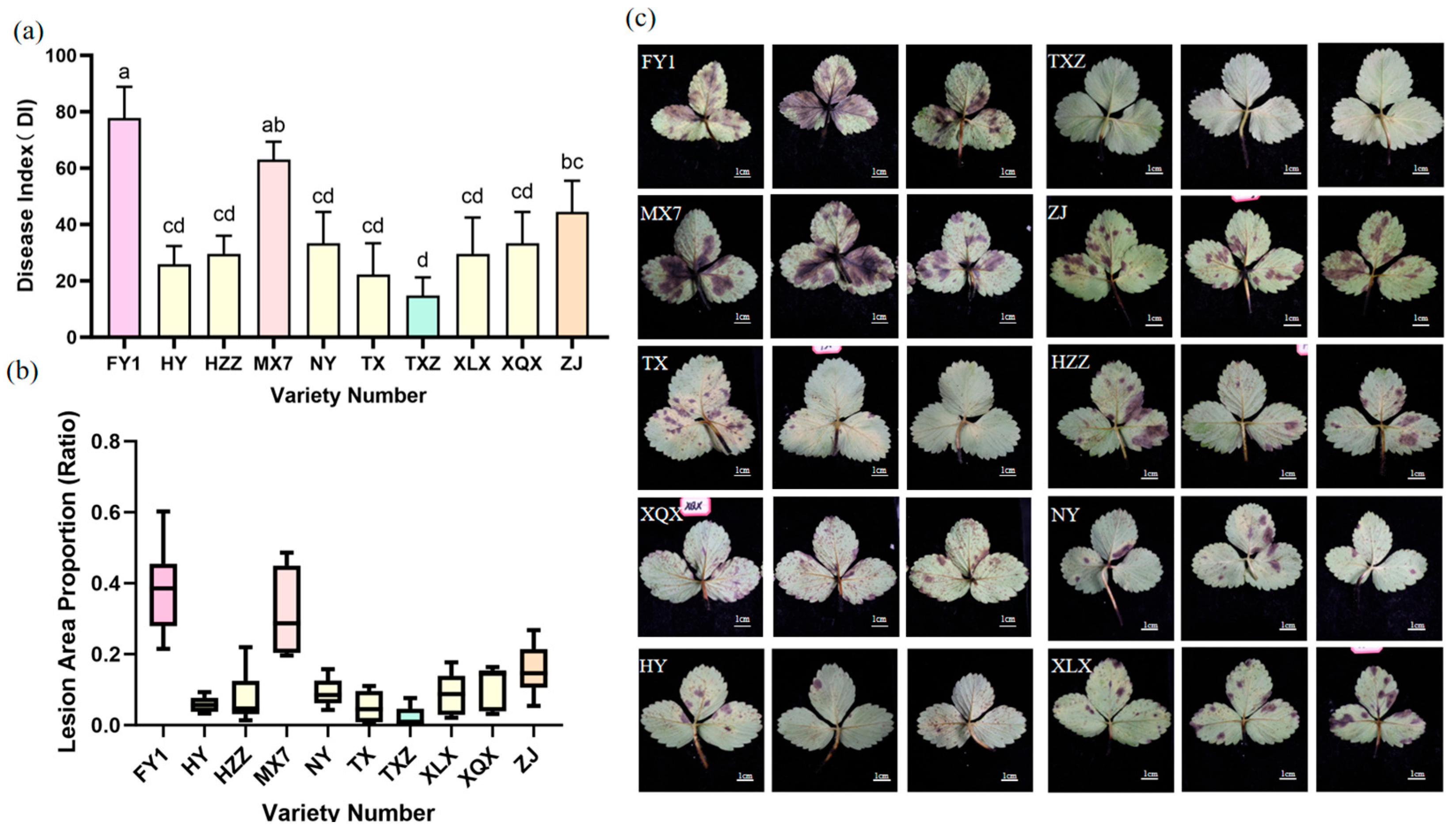
3.2. Resistance of Different Strawberry Varieties to Cs.4J
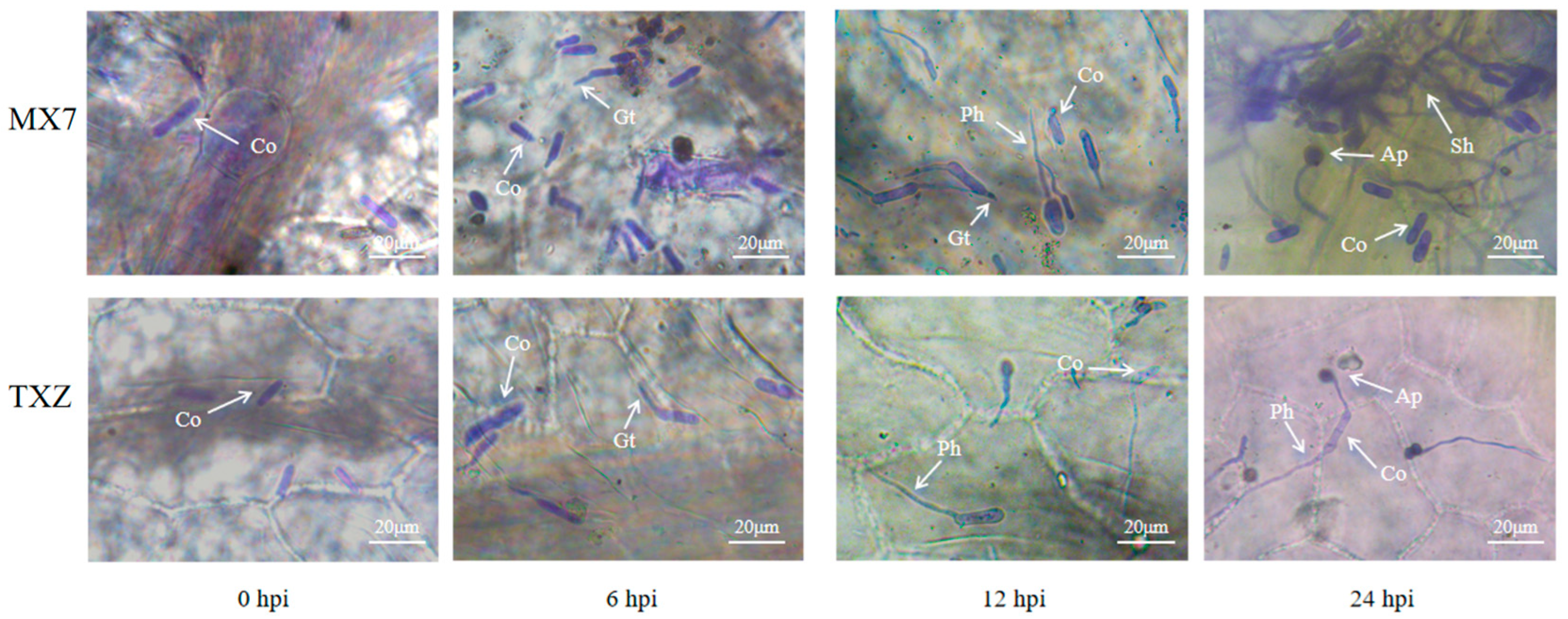
3.3. Microscopic Analysis of the Interaction of Cs.4J with ‘TXZ’ and ‘MX7’
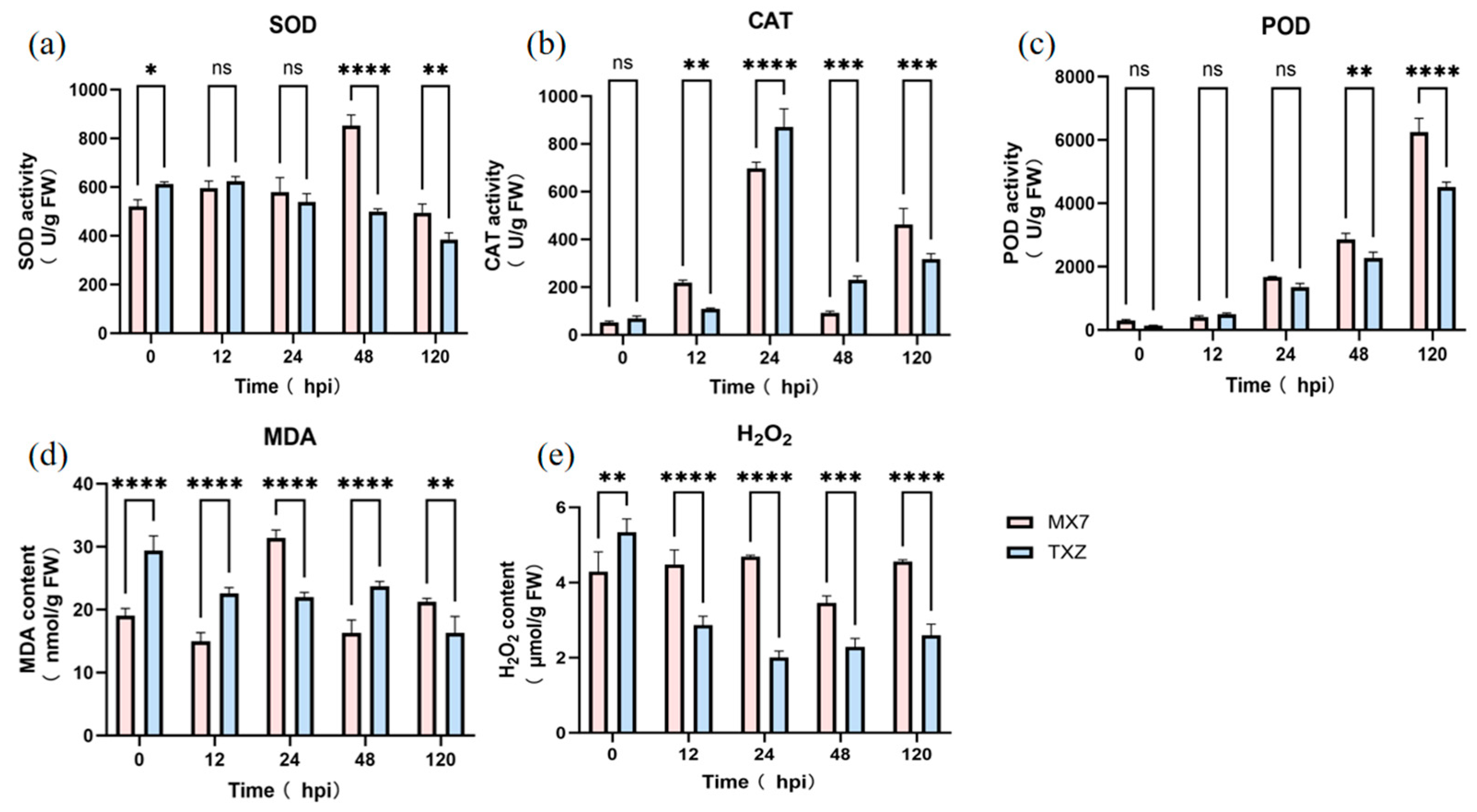
3.4. Comparative Oxidative Stress Responses and Antioxidant Defense Mechanisms in ‘TXZ’ and ‘MX7’ Infected with Cs.4J
3.5. Transcriptome Sequencing Data Statistics
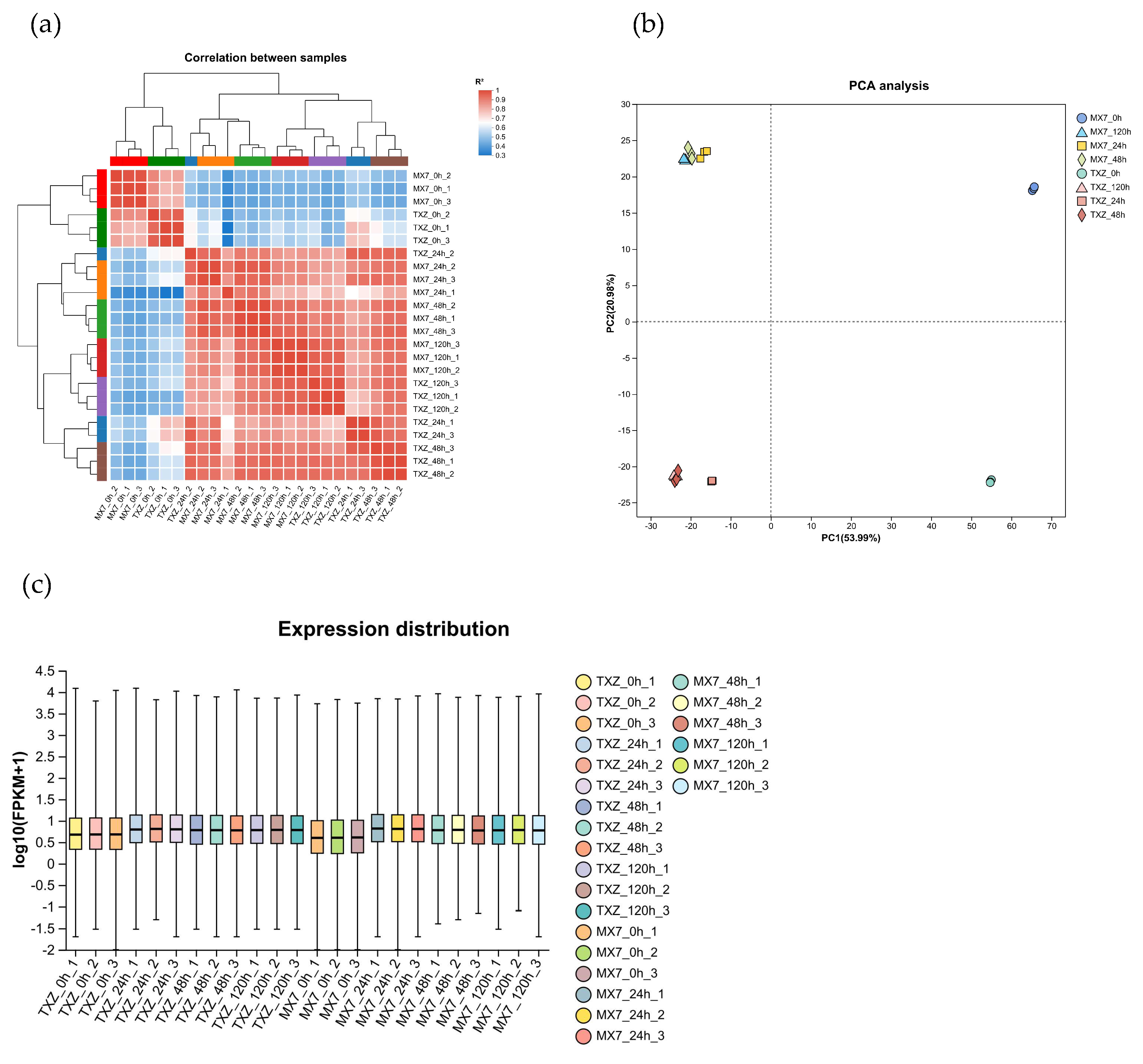
3.6. Gene Expression Level Analysis and Sample Correlation Analysis
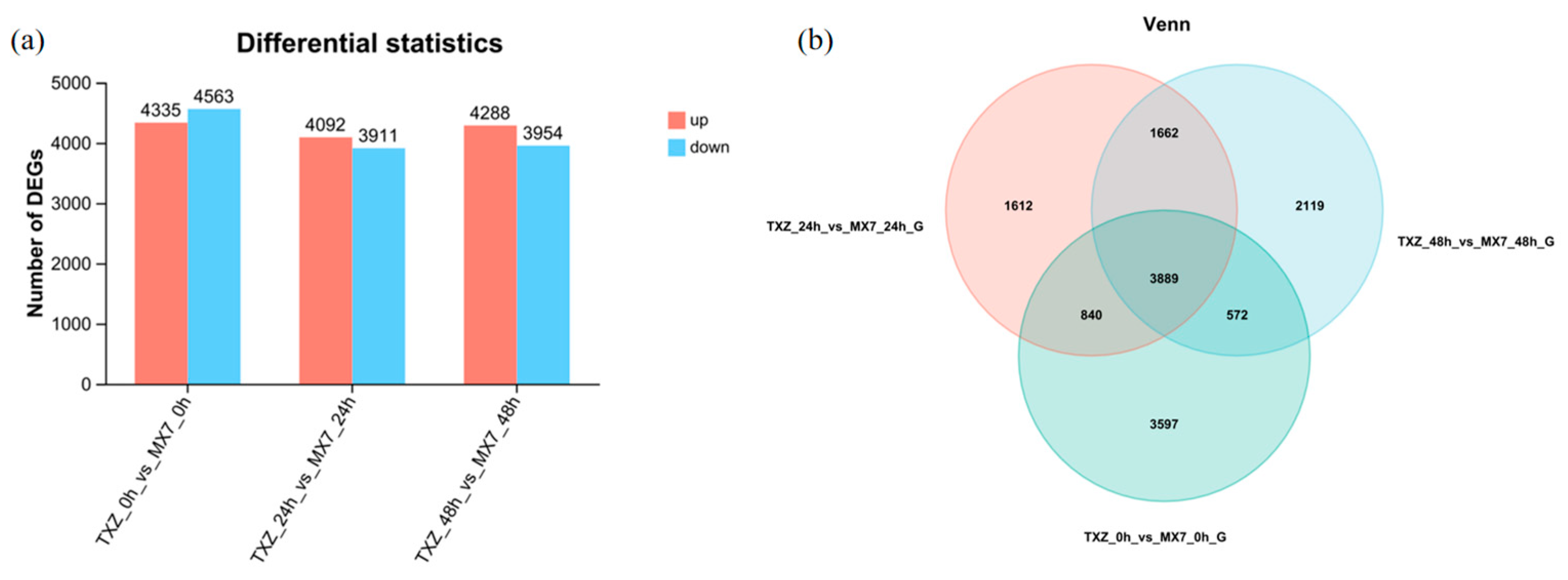
3.7. Differential Expression Gene Screening
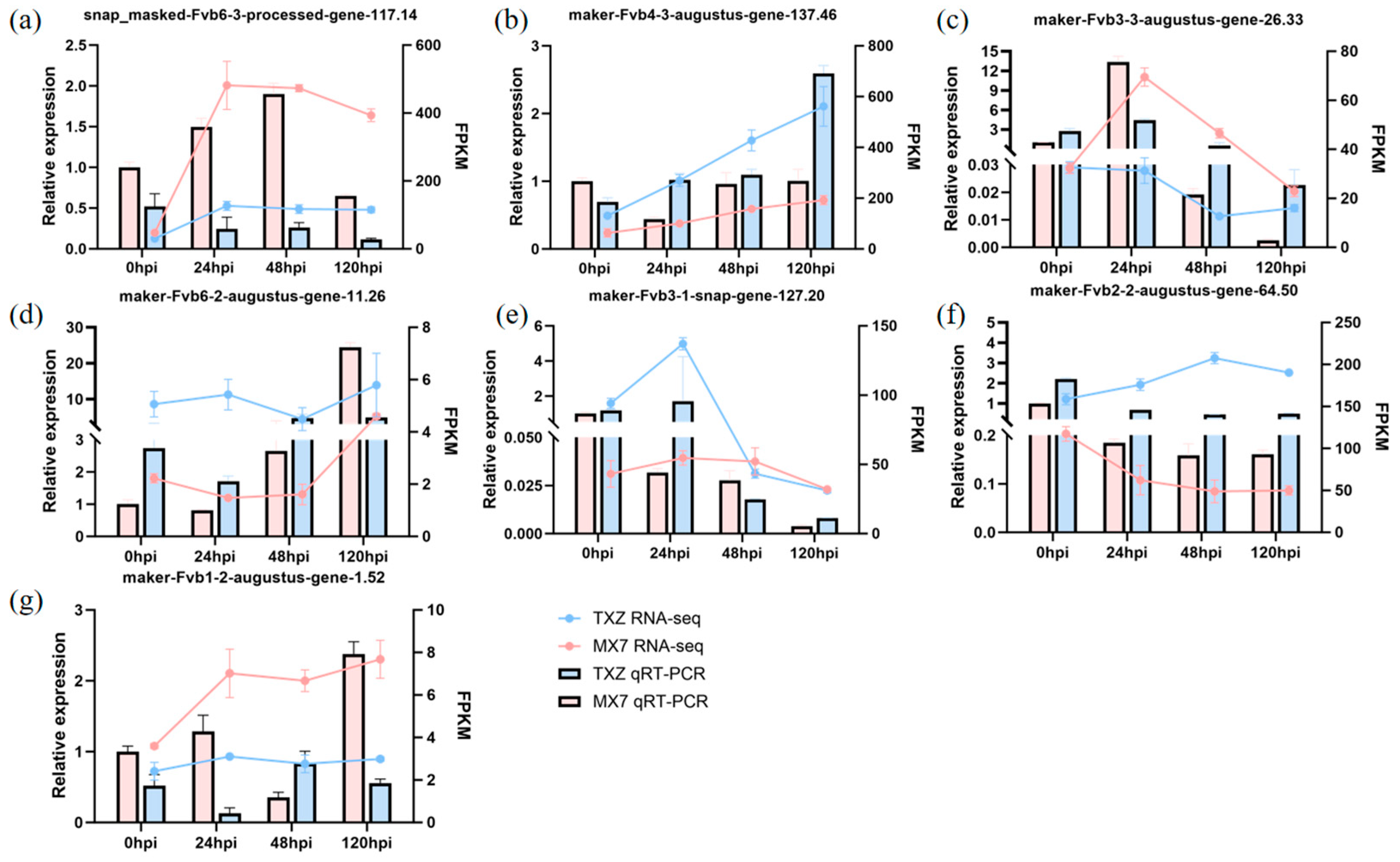
3.8. Validation of Differential Genes by RT–qPCR
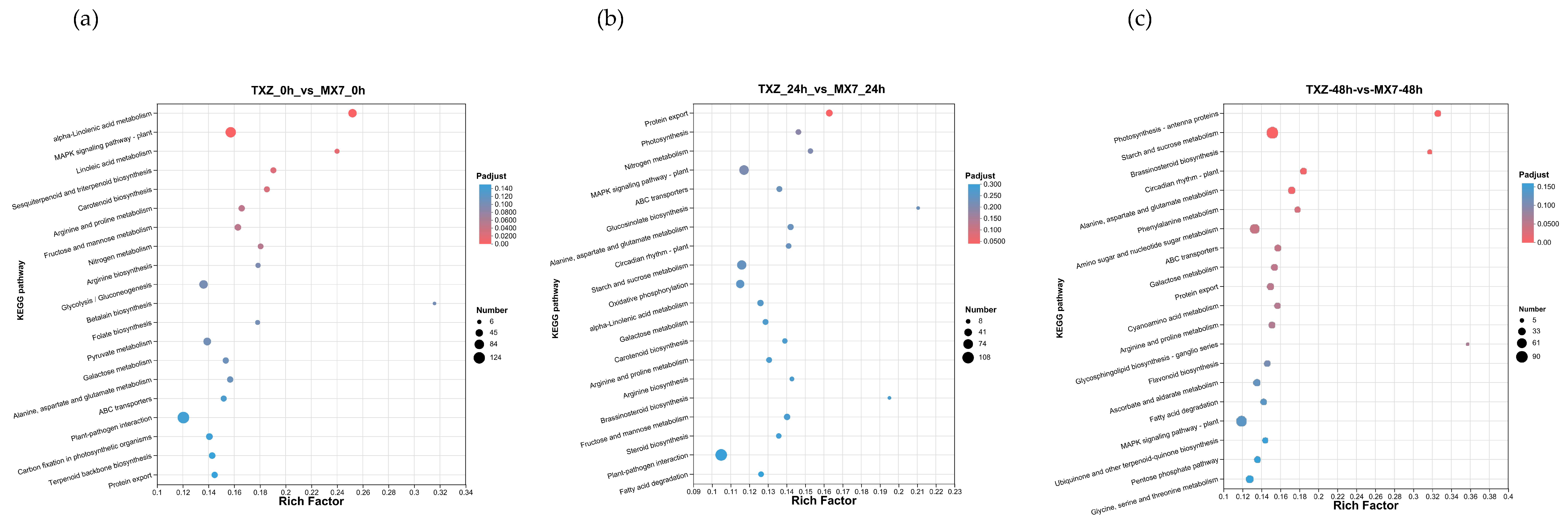
3.9. KEGG Pathway Enrichment Reveals Temporal Metabolic Reprogramming During Pathogen Invasion
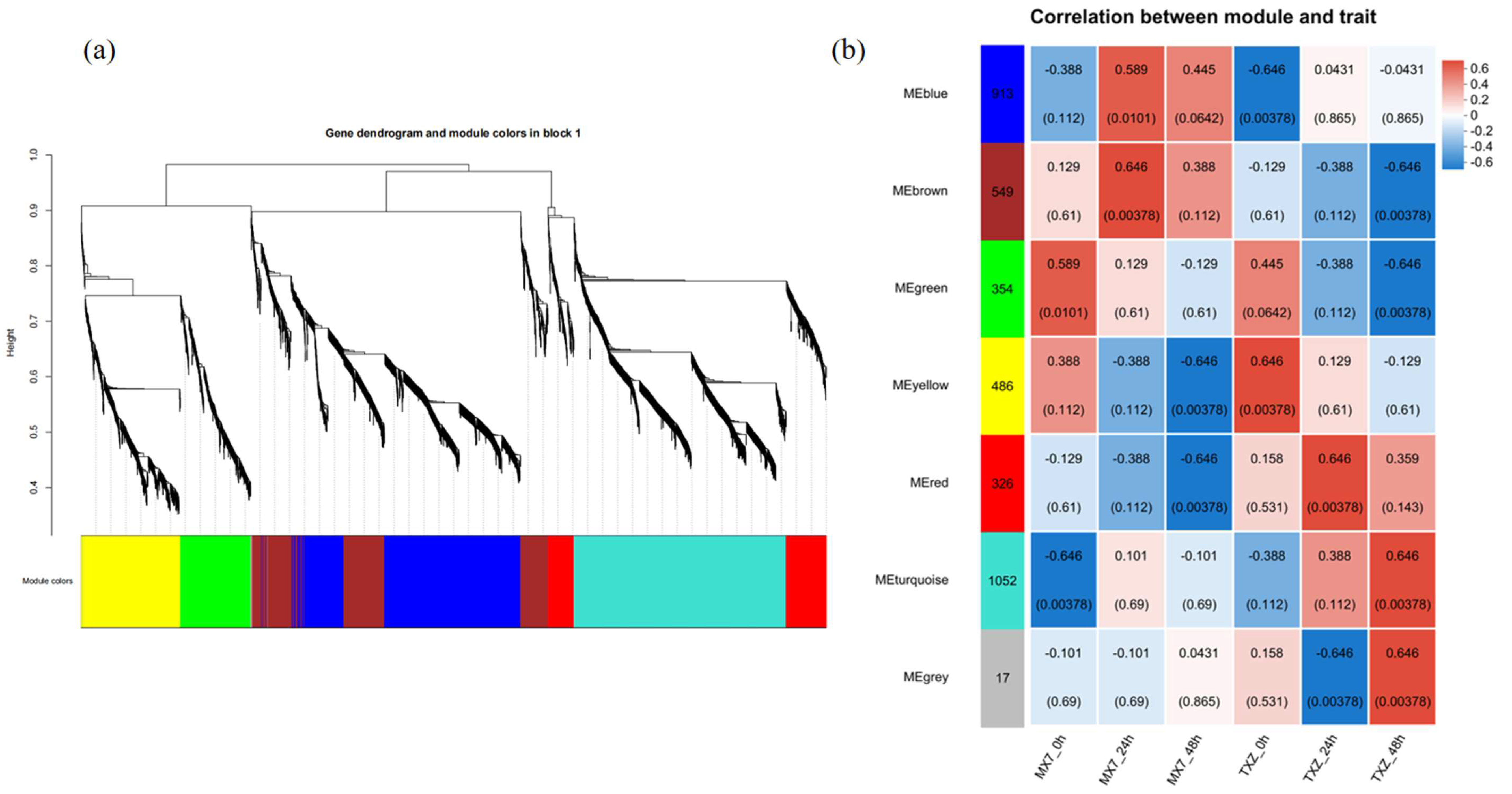
3.10. Construction of Coexpression Modules via WGCNA
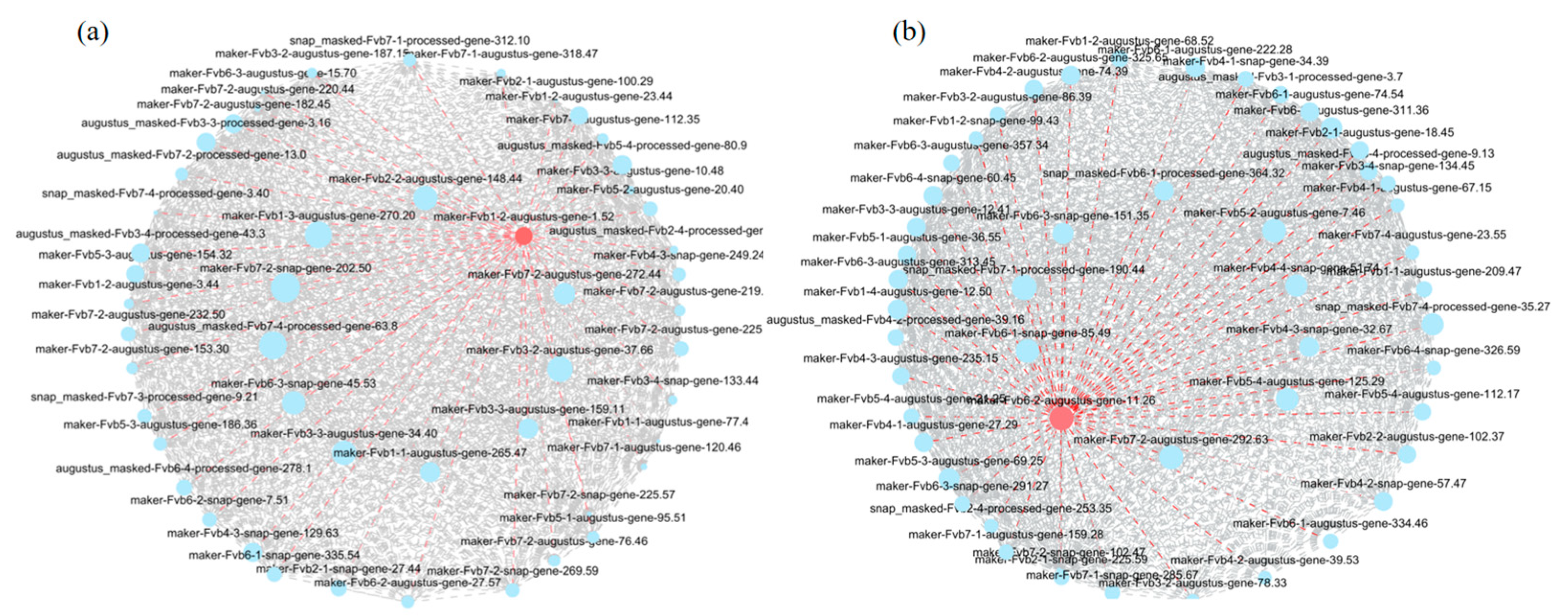
3.11. Searching for Hub Genes Related to Disease Resistance via Weighted Gene Coexpression Networks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amil-Ruiz, F.; Blanco-Portales, R.; Muñoz-Blanco, J.; Caballero, J.L. The strawberry plant defense mechanism: A molecular review. Plant Cell Physiol. 2011, 52, 1873–1903. [Google Scholar] [CrossRef]
- Fang, X.L.; Phillips, D.; Li, H.; Sivasithamparam, K.; Barbetti, M.J. Severity of crown and root diseases of strawberry and associated fungal and oomycete pathogens in Western Australia. Australas. Plant Pathol. 2011, 40, 109–119. [Google Scholar] [CrossRef]
- Adhikari, T.B.; Chacon, J.G.; Fernandez, G.E.; Louws, F.J. First Report of Anthracnose Causing Both Crown and Fruit Rot of Strawberry by Colletotrichum siamense in North Carolina. Plant Dis. 2019, 103, 1775. [Google Scholar] [CrossRef]
- Ji, Y.; Li, X.; Gao, Q.H.; Geng, C.; Duan, K. Colletotrichum species pathogenic to strawberry: Discovery history, global diversity, prevalence in China, and the host range of top two species. Phytopathol. Res. 2022, 4, 42. [Google Scholar] [CrossRef]
- Wang, C.H.; Jiang, Z.T.; Huang, R.J.; Shi, G.C.; Wang, P.P.; Xue, L. The occurrence and control to strawberry anthracnose in China. Acta Hortic. 2017, 1156, 797–800. [Google Scholar] [CrossRef]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum Species Responsible for Anthracnose Diseases of Various Fruits. Plant Dis. 1998, 82, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Godana, E.A.; Sui, Y.; Yang, Q.; Zhang, X.; Zhao, L. Biological control as an alternative to synthetic fungicides for the management of grey and blue mould diseases of table grapes: A review. Crit. Rev. Microbiol. 2020, 46, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Zeng, X.G.; Xiang, F.Y.; Zhang, Q.H.; Guo, C.; Chen, F.Y.; Gu, Y.C. Carbendazim sensitivity in populations of Colletotrichum gloeosporioides complex infecting strawberry and yams in Hubei Province of China. J. Integr. Agric. 2018, 17, 1391–1400. [Google Scholar] [CrossRef]
- Mangandi, J.; Peres, N.A.; Whitaker, V.M. Identifying resistance to crown rot caused by Colletotrichum gloeosporioides in strawberry. Plant Dis. 2015, 99, 954–961. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Rakwal, R.; Jwa, N.S. Rice (Oryza sativa L.) OsPR1b gene is phytohormonally regulated in close interaction with light signals. Mol. Genet. Genom. 2002, 278, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Súnico, V.; Higuera, J.J.; Amil-Ruiz, F.; Arjona-Girona, I.; López-Herrera, C.J.; Muñoz-Blanco, J.; Maldonado-Alconada, A.M.; Caballero, J.L. FaNPR3 Members of the NPR1-like Gene Family Negatively Modulate Strawberry Fruit Resistance against Colletotrichum acutatum. Plants 2024, 13, 2261. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, P.; Gong, Y.; Xu, L.L.; Wu, J.; Wang, A.N.; Wang, L.; Hu, J.B.; Dong, K.; Zhu, J.Y.; et al. A Positive Regulator CsPR10-9 Confers Resistance to Anthracnose (Colletotrichum gloeosporioides) Is Negatively Regulated by CsMYB72 in Tea Plants. Plant Cell Environ. 2025, 48, 6965–6981. [Google Scholar] [CrossRef]
- Ren, Z.; Tang, B.; Xing, J.; Liu, C.; Cai, X.; Hendy, A.; Kamran, M.; Liu, H.; Zheng, L.; Huang, J.; et al. MTA1-mediated RNA m6A modification regulates autophagy and is required for infection of the rice blast fungus. New Phytol. 2022, 235, 247–262. [Google Scholar] [CrossRef]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- Adam, L.; Ellwood, S.; Wilson, I.; Saenz, G.; Xiao, S.; Oliver, R.P.; Turner, J.G.; Somerville, S. Comparison of Erysiphe cichoracearum and E. cruciferarum and a survey of 360 Arabidopsis thaliana accessions for resistance to these two powdery mildew pathogens. Mol. Plant Microbe Interact. 1999, 12, 1031–1043. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.; Balajee, S.A.; Schroers, H.J.; Summerbell, R.C.; Robert, V.A.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef]
- Duarte, I.G.; Veloso, J.S.; Amaral, A.G.G.; Silva, A.C.D.; Silva, H.R.; Balbino, V.D.Q.; Vieira, W.A.D.S.; Castlebury, L.; Câmara, M.P.S. Colletotrichum siamense causing anthracnose on Etlingera elatior. Crop Prot. 2022, 162, 106092. [Google Scholar] [CrossRef]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Y.; Gao, X.; Li, J.; Zhang, S. Research on Identification Technology of Strawberry Varieties’ Resistance to Anthracnose. J. Fruit Sci. 2008, 25, 542–547. [Google Scholar] [CrossRef]
- Feng, W.; Shi, J.; Jin, J.; Duan, Z.Y.; Liang, X.; Luo, J.; Qiu, L.; Luo, J.; Xu, X.; Wen, Y.; et al. Comprehensive Evaluation of Resistance of Different Strawberry Varieties to Xanthomonas fragariae. Sci. Hortic. 2023, 325, 112647. [Google Scholar] [CrossRef]
- Hsu, K.C.; Hsu, P.F.; Chen, Y.C.; Lin, H.C.; Hung, C.C.; Chen, P.C.; Huang, Y.L. Oxidative stress during bacterial growth characterized through microdialysis sampling coupled with HPLC/fluorescence detection of malondialdehyde. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2016, 1019, 112–116. [Google Scholar] [CrossRef]
- Li, A.; Zhang, R.; Pan, L.; Tang, L.; Zhao, G.; Zhu, M.; Chu, J.; Sun, X.; Wei, B.; Zhang, X.; et al. Transcriptome analysis of H2O2-treated wheat seedlings reveals a H2O2-responsive fatty acid desaturase gene participating in powdery mildew resistance. PLoS ONE 2011, 6, e28810. [Google Scholar] [CrossRef]
- Ma, H.; Zou, F.; Li, D.; Wan, Y.; Zhang, Y.; Zhao, Z.; Wang, X.; Gao, H. Transcription Factor MdbHLH093 Enhances Powdery Mildew Resistance by Promoting Salicylic Acid Signaling and Hydrogen Peroxide Accumulation. Int. J. Mol. Sci. 2023, 24, 9390. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Sun, X.; Li, J.H.; Cao, X.Q.; Yao, S.Z.; Han, Y.H.; Chen, C.T.; Du, L.L.; Li, S.; et al. Perception of viral infections and initiation of antiviral defence in rice. Nature 2025, 641, 173–181. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, X.; Lin, T.; Zhang, Z.; Wu, X.; Zhang, Y.; Liu, Y.; Tian, Z. Accumulation of dually targeted StGPT1 in chloroplasts mediated by StRFP1, an E3 ubiquitin ligase, enhances plant immunity. Hortic. Res. 2024, 11, uhae241. [Google Scholar] [CrossRef]
- Wang, L.; Bian, L.; Shi, Q.L.; Li, X.; Sun, Y.D.; Li, M.; Zhao, A.Q.; Peng, X.Y.; Yu, Y.H. The Vitis yeshanensis U-box E3 ubiquitin ligase VyPUB21 enhances resistance to powdery mildew by targeting degradation of NIM1-interacting (NIMIN) protein. Plant Cell Rep. 2024, 43, 93. [Google Scholar] [CrossRef]
- Sun, C.; Liao, D.H.; Wu, S. The advances of PHR and SPX in the nutrition signaling pathway. J. Plant Nutr. Fertil. 2021, 27, 1080–1090. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Xu, X.; Zou, X.; Duan, K.; Gao, Q. Characterization and Fungicide Sensitivity of Colletotrichum Species Causing Strawberry Anthracnose in Eastern China. Plant Dis. 2020, 104, 1960–1968. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Hu, M.; Wu, J.; Zhang, C. Fungal Pathogens Associated with Strawberry Crown Rot Disease in China. J. Fungi 2022, 8, 1161. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Geng, C.; Song, L.; Zhang, L.Q.; Yang, J.; Gao, Q.H.; Han, Y.C.; Duan, K. Different responses to elevated temperature in the representative strains of strawberry pathogenic Colletotrichum spp. from eastern China. Mycol. Prog. 2023, 22, 3. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, Y.; Liang, X.; Yang, H.L.; Ma, Y.Y.; Wei, F.; Qiu, L.J.; Li, X.X.; Lu, L.J.; Zhao, W.J.; et al. Evaluation of host resistance and susceptibility to Podosphaera aphanis NWAU1 infection in 19 strawberry varieties. Sci. Hortic. 2023, 315, 111977. [Google Scholar] [CrossRef]
- Sun, J.; Lin, H.; Zhang, S.; Lin, Y.; Wang, H.; Lin, M.; Hung, Y.C.; Chen, Y. The roles of ROS production-scavenging system in Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-induced pericarp browning and disease development of harvested longan fruit. Food Chem. 2018, 247, 16–22. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Lin, Y.; Shi, J.; Lin, Y. A novel chitosan formulation treatment induces disease resistance of harvested litchi fruit to Peronophythora litchii in association with ROS metabolism. Food Chem. 2018, 266, 299–308. [Google Scholar] [CrossRef]
- Liang, X.; Wei, F.; Yang, H.; Fan, L.; Cai, X.; Ma, Y.; Shi, J.; Xing, K.; Qiu, L.; Li, X.; et al. Flagella-Driven Motility Is Critical to the Virulence of Xanthomonas fragariae in Strawberry. Plant Dis. 2023, 107, 3506–3516. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A.; Mhlongo, M.I. Metabolite profiling of susceptible and resistant wheat (Triticum aestivum) cultivars responding to Puccinia striiformis f. sp. tritici infection. BMC Plant Biol. 2023, 23, 293. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Lin, W.; Chai, J.; Shangguan, X.; Zhao, T. ZmDREB1A controls plant immunity via regulating salicylic acid metabolism in maize. Plant J. 2025, 121, e17226. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Cao, Q.; Huang, L.; Li, J.; Qu, P.; Tao, P.; Crabbe, M.J.C.; Zhang, T.; Qiao, Q. Integrated transcriptome and methylome analyses reveal the molecular regulation of drought stress in wild strawberry (Fragaria nilgerrensis). BMC Plant Biol. 2022, 22, 613. [Google Scholar] [CrossRef]
- Cheng, M.C.; Hsieh, E.J.; Chen, J.H.; Chen, H.Y.; Lin, T.P. Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol. 2012, 158, 363–375. [Google Scholar] [CrossRef]
- Chen, Y.T.; Liu, H.; Stone, S.; Callis, J. ABA and the ubiquitin E3 ligase KEEP ON GOING affect proteolysis of the Arabidopsis thaliana transcription factors ABF1 and ABF3. Plant J. 2013, 75, 965–976. [Google Scholar] [CrossRef]
- Bueso, E.; Rodriguez, L.; Lorenzo-Orts, L.; Gonzalez-Guzman, M.; Sayas, E.; Muñoz-Bertomeu, J.; Ibañez, C.; Serrano, R.; Rodriguez, P.L. The single-subunit RING-type E3 ubiquitin ligase RSL1 targets PYL4 and PYR1 ABA receptors in plasma membrane to modulate abscisic acid signaling. Plant J. 2014, 80, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, X.; Peirats-Llobet, M.; Belda-Palazon, B.; Wang, X.; Cui, S.; Yu, X.; Rodriguez, P.L.; An, C. Ubiquitin Ligases RGLG1 and RGLG5 Regulate Abscisic Acid Signaling by Controlling the Turnover of Phosphatase PP2CA. Plant Cell 2016, 28, 2178–2196. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L.; Williams, L.A.; Farmer, L.M.; Vierstra, R.D.; Callis, J. KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 2006, 18, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.B.; Han, H.; Lee, S. The role of WRKY transcription factors, FaWRKY29 and FaWRKY64, for regulating Botrytis fruit rot resistance in strawberry (Fragaria × ananassa Duch.). BMC Plant Biol. 2023, 23, 420. [Google Scholar] [CrossRef] [PubMed]
| Species | Variety Name | Variety Number | Chromosome Ploidy | Parents | Origin |
|---|---|---|---|---|---|
| Fragaria × ananassa Duch. | Akihime (Zhangji) | ZJ | 8× | Kunouwase × Nyoho | Japan |
| Benihoppe (Hongyan) | HY | 8× | Akihime × Sachinoka | Japan | |
| Daliangshanheizhenzhu | HZZ | 8× | ~ | China | |
| Fengyu 1 | FY1 | 8× | 2012-W-02 × Suizhu | China | |
| Miaoxiang 7 | MX7 | 8× | Benihoppe × Sweet Charlie | China | |
| Ningyu | NY | 8× | Sachinoka × Akihime | China | |
| Tianxianzui | TXZ | 8× | Akihime × Falandi | China | |
| Xuelixiang | XLX | 8× | ~ | China | |
| Xueqinxiang | XQX | 8× | Sulhyang × Yueli | China | |
| Tokun (Taoxun) | TX | 10× | K58N7-21 × Kurume IH1 | Japan |
| Gene | Primer | Primer (5′-3′) |
|---|---|---|
| rDNA-ITS | ITS1 | TCCGTAGGTGAACCTGCGG |
| ITS4 | TCCTCCGCTTATTGATATGC | |
| TUB | T1 | AACATGCGTGAGATTGTAAGT |
| Bt2b | ACCCTCAGTGTAGTGACCCTTGGC | |
| CAL | CL1C | GAATTCAAGGAGGCCTTCTC |
| CL2C | CTTCTGCATCATGAGCTGGAC | |
| ACT | ACT-512F | ATGTGCAAGGCCGGTTTCGC |
| ACT-783R | TACGAGTCCTTCTGGCCCAT | |
| CHS-1 | CHS-79F | TGGGGCAAGGATGCTTGGAAGAAG |
| CHS-354R | TGGAAGAACCATCTGTGAGAGTTG |
| Disease Grade | Severity of Symptoms |
|---|---|
| 0 | asymptomatic |
| 1 | 0 < percentage of spot area to leaf area ≤ 5% |
| 3 | 5% < percentage of spot area to leaf area ≤ 15% |
| 5 | 15% < percentage of spot area to leaf area ≤ 30% |
| 7 | 30% < percentage of spot area to leaf area ≤ 50% |
| 9 | 50% < percentage of spot area to leaf area ≤ 100% |
| Resistant | Disease Index (DI) |
|---|---|
| Immune, I | DI = 0 |
| High Resistance, HR | 0 < DI ≤ 10.0% |
| Resistance, R | 10.0% < DI ≤ 20.0% |
| Moderate Resistance, MR | 20.0% < DI ≤ 30.0% |
| Moderate Sensitive, MS | 30.0% < DI ≤ 50.0% |
| Sensitive, S | 50.0% < DI ≤ 80.0% |
| Highly Sensitive, HS | 80.0% < DI ≤ 100% |
| Variety Number | Disease Index (DI) ± SD | Resistance | |
|---|---|---|---|
| 1 | FY1 | 77.78 ± 6.414 a | S |
| 2 | HY | 25.93 ± 3.703 cd | MR |
| 3 | HZZ | 27.78 ± 3.703 cd | MR |
| 4 | MX7 | 61.90 ± 3.703 ab | S |
| 5 | NY | 33.33 ± 6.414 cd | MR |
| 6 | TX | 22.22 ± 6.414 cd | MR |
| 7 | TXZ | 14.81 ± 3.703 d | R |
| 8 | XLX | 26.98 ± 7.407 cd | MR |
| 9 | XQX | 33.33 ± 6.414 cd | MR |
| 10 | ZJ | 44.44 ± 6.417 bc | MS |
| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|---|
| TXZ_0h_1 | 45,948,472 | 6,938,219,272 | 45,535,814 | 6,754,656,273 | 0.0117 | 98.94 | 96.6 | 45.98 |
| TXZ_0h_2 | 51,956,742 | 7,845,468,042 | 51,461,878 | 7,653,823,942 | 0.0116 | 98.99 | 96.77 | 45.88 |
| TXZ_0h_3 | 40,741,774 | 6,152,007,874 | 40,316,428 | 6,017,651,569 | 0.0117 | 98.95 | 96.63 | 45.92 |
| TXZ_24h_1 | 43,340,244 | 6,544,376,844 | 42,806,818 | 6,346,496,003 | 0.0117 | 98.93 | 96.58 | 45.85 |
| TXZ_24h_2 | 44,990,548 | 6,793,572,748 | 44,578,378 | 6,632,968,154 | 0.0116 | 98.97 | 96.69 | 45.67 |
| TXZ_24h_3 | 47,095,362 | 7,111,399,662 | 46,558,442 | 6,934,461,630 | 0.0116 | 98.98 | 96.76 | 45.67 |
| TXZ_48h_1 | 43,238,730 | 6,529,048,230 | 42,783,002 | 6,369,735,811 | 0.0116 | 98.98 | 96.75 | 45.65 |
| TXZ_48h_2 | 46,877,944 | 7,078,569,544 | 46,341,678 | 6,889,158,059 | 0.0117 | 98.92 | 96.53 | 45.7 |
| TXZ_48h_3 | 43,849,952 | 6,621,342,752 | 43,326,976 | 6,419,185,076 | 0.0117 | 98.93 | 96.58 | 45.9 |
| TXZ_120h_1 | 52,475,490 | 7,923,798,990 | 51,973,460 | 7,711,031,806 | 0.0116 | 98.98 | 96.77 | 45.66 |
| TXZ_120h_2 | 43,645,226 | 6,590,429,126 | 43,240,576 | 6,462,671,064 | 0.0117 | 98.95 | 96.62 | 45.56 |
| TXZ_120h_3 | 43,916,166 | 6,631,341,066 | 43,494,546 | 6,488,817,503 | 0.0117 | 98.94 | 96.61 | 45.57 |
| MX7_0h_1 | 47,097,998 | 7,111,797,698 | 46,668,516 | 6,957,893,994 | 0.0117 | 98.93 | 96.57 | 45.97 |
| MX7_0h_2 | 41,333,248 | 6,241,320,448 | 40,891,150 | 6,089,514,671 | 0.0116 | 98.96 | 96.67 | 45.75 |
| MX7_0h_3 | 46,689,522 | 7,050,117,822 | 46,298,010 | 6,917,790,963 | 0.0118 | 98.9 | 96.44 | 45.86 |
| MX7_24h_1 | 42,454,020 | 6,410,557,020 | 42,041,832 | 6,275,218,513 | 0.0117 | 98.95 | 96.65 | 45.56 |
| MX7_24h_2 | 44,258,142 | 6,682,979,442 | 43,828,218 | 6,538,749,102 | 0.0117 | 98.95 | 96.64 | 45.53 |
| MX7_24h_3 | 46,732,768 | 7,056,647,968 | 46,267,100 | 6,916,961,270 | 0.0116 | 98.97 | 96.72 | 45.57 |
| MX7_48h_1 | 43,234,366 | 6,528,389,266 | 42,752,414 | 6,365,678,919 | 0.0117 | 98.94 | 96.64 | 45.76 |
| MX7_48h_2 | 40,651,472 | 6,138,372,272 | 40,220,550 | 6,007,501,355 | 0.0117 | 98.91 | 96.49 | 45.71 |
| MX7_48h_3 | 53,347,626 | 8,055,491,526 | 52,711,464 | 7,803,671,824 | 0.0116 | 98.96 | 96.69 | 45.66 |
| MX7_120h_1 | 43,977,184 | 6,640,554,784 | 43,534,596 | 6,511,006,817 | 0.0116 | 98.97 | 96.69 | 45.76 |
| MX7_120h_2 | 45,862,786 | 6,925,280,686 | 45,396,366 | 6,779,630,060 | 0.0117 | 98.95 | 96.64 | 45.69 |
| MX7_120h_3 | 46,059,100 | 6,954,924,100 | 45,524,368 | 6,823,682,895 | 0.0117 | 98.94 | 96.65 | 45.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Yang, J.; Huang, J.; Chen, W.; Zhong, Y.; Hu, W.; Ma, Y.; Mao, B. Resistance Phenotyping and WGCNA Identify Oxidative-Defense Hub Regulators in Strawberry Challenged by Colletotrichum siamense. Horticulturae 2025, 11, 1427. https://doi.org/10.3390/horticulturae11121427
Xie Y, Yang J, Huang J, Chen W, Zhong Y, Hu W, Ma Y, Mao B. Resistance Phenotyping and WGCNA Identify Oxidative-Defense Hub Regulators in Strawberry Challenged by Colletotrichum siamense. Horticulturae. 2025; 11(12):1427. https://doi.org/10.3390/horticulturae11121427
Chicago/Turabian StyleXie, Yulu, Jun Yang, Jiayu Huang, Weiliang Chen, Yuanxiang Zhong, Weizhen Hu, Yangyang Ma, and Bizeng Mao. 2025. "Resistance Phenotyping and WGCNA Identify Oxidative-Defense Hub Regulators in Strawberry Challenged by Colletotrichum siamense" Horticulturae 11, no. 12: 1427. https://doi.org/10.3390/horticulturae11121427
APA StyleXie, Y., Yang, J., Huang, J., Chen, W., Zhong, Y., Hu, W., Ma, Y., & Mao, B. (2025). Resistance Phenotyping and WGCNA Identify Oxidative-Defense Hub Regulators in Strawberry Challenged by Colletotrichum siamense. Horticulturae, 11(12), 1427. https://doi.org/10.3390/horticulturae11121427





