Genome-Wide Identification, Systematic Evolution, and Ethylene-Induced Response Characteristics of the Banana WRKY Gene Family During Fruit Ripening
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of WRKY Family Members and Analysis of Physicochemical Properties
2.2. Chromosomal Localization Analysis
2.3. Phylogenetic Analysis
2.4. Conserved Motif Analysis of Encoded Proteins
2.5. Gene Structure Analysis
2.6. Cis-Acting Element Analysis
2.7. Collinearity Analysis
2.8. Prediction of the MaWRKY Interaction Network
2.9. Experimental Materials and Treatment
2.10. High-Throughput Sequencing
2.11. Ethylene-Responsive Expression Pattern Analysis of WRKY Genes
3. Results
3.1. Analysis of Banana WRKY Gene Family Members and Their Physicochemical Properties
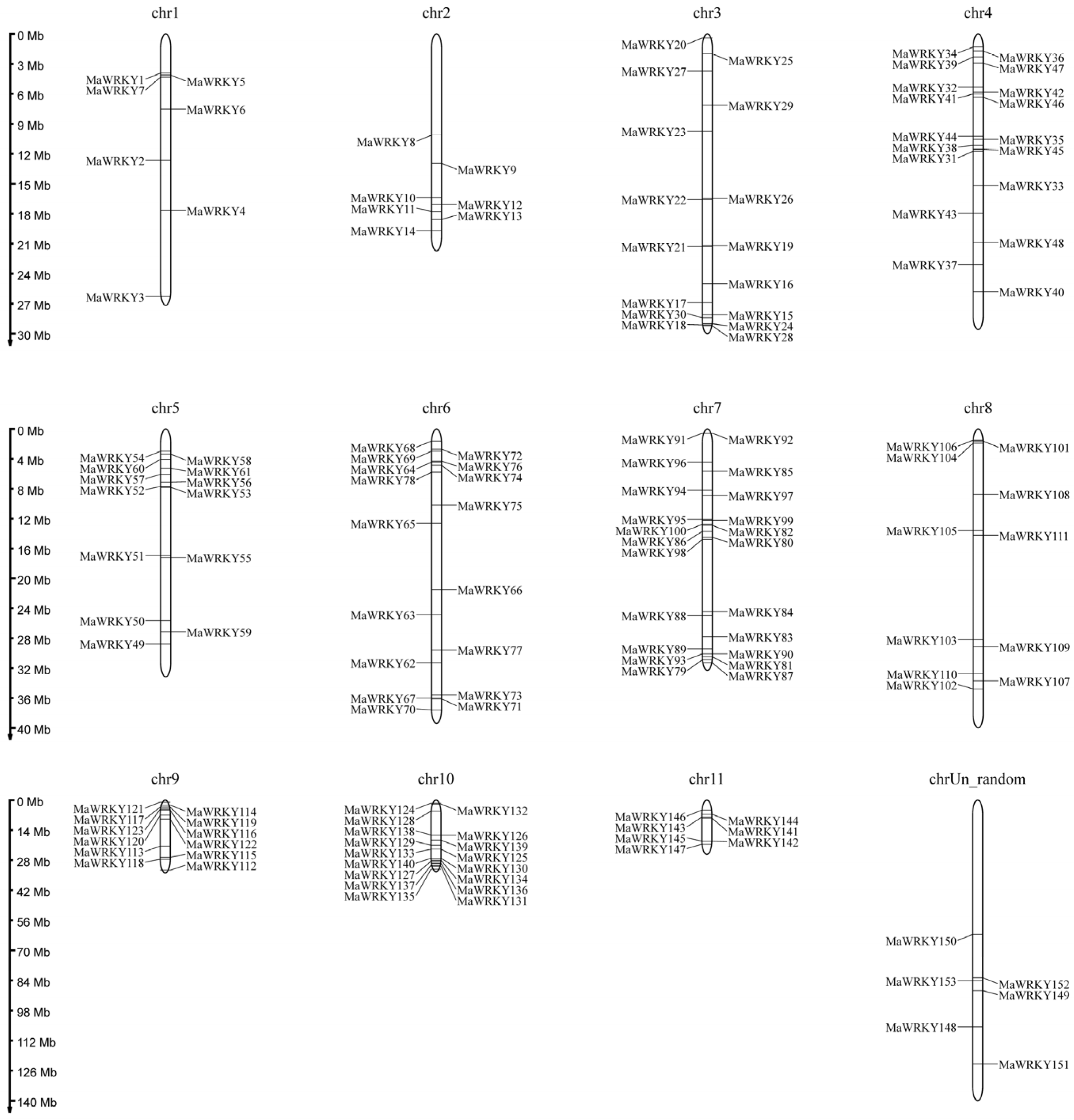
3.2. Chromosomal Localization Analysis of the Banana WRKY Gene Family
3.3. Conserved Motif Analysis of Encoded Proteins of Banana WRKY Gene Family Members
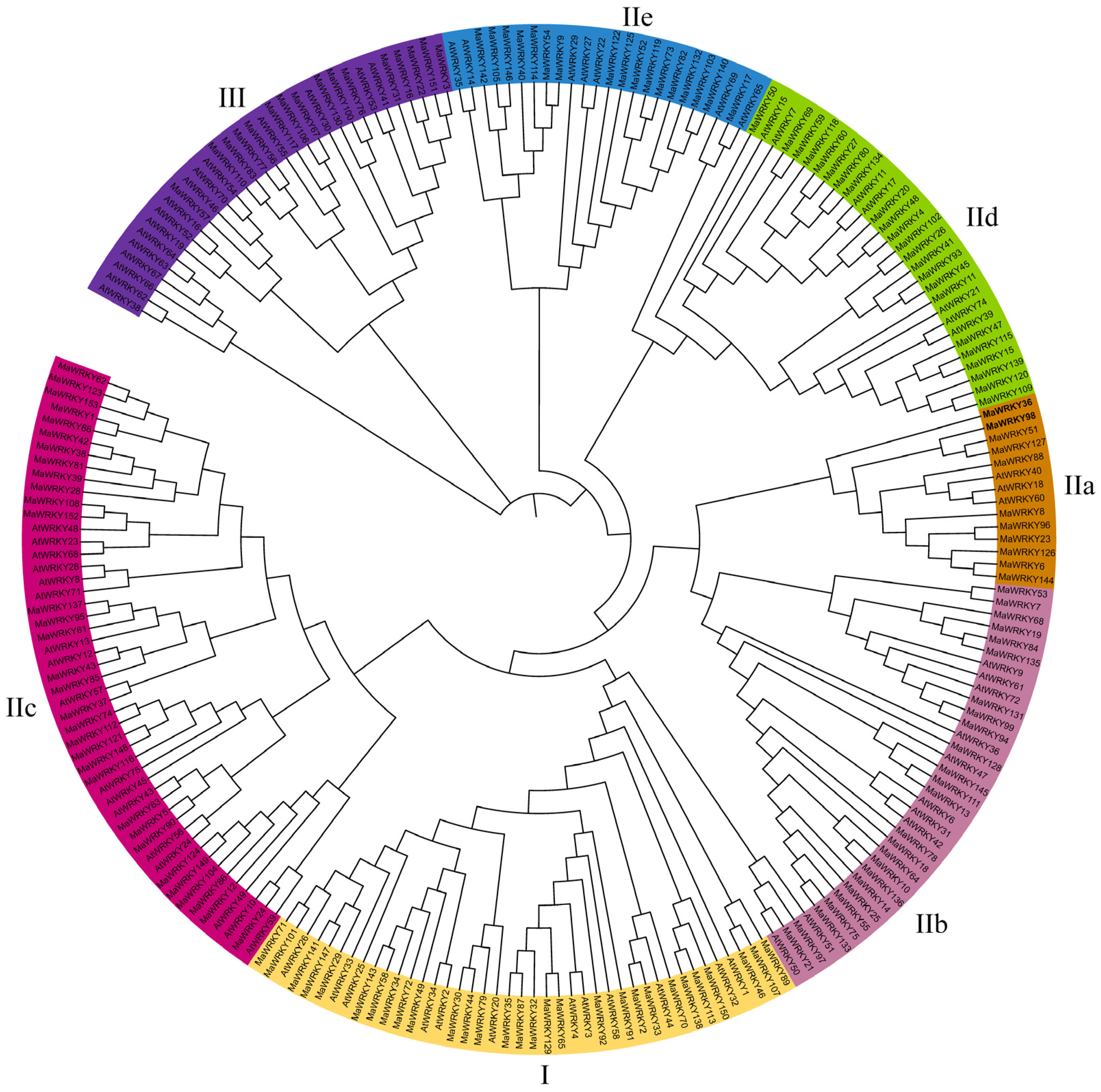
3.4. Phylogenetic Analysis of the Banana WRKY Gene Family
3.5. Gene Structure Analysis of Banana WRKY Gene Family Members
3.6. Cis-Acting Element Analysis of the Banana WRKY Gene Family
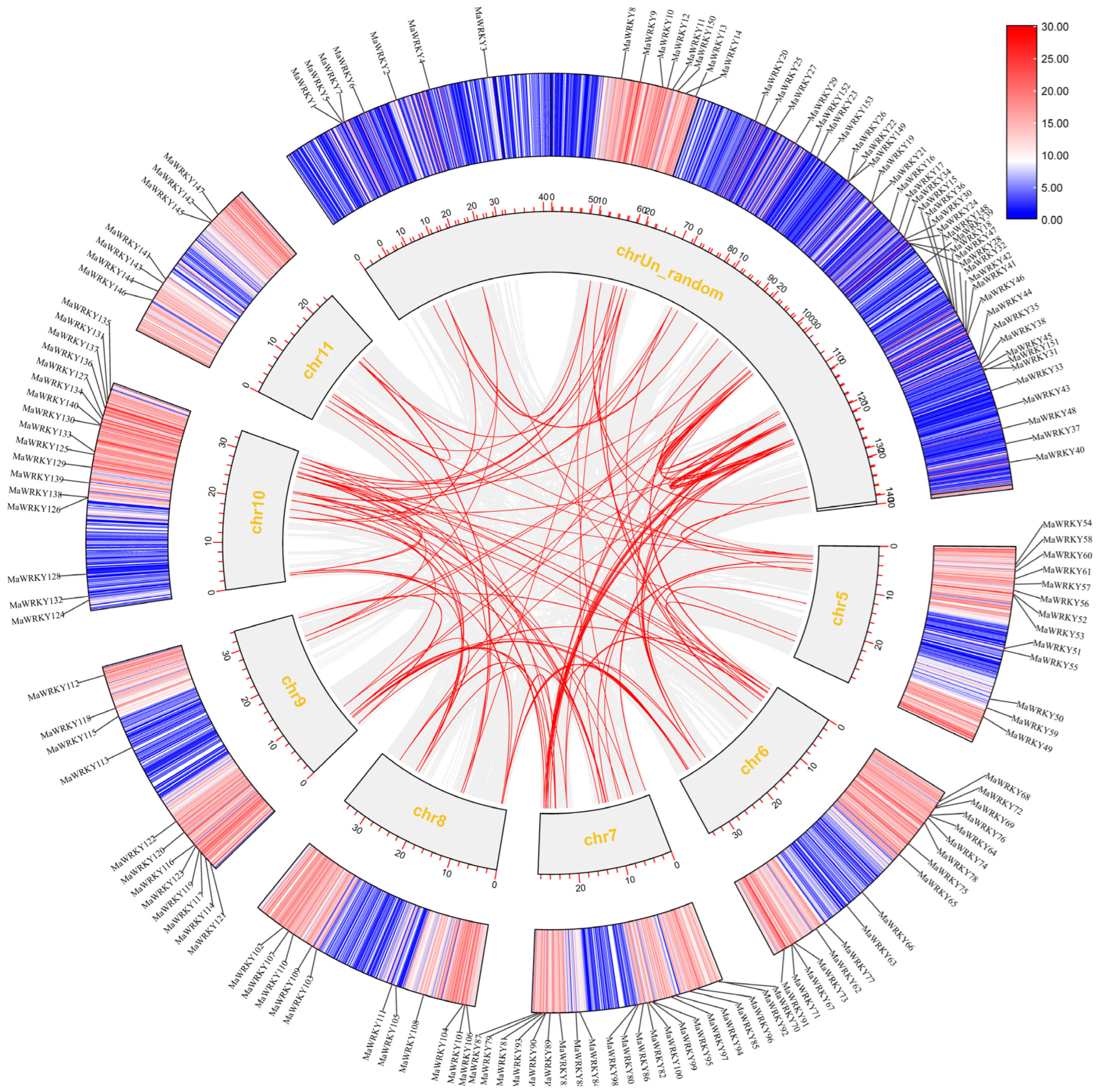
3.7. Collinearity Analysis Among Banana WRKY Gene Family Members
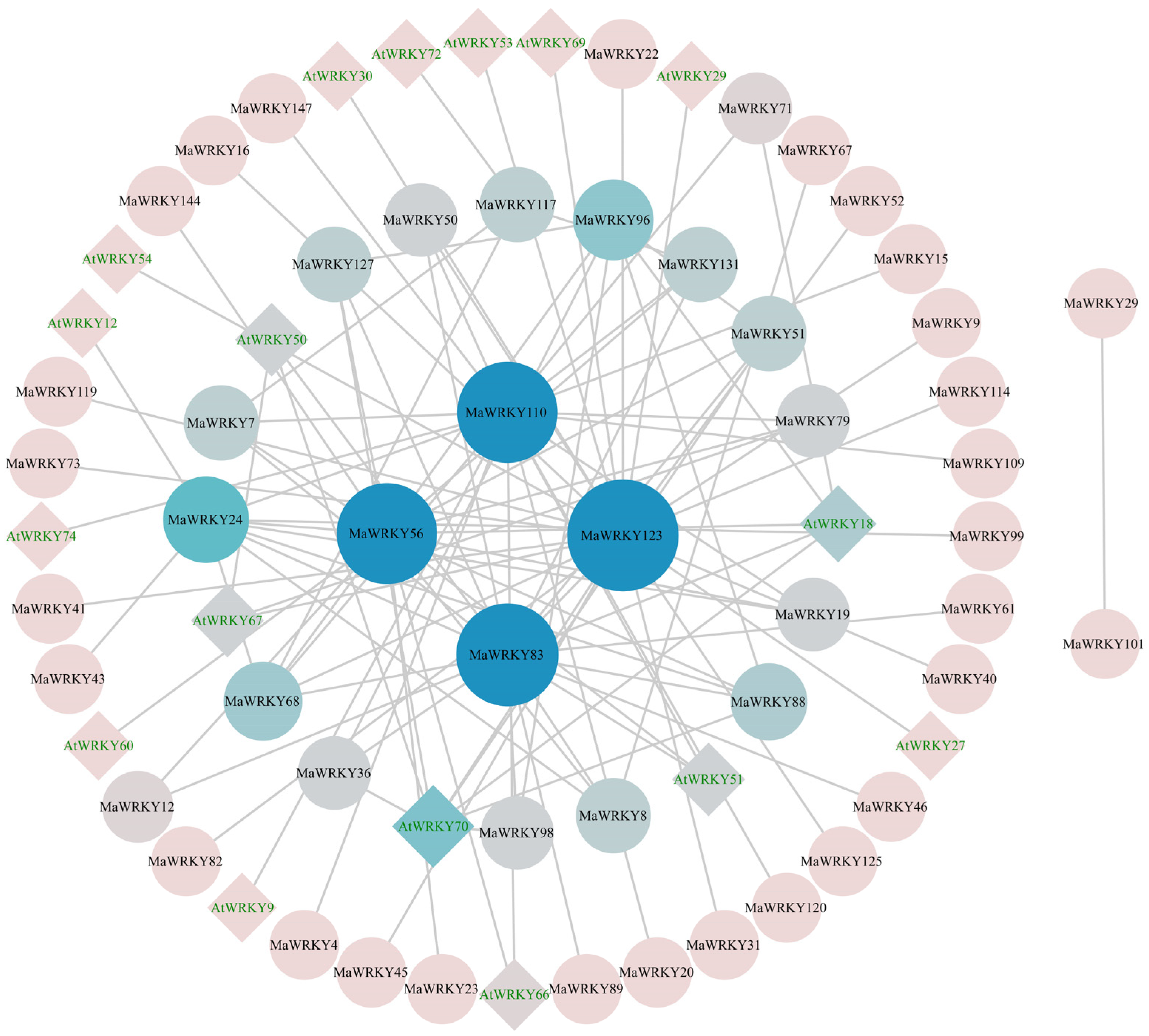
3.8. Prediction of the MaWRKY Interaction Network
3.9. Expression Pattern Analysis of the Banana WRKY Gene Family in Response to Ethylene Treatment
4. Discussion
4.1. Expansion and Functional Diversification of the Banana WRKY Gene Family
4.2. Conserved Motifs and Gene Structure Shape the Regulatory Plasticity of WRKY
4.3. The Central Hub Role of MaWRKY in Fruit Ripening
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Javed, T.; Gao, S.J. WRKY transcription factors in plant defense. Trends Genet. 2023, 39, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Tateno, M.; Yamasaki, T.; Yabuki, T.; Aoki, M.; Seki, E.; Matsuda, T.; Tomo, Y.; et al. Solution structure of an Arabidopsis WRKY DNA binding domain. Plant Cell 2005, 17, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1. [Google Scholar] [CrossRef]
- Wu, K.L.; Guo, Z.J.; Wang, H.H.; Li, J. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 2005, 12, 9–26. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Y.; An, L.; Yang, X.; Liu, J.; Dai, Z.; Zhang, Y.; Li, T.; Ahammed, G.J. Overexpression of SlWRKY6 enhances drought tolerance by strengthening antioxidant defense and stomatal closure via ABA signaling in Solanum lycopersicum L. Plant Physiol. Biochem. PPB 2024, 213, 108855. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Yu, L.; Jiang, H.; Guo, Z.; Xu, H.; Jiang, S.; Fang, H.; Zhang, J.; Su, M.; et al. MdWRKY11 participates in anthocyanin accumulation in red-fleshed apples by affecting MYB transcription factors and the photoresponse factor MdHY5. J. Agric. Food Chem. 2019, 67, 8783–8793. [Google Scholar] [CrossRef]
- Rosado, D.; Ackermann, A.; Spassibojko, O.; Rossi, M.; Pedmale, U.V. WRKY transcription factors and ethylene signaling modify root growth during the shade-avoidance response. Plant Physiol. 2022, 188, 1294–1311. [Google Scholar] [CrossRef]
- Chen, W.; Huang, B. Cytokinin or ethylene regulation of heat-induced leaf senescence involving transcriptional modulation of WRKY in perennial ryegrass. Physiol. Plant. 2022, 174, e13766. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.L.; Wang, L.; Tian, Y.; Jia, N.; Chen, S.; Shi, N.B.; Huang, X.; Zhou, C.; Yu, Y.; et al. Regulation of ethylene-responsive SlWRKYs involved in color change during tomato fruit ripening. Sci. Rep. 2017, 7, 16674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Sun, Q.; Ma, C.N.; Wei, M.M.; Wang, C.K.; Zhao, Y.W.; Wang, W.Y.; Hu, D.G. MdWRKY31-MdNAC7 regulatory network: Orchestrating fruit softening by modulating cell wall-modifying enzyme MdXTH2 in response to ethylene signalling. Plant Biotechnol. J. 2024, 22, 3244–3261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, W.; Liao, L.; Zheng, B.; Cao, Y.; Zhao, Y.; Zhang, R.X.; Han, Y. A PpEIL2/3-PpNAC1-PpWRKY14 module regulates fruit ripening by modulating ethylene production in peach. J. Integr. Plant Biol. 2024, 66, 2470–2489. [Google Scholar] [CrossRef]
- D’Hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar] [CrossRef]
- Seale, M. Banana ripening control: A non-canonical F-box protein links ethylene and ABA signaling. Plant Physiol. 2022, 188, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Y.; Chen, J.Y.; Kuang, J.F.; Shan, W.; Xie, H.; Jiang, Y.M.; Lu, W.J. Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J. Exp. Bot. 2013, 64, 2499–2510. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Mbéguié, A.M.D.; Hubert, O.; Baurens, F.C.; Matsumoto, T.; Chillet, M.; Fils-Lycaon, B.; Sidibé-Bocs, S. Expression patterns of cell wall-modifying genes from banana during fruit ripening and in relationship with finger drop. J. Exp. Bot. 2009, 60, 2021–2034. [Google Scholar] [CrossRef]
- Shan, W.; Kuang, J.F.; Wei, W.; Fan, Z.Q.; Deng, W.; Li, Z.G.; Bouzayen, M.; Pirrello, J.; Lu, W.J.; Chen, J.Y. MaXB3 modulates MaNAC2, MaACS1, and MaACO1 stability to repress ethylene biosynthesis during banana fruit ripening. Plant Physiol. 2020, 184, 1153–1171. [Google Scholar] [CrossRef]
- Belser, C.; Baurens, F.-C.; Noel, B.; Martin, G.; Cruaud, C.; Istace, B.; Yahiaoui, N.; Labadie, K.; Hřibová, E.; Doležel, J.; et al. Telomere-to-telomere gapless chromosomes of banana using nanopore sequencing. Commun. Biol. 2021, 4, 1047. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Z.; Wang, J.; Miao, H.; Zhang, J.; Xu, B.; Liu, J.; Jin, Z.; Liu, J. Genome-wide analysis of the banana WRKY transcription factor gene family closely related to fruit ripening and stress. Plants 2022, 11, 662. [Google Scholar] [CrossRef]
- Luo, D.L.; Ba, L.J.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Involvement of WRKY transcription factors in abscisic-acid-induced cold tolerance of banana fruit. J. Agric. Food Chem. 2017, 65, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Kan, B.; He, H.; Li, T.; Ding, Y.; Du, P.; Lai, W.; Hu, H.; Huang, J. Transcriptome analysis reveals MYB and WRKY transcription factors involved in banana (Musa paradisiaca AA) magnesium deficiency. Planta 2021, 254, 115. [Google Scholar] [CrossRef]
- Garcýa-Laynes, S.; Herrera-Valencia, V.A.; Tamayo-Torres, L.G.; Limones-Briones, V.; Barredo-Pool, F.A.; Baas-Espinola, F.M.; Alpuche-Solís, A.G.; Puch-Hau, C.; Peraza-Echeverria, S. The banana MaWRKY18, MaWRKY45, MaWRKY60 and MaWRKY70 genes encode functional transcription factors and display differential expression in response to defense phytohormones. Genes 2022, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, H.; Dong, P.; Ni, X.; Fan, M.; Yang, Y.; Xu, S.; Xu, Y.; Qian, Y.; Chen, Z.; et al. Low temperature-induced regulatory network rewiring via WRKY regulators during banana peel browning. Plant Physiol. 2023, 193, 855–873. [Google Scholar] [CrossRef]
- Liu, F.; Dou, T.; Hu, C.; Zhong, Q.; Sheng, O.; Yang, Q.; Deng, G.; He, W.; Gao, H.; Li, C.; et al. WRKY transcription factor MaWRKY49 positively regulates pectate lyase genes during fruit ripening of Musa acuminata. Plant Physiol. Biochem. PPB 2023, 194, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Andreeva, A.; Blum, M.; Chuguransky, S.R.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Llinares-López, F.; Meng-Papaxanthos, L.; et al. The Pfam protein families database: Embracing AI/ML. Nucleic Acids Res. 2025, 53, D523–D534. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular evolutionary genetic analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A "one for all, all for one" bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Majeed, A.; Mukhtar, S. Protein-Protein Interaction Network Exploration Using Cytoscape. Methods Mol. Biol. 2023, 2690, 419–427. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Garber, M.; Levin, J.Z.; Donaghey, J.; Robinson, J.; Adiconis, X.; Fan, L.; Koziol, M.J.; Gnirke, A.; Nusbaum, C.; et al. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotechnol 2010, 28, 503–510. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Cvijović, I.; Good, B.H.; Desai, M.M. The effect of strong purifying selection on genetic diversity. Genetics 2018, 209, 1235–1278. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; He, J.; Zhu, X.; Cai, J.; Fu, M.; Zhang, S.; Zeng, W.; Xing, F.; Mao, G. Genome wide identification of BjSWEET gene family and drought response analysis of BjSWEET12 and BjSWEET17 genes in Brassica juncea. BMC Plant Biol. 2024, 24, 1094. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, J.; Zhang, T.; Bai, S.; He, K.; Wang, Z. Genome-wide analysis of WRKY gene family and the dynamic responses of key WRKY genes involved in Ostrinia furnacalis attack in Zea mays. Int. J. Mol. Sci. 2021, 22, 3045. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Qu, J.; Li, F.; Du, W.; Weng, J. Genome-wide characterization of the maize (Zea mays L.) WRKY transcription factor family and their responses to Ustilago maydis. Int. J. Mol. Sci. 2023, 24, 4916. [Google Scholar] [CrossRef]
- Hu, W.; Ren, Q.; Chen, Y.; Xu, G.; Qian, Y. Genome-wide identification and analysis of WRKY gene family in maize provide insights into regulatory network in response to abiotic stresses. BMC Plant Biol. 2021, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Abdullah-Zawawi, M.R.; Ahmad-Nizammuddin, N.F.; Govender, N.; Harun, S.; Mohd-Assaad, N.; Mohamed-Hussein, Z.A. Comparative genome-wide analysis of WRKY, MADS-box and MYB transcription factor families in Arabidopsis and rice. Sci. Rep. 2021, 11, 19678. [Google Scholar] [CrossRef]
- Mirza, Z.; Haque, M.M.; Gupta, M. WRKY transcription factors: A promising way to deal with arsenic stress in rice. Mol. Biol. Rep. 2022, 49, 10895–10904. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Jiang, Y.; Guo, L.; Ling, Z.; Ye, S.; Duan, Y.; Li, C.; Luo, K. Isolation and characterization of a subgroup IIa WRKY transcription factor PtrWRKY40 from Populus trichocarpa. Tree Physiol. 2015, 35, 1129–1139. [Google Scholar] [CrossRef]
- Cao, H.; Chen, J.; Yue, M.; Xu, C.; Jian, W.; Liu, Y.; Song, B.; Gao, Y.; Cheng, Y.; Li, Z. Tomato transcriptional repressor MYB70 directly regulates ethylene-dependent fruit ripening. Plant J. Cell Mol. Biol. 2020, 104, 1568–1581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Acharya, T.P.; Malladi, A.; Tsai, H.J.; NeSmith, D.S.; Doyle, J.W.; Nambeesan, S.U. Atypical climacteric and functional ethylene metabolism and signaling during fruit ripening in blueberry (Vaccinium sp.). Front. Plant Sci. 2022, 13, 932642. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. Cell Mol. Biol. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, D.; Wang, B.; Khan, I.; Ni, Y. Ethylene control technologies in extending postharvest shelf life of climacteric fruit. J. Agric. Food Chem. 2017, 65, 7308–7319. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Jiang, M.; Zheng, H.; Miao, L. Genome-Wide Identification, Systematic Evolution, and Ethylene-Induced Response Characteristics of the Banana WRKY Gene Family During Fruit Ripening. Horticulturae 2025, 11, 1289. https://doi.org/10.3390/horticulturae11111289
Huang Y, Jiang M, Zheng H, Miao L. Genome-Wide Identification, Systematic Evolution, and Ethylene-Induced Response Characteristics of the Banana WRKY Gene Family During Fruit Ripening. Horticulturae. 2025; 11(11):1289. https://doi.org/10.3390/horticulturae11111289
Chicago/Turabian StyleHuang, Yuji, Ming Jiang, Haojun Zheng, and Lixiang Miao. 2025. "Genome-Wide Identification, Systematic Evolution, and Ethylene-Induced Response Characteristics of the Banana WRKY Gene Family During Fruit Ripening" Horticulturae 11, no. 11: 1289. https://doi.org/10.3390/horticulturae11111289
APA StyleHuang, Y., Jiang, M., Zheng, H., & Miao, L. (2025). Genome-Wide Identification, Systematic Evolution, and Ethylene-Induced Response Characteristics of the Banana WRKY Gene Family During Fruit Ripening. Horticulturae, 11(11), 1289. https://doi.org/10.3390/horticulturae11111289





