Comparative Evaluation of Organic and Synthetic Fertilizers on Lettuce Yield and Metabolomic Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Pot Experimental Design
2.2. Sampling and Analytical Methods
2.2.1. Soil Analysis
2.2.2. Plant Analysis
2.3. Statistical Analysis
3. Results
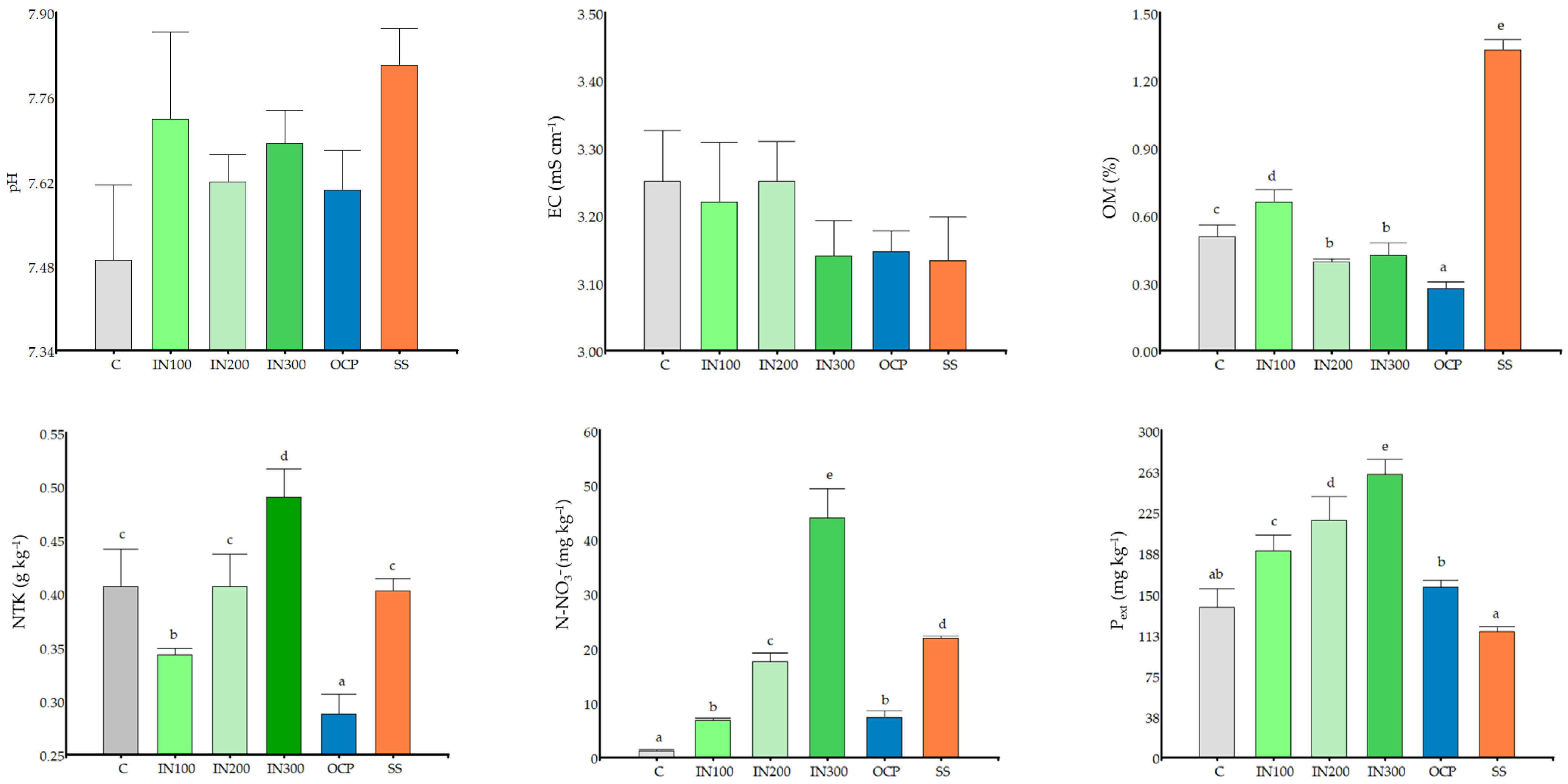
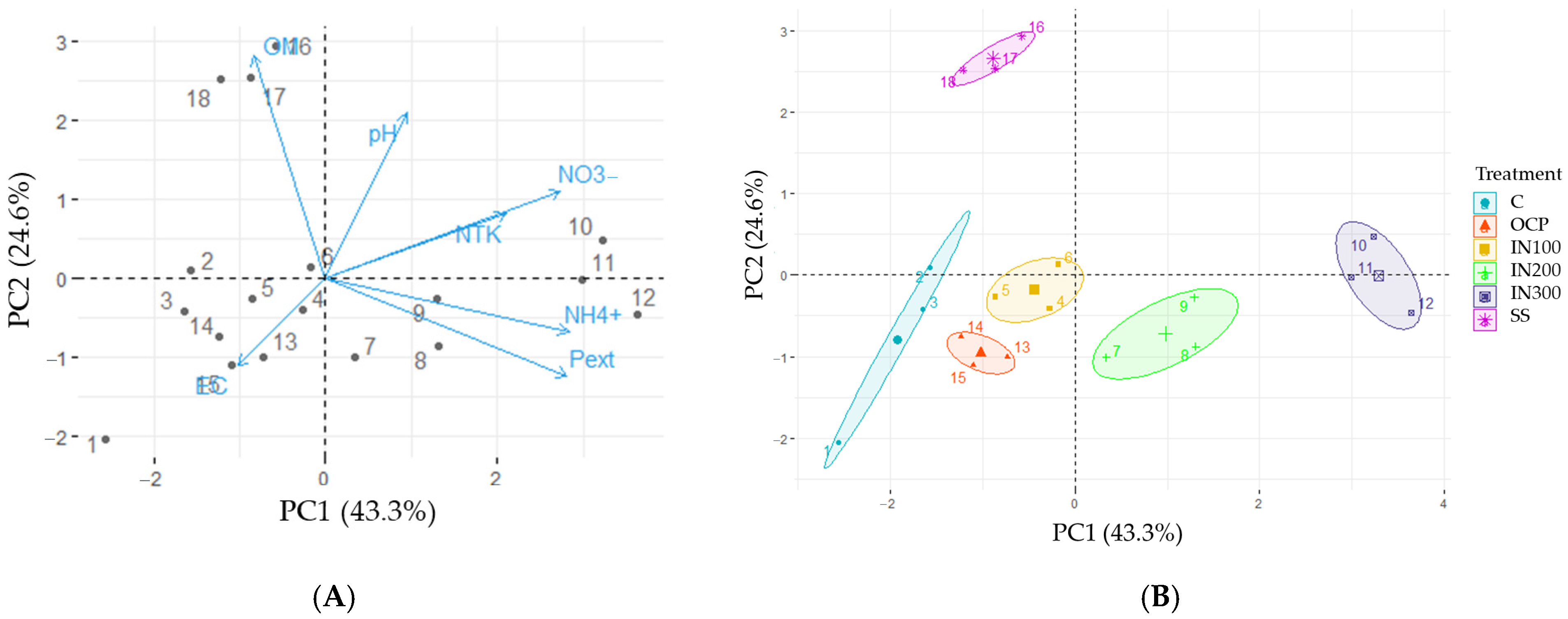
3.1. Soil Parameters
3.2. Crop Response to Fertilizer
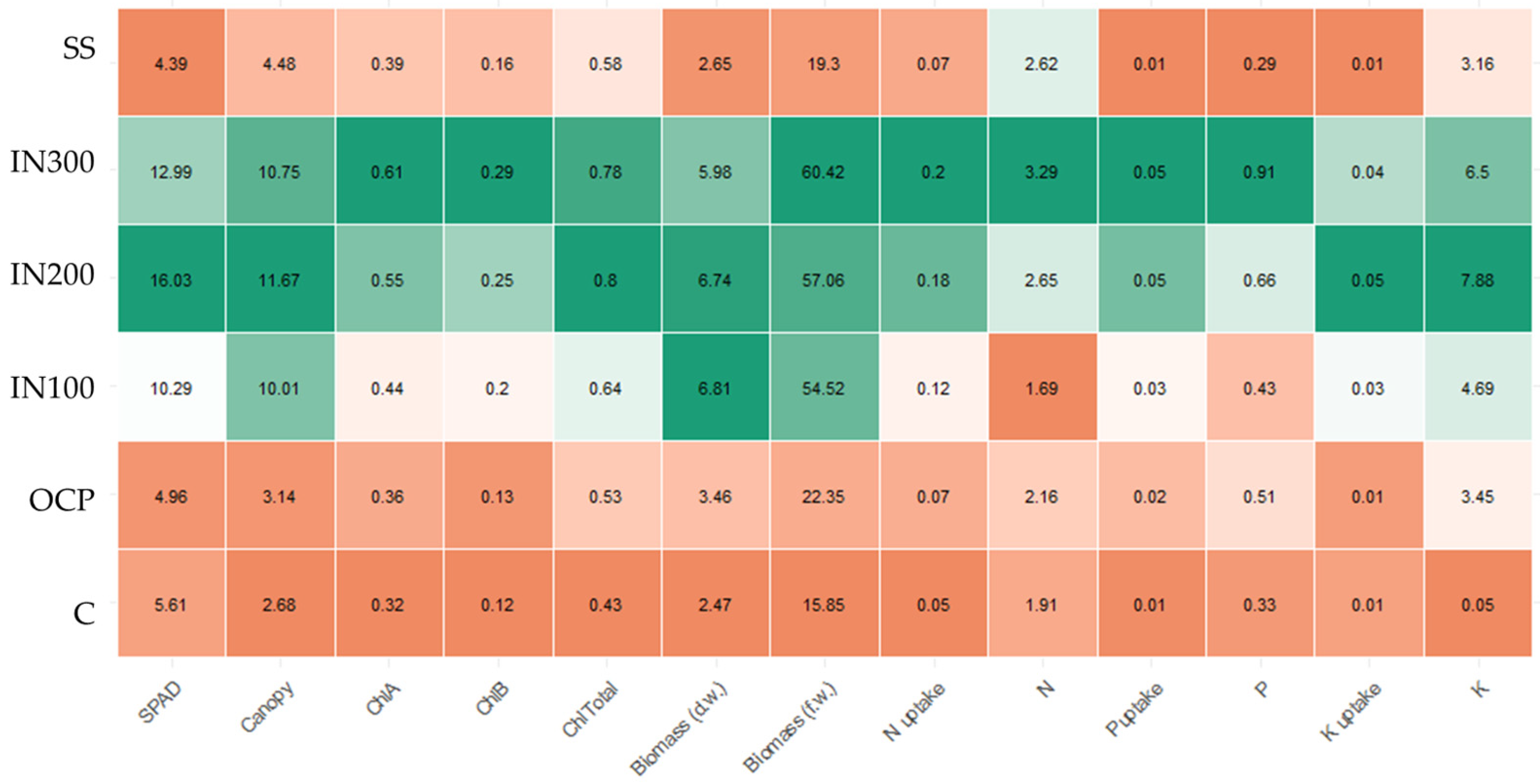
3.2.1. Biophysical Parameters and Biomass at Harvest
3.2.2. Nutrient Uptake
3.2.3. Yield and Nutrient Use Efficiency
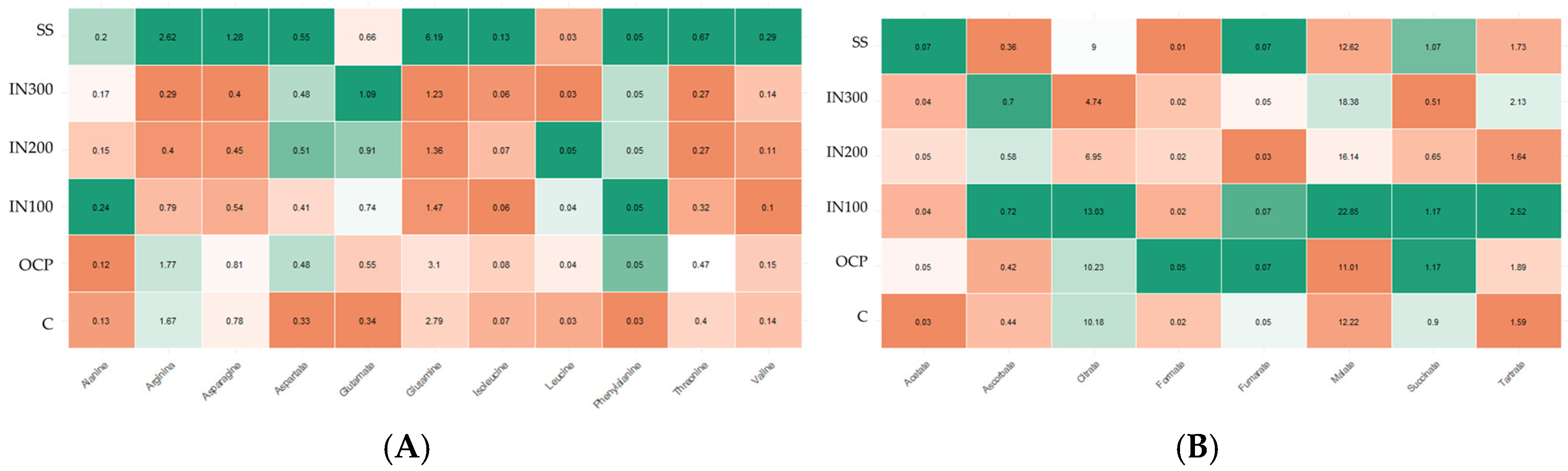
3.2.4. Nutrient Concentration Analysis
4. Discussion
4.1. Effects of Fertilization on Soil Properties
4.2. Effects of Fertilization on Crop Response
4.2.1. Effects of Fertilization on Yield and Morphological Parameters
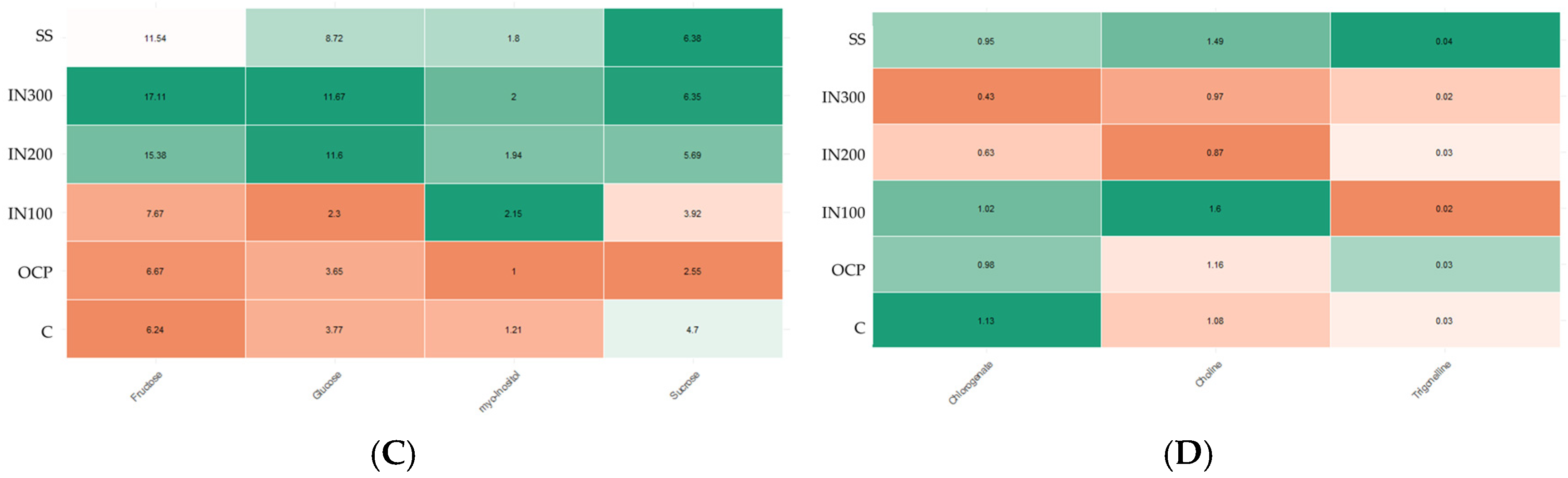
4.2.2. Effects of Fertilization on Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Zhao, Z.; Jiang, B.; Baoyin, B.; Cui, Z.; Wang, H.; Li, Q.; Cui, J. Effects of long-term application of nitrogen fertilizer on soil acidification and biological properties in China: A meta-analysis. Microorganisms 2024, 12, 1683. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elrahman, S.H.; Saudy, H.S.; Abd El-Fattah, D.A.; Hashem, F.A.-E.; Abou-Sreea, A.I. Effect of irrigation water and organic fertilizer on reducing nitrate accumulation and boosting lettuce productivity. J. Soil Sci. Plant Nutr. 2022, 22, 2144–2155. [Google Scholar] [CrossRef]
- Kerbiriou, P.J.; Stomph, T.J.; Lammerts van Bueren, E.T.; Struik, P.C. Modelling concept of lettuce breeding for nutrient efficiency. Euphytica 2014, 199, 167–181. [Google Scholar] [CrossRef]
- Gao, H.; Mao, H.; Ullah, I. Analysis of metabolomic changes in lettuce leaves under low nitrogen and phosphorus deficiencies stresses. Agriculture 2020, 10, 406. [Google Scholar] [CrossRef]
- Matamoros, V.; Rendón-Mera, A.M.; Piña, B.; Tadić, Đ.; Cànameras, N.; Carazo, N.; Bayona, J.M. Metabolomic and phenotypic implications of the application of fertilization products containing microcontaminants in lettuce (Lactuca sativa). Sci. Rep. 2021, 11, 9701. [Google Scholar] [CrossRef]
- Qadir, O.; Siervo, M.; Seal, C.J.; Brandt, K. Manipulation of contents of nitrate phenolic acids, chlorophylls, and carotenoids in lettuce (Lactuca sativa L.) via contrasting responses to nitrogen fertilizer when grown in a controlled environment. J. Agric. Food Chem. 2017, 65, 10003–10010. [Google Scholar] [CrossRef]
- Boletín Oficial del Estado (BOE). Ley 7/2022. de 8 de Abril. de Residuos y Suelos Contaminados para una Economía Circular. 2022. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2022-5809 (accessed on 1 October 2025).
- European Commission. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. Communication from the Commission to the European Parliament. The Council. The European Economic and Social Committee and the Committee of the Regions. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0098 (accessed on 1 October 2025).
- Sánchez-Méndez, S.; Valverde-Vozmediano, L.; Orden, L.; Andreu-Rodríguez, F.J.; Sáez-Tovar, J.A.; Martínez-Sabater, E.; Bustamante, M.Á.; Moral, R. Alternative Phosphorus Fertilisation with Bio-Based Pellet Fertilisers: A Case of Study on Ryegrass (Lollium perenne L.). Agronomy 2025, 15, 579. [Google Scholar] [CrossRef]
- García-Rández, A.; Orden, L.; Marks, E.A.N.; Andreu-Rodríguez, F.J.; Franco-Luesma, S.; Martínez-Sabater, E.; Sáez-Tovar, J.A.; Pérez-Murcia, M.D.; Agulló, E.; Bustamante, M.A.; et al. Monitoring of greenhouse gas emissions and compost quality during olive mill waste co-composting at industrial scale: The effect of N and C sources. J. Waste Manag. 2025, 193, 33–43. [Google Scholar] [CrossRef]
- Paredes, C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Bustamante, M.A.; Moreno-Caselles, J. Recycling of two-phase olive-mill cake “Alperujo” by co-composting with animal manures. Commun. Soil Sci. Plant Anal. 2015, 46, 238–247. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Moreno-Caselles, J.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Bernal, M.P. Co-composting of distillery and winery wastes with sewage sludge. Water Sci. Technol. 2007, 56, 187–192. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon. Organic Carbon. and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1996; pp. 961–1010. ISBN 978-0-89118-866-7. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1983; pp. 595–624. ISBN 978-0-89118-977-0. [Google Scholar]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In USDA Circular Nr 939; United States Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Bremmer, J.M.; Keeney, D.R. Steam distillation methods for determination of ammonium nitrate and nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A Powerful New Tool for Measuring Fractional Green Canopy Cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Wang, J.; Dimech, A.M.; Spangenberg, G.; Smith, K.; Badenhorst, P. Rapid screening of nitrogen use efficiency in perennial ryegrass (Lolium perenne L.) using automated image-based phenotyping. Front. Plant Sci. 2020, 11, 565361. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ritchie, R.J. Universal Chlorophyll Equations for Estimating Chlorophylls a. b. c. and d and Total Chlorophylls in Natural Assemblages of Photosynthetic Organisms Using Acetone. Methanol. or Ethanol Solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- López-Bellido, L.; López-Bellido, J.; Redondo, R. Nitrogen efficiency in wheat under rainfed Mediterranean conditions as affected by split nitrogen application. Field Crop. Res. 2005, 94, 86–97. [Google Scholar] [CrossRef]
- Van der Sar, S.; Kim, H.K.; Meissner, A.; Verpoorte, R.; Choi, Y.H. Nuclear Magnetic Resonance Spectroscopy for Plant Metabolite Profiling. In The Handbook of Plant Metabolomics; Wiley Blackwell: Hoboken, NJ, USA, 2013; pp. 57–76. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Cámara-Zapata, J.M.; Simón, I.; Martínez-Nicolás, J.J.; Lidón, V.; García-Sánchez, F. Physiological. Nutritional and Metabolomic Responses of Tomato Plants After the Foliar Application of Amino Acids Aspartic Acid. Glutamic Acid and Alanine. Front. Plant Sci. 2021, 11, 581234. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing (Version 4.3.1) Software; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 October 2025).
- Zhang, Y.; Zhang, S.; Wang, R.; Cai, J.; Zhang, Y.; Li, H.; Huang, S.; Jiang, Y. Impacts of fertilization practices on pH and the pH buffering capacity of calcareous soil. Soil Sci. Plant Nutr. 2016, 62, 432–439. [Google Scholar] [CrossRef]
- Bello, S.K.; Xu, H.; Abdelhafez, A.A.; Seneweera, S.; Tian, X. Mitigating soil salinity stress with gypsum and bio-organic amendments: A review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Debicka, M.; Spychaj-Fabisiak, E.; Gozdowski, D.; Telesiński, A. The influence of municipal solid waste compost on the transformations of phosphorus forms in soil. Agronomy 2023, 13, 1234. [Google Scholar] [CrossRef]
- Ladha, J.K.; Reddy, C.K.; Padre, A.T.; van Kessel, C. Role of nitrogen fertilization in sustaining organic matter in cultivated soils. J. Environ. Qual. 2011, 40, 1756–1766. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- de Soto, I.S.; Herencia, J.F.; Madejón, E. Twenty-five years of continuous sewage sludge application: Effects on soil quality and crop yield. Front. Soil Sci. 2022, 2, 960426. [Google Scholar] [CrossRef]
- Bernard, L.; Chapuis-Lardy, L.; Razafimbelo, T.; Razafindrakoto, M.; Razafimanantsoa, M.P.; Blagodatskaya, E.; Chotte, J.L. Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct. Ecol. 2022, 36, 1355–1377. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Gattinger, A.; Muller, A.; Haeni, M.; Skinner, C.; Fliessbach, A.; Buchmann, N.; Niggli, U. Enhanced top soil carbon stocks under organic farming. Proc. Natl. Acad. Sci. USA 2012, 109, 18226–18231. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, L.; de Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynthetica 2007, 45, 445–456. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants. 3rd ed. Edited by P. Marschner. Amsterdam, Netherlands: Elsevier/Academic Press (2011), pp. 684, ISBN 978-0-12-384905-2. Exp. Agric. 2012, 48, 305. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Ann. Rev. Plant. Biol. 2020, 2012, 153–182. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Mininni, C.; Parente, A.; Montesano, F.F.; Allegretta, I.; Terzano, R. Effects of municipal solid waste- and sewage sludge-compost-based growing media on the yield and heavy metal content of four lettuce cultivars. Environ. Sci. Pollut. Res. Int. 2017, 24, 25406–25415. [Google Scholar] [CrossRef]
- Hernández, T.; Chocano, C.; Moreno, J.L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Hefner, M.; Amery, F.; Denaeghel, H.; Loades, K.; Kristensen, H.L. Composts of diverse green wastes improve the soil biological quality, but do not alleviate drought impact on lettuce (Lactuca sativa L.) growth. Soil Use Manag. 2024, 40, e13016. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Zhang, F.S.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 2006, 157, 254–263. [Google Scholar] [CrossRef] [PubMed]
- González-Zamora, J.E.; Gamero-Monge, J.M.; Pérez-de la Luz, R. The use of olive mill pomace compost increases the population of certain ground/soil organisms in olive groves. Eur. J. Soil Biol. 2024, 122, 103668. [Google Scholar] [CrossRef]
- Hawkesford, M. Genetic variation for nitrogen use efficiency in crops. J. Exp. Bot. 2017, 68, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Mañas, M.P.; de las Heras, J. Nitrate content of lettuce (Lactuca sativa L.) after fertilization with sewage sludge and irrigation with treated wastewater. Food Addit. Contam. Part A 2009, 26, 172–179. [Google Scholar] [CrossRef]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef]
- Lanzotti, V.; Anzano, A.; Grauso, L.; Zotti, M.; Sacco, A.; Senatore, M.; Moreno, M.; Diano, M.; Parente, M.; Esposito, S.; et al. NMR Metabolomics and Chemometrics of Lettuce, Lactuca sativa L., under Different Foliar Organic Fertilization Treatments. Plants 2022, 11, 2164. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.K.; Umar, W.; Razzaq, A.; Aziz, T.; Maqsood, M.A.; Wei, S.; Niu, Q.; Huang, D.; Chang, L. Differential Metabolic Responses of Lettuce Grown in Soil, Substrate and Hydroponic Cultivation Systems under NH4+/NO3− Application. Metabolites 2022, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lyu, W.; Gao, Y.; Zhang, X.; Sun, Y.; Huang, B. Choline-Mediated Lipid Reprogramming as a Dominant Salt Tolerance Mechanism in Grass Species Lacking Glycine Betaine. Plant Cell Physiol. 2021, 61, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
| Treatments | Fertilizer Type | Acronym | Nutrient Dose |
|---|---|---|---|
| (kg N ha−1) | |||
| Control without fertilizer | Control | C | |
| Complex (15-15-15) | Synthetic | IN100 | 100 |
| Complex (15-15-15) | Synthetic | IN200 | 200 |
| Complex (15-15-15) | Synthetic | IN300 | 300 |
| Pelletized compost | Organic | OCP | 200 |
| Sewage Sludge | Organic | SS | 200 |
| Treatments | SPAD | Canopy | Chl | Biomass |
|---|---|---|---|---|
| (%) | (mg g−1) | (g pot −1) | ||
| C | 5.61 b | 2.68 a | 0.43 a | 15.85 a |
| IN100 | 10.29 c | 10.01 c | 0.64 c | 54.52 b |
| IN200 | 16.03 e | 11.67 c | 0.80 d | 57.06 b |
| IN300 | 12.99 d | 10.75 c | 0.78 d | 60.42 b |
| OCP | 4.96 ab | 3.14 ab | 0.53 b | 22.35 a |
| SS | 4.39 a | 4.48 b | 0.58 bc | 19.30 a |
| F-ANOVA | *** | *** | *** | *** |
| Treatments | N Uptake | NUE | P Uptake | PUE | K Uptake | KUE | Yield |
|---|---|---|---|---|---|---|---|
| (g N pot−1) | (%) | (g P pot−1) | (%) | (g K pot−1) | (%) | (g pot−1) | |
| C | 0.05 a | - | 0.01 a | - | 0.01 a | - | 2.47 a |
| IN100 | 0.12 b | 71.43 d | 0.03 c | 24.90 c | 0.03 b | 24.40 c | 6.81 d |
| IN200 | 0.18 c | 62.72 c | 0.05 d | 23.90 c | 0.05 d | 25.60 c | 6.07 d |
| IN300 | 0.20 e | 52.54 b | 0.05 e | 18.90 b | 0.04 c | 11.06 b | 5.98 c |
| OC P | 0.07 b | 14.48 a | 0.02 b | 23.93 c | 0.01 a | 0.00 a | 3.33 b |
| SS | 0.07 b | 11.72 a | 0.01 a | 8.23 a | 0.01 a | 0.00 a | 2.61 a |
| F-ANOVA | *** | *** | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rández, A.; Orden, L.; Sánchez-Méndez, S.; Andreu-Rodríguez, F.J.; Sáez-Tovar, J.A.; Martínez-Sabater, E.; Bustamante, M.d.l.Á.; Pérez-Murcia, M.D.; Moral, R. Comparative Evaluation of Organic and Synthetic Fertilizers on Lettuce Yield and Metabolomic Profiles. Horticulturae 2025, 11, 1421. https://doi.org/10.3390/horticulturae11121421
García-Rández A, Orden L, Sánchez-Méndez S, Andreu-Rodríguez FJ, Sáez-Tovar JA, Martínez-Sabater E, Bustamante MdlÁ, Pérez-Murcia MD, Moral R. Comparative Evaluation of Organic and Synthetic Fertilizers on Lettuce Yield and Metabolomic Profiles. Horticulturae. 2025; 11(12):1421. https://doi.org/10.3390/horticulturae11121421
Chicago/Turabian StyleGarcía-Rández, Ana, Luciano Orden, Silvia Sánchez-Méndez, Francisco Javier Andreu-Rodríguez, José Antonio Sáez-Tovar, Encarnación Martínez-Sabater, María de los Ángeles Bustamante, María Dolores Pérez-Murcia, and Raúl Moral. 2025. "Comparative Evaluation of Organic and Synthetic Fertilizers on Lettuce Yield and Metabolomic Profiles" Horticulturae 11, no. 12: 1421. https://doi.org/10.3390/horticulturae11121421
APA StyleGarcía-Rández, A., Orden, L., Sánchez-Méndez, S., Andreu-Rodríguez, F. J., Sáez-Tovar, J. A., Martínez-Sabater, E., Bustamante, M. d. l. Á., Pérez-Murcia, M. D., & Moral, R. (2025). Comparative Evaluation of Organic and Synthetic Fertilizers on Lettuce Yield and Metabolomic Profiles. Horticulturae, 11(12), 1421. https://doi.org/10.3390/horticulturae11121421







