Physiological Mechanisms of Drought-Induced Creasing in Citrus unshiu Marc: Roles of Antioxidant Dysregulation, Hormonal Imbalance, Cell Wall Degradation, and Mineral Redistribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Drought Treatments
2.2. Assessment of Creasing Incidence
2.3. Rind Microstructure Analysis
2.4. Antioxidant Parameter Assay
2.5. Phytohormone Quantification
2.6. Cell Wall Metabolite Assay
2.7. Mineral Element Assay
2.8. Fruit Quality Analysis
2.9. Statistical Analysis
3. Results
3.1. Soil Water Content and Creasing Incidence
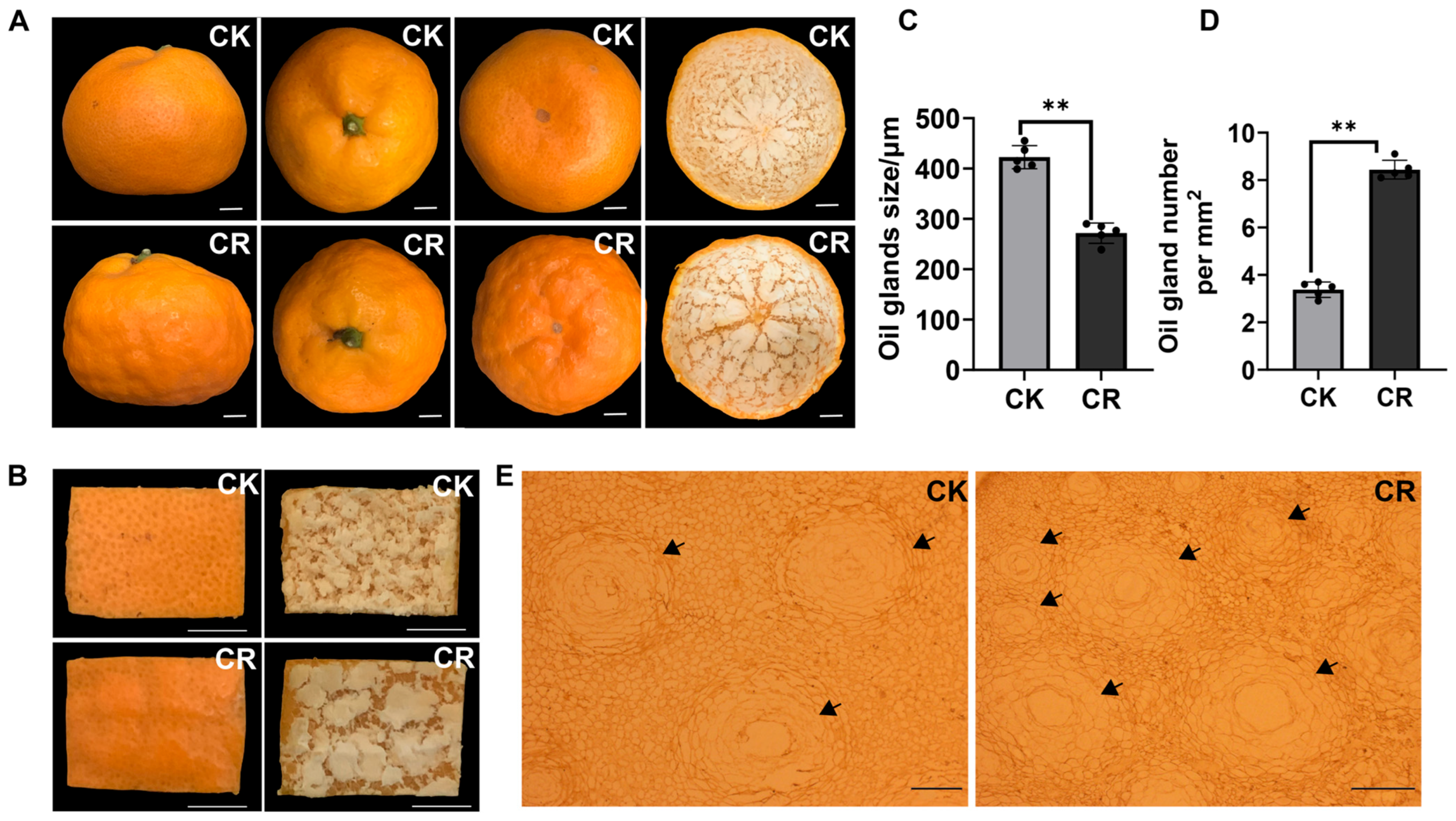
3.2. Morphological and Anatomical Alterations of the Rind
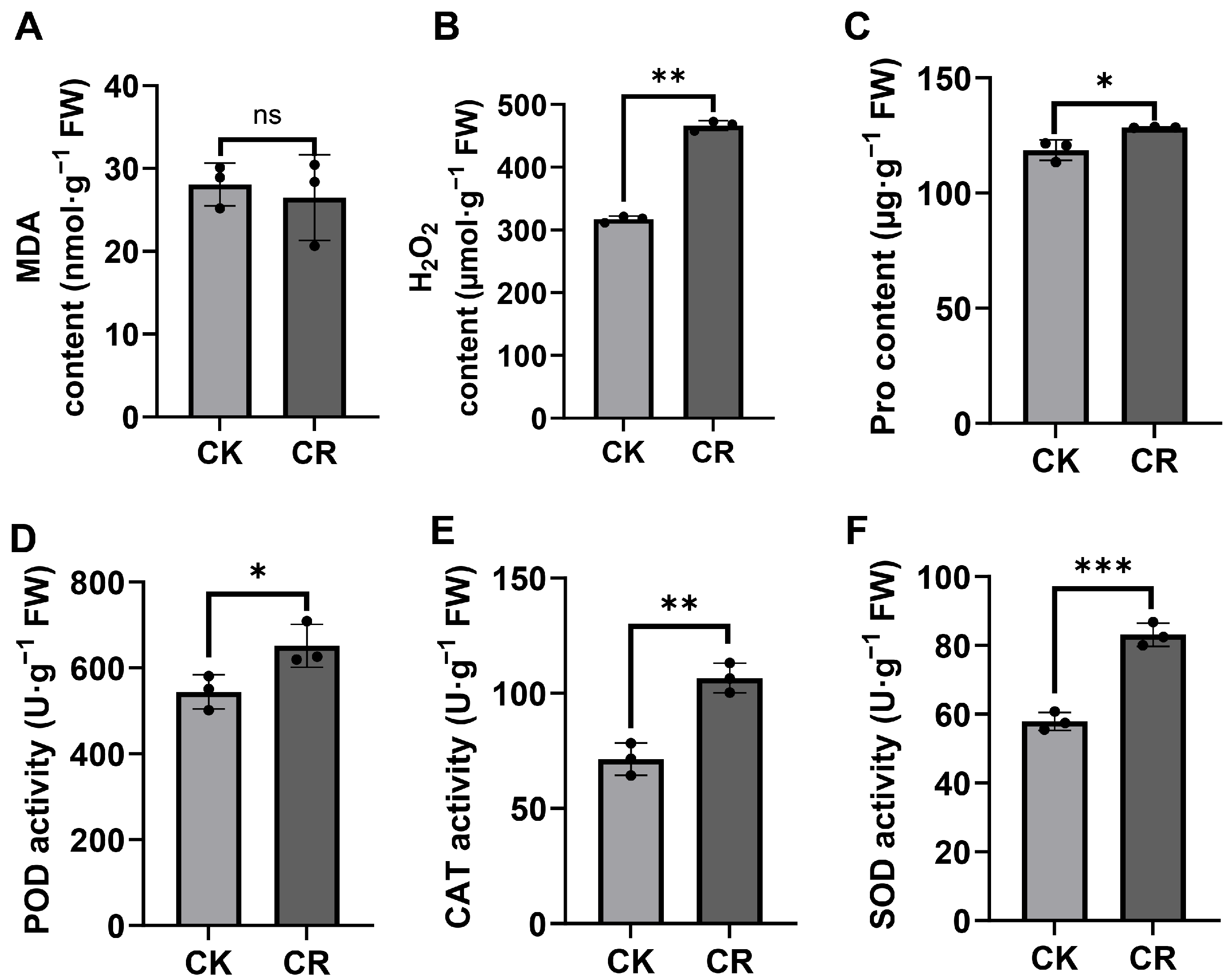
3.3. Alterations in Antioxidant Enzyme Activities and ROS Levels
3.4. Alterations in Phytohormone Levels
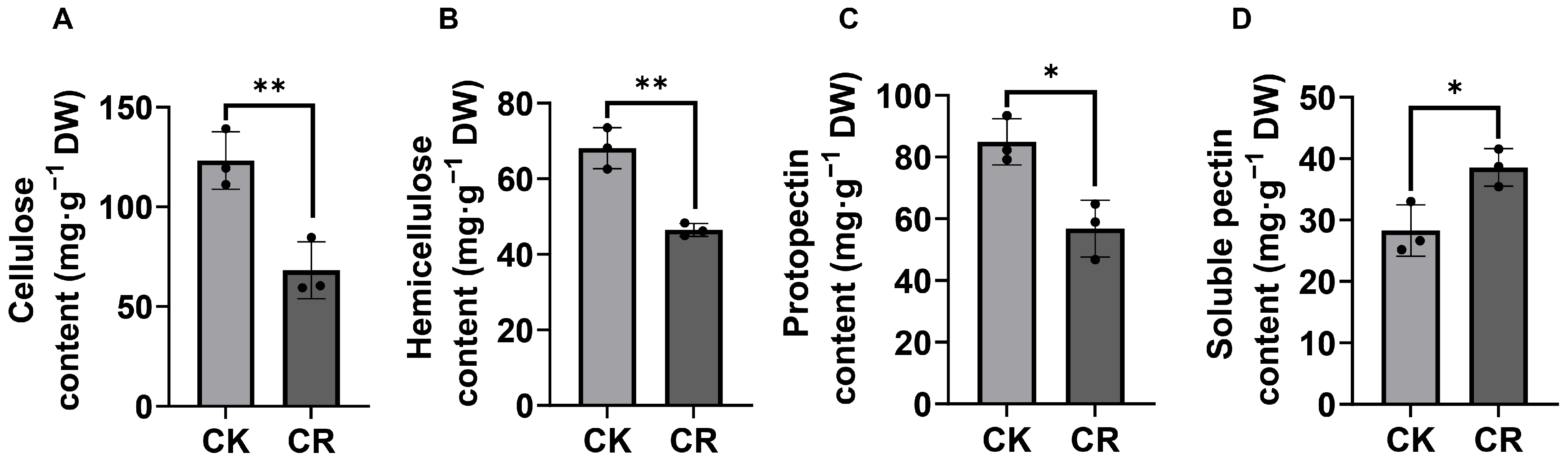
3.5. Alterations in Cell Wall Composition
3.6. Alterations in Mineral Elements
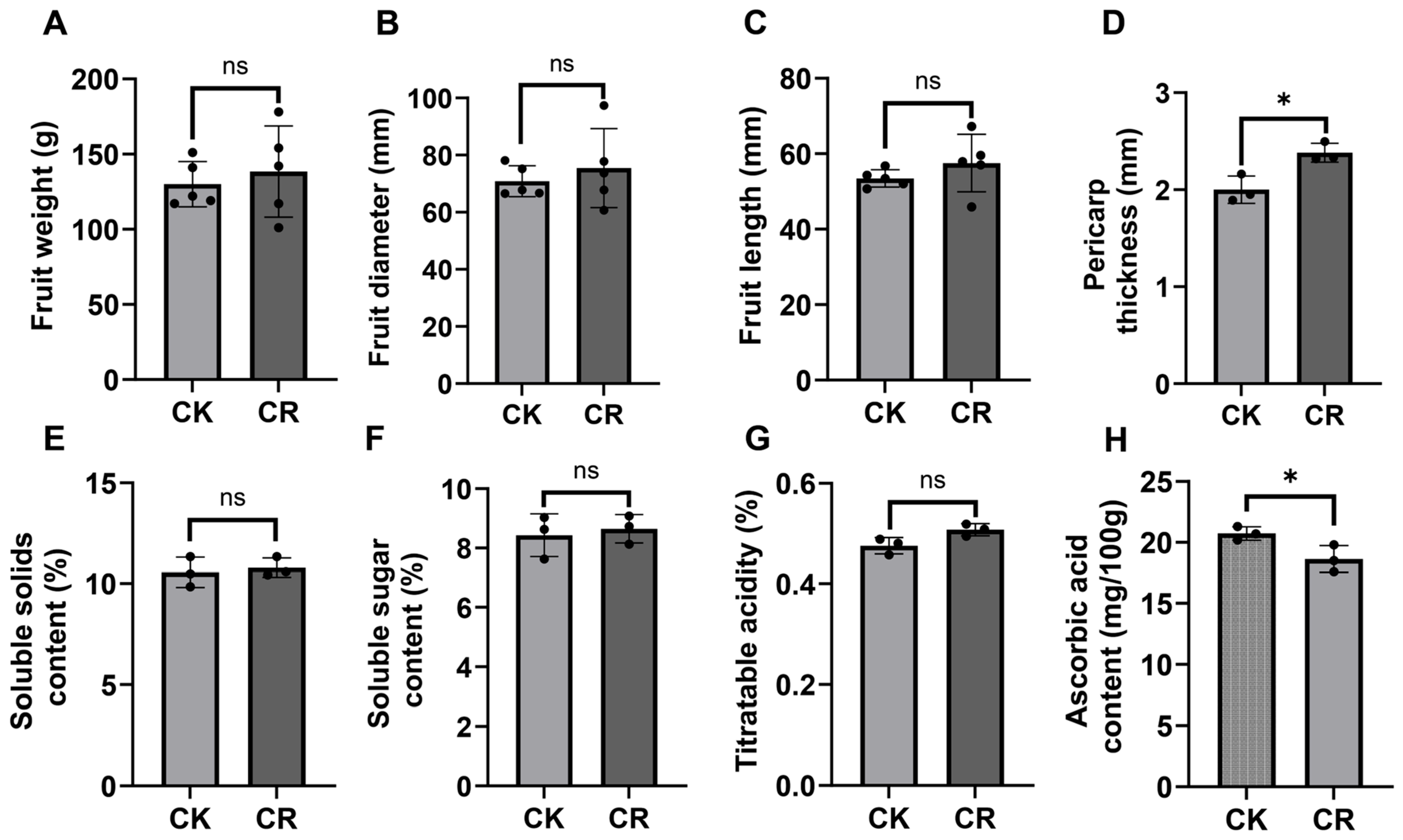
3.7. Alterations in Fruit Quality
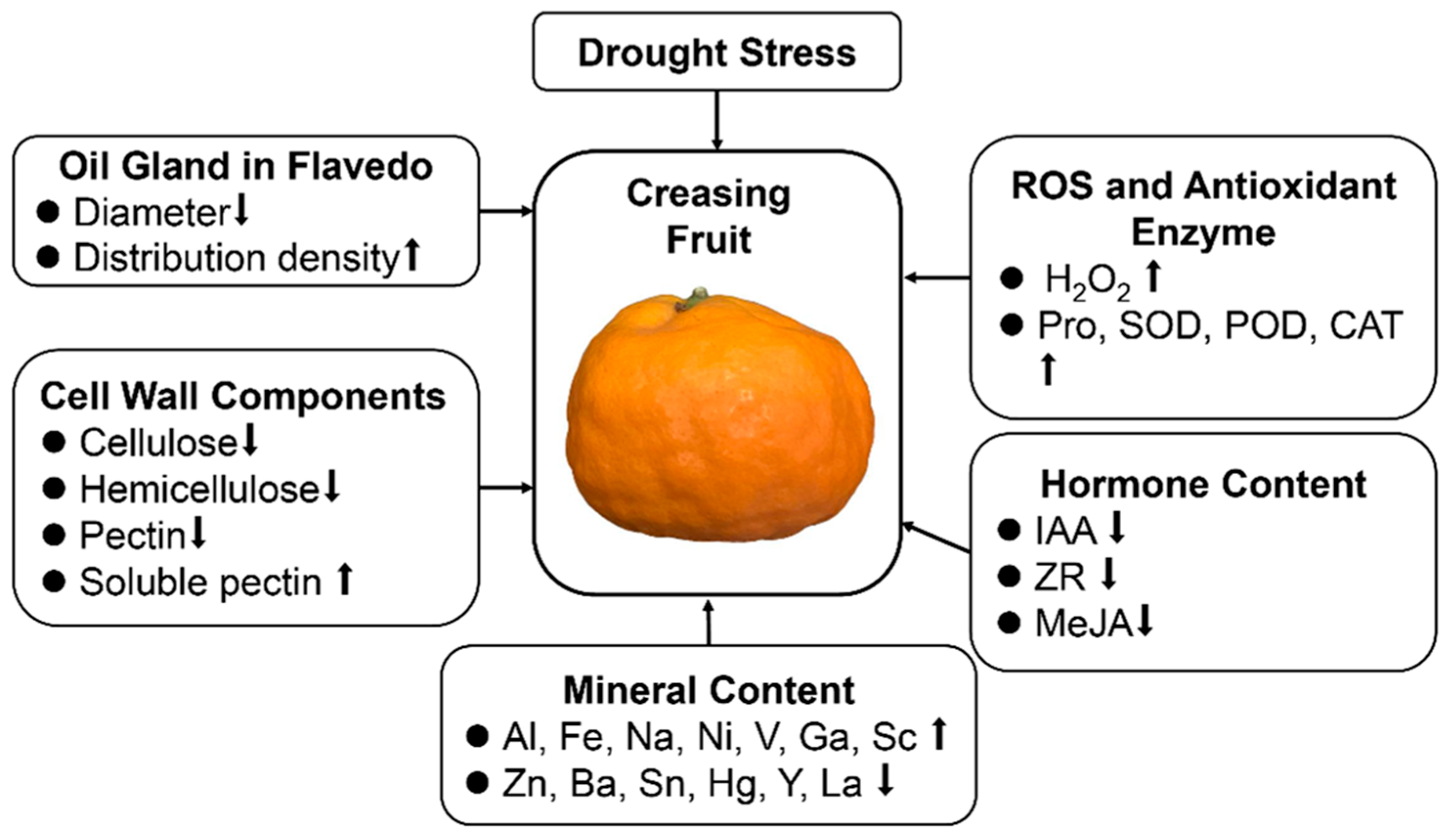
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, G.; Xu, Q.; Allan, A.C.; Xu, X. L-Ascorbic Acid Metabolism and Regulation in Fruit Crops. Plant Physiol. 2023, 192, 1684–1695. [Google Scholar] [CrossRef]
- Wang, L.; He, F.; Huang, Y.; He, J.; Yang, S.; Zeng, J.; Deng, C.; Jiang, X.; Fang, Y.; Wen, S. Genome of Wild Mandarin and Domestication History of Mandarin. Mol. Plant 2018, 11, 1024–1037. [Google Scholar] [CrossRef]
- El-Otmani, M.; Ait-Oubahou, A.; Zacarías, L. 21—Citrus spp.: Orange, Mandarin, Tangerine, Clementine, Grapefruit, Pomelo, Lemon and Lime. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 437–514, 515e–516e. [Google Scholar] [CrossRef]
- Huai, B.; Wu, Y.; Liang, C.; Tu, P.; Mei, T.; Guan, A.; Yao, Q.; Li, J.; Chen, J. Effects of Calcium on Cell Wall Metabolism Enzymes and Expression of Related Genes Associated with Peel Creasing in Citrus Fruits. PeerJ 2022, 10, e14574. [Google Scholar] [CrossRef]
- Treeby, M.T.; Storey, R. Calcium-Spray Treatments for Ameliorating Albedo Breakdown in Navel Oranges. Aust. J. Exp. Agric. 2002, 42, 495–502. [Google Scholar] [CrossRef]
- Jona, R.; Goren, R.; Marmora, M. Effect of Gibberellin on Cell-Wall Components of Creasing Peel in Mature ‘Valencia’ Orange. Sci. Hortic. 1989, 39, 105–115. [Google Scholar] [CrossRef]
- Phiri, Z.P. Creasing Studies in Citrus. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2010. [Google Scholar]
- Hussain, Z.; Singh, Z. Role of Aminoethoxyvinylglycine in Creasing of Sweet Orange [Citrus Sinensis (L.) Osbeck] Fruit. J. Pure Appl. Agric. 2020, 5, 1–10. [Google Scholar]
- Hussain, Z.; Singh, Z. Involvement of Polyamines in Creasing of Sweet Orange [Citrus Sinensis (L.) Osbeck] Fruit. Sci. Hortic. 2015, 190, 203–210. [Google Scholar] [CrossRef]
- Li, J.; Liang, C.; Liu, X.; Huai, B.; Chen, J.; Yao, Q.; Qin, Y.; Liu, Z.; Luo, X. Effect of Zn and NAA Co-Treatment on the Occurrence of Creasing Fruit and the Peel Development of ‘Shatangju’ Mandarin. Sci. Hortic. 2016, 201, 230–237. [Google Scholar] [CrossRef]
- Jones, W.W.; Embleton, T.W.; Clerber, M.J.; Cree, C.B. Creasing of Orange Fruit. Hilgardia 1967, 38, 231–244. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.; Chen, J.; Yao, Q.; Jiang, Y. Cellular Wall Metabolism in Citrus Fruit Pericarp and its Relation to Creasing Fruit Rate. Sci. Hortic. 2009, 122, 45–50. [Google Scholar] [CrossRef]
- Saleem, B.A.; Hassan, I.; Singh, Z.; Malik, A.U.; Pervez, M.A. Comparative Changes in the Rheological Properties and Cell Wall Metabolism in Rind of Healthy and Creased Fruit of Washington Navel and Navelina Sweet Orange (Citrus sinensis [L.] Osbeck). Aust. J. Crop Sci. 2014, 8, 62–70. [Google Scholar]
- Huang, S.; Yang, X.; Wang, T.; Li, H.; Deng, L.; Bi, X.; Hu, J.; Gong, Y.; Li, Y.; Qin, Z. Physiological Mechanisms of Citrus Fruit Cracking: Study on Cell Wall Components, Osmoregulatory Substances, and Antioxidant Enzyme Activities. Plants 2024, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, T.; Park, S.; Rezk, A.; Hur, M.; Obenland, D.; Arpaia, M.L.; El-kereamy, A. Metabolomic Analyses Provide Insights into the Preharvest Rind Disorder in Satsuma Owari Mandarin. Front. Plant Sci. 2023, 14, 1263354. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, A.M.; Martinelli, F.; Reagan, R.L.; Uratsu, S.L.; Vo, A.; Tinoco, M.A.; Phu, M.L.; Chen, Y.; Rocke, D.M.; Dandekar, A.M. Transcriptome and Metabolome Analysis of Citrus Fruit to Elucidate Puffing Disorder. Plant Sci. 2014, 217–218, 87–98. [Google Scholar] [CrossRef]
- Chen, J.; Lu, X.; Ye, Z.; Yao, Q.; Wu, L. Study on the Relation Between Mineral Nutrition Levels and Creasing Peel in Mature Orange. Plant Nutr. Fertitizer Sci. 2002, 8, 367–371. [Google Scholar] [CrossRef]
- Bower, J.P. The Physiological Control of Citrus Creasing. Acta Hortic. 2004, 632, 111–115. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zhang, X.; Sun, L.; Ke, F.; Huang, Y.; Song, L.; Ye, H.; Xu, J.; Xu, Y. Genomic Origin of Citrus reticulata “Unshiu”. Hortic. Res. 2025, 12, uhaf015. [Google Scholar] [CrossRef]
- Long, J.M.; Guo, W.W. Paraffin Embedding Citrus Tissues and Sectioning. Bio-101 2018, e1010196. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Hormonal Changes in the Grains of Rice Subjected to Water Stress During Grain Filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; Ping, X.; Wang, W.; Feng, X.; Yao, Z.; Wang, T. A Comprehensive Evaluation of Mineral Elements and Active Substances in Citrus Fruits. J. Fruit Sci. 2024, 41, 1592–1603. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Hu, W.; Chun, Y.; Zhang, J.; Liu, Y. Fruit Characteristics and Seed Anatomy of ‘Majia’ Pomelo Pollinated with Cobalt-60 Gamma-Ray-Irradiated Pollen. Sci. Hortic. 2020, 267, 109335. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; Prasad, M.N.V.; Gul, A.; Bhat, R.A.; Darvash, M.A.; Hasanuzzaman, M.; Nahar, K.; Unal, D. Role of Rare Earth Elements in Plants. Plant Mol. Biol. Report. 2023, 41, 345–368. [Google Scholar] [CrossRef]
- Sato, K. Chapter 6: Influence of Drought and High Temperature on Citrus. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 77–86. [Google Scholar] [CrossRef]
- Okuda, H.; Ichinokiyama, H.; Suzaki, N.; Hiratsuka, S.; Matsuba, K. Preference of Satsuma Mandarin Grown by Different Managing Methods. Hortic. Res. 2008, 7, 129–133. [Google Scholar] [CrossRef]
- Treeby, M.T.; Storey, R.M.J.; Bevington, K.B. Rootstock, seasonal, and fruit size influences on the incidence and severity of albedo breakdown in Bellamy navel oranges. Aust. J. Exp. Agric. 1995, 35, 103–108. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, M.; Liu, R.; Xue, C.; Xia, Z.; Hu, B.; Rennenberg, H. Drought-Mediated Oxidative Stress and its Scavenging Differ Between Citrus Hybrids With Medium and Late Fruit Maturation. Plant Stress 2024, 14, 100670. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional Regulators of Plant Adaptation to Abiotic Stress and Development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Allagulova, C.; Avalbaev, A.; Fedorova, K.; Shakirova, F. Methyl Jasmonate Alleviates Water Stress-Induced Damages by Promoting Dehydrins Accumulation in Wheat Plants. Plant Physiol. Biochem. 2020, 155, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Gao, H.; Yang, D.; Zhu, G.; Zhang, C.; Wang, B.; Jia, H.; Zhang, C.; Guo, J. Effects of Methyl Jasmonate on Physiological Characteristics and Drought Resistance of Alfalfa (Medicago sativa) and Ryegrass (Lolium perenne L.). J. Plant Growth Regul. 2025, 44, 335–352. [Google Scholar] [CrossRef]
- Wang, W.; Ji, D.; Yan, X.; Wei, Y.; Han, Y.; Zhang, W.; Liu, L.; Zhang, H.; Wang, Z.; Zhang, Z. Methyl Jasmonate Regulates Panicle Morphogenesis by Mediating the Negative Effects of High Temperature Stress on Carbon and Nitrogen Allocation and Utilization. Environ. Exp. Bot. 2025, 234, 106150. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The Molecular–Physiological Functions of Mineral Macronutrients and Their Consequences for Deficiency Symptoms in Plants. New Phytol. 2020, 229, 2446–2469. [Google Scholar] [CrossRef]
- Li, J.; Liang, C.; Chen, J.; Liu, X.; Zhou, W.; Yao, Q.; Zhou, B. Effects of Zn2+ Treatments on Cell Wall Metabolism in ‘Shatangju’Mandarin Fruits During Development and Ripening. Chin. J. Trop. Crops 2013, 34, 1982–1986. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, B. A Review of Zinc Nutrition and Plant Breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Kovarikova, M.; Tomaskova, I.; Soudek, P. Rare Earth Elements in Plants. Biol. Plant. 2019, 63, 20–32. [Google Scholar] [CrossRef]
- GB 2762-2022; China National Standard of Food Safety, Maximum Levels of Contaminants in Foods. Standards Press of China: Beijing, China, 2022.
- Scutarașu, E.C.; Trincă, L.C. Heavy Metals in Foods and Beverages: Global Situation, Health Risks and Reduction Methods. Foods 2023, 12, 3340. [Google Scholar] [CrossRef]


| Mineral Element (Unit) | Content | ||
|---|---|---|---|
| CK 1 | CR 1 | ||
| Macronutrients | P (g·kg−1) | 0.70 ± 0.03 | 0.71 ± 0.03 |
| K (g·kg−1) | 15.1 ± 1.73 | 16.23 ± 1.6 | |
| Ca (g·kg−1) | 1.07 ± 0.12 | 1.03 ± 0.03 | |
| Mg (g·kg−1) | 0.84 ± 0.16 | 0.98 ± 0.02 | |
| Micronutrients | Al (mg·kg−1) | 33.84 ± 1.25 | 45.37 ± 3.41 * |
| Fe (mg·kg−1) | 20.35 ± 1.33 | 36.76 ± 2.41 ** | |
| Zn (mg·kg−1) | 15.98 ± 3.11 * | 8.62 ± 0.67 | |
| Na (mg·kg−1) | 19.35 ± 2.22 | 27.92 ± 1.49 ** | |
| Mn (mg·kg−1) | 24.43 ± 1.96 | 23.02 ± 2.62 | |
| Sr (mg·kg−1) | 12.29 ± 1.54 | 10.72 ± 0.13 | |
| Ba (mg·kg−1) | 7.44 ± 0.51 ** | 3.98 ± 0.2 | |
| Cu (mg·kg−1) | 2.04 ± 0.2 | 2.91 ± 0.87 | |
| Ti (mg·kg−1) | 0.92 ± 0.21 | 1.37 ± 0.45 | |
| Ni (mg·kg−1) | 0.44 ± 0.20 | 1.42 ± 0.38 * | |
| V (μg·kg−1) | 41.28 ± 3.23 | 76.38 ± 10.43 ** | |
| Se (μg·kg−1) | 54.29 ± 49.63 | 67.74 ± 48.36 | |
| Ga (μg·kg−1) | 48.32 ± 8.79 | 105.70 ± 24.81 * | |
| Mo (μg·kg−1) | 0.085 ± 0.022 | 54.05 ± 14.54 | |
| Co (μg·kg−1) | 56.22 ± 5.37 | 69.47 ± 21.63 | |
| Sn (μg·kg−1) | 19.33 ± 0.43 ** | 12.67 ± 0.61 | |
| Zr (μg·kg−1) | 9.10 ± 6.01 | 13.97 ± 1.92 | |
| Be (μg·kg−1) | 2.92 ± 1.46 | 2.17 ± 0.70 | |
| Bi (μg·kg−1) | 1.73 ± 0.26 | 2.35 ± 0.47 | |
| Cr (mg·kg−1) | 1.80 ± 0.51 | 3.76 ± 1.94 | |
| As (μg·kg−1) | 42.71 ± 9.08 | 38.33 ± 7.58 | |
| Pb (μg·kg−1) | 93.57 ± 11.65 | 109.33 ± 21.16 | |
| Hg (μg·kg−1) | 6.54 ± 0.3 * | 5.29 ± 0.47 | |
| Cd (μg/kg) | 6.42 ± 4.01 | 4.35 ± 1.37 | |
| Rare earth elements | Y (mg·kg−1) | 1.99 ± 0.26 * | 0.97 ± 0.33 |
| Sc (μg·kg−1) | 65.71 ± 14.32 | 181.55 ± 38.39 ** | |
| La (mg·kg−1) | 1.28 ± 0.20 ** | 0.14 ± 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Fu, W.; Liu, D.; Xiong, Z.; Yang, L.; Kuang, L.; Song, J.; Xie, J.; Liu, Y. Physiological Mechanisms of Drought-Induced Creasing in Citrus unshiu Marc: Roles of Antioxidant Dysregulation, Hormonal Imbalance, Cell Wall Degradation, and Mineral Redistribution. Horticulturae 2025, 11, 1197. https://doi.org/10.3390/horticulturae11101197
Hu W, Fu W, Liu D, Xiong Z, Yang L, Kuang L, Song J, Xie J, Liu Y. Physiological Mechanisms of Drought-Induced Creasing in Citrus unshiu Marc: Roles of Antioxidant Dysregulation, Hormonal Imbalance, Cell Wall Degradation, and Mineral Redistribution. Horticulturae. 2025; 11(10):1197. https://doi.org/10.3390/horticulturae11101197
Chicago/Turabian StyleHu, Wei, Woxing Fu, Dechun Liu, Zhonghua Xiong, Li Yang, Liuqing Kuang, Jie Song, Jingheng Xie, and Yong Liu. 2025. "Physiological Mechanisms of Drought-Induced Creasing in Citrus unshiu Marc: Roles of Antioxidant Dysregulation, Hormonal Imbalance, Cell Wall Degradation, and Mineral Redistribution" Horticulturae 11, no. 10: 1197. https://doi.org/10.3390/horticulturae11101197
APA StyleHu, W., Fu, W., Liu, D., Xiong, Z., Yang, L., Kuang, L., Song, J., Xie, J., & Liu, Y. (2025). Physiological Mechanisms of Drought-Induced Creasing in Citrus unshiu Marc: Roles of Antioxidant Dysregulation, Hormonal Imbalance, Cell Wall Degradation, and Mineral Redistribution. Horticulturae, 11(10), 1197. https://doi.org/10.3390/horticulturae11101197







