An Endogenous, Flavor-Enhancing TRV/Agrobacterium System for Edible Tomato Fruits with the Sweet Protein Thaumatin II
Abstract
1. Introduction
| Sweet Protein | Source | Size (AA/MW) | Active Form | Variants | Sweetness Factor (Based on Weight) | Stability | Applications | References |
|---|---|---|---|---|---|---|---|---|
| Thaumatin | Thaumatococcus daniellii Benth. | 207AA | Monomer | I, II, a, b, c (a) | 3000 (licorice-like aftertaste) | -Soluble -Extreme acid/heat tolerance (80 °C, pH 2.0 for 4 h) -Aggregates > 70 °C at pH 7.0 | -FDA-approved natural flavor enhancer and high-intensity sweetener -Approved in multiple countries | [10,11,13,14,22] |
| Monellin | Dioscoreophyllum cumminsii Diels | 45AA (A chain) 50AA (B chain) | Dimer (A + B) | - | 3000 | -Heat-labile (>50 °C) -Low pH tolerance | -Safety not fully evaluated -Not approved as sweetener | [23,24,25,26] |
| Brazzein | Pentadiplandra brazzeana Baillon | 54AA | Monomer | I, II, III | 2000 (Sucrose-like taste profile, sweetness potency varies by isoform [27]) | -Solubility: Good -Best thermostability among sweet proteins: -No property loss after 2 h at 98 °C -Stable at pH 2.5–8 (4 h at 80 °C) | -Traditionally used by indigenous peoples [28] -Potential allergenicity concerns prevent approval [7] | [29,30] |
| Curculin | Curculligo latifolia | 114AA | Monomer | - | 550 (taste-modifying) | -Good stability -Taste-modifying activity remains after 1 h at 50 °C (pH 3–11) | -Recognized as safe food additive in Japan [31] -Not approved in US/EU [32] | [33,34] |
| Mabinlin | Capparis masailai Levi | 33AA (A chain) 72AA (B chain) | Dimer (A + B) | I, II-a, III, IV (a) | 100 | -High thermostability: -Mabinlin-II-a: Sweetness unchanged after 48 h at boiling point [35] -Mabinlin-III/IV (a): Sweetness unchanged after 1 h at 80 °C [36] | Not FDA-approved | [36,37] |
| Pentadin | Pentadiplandra brazzeana Baillon | - | Putative cross-linked brazzein analog | - | 500 (synergistic with saccharin to suppress bitter aftertaste) | Stable at 100 °C for 5 h | - | [7,38,39,40] |
2. Materials and Methods
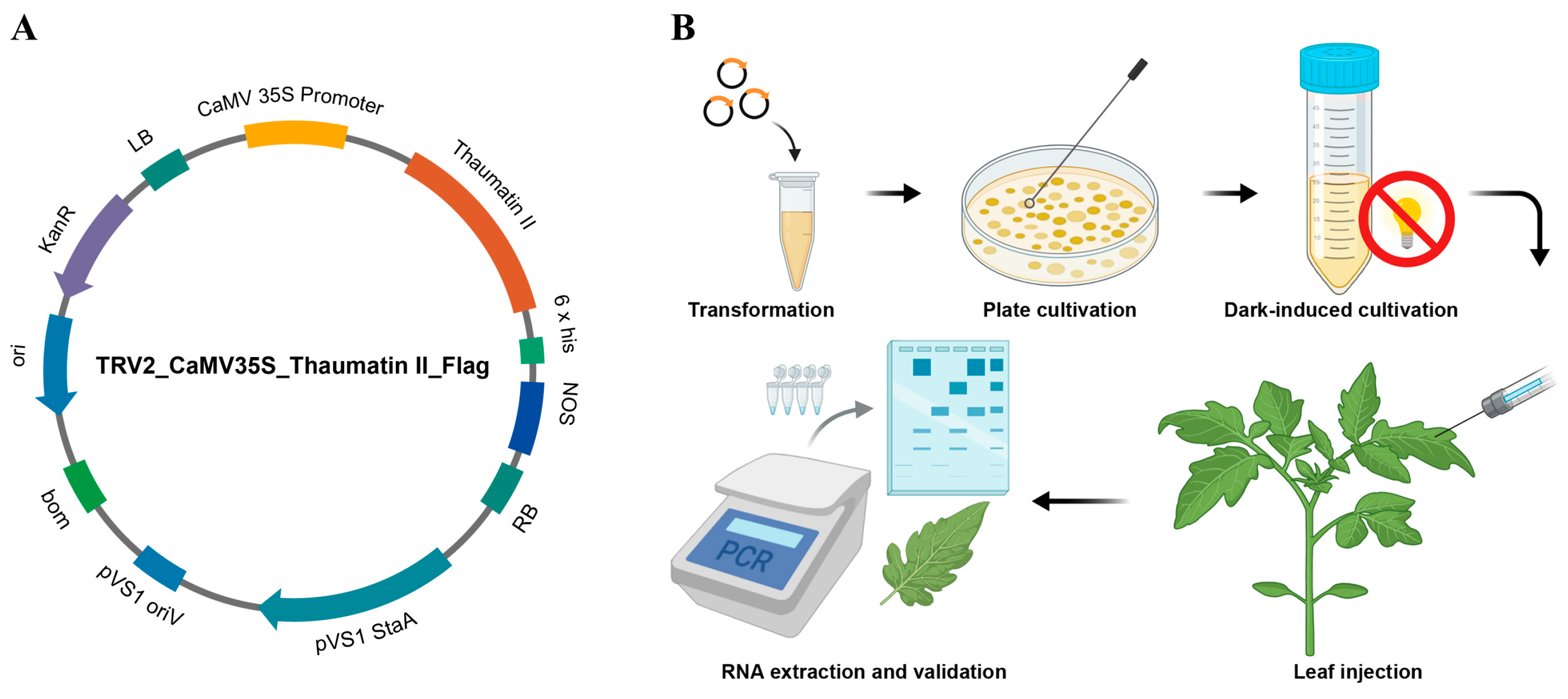
2.1. Plasmid Construction
2.2. Plant Materials
2.3. RNA Extraction and RT-PCR Analysis
2.4. Protein Extraction and Detection
2.5. Thaumatin Purification and Co-Immunoprecipitation
2.6. Tomato Metabolomic Analysis
2.6.1. UPLC Conditions
2.6.2. MS Conditions
2.7. Statistical Analysis
3. Results
3.1. Molecular Analysis of Transiently Transfected Plants with Viruses
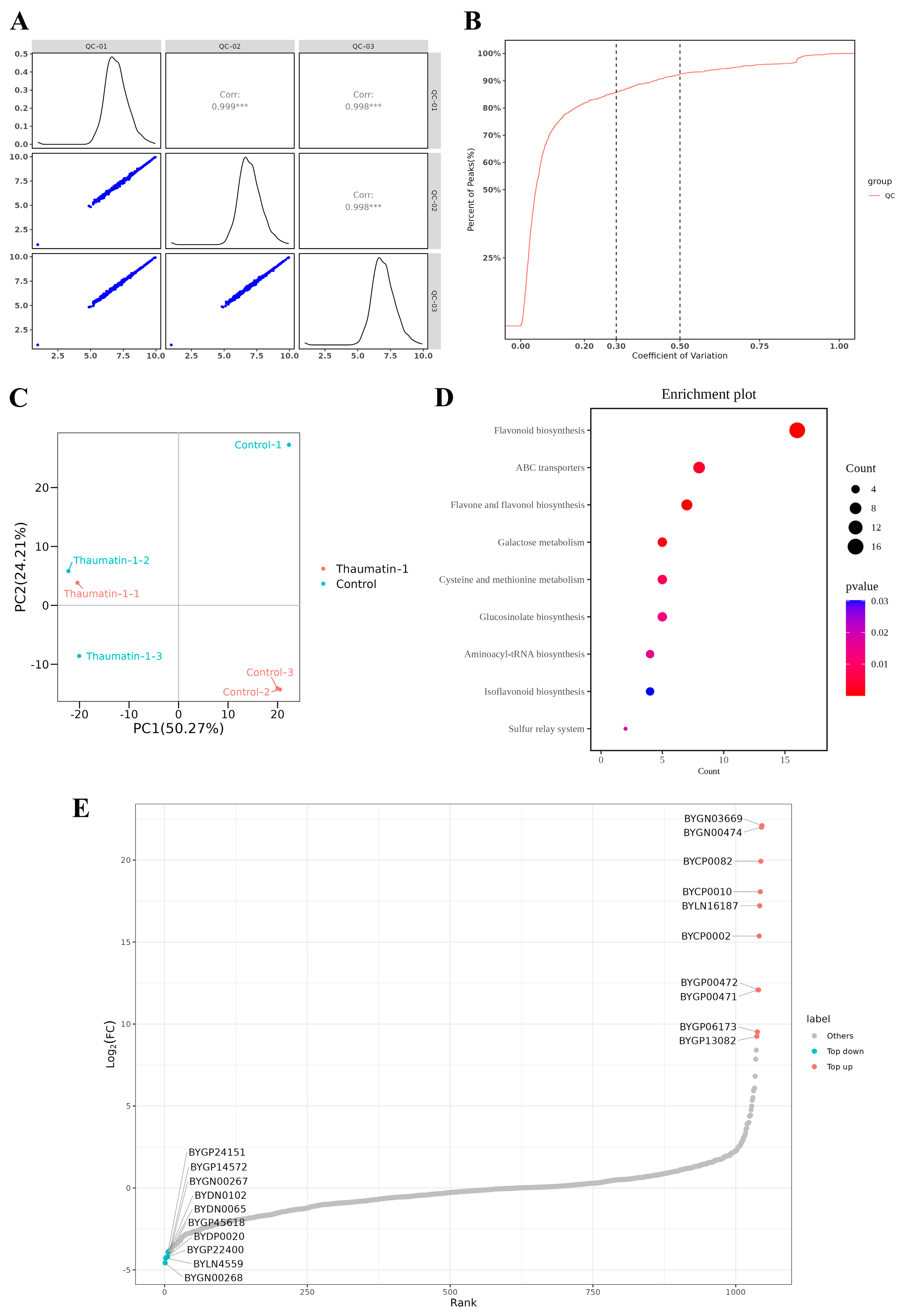
3.2. Metabolomic Analysis of Infected Tomato Plants
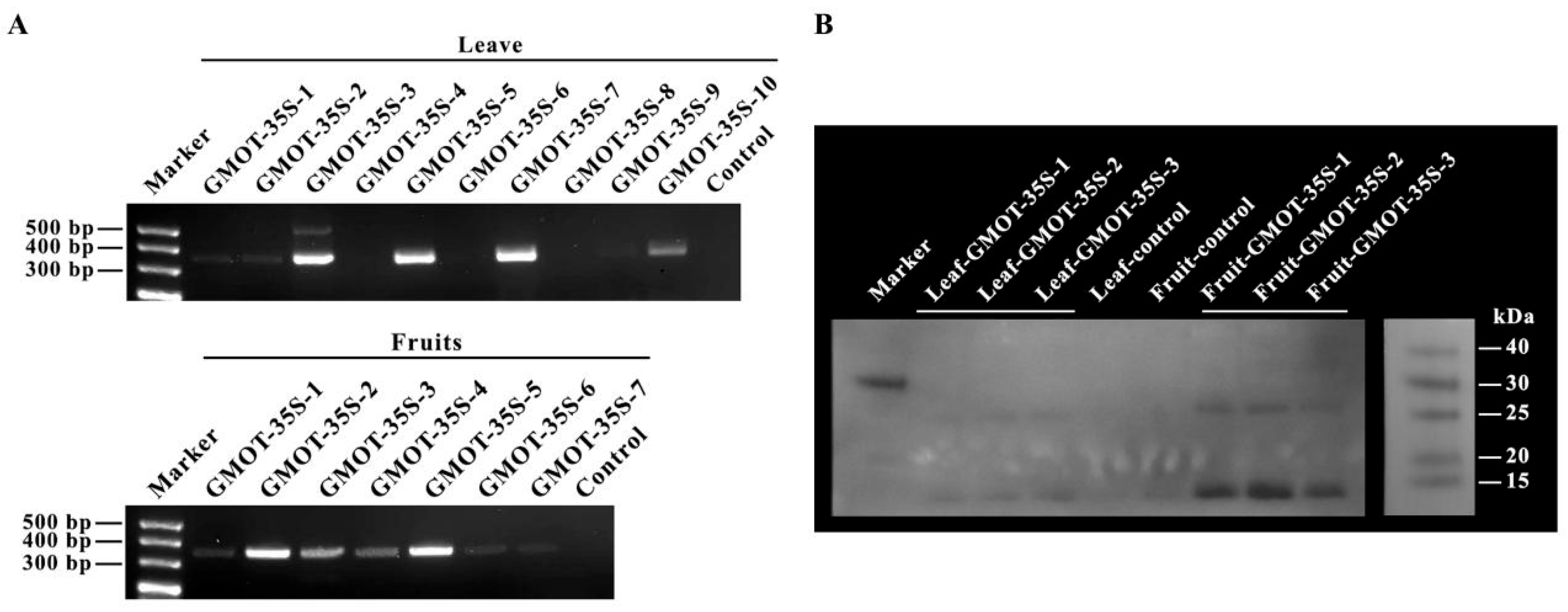
3.3. Transgenics and Regeneration
3.4. Characterization of Transgenic Plant Thaumatin Ⅱ
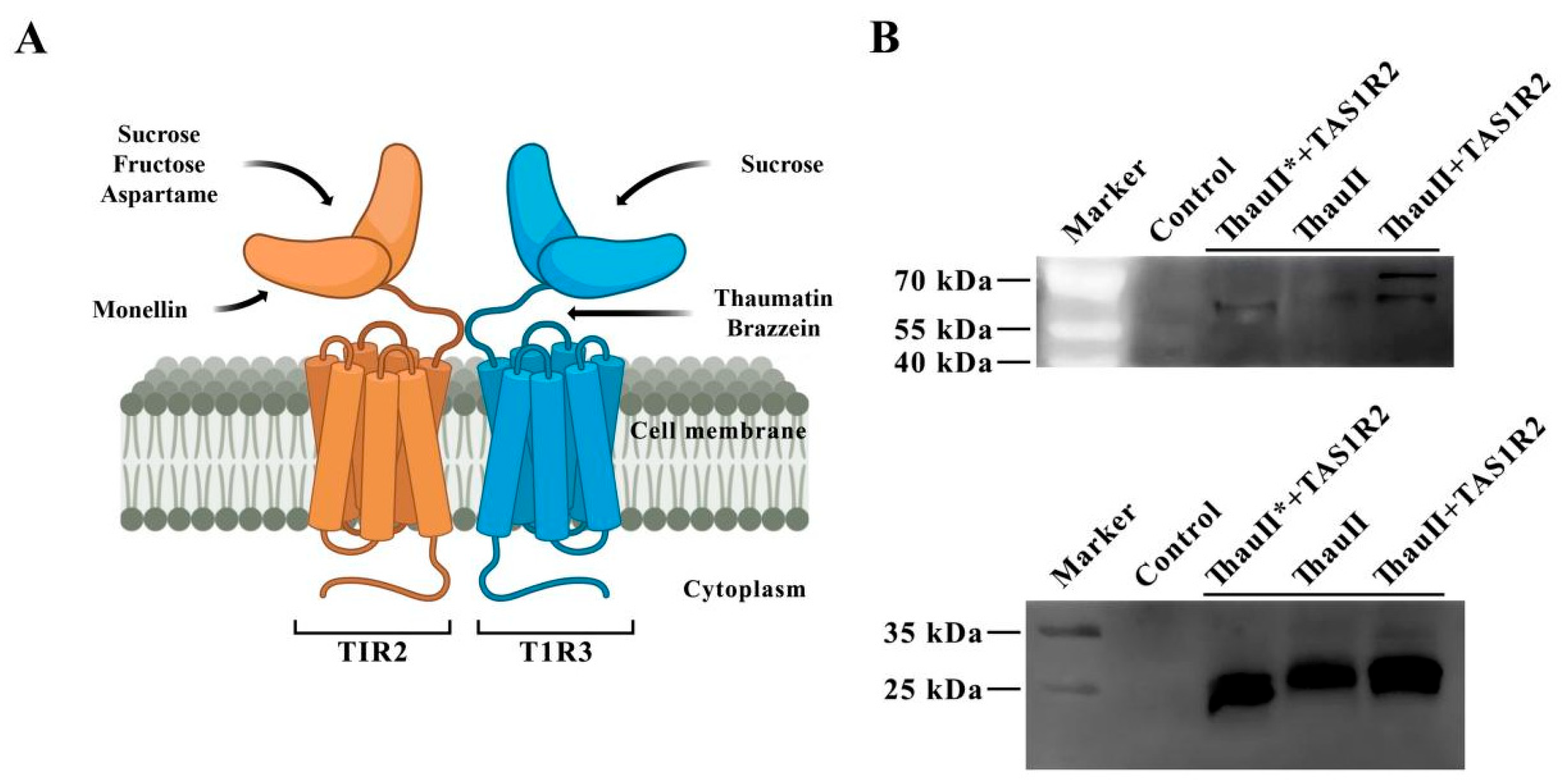
3.5. Interaction Analysis of Recombinant Thaumatin Ⅱ-T1RS2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnone, D.; Chabot, C.; Heba, A.C.; Kökten, T.; Caron, B.; Hansmannel, F.; Dreumont, N.; Ananthakrishnan, A.N.; Quilliot, D.; Peyrin-Biroulet, L. Sugars and Gastrointestinal Health. Clin. Gastroenterol. Hepatol. 2022, 20, 1912–1924.e7. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.X.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Artificial Sweeteners. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/artificial-sweeteners (accessed on 1 August 2025).
- Sun, Y.Y.; Liang, J.; Zhang, Z.R.; Sun, D.J.; Li, H.; Chen, L.X. Extraction, physicochemical properties, bioactivities and application of natural sweeteners: A review. Food Chem. 2024, 457, 140103. [Google Scholar] [CrossRef]
- Kant, R. Sweet proteins—Potential replacement for artificial low calorie sweeteners. Nutr. J. 2005, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Rezk, M.M.; Elashal, M.H.; El-Araby, M.; Khalifa, S.A.M.; El-Seedi, H.R. An updated multifaceted overview of sweet proteins and dipeptides as sugar substitutes; the chemistry, health benefits, gut interactions, and safety. Food Res. Int. 2022, 162, 111853. [Google Scholar] [CrossRef] [PubMed]
- Assadi-Porter, F.M.; Aceti, D.J.; Cheng, H.; Markley, J.L. Efficient production of recombinant brazzein, a small, heat-stable, sweet-tasting protein of plant origin. Arch. Biochem. Biophys. 2000, 376, 252–258. [Google Scholar] [CrossRef]
- Chen, Z.J.; Cai, H.; Lu, F.P.; Du, L.X. High-level expression of a synthetic gene encoding a sweet protein, monellin, in Escherichia coli. Biotechnol. Lett. 2005, 27, 1745–1749. [Google Scholar] [CrossRef]
- Masuda, T.; Okubo, K.; Baba, S.; Suzuki, M.; Tani, F.; Yamasaki, M.; Mikami, B. Structure of thaumatin under acidic conditions: Structural insight into the conformations in lysine residues responsible for maintaining the sweetness after heat-treatment. Food Chem. 2022, 389, 132996. [Google Scholar] [CrossRef]
- Kaneko, R.; Kitabatake, N. Sweetness of sweet protein thaumatin is more thermoresistant under acid conditions than under neutral or alkaline conditions. Biosci. Biotechnol. Biochem. 2001, 65, 409–413. [Google Scholar] [CrossRef]
- Teratogenesis. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/teratogenesis (accessed on 28 July 2025).
- Kelada, K.D.; Tusé, D.; Gleba, Y.; McDonald, K.A.; Nandi, S. Process Simulation and Techno-Economic Analysis of Large-Scale Bioproduction of Sweet Protein Thaumatin II. Foods 2021, 10, 838. [Google Scholar] [CrossRef]
- Zemanek, E.C.; Wasserman, B.P. Issues and Advances in the Use of Transgenic Organisms for the Production of Thaumatin, the Intensely Sweet Protein from Thaumatococcus-Danielli. Crit. Rev. Food Sci. Nutr. 1995, 35, 455–466. [Google Scholar] [CrossRef]
- Kiełkiewicz, M.; Gajc-Wolska, J.; Ślusarz, S.; Szwacka, M. Growth, development and yield of transgenic 35S-thaumatin II-expressing cucumber plants—Open field evaluation. Sci. Hortic. 2012, 143, 82–91. [Google Scholar] [CrossRef]
- Edens, L.; Heslinga, L.; Klok, R.; Ledeboer, A.M.; Maat, J.; Toonen, M.Y.; Visser, C.; Verrips, C.T. Cloning of cDNA encoding the sweet-tasting plant protein thaumatin and its expression in Escherichia coli. Gene 1982, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Daniell, S.; Mellits, K.H.; Faus, I.; Connerton, I. Refolding the sweet-tasting protein thaumatin II from insoluble inclusion bodies synthesised in Escherichia coli. Food Chem. 2000, 71, 105–110. [Google Scholar] [CrossRef]
- Illingworth, C.; Larson, G.; Hellekant, G. Secretion of the sweet-tasting plant protein thaumatin by Bacillus subtilis. Biotechnol. Lett. 1988, 10, 587–592. [Google Scholar] [CrossRef]
- Lombrana, M.; Moralejo, F.J.; Pinto, R.; Martín, J.F. Modulation of Aspergillus awamori thaumatin secretion by modification of bipA gene expression. Appl. Environ. Microbiol. 2004, 70, 5145–5152. [Google Scholar] [CrossRef]
- Jahic, M.; Wallberg, F.; Bollok, M.; Garcia, P.; Enfors, S.-O. Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb. Cell Factories 2003, 2, 6. [Google Scholar] [CrossRef]
- Guo, G.; Li, M.-J.; Lai, J.-L.; Du, Z.-Y.; Liao, Q.-S. Development of tobacco rattle virus-based platform for dual heterologous gene expression and CRISPR/Cas reagent delivery. Plant Sci. 2022, 325, 111491. [Google Scholar] [CrossRef] [PubMed]
- Vanderwel, H.; Loeve, K. Isolation and Characterization of Thaumatin I and II, Sweet-Tasting Proteins from Thaumatococcus-Daniellii Benth. Eur. J. Biochem. 1972, 31, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Sawa, A.; Nio, N.; Ariyoshi, Y. Enzymatic ligation for synthesis of single-chain analogue of monellin by transglutaminase. Biopolymers 1999, 50, 193–200. [Google Scholar] [CrossRef]
- Ogata, C.; Hatada, M.; Tomlinson, G.; Shin, W.C.; Kim, S.H. Crystal-Structure of the Intensely Sweet Protein Monellin. Nature 1987, 328, 739–742. [Google Scholar] [CrossRef]
- Morris, J.A.; Martenson, R.; Deibler, G.; Cagan, R.H. Characterization of Monellin, A Protein That Tastes Sweet. J. Biol. Chem. 1973, 248, 534–539. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, C.H.; Kim, R.; Cho, J.M.; Lee, Y.B.; Lee, T.K. Redesigning a Sweet Protein—Increased Stability and Renaturability. Protein Eng. 1989, 2, 571–575. [Google Scholar] [CrossRef]
- Lamphear, B.J.; Barker, D.K.; Brooks, C.A.; Delaney, D.E.; Lane, J.R.; Beifuss, K.; Love, R.; Thompson, K.; Mayor, J.; Clough, R.; et al. Expression of the sweet protein brazzein in maize for production of a new commercial sweetener. Plant Biotechnol. J. 2005, 3, 103–114. [Google Scholar] [CrossRef]
- Zeece, M. Chapter six—Flavors. In Introduction to the Chemistry of Food; Zeece, M., Ed.; Academic Press: London, UK, 2020; pp. 213–250. [Google Scholar]
- Izawa, H.; Ota, M.; Kohmura, M.; Ariyoshi, Y. Synthesis and characterization of the sweet protein brazzein. Biopolymers 1996, 39, 95–101. [Google Scholar] [CrossRef]
- Ming, D.; Hellekant, G. Brazzein, a New High-Potency Thermostable Sweet Protein from Pentadiplandra-Brazzeana-B. Febs Lett. 1994, 355, 106–108. [Google Scholar] [CrossRef]
- Bahadur, B.; Pal, A.K. Natural sweetening plants: A global review. LS Int. J. Life Sci. 2020, 9, 134–157. [Google Scholar] [CrossRef]
- Tafazoli, S.; Vo, T.D.; Roberts, A.; Rodriguez, C.; Viñas, R.; Madonna, M.E.; Chiang, Y.-H.; Noronha, J.W.; Holguin, J.C.; Ryder, J.A.; et al. Safety assessment of miraculin using in silico and in vitro digestibility analyses. Food Chem. Toxicol. 2019, 133, 110762. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Theerasilp, S.; Aiuchi, T.; Nakaya, K.; Nakamura, Y.; Kurihara, Y. Purification and complete amino acid sequence of a new type of sweet protein taste-modifying activity, curculin. J. Biol. Chem. 1990, 265, 15770–15775. [Google Scholar] [CrossRef]
- Yamashita, H.; Akabane, T.; Kurihara, Y. Activity and stability of a new sweet protein with taste-modifying action, curculin. Chem. Senses 1995, 20, 239–243. [Google Scholar] [CrossRef]
- Liu, X.; Maeda, S.; Hu, Z.; Aiuchi, T.; Nakaya, K.; Kurihara, Y. Purification, complete amino acid sequence and structural characterization of the heat-stable sweet protein, mabinlin II. Eur. J. Biochem. 1993, 211, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Nirasawa, S.; Nishino, T.; Katahira, M.; Uesugi, S.; Hu, Z.; Kurihara, Y. Structures of heat-stable and unstable homologues of the sweet protein mabinlin. The difference in the heat stability is due to replacement of a single amino acid residue. Eur. J. Biochem. 1994, 223, 989–995. [Google Scholar] [CrossRef]
- Nirasawa, S.; Liu, X.; Nishino, T.; Kurihara, Y. Disulfide bridge structure of the heat-stable sweet protein mabinlin II. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1993, 1202, 277–280. [Google Scholar] [CrossRef]
- Wel, H.v.d.; Larson, G.; Hladik, A.; Hladik, C.M.; Hellekant, G.; Glaser, D. Isolation and characterization of pentadin, the sweet principle of Pentadiplandra brazzeana Baillon. Chem. Senses 1989, 14, 75–79. [Google Scholar] [CrossRef]
- Faus, I. Recent developments in the characterization and biotechnological production of sweet-tasting proteins. Appl. Microbiol. Biotechnol. 2000, 53, 145–151. [Google Scholar] [CrossRef]
- Kashani-Amin, E.; Faraji, H.; Nouriyengejeh, S.; Ebrahim-Habibi, A. Structure-sweetness relationship of sweet proteins: A systematic review on “sweet protein” studies as a sub-group of “sweetener” investigations. Mosc. Univ. Biol. Sci. Bull. 2021, 76, 175–190. [Google Scholar] [CrossRef]
- Firsov, A.; Shaloiko, L.; Kozlov, O.; Vinokurov, L.; Vainstein, A.; Dolgov, S. Purification and characterization of recombinant supersweet protein thaumatin II from tomato fruit. Protein Expr. Purif. 2016, 123, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wuhan MetWare Biotechnology Co., Ltd. Available online: https://www.metware.cn/ (accessed on 25 June 2025).
- ANPEL. Available online: https://www.anpel.com.cn/ (accessed on 25 June 2025).
- MS-DIAL Software. Available online: https://lipidmaps.org/resources/tools/16?task=4.3 (accessed on 25 July 2025).
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 30 July 2025).
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- DuBois, G.E. Molecular mechanism of sweetness sensation. Physiol. Behav. 2016, 164, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Sui, X.; Zhang, Y. Amino Acid Metabolism and Transporters in Plant−Pathogen Interactions: Mechanisms and Implications. Plant Cell Environ. 2025, 48, 6086–6098. [Google Scholar] [CrossRef]
- Bauer, S.; Mekonnen, D.W.; Geist, B.; Lange, B.; Ghirardo, A.; Zhang, W.; Schäffner, A.R. The isoleucic acid triad: Distinct impacts on plant defense, root growth, and formation of reactive oxygen species. J. Exp. Bot. 2020, 71, 4258–4270. [Google Scholar] [CrossRef] [PubMed]
- Schestibratov, K.A.; Dolgov, S.V. Transgenic strawberry plants expressing a thaumatin II gene demonstrate enhanced resistance to Botrytis cinerea. Sci. Hortic. 2005, 106, 177–189. [Google Scholar] [CrossRef]
- Kawakami, C.A.; Selani, M.M.; Saldaña, E.; Pimentel-Filho, N.d.J.; Fontenele Domingues, M.A. Sensory dynamic profile and consumer acceptance of short-dough biscuits with reduced sucrose and thaumatin addition. Food Res. Int. 2025, 200, 115524. [Google Scholar] [CrossRef]
- Suckling, J.; Morse, S.; Murphy, R.; Astley, S.; Boy, C.; Halford, J.C.G.; Harrold, J.A.; Le-Bail, A.; Koukouna, E.; Musinovic, H.; et al. Life cycle assessment of the sweetness enhancer thaumatin (E957) produced from Thaumatococcus daniellii fruit foraged from West Africa: The SWEET project. J. Clean. Prod. 2023, 411, 137226. [Google Scholar] [CrossRef]
- Pallante, L.; Malavolta, M.; Grasso, G.; Korfiati, A.; Mavroudi, S.; Mavkov, B.; Kalogeras, A.; Alexakos, C.; Martos, V.; Amoroso, D.; et al. On the human taste perception: Molecular-level understanding empowered by computational methods. Trends Food Sci. Technol. 2021, 116, 445–459. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, W.; Wu, L.; Yue, X.; Liu, S.; Ding, W.; Zhang, J.; Meng, B.; Zhao, L.; Liu, X.; et al. Structural and functional characterization of human sweet taste receptor. Nature 2025, 645, 801–808. [Google Scholar] [CrossRef]
- Ohta, K.; Masuda, T.; Tani, F.; Kitabatake, N. The cysteine-rich domain of human T1R3 is necessary for the interaction between human T1R2–T1R3 sweet receptors and a sweet-tasting protein, thaumatin. Biochem. Biophys. Res. Commun. 2011, 406, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, W. Molecular basis and detection technologies of sweetness. Food Res. Int. 2025, 219, 116965. [Google Scholar] [CrossRef]

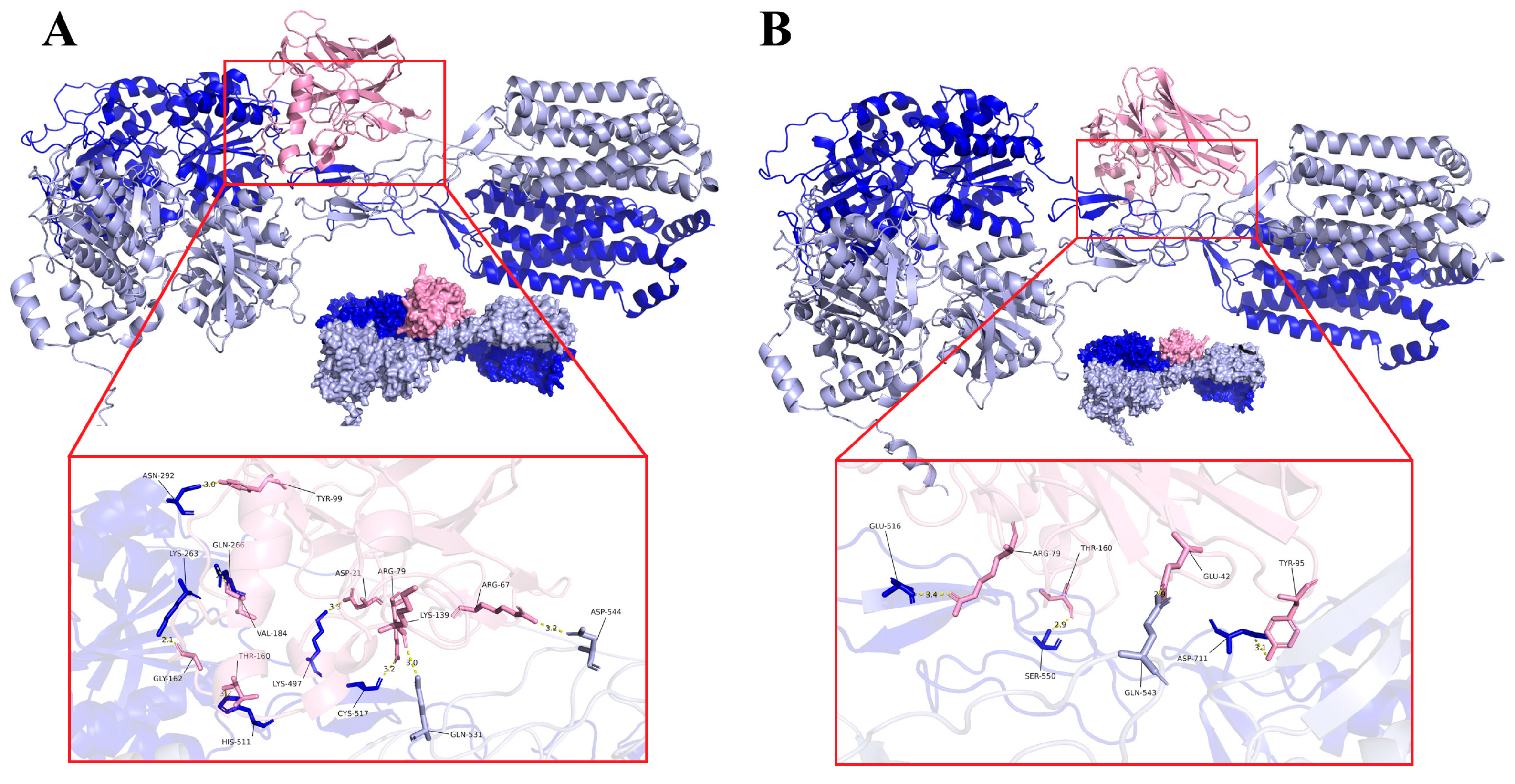
| Thaumatin II | TAS1R2 | Distance (Å) | Interface Area (Å2) | ΔiG (kcal/mol) |
| SP-21 | LYS-497 | 3.3 | 584.3 | −0.7 |
| ARG-79 | CYS-517 | 3.2 | ||
| TYR-99 | ASN-292 | 3.0 | ||
| LYS-163 | GLN-237 | 3.7 | ||
| GLY-162 | LYS-263 | 2.1 | ||
| PRO-188 | ASP-262 | 2.9 | ||
| Thaumatin II | TAS1R3 | Distance (Å) | Interface Area (Å2) | ΔiG (kcal/mol) |
| ARG-67 | ASP-544 | 3.2 | 442.1 | 0.1 |
| LYS-139 | GLN-531 | 3.0 | ||
| PRO-141 | BLN-531 | 3.0 |
| Thaumatin Ⅱ | TAS1R2 | Distance (Å) | Interface Area (Å2) | ΔiG (kcal/mol) |
| ARG-79 | GLU-516 | 3.4 | 731.5 | −0.1 |
| TYR-95 | ASP-711 | 3.1 | ||
| THR-160 | SER-550 | 2.9 | ||
| Thaumatin Ⅱ | TAS1R3 | Distance (Å) | Interface Area (Å2) | ΔiG (kcal/mol) |
| GLU-42 | GLN-543 | 3.2 | 56.0 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, Q.; Guo, S.; Li, Y.; Chen, R.; Li, K.; An, G.; Liu, Y.; Hong, Z.; Mo, B.; et al. An Endogenous, Flavor-Enhancing TRV/Agrobacterium System for Edible Tomato Fruits with the Sweet Protein Thaumatin II. Horticulturae 2025, 11, 1284. https://doi.org/10.3390/horticulturae11111284
Chen J, Liu Q, Guo S, Li Y, Chen R, Li K, An G, Liu Y, Hong Z, Mo B, et al. An Endogenous, Flavor-Enhancing TRV/Agrobacterium System for Edible Tomato Fruits with the Sweet Protein Thaumatin II. Horticulturae. 2025; 11(11):1284. https://doi.org/10.3390/horticulturae11111284
Chicago/Turabian StyleChen, Jiachun, Qizheng Liu, Siyuan Guo, Yitong Li, Ruohan Chen, Kexin Li, Guangbin An, Yuanrun Liu, Zhengyue Hong, Beixin Mo, and et al. 2025. "An Endogenous, Flavor-Enhancing TRV/Agrobacterium System for Edible Tomato Fruits with the Sweet Protein Thaumatin II" Horticulturae 11, no. 11: 1284. https://doi.org/10.3390/horticulturae11111284
APA StyleChen, J., Liu, Q., Guo, S., Li, Y., Chen, R., Li, K., An, G., Liu, Y., Hong, Z., Mo, B., Liu, X., & Chen, W. (2025). An Endogenous, Flavor-Enhancing TRV/Agrobacterium System for Edible Tomato Fruits with the Sweet Protein Thaumatin II. Horticulturae, 11(11), 1284. https://doi.org/10.3390/horticulturae11111284







