Foliar Application of Ca-Based Fertilizers (Conventional vs. Nanofertilizers): Effects on Fruit Traits, Seed Quality Parameters and Initial Plant Growth of Tomato Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Trial
2.3. Tomato Fruit Characterization
2.4. Seed Extraction
2.5. The Germination Test
2.5.1. Assessment of Seed Germination and Germination-Related Parameters
2.5.2. Assessment of Seedling Growth Parameters and Biomass Accumulation
2.6. Statistical Data Analysis
3. Results
3.1. Characteristics of Tomato Fruit
3.2. Seed Germination and Germination-Related Parameters
3.3. Initial Plant Growth
3.4. PCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Renna, M.; Durante, M.; Gonnella, M.; Buttaro, D.; D’Imperio, M.; Mita, G.; Serio, F. Quality and Nutritional Evaluation of Regina Tomato, a Traditional Long-Storage Landrace of Puglia (Southern Italy). Agriculture 2018, 8, 83. [Google Scholar] [CrossRef]
- Casals, J.; Romero Del Castillo, R.; Pons, C.; Mazzucato, A.; Tringovska, I.; Pasev, G.; Barone, A.; Soler, S.; Fumelli, L.; Grozeva, S.; et al. European Fresh-Market Tomato Sensory Ideotypes Based on Consumer Preferences. Sci. Hortic. 2024, 335, 113351. [Google Scholar] [CrossRef]
- FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 14 November 2024).
- Farooq, A.; Javad, S.; Jabeen, K.; Ali Shah, A.; Ahmad, A.; Noor Shah, A.; Nasser Alyemeni, M.; Mosa, W.F.A.; Abbas, A. Effect of Calcium Oxide, Zinc Oxide Nanoparticles and Their Combined Treatments on Growth and Yield Attributes of Solanum lycopersicum L. J. King Saud Univ. Sci. 2023, 35, 102647. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of Nano-SiO2 in Germination of Tomato (Lycopersicum esculentum Seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Souri, M.K.; Dehnavard, S. Tomato Plant Growth, Leaf Nutrient Concentrations and Fruit Quality under Nitrogen Foliar Applications. Adv. Hortic. Sci. 2017, 32, 41–47. [Google Scholar] [CrossRef]
- Le, V.T.; Bui, B.T. Effects of Gibberellic Acid, Micronutrient Fertilizer and Calcium Nitrate Foliar Fertilizer on Growth and Yield of Tomato Solanum lycopersicum L. Cultivated in Vietnam. RUDN J. Agron. Anim. Ind. 2019, 14, 306–318. [Google Scholar] [CrossRef]
- Mazumder, M.N.N.; Misran, A.; Ding, P.; Wahab, P.E.M.; Mohamad, A. Preharvest Foliar Spray of Calcium Chloride on Growth, Yield, Quality, and Shelf Life Extension of Different Lowland Tomato Varieties in Malaysia. Horticulturae. 2021, 7, 466. [Google Scholar] [CrossRef]
- Abdelhameed, A.; Abd El-Hady, M. Response of Tomato Plant to Foliar Application of Calcium and Potassium Nitrate Integrated with Different Phosphorus Rates under Sandy Soil Conditions. Egypt. J. Soil Sci. 2018, 58, 45–55. [Google Scholar] [CrossRef]
- Islam, M.M.; Jahan, K.; Sen, A.; Urmi, T.A.; Haque, M.M.; Ali, H.M.; Siddiqui, M.H.; Murata, Y. Exogenous Application of Calcium Ameliorates Salinity Stress Tolerance of Tomato (Solanum lycopersicum L.) and Enhances Fruit Quality. Antioxidants 2023, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Batistič, O.; Kudla, J. Analysis of Calcium Signaling Pathways in Plants. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Demarty, M.; Morvan, C.; Thellier, M. Calcium and the Cell Wall. Plant Cell Environ. 1984, 7, 441–448. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Haider, S.T.-A.; Anjum, M.A.; Shah, M.N.; Hassan, A.U.; Parveen, M.; Danish, S.; Alharbi, S.A.; Alfarraj, S. Deciphering the Effects of Different Calcium Sources on the Plant Growth, Yield, Quality, and Postharvest Quality Parameters of ‘Tomato’. Horticulturae 2024, 10, 1003. [Google Scholar] [CrossRef]
- And, X.H.; Papadopoulos, A.P. Effects of Calcium and Magnesium on Growth, Fruit Yield and Quality in a Fall Greenhouse Tomato Crop Grown on Rockwool. Can. J. Plant Sci. 2003, 83, 903–912. [Google Scholar] [CrossRef]
- Jiang, J.-F.; Li, J.-G.; Dong, Y.-H. Effect of Calcium Nutrition on Resistance of Tomato against Bacterial Wilt Induced by RalstoniaSolanacearum. Eur. J. Plant Pathol. 2013, 136, 547–555. [Google Scholar] [CrossRef]
- Yan, S.; Hu, Q.; Wei, Y.; Jiang, Q.; Yin, M.; Dong, M.; Shen, J.; Du, X. Calcium Nutrition Nanoagent Rescues Tomatoes from Mosaic Virus Disease by Accelerating Calcium Transport and Activating Antiviral Immunity. Front. Plant Sci. 2022, 13, 1092774. [Google Scholar] [CrossRef] [PubMed]
- Wasti, S.; Manaa, A.; Mimouni, H.; Nsairi, A.; Ibtissem, M.; Gharbi, E.; Gautier, H.; Ben Ahmed, H. Exogenous Application of Calcium Silicate Improves Salt Tolerance in Two Contrasting Tomato (Solanum lycopersicum) Cultivars. J. Plant Nutr. 2017, 40, 673–684. [Google Scholar] [CrossRef]
- White, P.J. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.V.; Meza-Figueroa, D.; Rivera-Cruz, M.C.; Cadenas-Pliego, G.; Juárez-Maldonado, A. SiO2 Nanoparticles Improve Nutrient Uptake in Tomato Plants Developed in the Presence of Arsenic. Rev. Bio Cienc. 2021, 8, e1084. [Google Scholar] [CrossRef]
- Marchiol, L.; Filippi, A.; Adamiano, A.; Degli Esposti, L.; Iafisco, M.; Mattiello, A.; Petrussa, E.; Braidot, E. Influence of Hydroxyapatite Nanoparticles on Germination and Plant Metabolism of Tomato (Solanum lycopersicum L.): Preliminary Evidence. Agronomy 2019, 9, 161. [Google Scholar] [CrossRef]
- Mastronardi, E.; Tsae, P.; Zhang, X.; Monreal, C.; DeRosa, M.C. Strategic Role of Nanotechnology in Fertilizers: Potential and Limitations. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 25–67. ISBN 978-3-319-14023-0. [Google Scholar]
- Ditta, A.; Arshad, M. Applications and Perspectives of Using Nanomaterials for Sustainable Plant Nutrition. Nanotechnol. Rev. 2016, 5, 209–229. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Hasan, M.R.; Ahommed, M.S.; Bacchu, M.S.; Ali, M.R.; Khan, M.Z.H. Nanofertilizers towards Sustainable Agriculture and Environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Avila-Quezada, G.D.; Ingle, A.P.; Golińska, P.; Rai, M. Strategic Applications of Nano-Fertilizers for Sustainable Agriculture: Benefits and Bottlenecks. Nanotechnol. Rev. 2022, 11, 2123–2140. [Google Scholar] [CrossRef]
- Tantawy, A.S.; Salama, Y.A.M.; Abdel-Mawgoud, M.R.; Ghoname, A.A. Comparison of chelated calcium with nano calcium on alleviation of salinity negative effects on tomato plants. Middle East J. Agric. Res. 2014, 3, 912–916. [Google Scholar]
- Rahman, M.H.; Hasan, M.N.; Nigar, S.; Ma, F.; Aly Saad Aly, M.; Khan, M.Z.H. Synthesis and Characterization of a Mixed Nanofertilizer Influencing the Nutrient Use Efficiency, Productivity, and Nutritive Value of Tomato Fruits. ACS Omega 2021, 6, 27112–27120. [Google Scholar] [CrossRef]
- Roushan, K.; Deepanshu; Singh, D. Effect of Traditional Fertilizer, Nano-Fertilizer and Micronutrient on Growth, Yield and Quality of Tomato (Solanum lycopersicum L.). IJECC 2023, 13, 3154–3162. [Google Scholar] [CrossRef]
- Takač, A. Proizvodnja paradajza. In Semenarstvo 3; Milošević, M., Kobiljski, B., Eds.; Institut za ratarstvo i povrtarstvo: Novi Sad, Serbia, 2011; Volume 3, pp. 123–172. ISBN 978-86-80417-34-9. [Google Scholar]
- ISTA. International Rules for Seed Testing; Seed Science and Technology: Zurich, Switzerland, 2021. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Channaoui, S.; El Idrissi, I.S.; Mazouz, H.; Nabloussi, A. Reaction of Some Rapeseed ( Brassica napus L.) Genotypes to Different Drought Stress Levels during Germination and Seedling Growth Stages. OCL 2019, 26, 23. [Google Scholar] [CrossRef]
- Vojnović, Đ.; Maksimović, I.; TepićHorecki, A.; Milić, A.; Šumić, Z.; Žunić, D.; Adamović, B.; Ilin, Ž. Biostimulants Improve Bulb Yield, Concomitantly Affecting the Total Phenolics, Flavonoids, and Antioxidant Capacity of Onion (Allium cepa). Horticulturae 2024, 10, 391. [Google Scholar] [CrossRef]
- Kamal, A.; Abd Al-Gaid, M. Enhancing Tomato Fruits Yield and Quality Using Foliar Spray with Calcium. J. Plant Prod. 2008, 33, 8723–8734. [Google Scholar] [CrossRef]
- Birgin, Ö.; Akhoundnejad, Y.; Dasgan, H.Y. The Effect of Foliar Calcium Application in Tomato(Solanum lycopersicum L.) Under Drought Stress in Greenhouse Conditions. Appl. Ecol. Env. Res. 2021, 19, 2971–2982. [Google Scholar] [CrossRef]
- Santos, E.; Montanha, G.S.; Agostinho, L.F.; Polezi, S.; Marques, J.P.R.; De Carvalho, H.W.P. Foliar Calcium Absorption by Tomato Plants: Comparing the Effects of Calcium Sources and Adjuvant Usage. Plants 2023, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Haleema, B.; Shah, S.T.; Basit, A.; Hikal, W.M.; Arif, M.; Khan, W.; Said-Al Ahl, H.A.H.; Fhatuwani, M. Comparative Effects of Calcium, Boron, and Zinc Inhibiting Physiological Disorders, Improving Yield and Quality of Solanum lycopersicum. Biology 2024, 13, 766. [Google Scholar] [CrossRef]
- Soundharya, N.; Srinivasan, S.; Sivakumar, T.; Kamalkumaran, P. Effect of Foliar Application of Nutrients and Silicon on Yield and Quality Traits of Tomato (Lycopersicon esculentum L.). Int. J. Pure App. Biosci. 2019, 7, 526–531. [Google Scholar] [CrossRef]
- Chaudhary, J.N.; Srivastava, A.; Sharma, M.D.; Gautam, I.P. Response of Organic and Inorganic Sources of Nitrogen on Tomato Production in Parwanipur, Bara, Nepal. J. Agric. Nat. Res. 2024, 7, 81–91. [Google Scholar] [CrossRef]
- Ayodele Ige, S.; Christopher, A.; Faith, A.; Abolusoro, S.; Aremu, C.; Omolaran Bello, B.; Ojo, A.; Victoria, A.; Favour, C. Tomato Genotype Response to Organic and Synthetic Fertilizers. Int. J. Recycl. Org. Waste Agric. 2024, 13, 132446. [Google Scholar] [CrossRef]
- Wang, L.; Jin, N.; Xie, Y.; Zhu, W.; Yang, Y.; Wang, J.; Lei, Y.; Liu, W.; Wang, S.; Jin, L.; et al. Improvements in the Appearance and Nutritional Quality of Tomato Fruits Resulting from Foliar Spraying with Silicon. Foods 2024, 13, 223. [Google Scholar] [CrossRef]
- Sakya, A.T.; Sulandjari. Foliar Iron Application on Growth and Yield of Tomato. IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012001. [Google Scholar] [CrossRef]
- Suman, M.; Sangma, P.D.; Singh, D. Role of Micronutrients (Fe, Zn, B, Cu, Mg, Mn and Mo) in Fruit Crops. Int. J. Curr. Microbiol. App. Sci 2017, 6, 3240–3250. [Google Scholar] [CrossRef]
- Zamban, D.T.; Prochnow, D.; Caron, B.O.; Turchetto, M.; Fontana, D.C.; Schmidt, D. Applications of Calcium and Boron Increase Yields of Italian Tomato Hybrids (Solanum lycopersicum) in Two Growing Seasons. Rev. Colomb. Cienc. Hortic. 2018, 12, 82–93. [Google Scholar] [CrossRef]
- Ku, H.-M.; Doganlar, S.; Chen, K.-Y.; Tanksley, S.D. The Genetic Basis of Pear-Shaped Tomato Fruit. Theor. Appl. Genet. 1999, 99, 844–850. [Google Scholar] [CrossRef]
- Gan, L.; Song, M.; Wang, X.; Yang, N.; Li, H.; Liu, X.; Li, Y. Cytokinins Are Involved in Regulation of Tomato Pericarp Thickness and Fruit Size. Hortic. Res. 2022, 9, uhab041. [Google Scholar] [CrossRef] [PubMed]
- Oko-Ibom, G.O.; Asiegbu, J.E. Aspects of Tomato Fruit Quality as Influenced by Cultivar and Scheme of Fertilizer Appication. Agro-Science 2007, 6, 71–81. [Google Scholar] [CrossRef]
- Sajid, M. Foliar Application of Calcium Improves Growth, Yield and Quality of Tomato Cultivars. PAB 2020, 9, 10–19. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, S.K. Effect of micmnutrient sprays on tomato (Lycopersicon esculentum) seed production. IJAS 2006, 76, 676–678. [Google Scholar]
- Gupta, N.; Jain, S.; Tomar, B.; Anand, A.; Singh, J.; Sagar, V.; Kumar, R.; Singh, V.; Chaubey, T.; Abd-Elsalam, K.; et al. Impact of Foliar Application of ZnO and Fe3O4 Nanoparticles on Seed Yield and Physio-Biochemical Parameters of Cucumber (Cucumis sativus L.) Seed under Open Field and Protected Environment Vis a Vis during Seed Germination. Plants 2022, 11, 3211. [Google Scholar] [CrossRef]
- Prodhan, M.M.; Sarker, U.; Hoque, M.A.; Biswas, M.S.; Ercisli, S.; Assouguem, A.; Ullah, R.; Almutairi, M.H.; Mohamed, H.R.H.; Najda, A. Foliar Application of GA3 Stimulates Seed Production in Cauliflower. Agronomy 2022, 12, 1394. [Google Scholar] [CrossRef]
- Fu, Y.; Ma, L.; Li, J.; Hou, D.; Zeng, B.; Zhang, L.; Liu, C.; Bi, Q.; Tan, J.; Yu, X.; et al. Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology. Plants 2024, 13, 1319. [Google Scholar] [CrossRef]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An Updated Overview on the Regulation of Seed Germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef]
- Sembada, A.A.; Maki, S.; Faizal, A.; Fukuhara, T.; Suzuki, T.; Lenggoro, I.W. The Role of Silica Nanoparticles in Promoting the Germination of Tomato (Solanum lycopersicum) Seeds. Nanomaterials 2023, 13, 2110. [Google Scholar] [CrossRef]
- Ulfianida, D.; Rachmawati, D. Effect of Silicon Priming on Germination and Growth of Rice (Oryza sativa L.) in Drought Condition. BIO Web Conf. 2024, 94, 06007. [Google Scholar] [CrossRef]
- Wang, T.; Long, H.; Mao, S.; Jiang, Z.; Liu, Y.; He, Y.; Zhu, Z.; Yan, G. Silicon Nanoparticles Improve Tomato Seed Germination More Effectively than Conventional Silicon under Salt Stress via Regulating Antioxidant System and Hormone Metabolism. Horticulturae 2024, 10, 785. [Google Scholar] [CrossRef]
- Geshnizjani, N.; Sarikhani Khorami, S.; Willems, L.A.J.; Snoek, B.L.; Hilhorst, H.W.M.; Ligterink, W. The Interaction between Genotype and Maternal Nutritional Environments Affects Tomato Seed and Seedling Quality. J. Exp. Bot. 2019, 70, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C. Foliar Boron Application Improves Seed Set, Seed Yield, and Seed Quality of Alfalfa. Agron. J. 2006, 98, 907–913. [Google Scholar] [CrossRef]
- Alshaal, T.; Alsaeedi, A.; El-Ramady, H.; Almohsen, M. Enhancing Seed Germination and Seedlings Development of Common Bean (Phaseolus vulgaris) by SiO2 Nanoparticles. Egypt. J. Soil Sci. 2017, 57, 407–415. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic. Agronomy 2022, 12, 2366. [Google Scholar] [CrossRef]
- Akram, S.; Khan, A.R.; Junaid, J.A. A Multivariate Analysis of Seed Priming Agents and Dosage on Germination Performance Seedling Growth and Biochemical Profiling in Tomato. Sci. Rep. 2025, 15, 22991. [Google Scholar] [CrossRef]
- Markovic, V.; Djurovka, M.; Ilin, Z. The Effect of Seedling Quality on Tomato Yield, Plant and Fruit Characteristics. Acta Hortic. 1997, 462, 163–170. [Google Scholar] [CrossRef]
- Abbas, M.A.; Ibraheem, F.F.R. A Comparison between the Effect of Nano and Traditional Calcium Fertilizer on Growth and Anatomical Traits of Two Tomato Cultivars. Int. J. Environ. Sci. 2025, 11, 461–471. [Google Scholar] [CrossRef]
- Ali, Y.; Zamin, M.; Jan, I.; Shah, S.; Hussain, M.M.; Rabbi, F.; Amin, M. Impact of Different Media on Germination and Emergence of Tomato Genotypes. Sarhad J. Agric. 2020, 35, 230–235. [Google Scholar] [CrossRef]
- Suchoff, D.H.; Gunter, C.C.; Louws, F.J. Comparative Analysis of Root System Morphology in Tomato Rootstocks. HortTechnology 2017, 27, 319–324. [Google Scholar] [CrossRef]
- Miroshnychenko, M.; Hladkikh, Y.; Revtye-Uvarova, A.; Siabryk, O.; Voitovych, O. Beneficial Effects of Silicon Fertilizers on Indicators of Seed Germination, Grain Yield of Barley and Soybean and Silage Corn Biomass. J. Agric. Sci. 2023, 68, 43–57. [Google Scholar] [CrossRef]
- Gupta, S.; Kant, K.; Kaur, N.; Jindal, P.; Ali, A.; Naeem, M. Nano-Calcium Applications in Modern Agriculture: A Review. Plant Nano Biol. 2025, 12, 100147. [Google Scholar] [CrossRef]
- Ronga, D.; Zaccardelli, M.; Lovelli, S.; Perrone, D.; Francia, E.; Milc, J.; Ulrici, A.; Pecchioni, N. Biomass Production and Dry Matter Partitioning of Processing Tomato under Organic vs Conventional Cropping Systems in a Mediterranean Environment. Sci. Hortic. 2017, 224, 163–170. [Google Scholar] [CrossRef]
- Włodarczyk, K.; Smolińska, B. The Effect of Nano-ZnO on Seeds Germination Parameters of Different Tomatoes (Solanum lycopersicum L.) Cultivars. Molecules 2022, 27, 4963. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, X.; Guan, X.; Sun, L.; Yang, Z.; Wang, D.; Chen, Y.; Li, P.; Xie, Z. Nano-Silicon Enhances Tomato Growth and Antioxidant Defense under Salt Stress. Environ. Sci. Nano 2025, 12, 315–324. [Google Scholar] [CrossRef]
- Klarod, K.; Dongsansuk, A.; Piepho, H.P.; Siri, B. Seed Coating with Plant Nutrients Enhances Germination and Seedling Growth, and Promotes Total Dehydrogenase Activity during Seed Germination in Tomato (Lycopersicon esculentum). Seed Sci. Technol. 2021, 49, 107–124. [Google Scholar] [CrossRef]
- Souza, L.M.D.; Melo, P.C.T.; Luders, R.R.; Melo, A.M. Correlations between Yield and Fruit Quality Characteristics of Fresh Market Tomatoes. Hortic. Bras. 2012, 30, 627–631. [Google Scholar] [CrossRef]
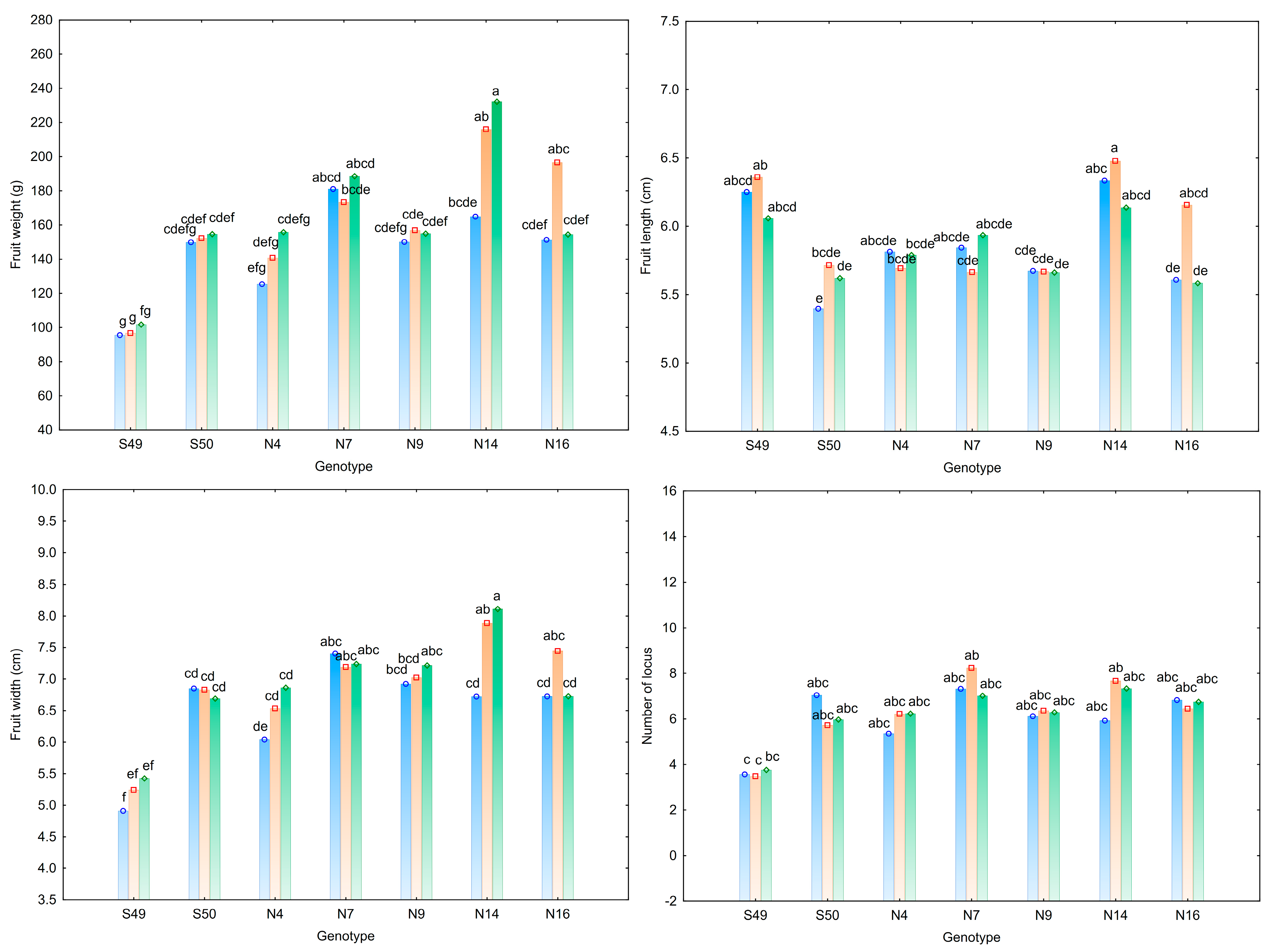
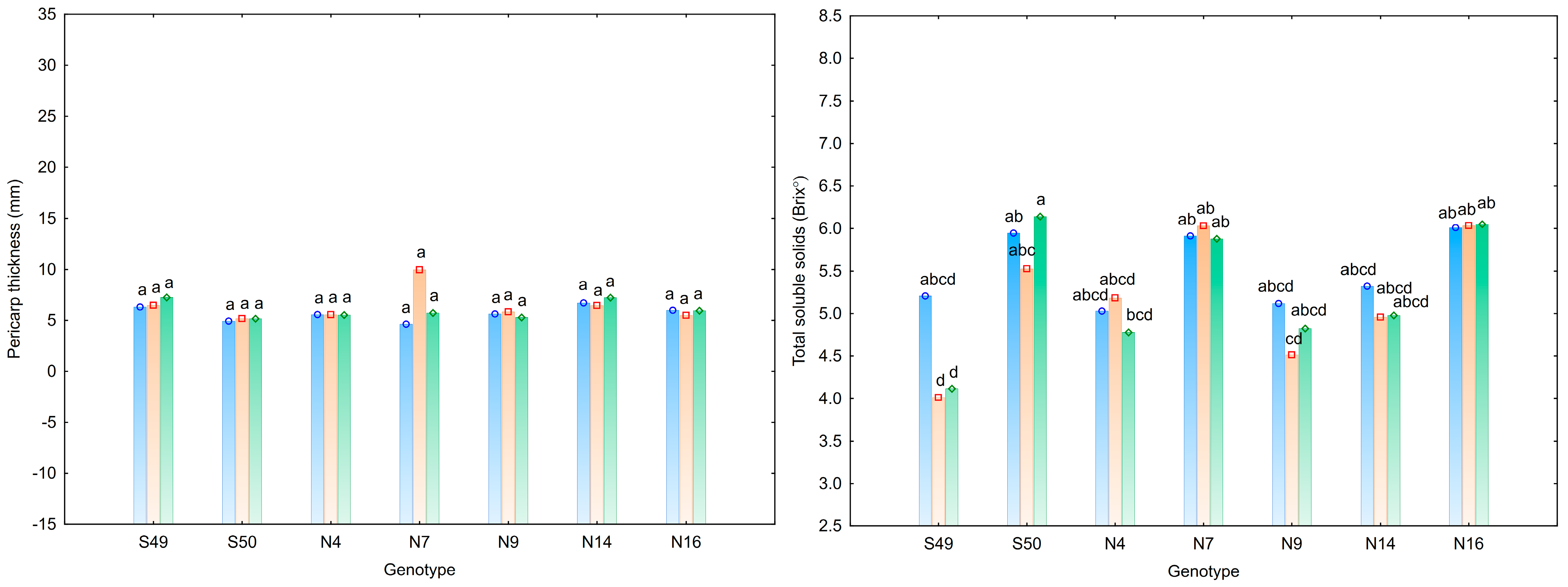
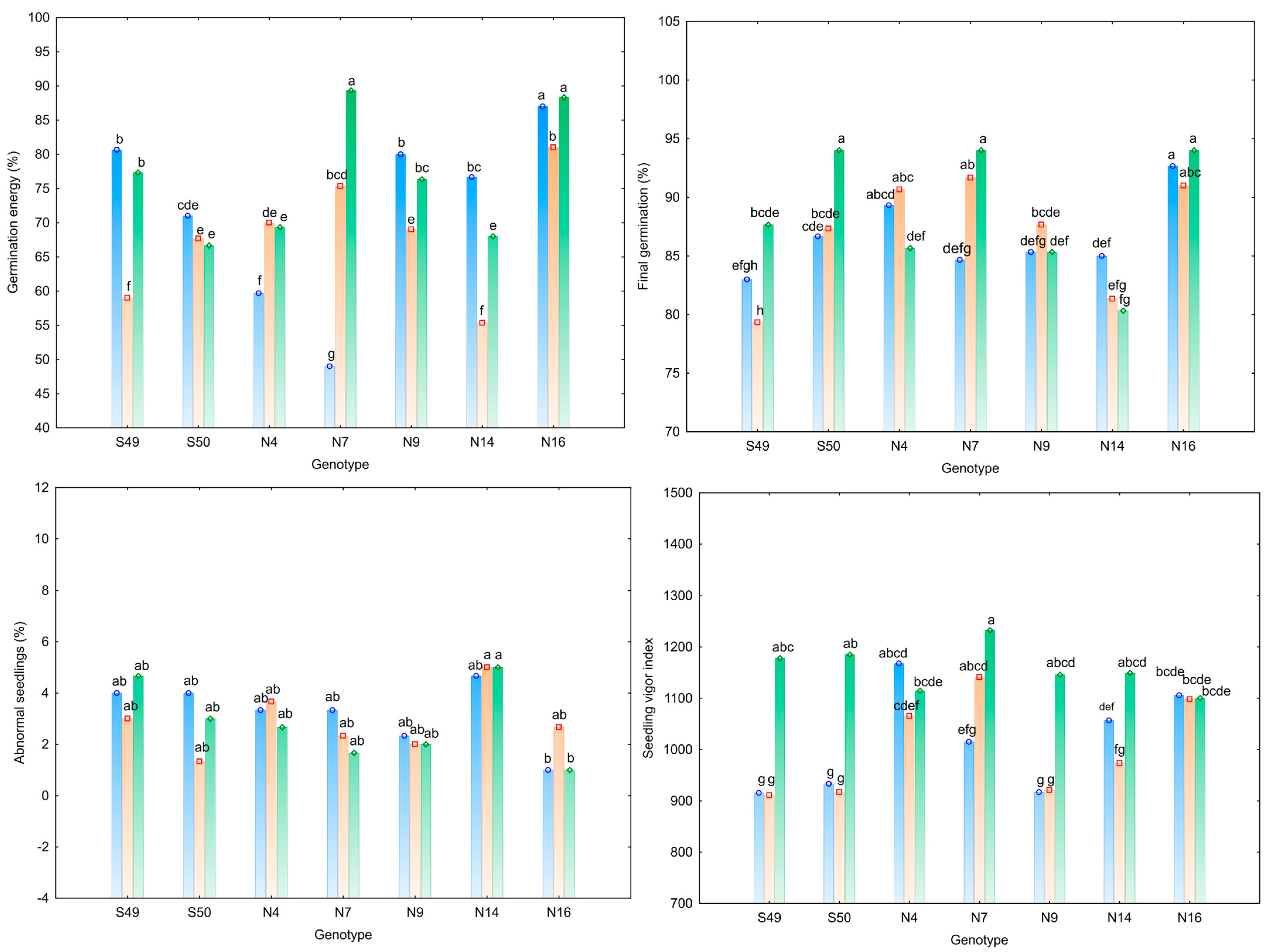



| Trait | Fruit Weight (g) | Fruit Length (cm) | Fruit Width (cm) | Number of Locules | Pericarp Thickness (mm) | Total Soluble Solids (°Brix) |
|---|---|---|---|---|---|---|
| G (p) | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.230 ns | 0.000 *** |
| S49 | 97.89 e | 6.22 a | 5.19 e | 3.61 b | 6.67 a | 4.44 c |
| S50 | 152.23 cd | 5.58 b | 6.79 cd | 6.25 a | 5.09 a | 5.87 a |
| N4 | 140.60 d | 5.77 b | 6.48 d | 5.94 a | 5.55 a | 5.00 bc |
| N7 | 180.94 ab | 5.81 b | 7.28 ab | 7.53 a | 6.78 a | 5.94 a |
| N9 | 153.93 cd | 5.67 b | 7.05 bc | 6.25 a | 5.58 a | 4.82 bc |
| N14 | 204.34 a | 6.32 a | 7.57 a | 6.97 a | 6.79 a | 5.08 b |
| N16 | 167.42 bc | 5.78 b | 6.96 bc | 6.67 a | 5.81 a | 6.03 a |
| T (p) | 0.003 ** | 0.103 ns | 0.000 *** | 0.768 ns | 0.402 ns | 0.048 * |
| T0 | 145.40 b | 5.85 a | 6.51 b | 6.02 a | 5.68 a | 5.51 a |
| T1 | 161.73 a | 5.96 a | 6.88 a | 6.31 a | 6.42 a | 5.18 b |
| T2 | 163.16 a | 5.83 a | 6.90 a | 6.19 a | 6.02 a | 5.25 b |
| RC T1 (%) | +10.23 | +1.88 | +5.68 | +4.82 | +13.03 | −5.99 |
| RC T2 (%) | +12.22 | −0.34 | +5.99 | +2.82 | +5.99 | −4.72 |
| G × T(p) | 0.016 * | 0.074 ns | 0.002 ** | 0.830 ns | 0.302 ns | 0.217 ns |
| Traits | Germination Energy (%) | Final Germination (%) | Abnormal Seedlings (%) | Seedling Vigor Index |
|---|---|---|---|---|
| G (p) | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** |
| S49 | 72.33 bc | 83.33 d | 3.89 ab | 1001.5 d |
| S50 | 68.44 de | 89.33 b | 2.78 bc | 1011.8 cd |
| N4 | 66.33 e | 88.56 b | 3.22 abc | 1115.9 a |
| N7 | 71.22 cd | 90.11 b | 2.44 bc | 1129.6 a |
| N9 | 75.11 b | 86.11 c | 2.11 bc | 994.5 d |
| N14 | 66.67 e | 82.22 d | 4.89 a | 1059.8 bc |
| N16 | 85.44 a | 92.56 a | 1.56 c | 1101.5 ab |
| T (p) | 0.000 *** | 0.000 *** | 0.515 ns | 0.000 *** |
| T0 | 72.00 b | 86.67 b | 3.24 a | 1016.0 b |
| T1 | 68.19 c | 87.00 b | 2.86 a | 1003.8 b |
| T2 | 76.48 a | 88.71 a | 2.86 a | 1157.9 a |
| RC T1 (%) | −5.29 | +0.38 | −11.73 | −1.20 |
| RC T2 (%) | +6.22 | +2.35 | −11.73 | +13.97 |
| G × T (p) | 0.000 *** | 0.000 *** | 0.217 ns | 0.000 *** |
| Traits | Shoot Length (mm) | Root Length (mm) | Shoot Elongation Rate (mm day−1) | Root Elongation Rate (mm day−1) | Fresh Seedling Weight (g) | Dry Seedlings Weight (g) | R/S Ratio |
|---|---|---|---|---|---|---|---|
| G (p) | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** |
| S49 | 45.29 bc | 74.54 bc | 2.66 b | 1.98 c | 0.35 c | 0.017 b | 1.64 a |
| S50 | 41.71 d | 71.22 c | 2.68 b | 1.60 c | 0.29 d | 0.014 c | 1.71 a |
| N4 | 46.94 ab | 79.16 a | 2.60 b | 1.68 c | 0.40 a | 0.022 a | 1.67 a |
| N7 | 45.93 bc | 79.21 a | 3.25 ab | 2.13 bc | 0.30 d | 0.015 c | 1.73 a |
| N9 | 49.23 a | 66.34 d | 3.68 a | 3.04 a | 0.37 b | 0.017 b | 1.34 b |
| N14 | 46.72 ab | 82.30 a | 3.07 ab | 2.84 ab | 0.40 a | 0.017 b | 1.76 a |
| N16 | 43.67 cd | 75.37 b | 3.01 ab | 2.12 bc | 0.34 c | 0.014 c | 1.73 a |
| T (p) | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** |
| T0 | 45.12 b | 71.98 b | 3.55 a | 2.05 b | 0.34 b | 0.016 b | 1.60 b |
| T1 | 44.50 b | 70.81 b | 2.77 b | 1.81 b | 0.34 b | 0.016 b | 1.60 b |
| T2 | 47.31 a | 83.56 a | 2.65 b | 2.74 a | 0.37 a | 0.017 a | 1.77 a |
| RC T1 (%) | −1.37 | −1.63 | −21.97 | −11.71 | 0.00 | 0.00 | 0.00 |
| RC T2 (%) | +4.85 | +16.09 | −25.35 | +33.66 | +8.82 | +6.25 | +10.63 |
| G × T (p) | 0.042 * | 0.000 *** | 0.001 *** | 0.000 *** | 0.000 *** | 0.001 *** | 0.000 *** |
| Trait | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
|---|---|---|---|---|---|---|
| FW | 0.529 | 0.488 | 0.633 | |||
| FL | −0.572 | 0.334 | −0.421 | |||
| FWI | 0.599 | 0.375 | 0.673 | |||
| NL | 0.755 | 0.506 | ||||
| PT | 0.319 | −0.303 | 0.838 | |||
| TSS | 0.880 | |||||
| EG | 0.360 | −0.475 | 0.356 | 0.343 | 0.349 | |
| FG | 0.704 | −0.548 | ||||
| AS | −0.553 | 0.386 | −0.403 | |||
| SL | 0.525 | 0.627 | ||||
| RL | 0.913 | |||||
| FSW | −0.468 | 0.556 | 0.431 | |||
| DSW | −0.525 | 0.437 | 0.450 | −0.423 | ||
| SER | −0.360 | 0.469 | 0.390 | 0.464 | ||
| RER | 0.387 | 0.759 | ||||
| SVI | 0.352 | 0.775 | −0.428 | |||
| R/S RATIO | 0.669 | −0.387 | −0.477 | |||
| Eigenvalue | 4.02 | 3.76 | 2.47 | 1.93 | 1.38 | 1.06 |
| % Total variance | 23.62 | 22.10 | 14.54 | 11.33 | 8.14 | 6.24 |
| Cumulative variance % | 23.62 | 45.72 | 60.26 | 71.59 | 79.73 | 85.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zec, S.; Tamindžić, G.; Azizbekian, S.; Ignjatov, M.; Danojević, D.; Červenski, J.; Vlajić, S.; Vojnović, Đ.; Banjac, B. Foliar Application of Ca-Based Fertilizers (Conventional vs. Nanofertilizers): Effects on Fruit Traits, Seed Quality Parameters and Initial Plant Growth of Tomato Genotypes. Horticulturae 2025, 11, 1303. https://doi.org/10.3390/horticulturae11111303
Zec S, Tamindžić G, Azizbekian S, Ignjatov M, Danojević D, Červenski J, Vlajić S, Vojnović Đ, Banjac B. Foliar Application of Ca-Based Fertilizers (Conventional vs. Nanofertilizers): Effects on Fruit Traits, Seed Quality Parameters and Initial Plant Growth of Tomato Genotypes. Horticulturae. 2025; 11(11):1303. https://doi.org/10.3390/horticulturae11111303
Chicago/Turabian StyleZec, Srđan, Gordana Tamindžić, Sergei Azizbekian, Maja Ignjatov, Dario Danojević, Janko Červenski, Slobodan Vlajić, Đorđe Vojnović, and Borislav Banjac. 2025. "Foliar Application of Ca-Based Fertilizers (Conventional vs. Nanofertilizers): Effects on Fruit Traits, Seed Quality Parameters and Initial Plant Growth of Tomato Genotypes" Horticulturae 11, no. 11: 1303. https://doi.org/10.3390/horticulturae11111303
APA StyleZec, S., Tamindžić, G., Azizbekian, S., Ignjatov, M., Danojević, D., Červenski, J., Vlajić, S., Vojnović, Đ., & Banjac, B. (2025). Foliar Application of Ca-Based Fertilizers (Conventional vs. Nanofertilizers): Effects on Fruit Traits, Seed Quality Parameters and Initial Plant Growth of Tomato Genotypes. Horticulturae, 11(11), 1303. https://doi.org/10.3390/horticulturae11111303








