The Agronomic Traits Differences in Hericium erinaceus Cultivated with Different Straw Formulations by Replacing Wood with Straw
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Plate Culture Experiment
2.3. Cultivation Experiment
2.4. Agronomic Trait Measurement
2.5. Data Processing
3. Results
3.1. The Result of Plate Culture Experiment
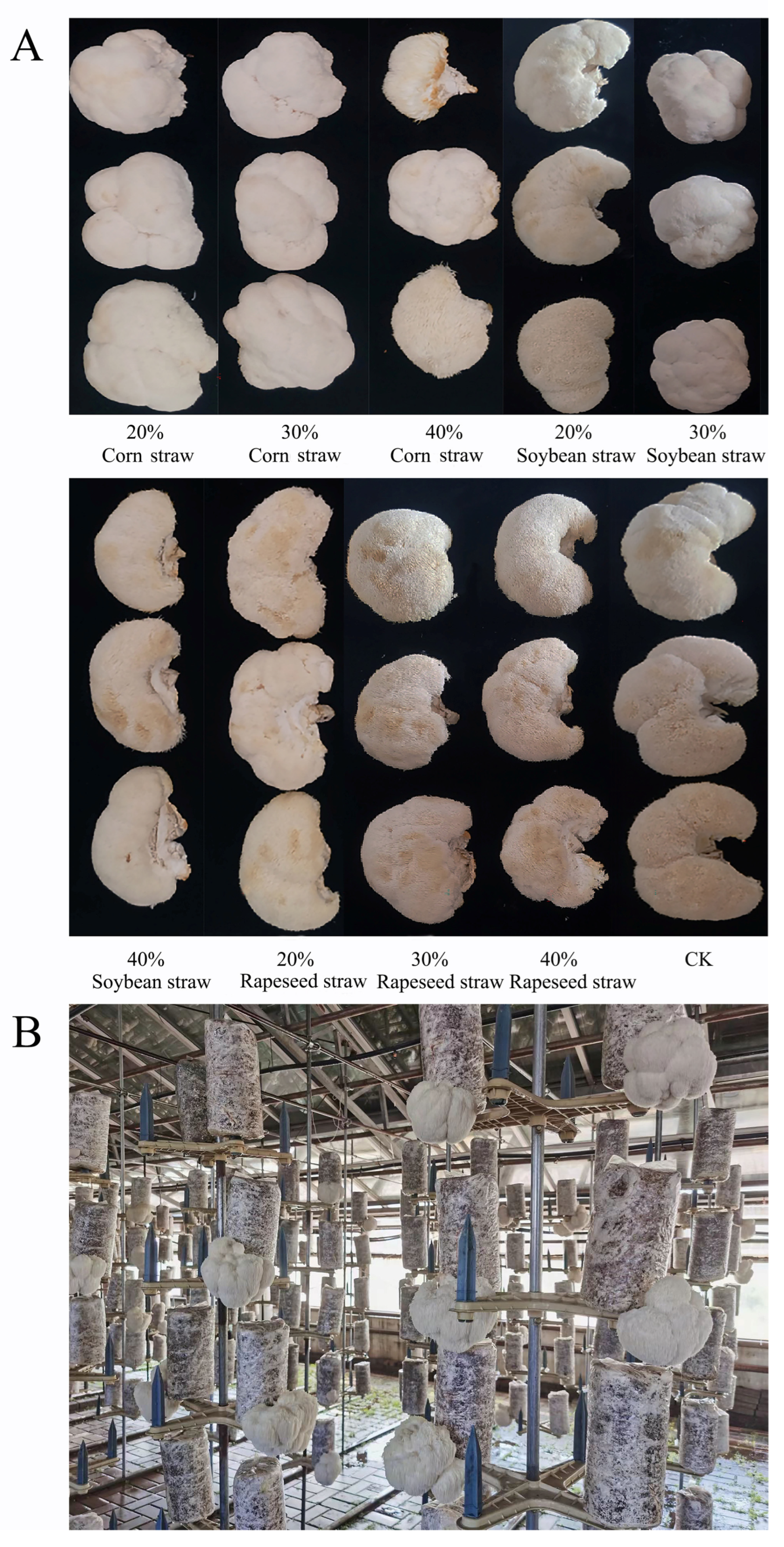
3.2. The Growth Status of Hericium erinaceus Hyphae in the Cultivation Bag
3.3. Comparison of Agronomic Traits of Hericium erinaceus with Different Formulas
| Number | Primordia Formation Time (d) | Length of the Fruiting Body (cm) | Width of the Fruiting Body (cm) | Firmness | Antimicrobial Resistance | Biological Efficiency (%) | Fresh Weight per Mushroom (g) | Average Fresh Weight (g) |
|---|---|---|---|---|---|---|---|---|
| CK | 7 ± 1 | 6–12 | 8–13 | Firm | High resistance | 90.91 ± 0.33 b* | 210–275 | 250.00 ± 0.38 c |
| 1 | 7 ± 1 | 7–13 | 8–14 | Firm | High resistance | 92.31 ± 0.60 a | 209–278 | 253.85 ± 0.70 a |
| 2 | 8 ± 1 | 7–12 | 8–13 | Moderate | High resistance | 87.56 ± 0.61 d | 210–260 | 240.79 ± 0.69 e |
| 3 | 8 ± 2 | 5–10 | 7–11 | Loose | High resistance | 70.47 ± 0.55 g | 180–240 | 193.79 ± 0.51 h |
| 4 | 7 ± 1 | 5–12 | 8–13 | Firm | High resistance | 91.64 ± 0.54 ab | 190–280 | 252.01 ± 0.86 b |
| 5 | 8 ± 1 | 6–12 | 8–13 | Moderate | High resistance | 88.89 ± 0.85 c | 185–269 | 244.45 ± 0.42 d |
| 6 | 8 ± 3 | 5–10 | 6–12 | Loose | High resistance | 79.79 ± 0.53 e | 180–235 | 219.42 ± 0.46 f |
| 7 | 7 ± 1 | 5–12 | 8–13 | Firm | High resistance | 90.89 ± 0.83 b | 200–265 | 249.95 ± 0.61 c |
| 8 | 8 ± 3 | 6–13 | 8–13 | Loose | High resistance | 87.69 ± 0.66 cd | 189–267 | 241.15 ± 0.81 e |
| 9 | 8 ± 2 | 5–10 | 6–12 | Loose | High resistance | 76.46 ± 0.45 f | 175–243 | 210.27 ± 0.59 g |
3.4. Nutritional Components
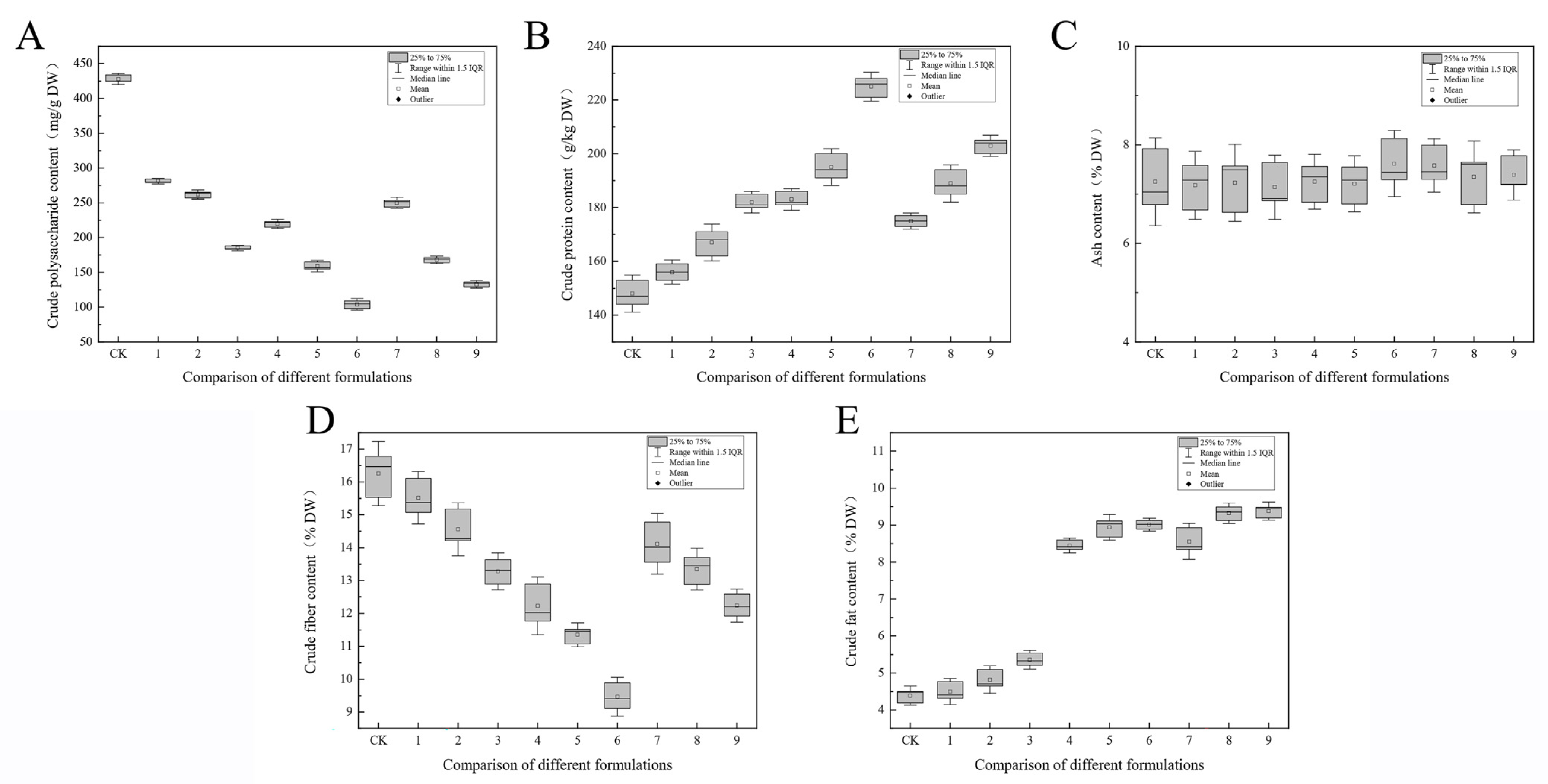
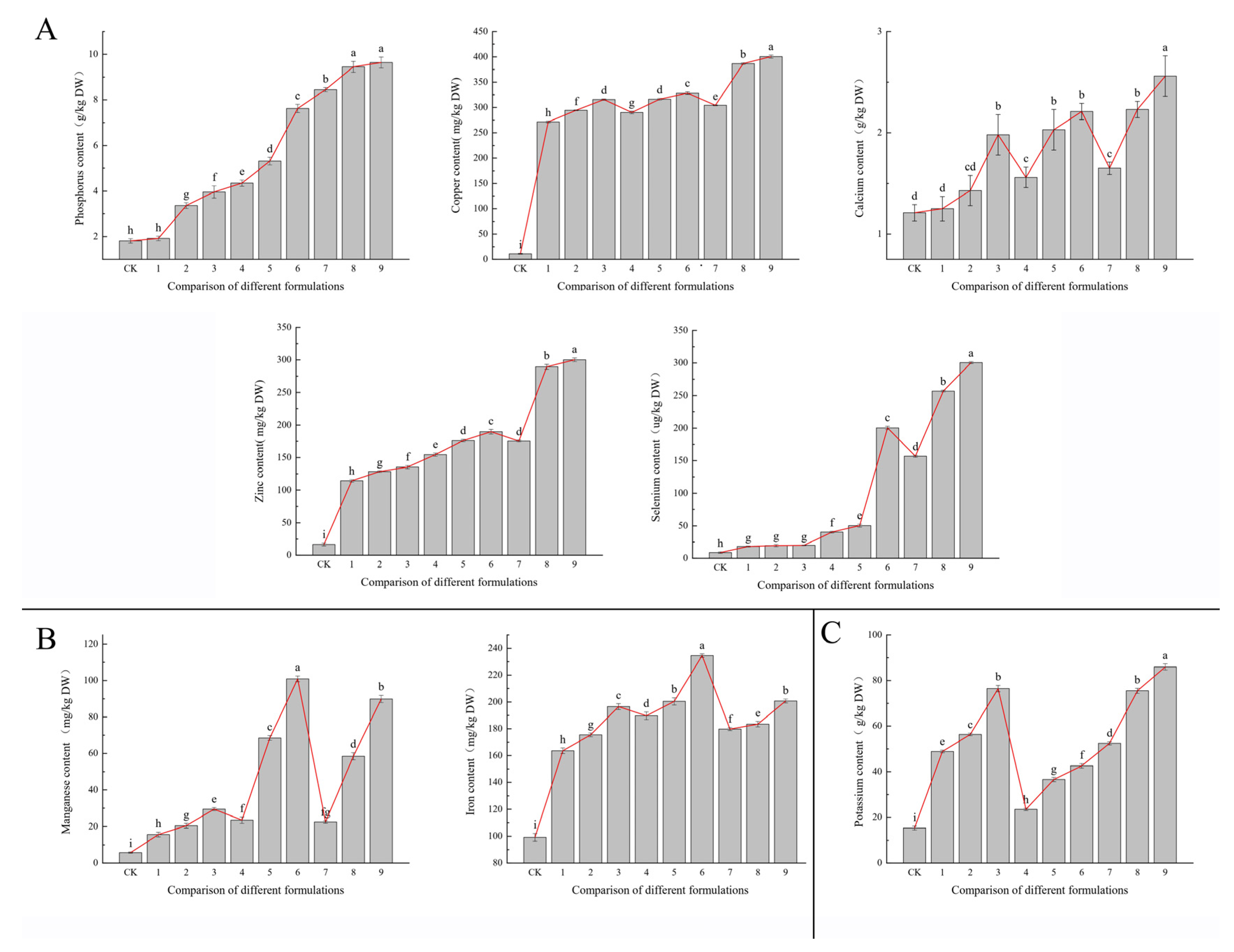
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T. Bioactive substances in Hericium erinaceus (Bull.: Fr.) Pers. (Yamabushitake), and its medicinal utilization. Int. J. Med. Mushrooms 1999, 1, 105–119. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, G.L.; Liu, W.M.; Zhang, F.M.; Linhardt, R.J.; Wang, X.L.; Zhang, A.Q. Bioactive substances in Hericium erinaceus and their biological properties: A review. Food Sci. Hum. Wellness 2024, 13, 1825–1844. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Song, J.; Jo, M.; Yu, J.; Kim, K.; Kim, Y.; Oh, J.; Choi, S. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf. B Biointerfaces 2017, 150, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Zhang, J.C.; Dong, C.; Zhuang, C.; Hirota, S.; Inanaga, K.; Hashimoto, K. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2015, 136, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawahara, K.; Osawa, H.; Song, L.T.; Numazaki, K.; Kawai, J.; Onoue, S.; Nishioka, T.; Nemoto, E.; Matsushita, K.; et al. Hericium erinaceus ethanol extract and ergosterol exert anti-inflammatory activities by neutralizing lipopolysaccharide-induced pro-inflammatory cytokine production in human monocytes. Biochem. Biophys. Res. Commun. 2022, 636, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, E.; Nie, S.; Liu, L.; Ren, J. Study on the interaction of Hericium erinaceus mycelium polysaccharides and its degradation products with food additive silica nanoparticles. Food Chem. X 2021, 12, 100172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Zhou, W.; Kim, E.; Shim, S.; Kang, H.; Kim, Y. Isolation and identification of aromatic compounds in Lion’s Mane Mushroom and their anticancer activities. Food Chem. 2015, 170, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, X.; Li, Z.; Shan, S.; Chang, M.; Feng, C.; Wei, Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. Int. J. Biol. Macromol. 2020, 157, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Gao, X.; Zhang, J.; Liu, M.; Zhang, C.; Xu, N.; Zhao, H.; Lin, L.; Zhou, M.; Jia, L. Protective effects of extracellular and intracellular polysaccharides on hepatotoxicity by Hericium erinaceus SG-02. Curr. Microbiol. 2016, 73, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, Y.; Zhang, X. Comparative studies on extracts from Hericium erinaceus by different polarity reagents to gain higher antioxidant activities. Exp. Ther. Med. 2016, 12, 513–517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, S.; Chen, L.; Xiong, R.; Jiang, J.; Liu, Y.; Tan, X.; Liu, T.; Zeng, Y.; Pan, X.; Zeng, Y. Long-term straw return improves cooked indica rice texture by altering starch structural, physicochemical properties in South China. Food Chem. X 2023, 20, 100965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dedousi, M.; Melanouri, E.M.; Karayannis, D.; Kaminarides, E.I.; Diamantopoulou, P. Utilization of spent substrates and waste products of mushroom cultivation to produce new crops of Pleurotus ostreatus, Pleurotus eryngii and Agaricus bisporus. Carbon Resour. Convers. 2024, 7, 100196. [Google Scholar] [CrossRef]
- Liu, F.; Xiang, M.; Guo, Y.; Lu, G.X.; Yang, Y.; Liu, X.Z.; Chen, S.J.; Zhang, G.Z.; Shi, W.P. Culture conditions and nutrition requirements for the mycelial growth of Isaria farinosa (Hypocreales: Cordycipitaceae) and the altitude effect on its growth and metabolome. Sci. Rep. 2018, 8, 15623. [Google Scholar] [CrossRef]
- Wu, N.; Tian, F.; Moodley, O.; Song, B.; Jia, C.; Ye, J.; Lv, R.; Qin, Z.; Li, C. Optimization of agro-residues as substrates for Pleurotus pulmonarius production. AMB Express 2019, 9, 184. [Google Scholar] [CrossRef]
- Pedri, Z.; Lozano, L.; Hermann, K.; Helm, C.; Peralta, R.; Tavares, L. Influence of nitrogen sources on the enzymatic activity and grown by Lentinula edodes in biomass Eucalyptus benthamii. Braz. J. Biol. 2015, 75, 940–947. [Google Scholar] [CrossRef]
- Ren, J.; Yu, P.; Xu, X. Straw Utilization in China—Status and Recommendations. Sustainability 2019, 11, 1762. [Google Scholar] [CrossRef]
- Árvay, J.; Tomáš, J.; Hauptvogl, M.; Kopernická, M.; Kováčik, A.; Bajčan, D.; Massányi, P. Contamination of wild-grown edible mushrooms by heavy metals in a former mercury-mining area. J. Environ. Sci. Health B 2014, 49, 815–827. [Google Scholar] [CrossRef]
- Širić, I.; Humar, M.; Kasap, A.; Kos, I.; Mioč, B.; Pohleven, F. Heavy metal bioaccumulation by wild edible saprophytic and ectomycorrhizal mushrooms. Environ. Sci. Pollut. Res. Int. 2016, 23, 18239–18252. [Google Scholar] [CrossRef]
- Rzymski, P.; Mleczek, M.; Siwulski, M.; Jasińska, A.; Budka, A.; Niedzielski, P.; Kalač, P.; Gąsecka, M.; Budzyńska, S. Multielemental analysis of fruit bodies of three cultivated commercial Agaricus species. J. Food Compos. Anal. 2017, 59, 170–178. [Google Scholar] [CrossRef]
- Elkanah, F.; Oke, M.; Adebayo, E. Substrate composition effect on the nutritional quality of Pleurotus ostreatus (MK751847) fruiting body. Heliyon 2022, 8, e11841. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P. Is the yellow knight mushroom edible or not? A systematic review and critical viewpoints on the toxicity of Tricholoma equestre. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1309–1324. [Google Scholar] [CrossRef]
- Meng, L.; Fu, Y.; Li, D.; Sun, X.; Chen, Y.; Li, X.; Xu, S.; Li, X.; Li, C.; Song, B.; et al. Effects of corn stalk cultivation substrate on the growth of the slippery mushroom (Pholiota microspora). RSC Adv. 2019, 9, 5347–5353. [Google Scholar] [CrossRef]
- Mshandete, A.; Cuff, J. Proximate and Nutrient Composition of Three Types of Indigenous Edible Wild Mushrooms Grown in Tanzania and Their Utilization Prospects. Afr. J. Food Agric. Nutr. Dev. 2007, 7, 1684–5374. [Google Scholar]
- Bhattacharjya, D.K.; Paul, R.K.; Miah, M.N.; Ahmed, K.U. Comparative Study on Nutritional Composition of Oyster Mushroom (Pleurotus ostreatus Fr.) Cultivated on Different Sawdust Substrates. BRC 2015, 1, 93–98. [Google Scholar]
- Yin, Y.; Chen, B.B.; Xu, S.; Zuo, J.C.; Xu, Y.; Xiong, S.J.; Chen, F. Investigation of crop straw for edible and medicinal fungi cultivation: Assessment of lignocellulose preprocessing and spent substrate biofuel properties. Ind. Crop. Prod. 2025, 223, 120004. [Google Scholar] [CrossRef]
- Jahedi, A.; Ahmadifar, S.; Mohammadigoltapeh, E. Revival of wild edible-medicinal mushroom (Hericium erinaceus) based on organic agro-industrial waste-achieving a commercial protocol with the highest yield; optimum reuse of organic waste. Sci. Hortic. 2024, 323, 112510. [Google Scholar] [CrossRef]
- Bunroj, A.; Jiraporn, S.; Watcharawit, R. Research and development project of monkey’s head mushroom (Hericium erinaceus) cultivation in east of Thailand. Int. J. Agric. Technol. 2017, 13, 1529–1535. [Google Scholar]
- Aswathy, S.; Shyamalagowri, S.; Hari, S.; Kanimozhi, M.; Meenambiga, S.; Thenmozhi, M.; Karthiyayini, R.; Suresh, D.; Manjunathan, J. Comparative studies on the cultivation, yield, and nutritive value of an edible mushroom, Pleurotus tuber-regium (Rumph. ex Fr.) Singer, grown under different agro waste substrates. 3 Biotech 2024, 14, 123. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, L.; Ren, Z.; Hu, S.; Wang, Y.; Ji, S.; Wang, X.; Du, Z.; Liu, Y.; Yang, Y.; et al. Optimization of substrate formulation for Hericium erinaceus by replacing wood by straw and their effect on enzyme activities. Front. Plant Sci. 2024, 15, 1436385. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Mikołajczak, P.; Gąsecka, M.; Rissmann, I.; Goliński, P.; Sobieralski, K. Differences in Cu content in selected mushroom species growing in the same unpolluted areas in Poland. J. Environ. Sci. Health B 2015, 50, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Lee, W.; Han, S.; Sung, J. Observations on some of the mycelial growth and pigmentation characteristics of Cordyceps militaris isolates. Mycobiology 2006, 34, 83–91. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.H.; Kim, H.R.; Chun, Y.S.; Lee, J.H.; Park, C.; Yoo, H.Y.; Kim, S.W. Rapid and concise quantification of mycelial growth by microscopic image intensity model and application to mass cultivation of fungi. Sci. Rep. 2021, 11, 24157. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, F.; Chen, H.; Chen, L.; Liu, Y. Integrated nutritional and functional components analyses reveal insights into the peel and pulp quality at different harvest times of ‘Dahongpao’ tangerine (Citrus reticulata Blanco). Food Chem. 2024, 463, 141263. [Google Scholar] [CrossRef] [PubMed]
- Hueso, D.; Fontecha, J.; Gómez-Cortés, P. Comparative study of the most commonly used methods for total protein determination in milk of different species and their ultrafiltration products. Front. Nutr. 2022, 9, 925565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, S.; Qian, Z.; He, Z.; Zhou, W.; Xu, H. Analysis of nutrients in Apriona germari and treatment of diarrhea in mice fed with insect powder. Biochem. Biophys. Rep. 2023, 33, 101368. [Google Scholar] [CrossRef]
- Tian, T.; Hu, H.; Ma, Y.; Qin, J.; Li, C.; Li, Y. Effects of light quality on agronomic traits, antioxidant capacity and nutritional composition of Sarcomyxa edulis. Sci. Rep. 2024, 14, 24762. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, J.; Li, Z.; Weng, M.; Zhang, X.; Tang, X.; Pan, Y. Effects of ultra-high pressure, thermal pasteurization, and ultra-high temperature sterilization on color and nutritional components of freshly-squeezed lettuce juice. Food Chem. 2024, 435, 137524. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Fang, H.; Jia, Y.; Yun, Z.; Wang, L.; Wang, X. Comparative evaluation of physical and chemical properties, nutritional components and volatile substances of 24 chestnut varieties in Yanshan area. Food Chem. 2025, 477, 143624. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, L.; Wang, X.; Zhao, W.; Shi, X.; Wang, W.; Li, Z.; Wang, L. The yield, nutritional value, umami components and mineral contents of the first-flush and second-flush Pleurotus pulmonarius mushrooms grown on three forestry wastes. Food Chem. 2022, 397, 133714. [Google Scholar] [CrossRef]
- Schmitz, C.; Grambusch, I.; Neutzling Lehn, D.; Hoehne, L.; Volken de Souza, C. A systematic review and meta-analysis of validated analytical techniques for the determination of total selenium in foods and beverages. Food Chem. 2023, 429, 136974. [Google Scholar] [CrossRef]
- Aicha, A.H.; Nico, A.; Antje, C.S.; Petr, B.; Said, B. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar]
- Zhang, Y.; Yao, A.; Song, K. Torrefaction of cultivation residue of Auricularia auricula-judae to obtain biochar with enhanced fuel properties. Bioresour. Technol. 2016, 206, 211–216. [Google Scholar] [CrossRef]
- Moonmoon, M.; Uddin, M.; Ahmed, S.; Shelly, N.; Khan, M. Cultivation of different strains of king oyster mushroom (Pleurotus eryngii) on saw dust and rice straw in Bangladesh. Saudi J. Biol. Sci. 2010, 17, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Munna, J.; Simon, D.S.; Lal, D.A.; Baboo, A.; Singh, P.K.; Singh, K. Cultivation of Blue Oyster Medicinal Mushroom (Hypsizygus ulmarius (Bull.: Fr.) Redhead) on Various Agricultural Residue for Growth, Yield and Nutrient Content. J. Pharmacogn. Phytochem. 2021, 10, 842–845. [Google Scholar]
- Atila, F. Lignocellulosic and proximate based compositional changes in substrates during cultivation of Hericium erinaceus mushroom. Sci. Hortic. 2019, 258, 108779. [Google Scholar] [CrossRef]
- Hoa, H.; Wang, C.; Wang, C. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Zied, D.; Pardo, J.; Tomaz, R.; Miasaki, C.; Pardo-Giménez, A. Mycochemical characterization of Agaricus subrufescens considering their morphological and physiological stage of maturity on the traceability process. Biomed Res. Int. 2017, 2017, 2713742. [Google Scholar] [CrossRef]
- Yao, H.; Liu, Y.; Ma, Z.F.; Zhang, H.; Fu, T.; Li, Z.; Li, Y.; Hu, W.; Han, S.; Zhao, F.; et al. Analysis of nutritional quality of black fungus cultivated with corn stalks. J. Food Qual. 2019, 2019, 9590251. [Google Scholar] [CrossRef]
- Siwulski, M.; Rzymski, P.; Budka, A.; Kalaě, P.; Budzyńska, S.; Dawidowicz, L.; Hajduk, E.; Kozak, L.; Budzulak, J.; Sobieralski, K.; et al. The effect of different substrates on the growth of six cultivated mushroom species and composition of macro and trace elements in their fruiting bodies. Eur. Food Res. Technol. 2019, 245, 419–431. [Google Scholar] [CrossRef]
- Ayodele, S.; Ojoghoro, O. Salt stress effects on the vegetative growth of Pleurotus tuberregium (FR) Sing. J. Biol. Sci. 2007, 7, 1278–1281. [Google Scholar] [CrossRef]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Liu, X.; Huang, G.; Xiao, W.; Han, L. The composition characteristics of different crop straw types and their multivariate analysis and comparison. Waste Manag. 2020, 110, 87–97. [Google Scholar] [CrossRef]
- Muswati, C.; Simango, K.; Tapfumaneyi, L.; Mutetwa, M.; Ngezimana, W. The effects of different substrate combinations on growth and yield of oyster mushroom (Pleurotus ostreatus). Int. J. Agron. 2021, 2021, 9962285. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, Q.; Ai, C.; Liang, G.; He, P.; Zhou, W. Nitrogen enrichment regulates straw decomposition and its associated microbial community in a double-rice cropping system. Sci. Rep. 2018, 8, 1847. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Griensven, L. Biodegradation of lignocellulosic wastes through cultivation of Pleurotus sajor-caju. In Proceedings of the 15th International Congress on the Science & Cultivation of Edible Fungi, Maastricht, The Netherlands, 15–19 May 2000; pp. 517–521. [Google Scholar]

| Number | Formula |
|---|---|
| CK | Sawdust (79%) |
| 1 | Rice Straw (10%) + Sawdust (69%) |
| 2 | Rice Straw (20%) + Sawdust (59%) |
| 3 | Rice Straw (30%) + Sawdust (49%) |
| 4 | Rice Straw (40%) + Sawdust (39%) |
| 5 | Rice Straw (50%) + Sawdust (29%) |
| 6 | Rape Straw (10%) + Sawdust (69%) |
| 7 | Rape Straw (20%) + Sawdust (59%) |
| 8 | Rape Straw (30%) + Sawdust (49%) |
| 9 | Rape Straw (40%) + Sawdust (39%) |
| 10 | Rape Straw (50%) + Sawdust (29%) |
| 11 | Wheat Straw (10%) + Sawdust (69%) |
| 12 | Wheat Straw (20%) + Sawdust (59%) |
| 13 | Wheat Straw (30%) + Sawdust (49%) |
| 14 | Wheat Straw (40%) + Sawdust (39%) |
| 15 | Wheat Straw (50%) + Sawdust (29%) |
| 16 | Soybean Straw (10%) + Sawdust (69%) |
| 17 | Soybean Straw (20%) + Sawdust (59%) |
| 18 | Soybean Straw (30%) + Sawdust (49%) |
| 19 | Soybean Straw (40%) + Sawdust (39%) |
| 20 | Soybean Straw (50%) + Sawdust (29%) |
| 21 | Peanut Straw (10%) + Sawdust (69%) |
| 22 | Peanut Straw (20%) + Sawdust (59%) |
| 23 | Peanut Straw (30%) + Sawdust (49%) |
| 24 | Peanut Straw (40%) + Sawdust (39%) |
| 25 | Peanut Straw (50%) + Sawdust (29%) |
| 26 | Corn Straw (10%) + Sawdust (69%) |
| 27 | Corn Straw (20%) + Sawdust (59%) |
| 28 | Corn Straw (30%) + Sawdust (49%) |
| 29 | Corn Straw (40%) + Sawdust (39%) |
| 30 | Corn Straw (50%) + Sawdust (29%) |
| 31 | Corn Cob (10%) + Sawdust (69%) |
| 32 | Corn Cob (20%) + Sawdust (59%) |
| 33 | Corn Cob (30%) + Sawdust (49%) |
| 34 | Corn Cob (40%) + Sawdust (39%) |
| 35 | Corn Cob (50%) + Sawdust (29%) |
| Number | Formula Composition | Mycelial Germination Time (d) | Mycelium Full Bag Days (d) |
|---|---|---|---|
| CK | CK | 3 ± 1 | 33 ± 2 |
| 1 | Corn straw 20% | 3 ± 1 | 31 ± 2 |
| 2 | Corn straw 30% | 3 ± 1 | 29 ± 2 |
| 3 | Corn straw 40% | 2 ± 1 | 28 ± 2 |
| 4 | Soybean straw 20% | 3 ± 1 | 32 ± 2 |
| 5 | Soybean straw 30% | 3 ± 1 | 30 ± 2 |
| 6 | Soybean straw 40% | 2 ± 1 | 28 ± 2 |
| 7 | Rapeseed straw 20% | 3 ± 1 | 32 ± 2 |
| 8 | Rapeseed straw 30% | 2 ± 1 | 31 ± 2 |
| 9 | Rapeseed straw 40% | 2 ± 1 | 30 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Yang, Y.; Hu, S.; Ma, Y.-K.; Ren, Z.-M.; Wang, Y.; Yang, Y.-K.; Ji, S.-J.; Wang, H.; Huang, X. The Agronomic Traits Differences in Hericium erinaceus Cultivated with Different Straw Formulations by Replacing Wood with Straw. Horticulturae 2025, 11, 1220. https://doi.org/10.3390/horticulturae11101220
Lu Z, Yang Y, Hu S, Ma Y-K, Ren Z-M, Wang Y, Yang Y-K, Ji S-J, Wang H, Huang X. The Agronomic Traits Differences in Hericium erinaceus Cultivated with Different Straw Formulations by Replacing Wood with Straw. Horticulturae. 2025; 11(10):1220. https://doi.org/10.3390/horticulturae11101220
Chicago/Turabian StyleLu, Zhu, Yang Yang, Shuang Hu, Yu-Kun Ma, Zi-Ming Ren, Yue Wang, Ying-Kun Yang, Shu-Juan Ji, Huan Wang, and Xiao Huang. 2025. "The Agronomic Traits Differences in Hericium erinaceus Cultivated with Different Straw Formulations by Replacing Wood with Straw" Horticulturae 11, no. 10: 1220. https://doi.org/10.3390/horticulturae11101220
APA StyleLu, Z., Yang, Y., Hu, S., Ma, Y.-K., Ren, Z.-M., Wang, Y., Yang, Y.-K., Ji, S.-J., Wang, H., & Huang, X. (2025). The Agronomic Traits Differences in Hericium erinaceus Cultivated with Different Straw Formulations by Replacing Wood with Straw. Horticulturae, 11(10), 1220. https://doi.org/10.3390/horticulturae11101220





