Secretory Structures and Essential Oil Composition in Santolina chamaecyparissus L. Cultivated in Northern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Micromorphological Survey
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Light Microscopy (LM) and Fluorescence Microscopy (FM)
2.4. Phytochemical Survey
2.4.1. Preparation of Essential Oils (EOs)
2.4.2. GC–MS Analysis of EOs
2.4.3. GC–FID Analysis of EOs
2.5. Scientific Dissemination
3. Results
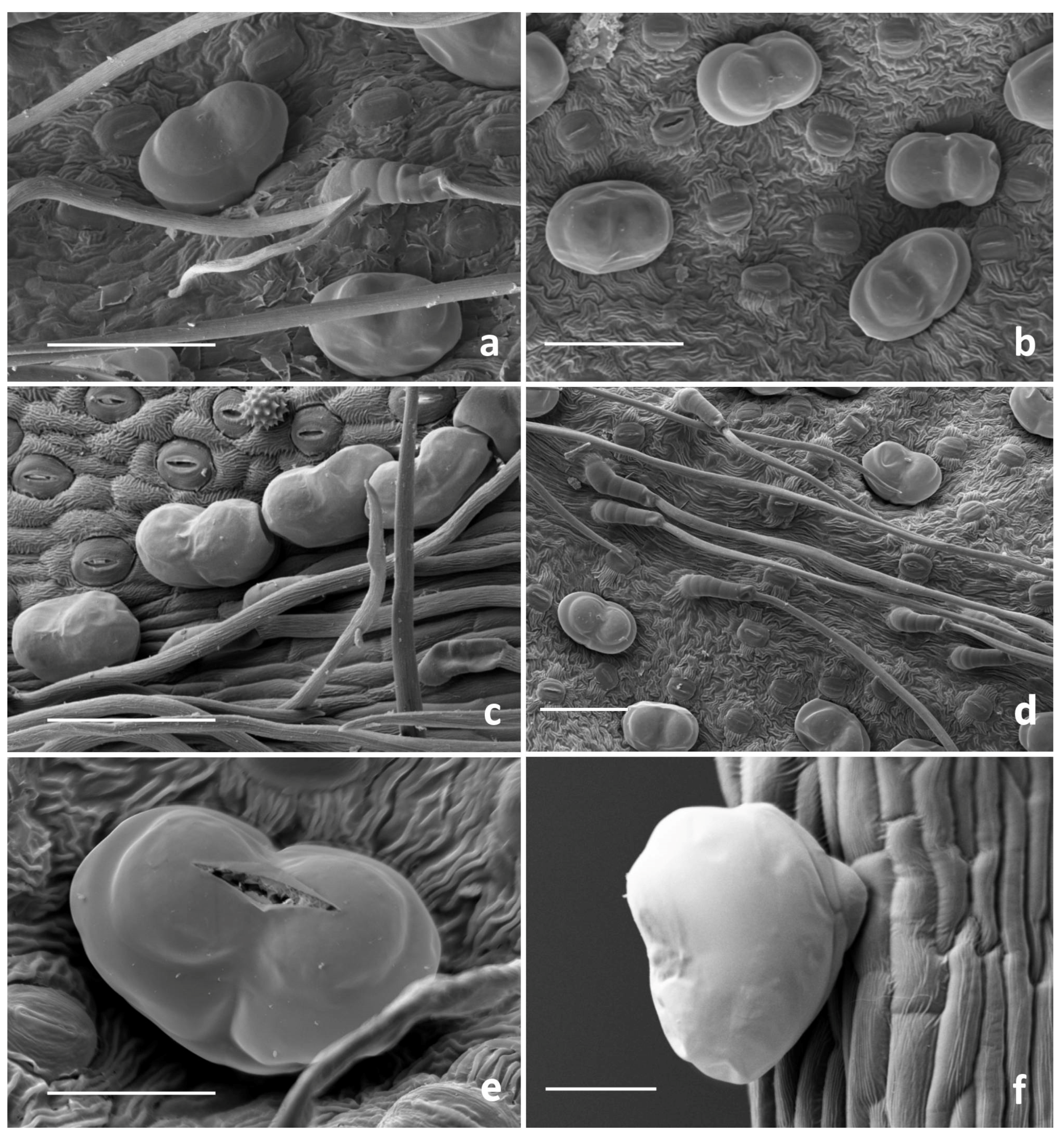
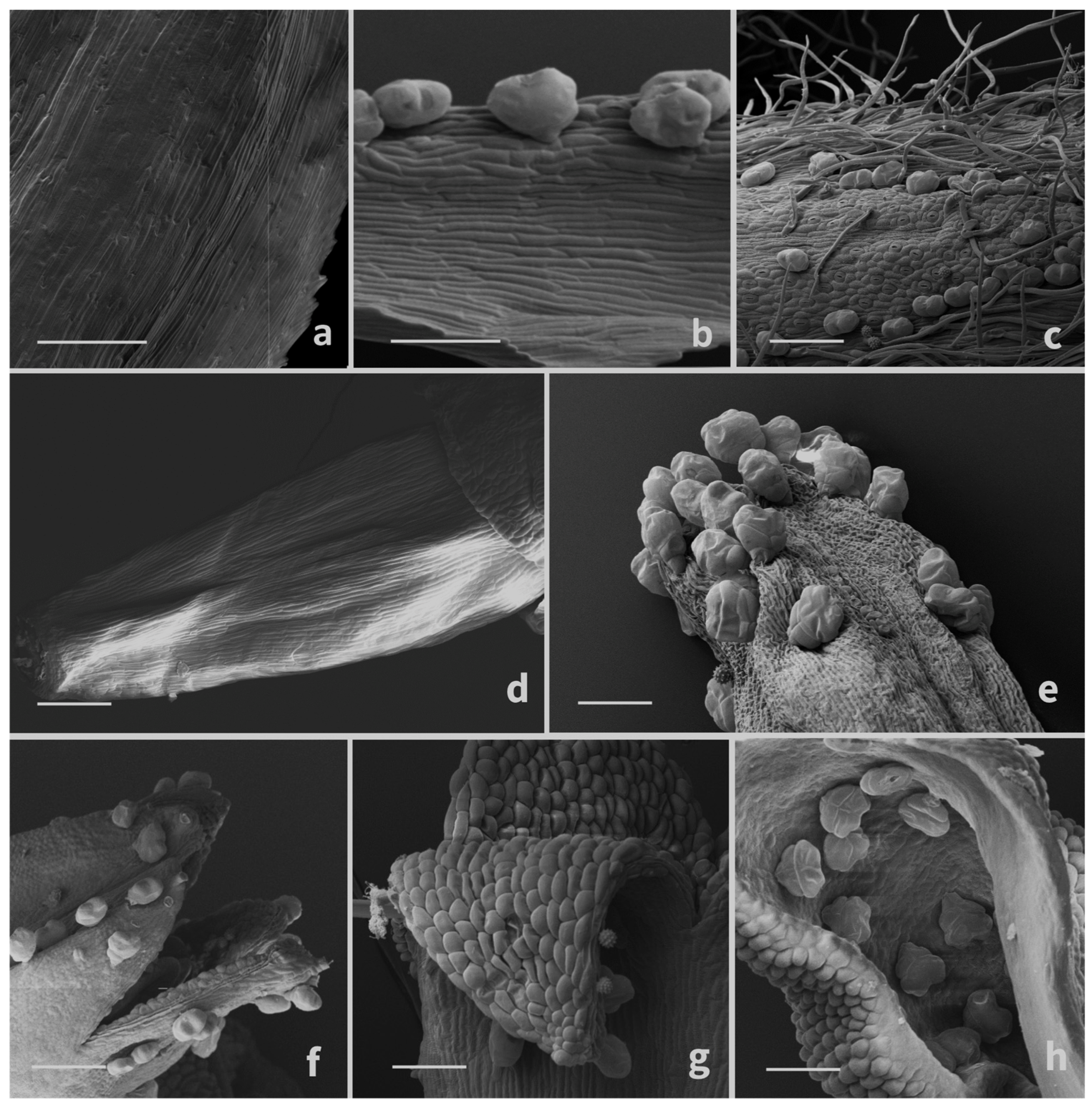
3.1. Micromorphological Survey
3.1.1. Glandular Trichomes
3.1.2. Secretory Ducts
3.1.3. Histochemistry of the Secretory Material
3.2. Phytochemical Survey
3.3. Scientific Dissemination
4. Discussion
4.1. Micromorphological Survey
4.2. Phytochemical Survey
- ✓
- Camphor-rich profiles, such as the one observed in the present study, were also reported in Turkish samples (17.7%) [14];
- ✓
- ✓
- Mixed artemisia ketone and camphor profiles, resembling our findings, characterized the Tunisian samples [19];
- ✓
- ✓
- ✓
- Profiles with trans-p-mentha-2,8-dienol, detected exclusively in Saudi Arabian samples [23], suggested a clearly distinct chemical profile compared to those reported elsewhere.
4.3. Scientific Dissemination
5. Conclusions
- (i)
- The micromorphological approach, combining digital light and fluorescence microscopy with scanning electron microscopy, allowed us to describe for the first time the non-glandular indumentum and the glandular structures of both the vegetative and reproductive organs; specifically, our survey demonstrated a great affinity regarding the micromorphology and the distribution pattern of the secretory structures between the target species and the congeneric taxa known in literature, whereas the histochemistry proved to be divergent, with the homogeneous production of oleoresin and polyphenols in both the glandular hairs and the internal ducts.
- (ii)
- The characterization of the EO profiles from Italian samples represented an element of novelty in the panorama of the literature. The EOs composition proved to be repeatable in the analyzed samples and confirmed the dominance of oxygenated monoterpenes, with artemisia ketone and camphor as key single components. The literature data on the bio-ecology of these two compounds, along with other major molecules, supported the potential of the Italian oil in the therapeutical and agricultural fields.
- (iii)
- The scientific results were combined into the creation of an appealing interpretative outdoor facility for the target species at the study site; this strategy falls within the Open Science policies, aimed at promoting practices that make scientific research more transparent, accessible, and collaborative.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacò, A.; De Giorgi, P.; Astuti, G.; Caputo, P.; Serrano, M.; Carballal, R.; Sáez, L.; Bacchetta, G.; Peruzzi, L. A morphometric analysis of the Santolina chamaecyparissus complex (Asteraceae). Plants 2022, 11, 3458. [Google Scholar] [CrossRef]
- Giacò, A.; Astuti, G.; Peruzzi, L. Typification and nomenclature of the names in the Santolina chamaecyparissus species complex (Asteraceae). Taxon 2021, 70, 189–201. [Google Scholar] [CrossRef]
- Giacò, A.; De Giorgi, P.; Astuti, G.; Varaldo, L.; Minuto, L.; Peruzzi, L. Taxonomy and distribution of the genus Santolina (Asteraceae) in Italy. Biogeogr. J. Integr. Biogeogr. 2022, 37, a021. [Google Scholar] [CrossRef]
- Arrigoni, P.V. Santolina L. In Flora D’Italia; Pignatti, S., Ed.; Edagricole: Milano, Italy, 2018; Volume 3, pp. 874–878. [Google Scholar]
- Carbajal, R.; Ortiz, S.; Sáez, L. Santolina L. In Flora Iberica; Castroviejo, S.B., Benedí, C., Buira, A., Rico, E., Crespo, M.B., Quintanar, A., Aedo, C., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 2019; Volume 16, pp. 1938–1962. [Google Scholar]
- Tundis, R.; Loizzo, M. A review of the traditional uses, phytochemistry and biological activities of the genus Santolina. Planta Med. 2018, 84, 627–637. [Google Scholar] [CrossRef]
- Missouri Botanical Garden. Santolina Chamaecyparissus-Plant Finder. Available online: https://www.missouribotanicalgarden.org/plantfinder/PlantFinderDetails.aspx?taxonid=277227 (accessed on 7 August 2025).
- Araújo-Alves, J.P.L.; Torres-Pereira, J.M.; Biel, C.; de Herralde, F.; Savé, R. Effects of minimum irrigation technique on ornamental parameters of two Mediterranean species used in xerigardening and landscaping. Acta Hortic. 2000, 541, 353–358. [Google Scholar] [CrossRef]
- Guarrera, P.; Forti, G.; Marignoli, S. Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444. [Google Scholar] [CrossRef]
- Cotton Lavender (Santolina). Available online: https://herbgarden.co.za/mountainherb/article.php?tag=CottonLavender (accessed on 1 August 2025).
- Sánchez-Hernández, E.; Martín-Gil, J.; González-García, V.; Casanova-Gascón, J.; Martín-Ramos, P. Bioactive Sesquiterpenoids from Santolina chamaecyparissus L. Flowers: Chemical Profiling and Antifungal Activity Against Neocosmospora Species. Plants 2025, 14, 235. [Google Scholar] [CrossRef]
- Schmidt, J.; Juhasz, K.; Bona, A. Exploring the Chemical Profile, In Vitro Antioxidant and Anti-Inflammatory Activities of Santolina rosmarinifolia Extracts. Molecules 2024, 29, 1515. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Husain Warsi, M.; Ahmad, W.; Tahir, A. Chemical characterization, antidiabetic and anticancer activities of Santolina chamaecyparissus. Saudi J. Biol. Sci. 2021, 28, 4575–4580. [Google Scholar] [CrossRef]
- Demirci, B.; Özek, T.; Baser, K.H.C. Chemical composition of Santolina chamaecyparissus L. essential oil. J. Essent. Oil Res. 2000, 12, 625–627. [Google Scholar] [CrossRef]
- Djeddi, S.; Djebile, K.; Hadjbourega, G.; Achour, Z.; Argyropoulou, C.; Skaltsa, H. In vitro antimicrobial properties and chemical composition of Santolina chamaecyparissus essential oil from Algeria. Nat. Prod. Commun. 2012, 7, 937–940. [Google Scholar] [CrossRef]
- Khubeiz, M.J.; Mansour, G. In vitro antifungal, antimicrobial properties and chemical composition of Santolina chamaecyparissus essential oil in Syria. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 372–378. [Google Scholar]
- Vernin, G. Volatile Constituents of the essential oil of Santolina chamaecyparissus L. J. Essent. Oil Res. 1991, 3, 49–53. [Google Scholar] [CrossRef]
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms. Plants 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Salah-Fatnassi, K.B.; Hassayoun, F.; Cheraif, I.; Khan, S.; Jannet, H.B.; Hammami, M.; Aouni, M.; Harzallah-Skhiri, F. Chemical Composition, Antibacterial and Antifungal Activities of Flowerhead and Root Essential Oils of Santolina chamaecyparissus L., Growing Wild in Tunisia. Saudi J. Biol. Sci. 2017, 24, 875–882. [Google Scholar] [CrossRef]
- Niu, L.-L.; Qin, Q.-P.; Wang, L.-T.; Gai, Q.-Y.; Jiao, J.; Zhao, C.-J.; Fu, Y.-J. Chemical profiling of volatile components of micropropagated Santolina chamaecyparissus L. Ind. Crops Prod. 2019, 137, 162–170. [Google Scholar] [CrossRef]
- Azevedo, T.; Faustino-Rocha, A.I.; Barros, L.; Finimundy, T.C.; Matos, M.; Oliveira, P.A. Santolina chamaecyparissus L.: A Brief Overview of Its Medicinal Properties. Med. Sci. Forum 2023, 21, 8. [Google Scholar]
- Alves-Silva, J.M.; Gonçalves, M.J.; Silva, A.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical Profile, Anti-Microbial and Anti-Inflammaging Activities of Santolina rosmarinifolia L. Essential Oil from Portugal. Antibiotics 2023, 12, 179. [Google Scholar] [CrossRef]
- Aati, H.Y.; Sarawi, W.; Attia, H.; Ghazwani, R.; Aldmaine, L. Exploring the Phytochemical Profile and Therapeutic Potential of Saudi Native Santolina chamaecyparissus L. Essential Oil. Pharmaceutics 2025, 17, 830. [Google Scholar] [CrossRef]
- Giacò, A.; De Giorgi, P.; Astuti, G.; Varaldo, L.; Sáez, L.; Carballal, R.; Serrano, M.; Casazza, G.; Caputo, P.; Bacchetta, G.; et al. Diploids and polyploids in the Santolina chamaecyparissus complex (Asteraceae) show different karyotype asymmetry. Plant Biosyst. 2022, 156, 1237–1246. [Google Scholar] [CrossRef]
- El-Sahhar, K.F.; Nassar, D.M.; Farag, H.M. Morphological and anatomical studies of Santolina chamaecyparissus L. (Asteraceae). I. Morphological characteristics. Res. J. Agric. Biol. Sci. 2011, 7, 294–302. [Google Scholar]
- El-Sahhar, K.F.; Nassar, D.M.; Farag, H.M. Morphological and anatomical studies of Santolina chamaecyparissus L. (Asteraceae) II. Anatomical characteristics and volatile oil. Res. J. Agric. Biol. Sci. 2011, 7, 413–422. [Google Scholar]
- Pagni, A.M. Secretory structures in the capitula of Santolina leucantha Bertol. (Asteraceae). Morphology and histochemistry. Ann. Bot. 1995, 53, 239–249. [Google Scholar]
- Pagni, A.M.; Masini, A. Morphology, distribution, and histochemistry of secretory structures in vegetative organs of Santolina leucantha Bertol. (Asteraceae). Isr. J. Plant Sci. 1999, 47, 257–263. [Google Scholar] [CrossRef]
- Pagni, A.M.; Orlando, R.; Masini, A.; Ciccarelli, D. Secretory structures of Santolina ligustica Arrigoni (Asteraceae), an Italian endemic species. Isr. J. Plant Sci. 2003, 51, 185–192. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Mendes, A.R.; Caeiro, M.F.; Figueiredo, A.C.; Ascensão, L. New reports on the Portuguese endemic species, Santolina impressa: Secretory structures, essential oil composition and antiviral activity. Plants 2023, 12, 2391. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Milano, Italy, 2019; Volume 2. [Google Scholar]
- Bottoni, M.; Baron, G.; Gado, F.; Milani, F.; Santagostini, L.; Colombo, L.; Colombo, P.S.; Caporali, E.; Spada, A.; Biagi, M.; et al. Achillea moschata Wulfen: From ethnobotany to phytochemistry, morphology, and biological activity. Molecules 2022, 27, 8318. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Milani, F.; Spada, A.; Falsini, S.; Papini, A.; Santagostini, L.; Fico, G. An integrative approach to selected species of Tanacetum L. (Asteraceae): Insights into morphology and phytochemistry. Plants 2024, 13, 155. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured: Carol Stream, IL, USA, 2007. [Google Scholar]
- Martínez-Quezada, D.M.; Rivera, P.; Rojas-Leal, A.; Villaseñor, J.L.; Terrazas, T. Leaf secretory structures in Asteraceae: A synthesis of their diversity and evolution. Bot. Rev. 2023, 89, 59–90. [Google Scholar] [CrossRef]
- Martínez-Quezada, D.M.; Rojas-Leal, A.; Villaseñor, J.L.; Terrazas, T. Structural considerations and differences between leaf canals and secretory cavities in Asteraceae. Protoplasma 2025, 262, 707–720. [Google Scholar] [CrossRef]
- Sacchetti, G.; Romagnoli, C.; Ballero, M.; Tosi, B.; Poli, F. Internal secretory structures and preliminary phytochemical investigation on flavonoid and coumarin content in Santolina insularis (Asteraceae). Phyton-Ann. Rei. Bot. 1997, 32, 219–228. [Google Scholar]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Perez-Alvarez, J.A.; Sendra, E.; Fernandez-Lopez, J. Chemical characterization and antibacterial activity of two aromatic herbs (Santolina chamaecyparissus and Sideritis angustifolia) widely used in the folk medicine. J. Food Saf. 2012, 32, 426–434. [Google Scholar] [CrossRef]
- Grosso, C.; Figuerdo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M.F. Supercritical fluid extraction of the volatile oil from Santolina chamaecyparissus. J. Sep. Sci. 2009, 32, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alonso, M.J.; Velasco-Negueruela, A. Essential oil components of Santolina chamaecyparissus L. Flavour Fragr. J. 1992, 7, 37–41. [Google Scholar]
- Nikolic, M.; Radulovic, N. Chemical composition of the essential oil from the aboveground parts of Santolina chamaecyparissus L. from Greece: NMR determination of the exocyclic double bond geometry of the major spiroketal-enol ether polyynic constituent. FU Phys. Chem. Technol. 2018, 16, 130. [Google Scholar]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzuspersicae (Sulzer). Pestic. Biochem. Physiol. 2018, 145, 84–92. [Google Scholar] [CrossRef]
- Lončar, B.; Cvetković, M.; Rat, M.; Jeremić, J.S.; Filipović, J.; Pezo, L.; Aćimović, M. Chemical composition, chemometric analysis, and sensory profile of Santolina chamaecyparissus L. (Asteraceae) essential oil: Insights from a case study in Serbia and literature-based review. Separations 2025, 12, 115. [Google Scholar] [CrossRef]
- Garg, S.N.; Gupta, D.; Mehta, V.K.; Kumar, S. Volatile constituents of the essential oil of Santolina chamaecyparissus Linn, from the Southern Hills of India. J. Essent. Oil Res. 2001, 13, 234–235. [Google Scholar] [CrossRef]
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef]
- Villar, A.; Giner, R.M.; Rios, J.L. Chemical composition of Santolina chamaecyparissus ssp. squarrosa essential oil. J. Nat. Prod. 1986, 49, 1143–1144. [Google Scholar]
- Chirane, M.S.; Benchabane, O.; Bousbia, N.; Zenia, S. Antioxydant and antimicrobial activities of essential oil and ethanol extract of Santolina chamaecyparissus L. Agrobiologia 2019, 9, 1660–1668. [Google Scholar]
- Zaiter, L.; Benayache, F.; Beghidja, N.; Figuerdo, G.; Chalard, P.; Chalcat, J.C.; Marchioni, E.; Benayache, S. Essential oils of Santolina africana Jord. & Fourr. and Santolina chamaecyparissus L. J. Essent. Oil-Bear. Plants 2015, 18, 1338–1342. [Google Scholar]
- Al Motwaa, S.M.; Al-Otaibi, W.A. Formulation design, statistical optimization and in vitro biological activities of nano-emulsion containing essential oil from cotton-lavender (Santolina chamaecyparissus L.). J. Drug Deliv. Sci. Technol. 2022, 75, 103664. [Google Scholar] [CrossRef]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor Activates and Strongly Desensitizes the Transient Receptor Potential Vanilloid Subtype 1 Channel in a Vanilloid-Independent Mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Grabarczyk, M.; Topola, E.; Kozłowska, J.; Mączka, W.; Wińska, K. Is Camphor the Future in Supporting Therapy for Skin Infections? Pharmaceuticals 2024, 17, 715. [Google Scholar] [CrossRef]
- Stinson, R.J.; Morice, A.H.; Sadofsky, L.R. Modulation of Transient Receptor Potential (TRP) Channels by Plant Derived Substances Used in over-the-Counter Cough and Cold Remedies. Respir. Res. 2023, 24, 45. [Google Scholar] [CrossRef]
- Kumar, N.; Nepali, K.; Sapra, S.; Bijjem, K.R.V.; Kumar, R.; Suri, O.P.; Dhar, K.L. Effect of Nitrogen Insertion on the Antitussive Properties of Menthol and Camphor. Med. Chem. Res. 2012, 21, 531–537. [Google Scholar] [CrossRef]
- Ahuja, A.; Bakshi, S.K.; Sharma, S.K.; Thappa, R.K.; Agarwal, S.G.; Kichlu, S.K.; Paul, R.; Kaul, M.K. Production of volatile terpenes by proliferating shoots and micropropagated plants Santolina chamaecyparissus L. (cotton lavender). Flavour Fragr. J. 2005, 20, 403–406. [Google Scholar] [CrossRef]
- Anjaneyulu, B.; Saini, N. A study on camphor derivatives and its applications: A review. Curr. Org. Chem. 2021, 25, 1404–1428. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434. [Google Scholar] [CrossRef]
- Kong, W.B.; Huo, H.R.; Gu, Y.; Cao, Y.Q.; Wang, J.L.; Liang, J.Y.; Niu, S.Q. Antifungal activity of camphor against four phytopathogens of Fusarium. S. Afr. J. Bot. 2022, 148, 437–445. [Google Scholar] [CrossRef]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical Composition, Antimicrobial, Cytotoxic and Antioxidant Activities of the Essential Oil of Artemisia Indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and biological effects of alpha-bisabolol: An updated review of the molecular mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.T.L.; Dias, G.D.S.; Pereira, M.M.; Bastos, L.D.S.; Souza, M.I.A.; Vieira, L.F.A.; de Paula, A.C.C.F.F.; Marco, C.A.; Marchiori, P.E.R.; Bicalho, E.M. New perspective on the use of α-Bisabolol for weed control. J. Agric. Food Chem. 2024, 72, 6289–6301. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What are the potential health benefits of this flavouring and aroma agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Soares-Castro, P.; Soares, F.; Reis, F.; Lino-Neto, T.; Santos, P.M. Bioprospection of the bacterial β-myrcene-biotransforming trait in the rhizosphere. Appl. Microbiol. Biotechnol. 2023, 107, 5209–5224. [Google Scholar] [CrossRef]
- Hachlafi, N.E.; Aanniz, T.; Menyiy, N.E.; Baaboua, A.E.; Omari, N.E.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In vitro and in vivo biological investigations of camphene and its mechanism insights: A review. Food Rev. Int. 2023, 39, 1799–1826. [Google Scholar] [CrossRef]
- Benelli, G.; Govindarajan, M.; Rajeswary, M.; Vaseeharan, B.; Alyahya, S.A.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Maggi, F. Insecticidal activity of camphene, zerumbone and α-humulene from Cheilocostus speciosus rhizome essential oil against the Old-World bollworm, Helicoverpa armigera. Ecotoxicol. Environ. Saf. 2018, 148, 781–786. [Google Scholar] [CrossRef]
- Chadha, J.; Khullar, L.; Mudgil, U.; Harjai, K. A comprehensive review on the pharmacological prospects of Terpinen-4-ol: From nature to medicine and beyond. Fitoterapia 2024, 176, 106051. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Milani, F.; Todero, S.; Berera, P.; Maggi, F.; Santagostini, L.; Fico, G. Botanic Garden as a Factory of Molecules: Myrtus communis L. subsp. communis as a Case Study. Plants 2022, 11, 754. [Google Scholar] [PubMed]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Milani, F.; Flamini, G.; Fico, G. Scutellaria caucasica A. Ham: Morphological features and headspace characterization. Flora 2020, 269, 151638. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Milani, F.; Spada, A.; Papini, A.; Flamini, G.; Fico, G. Insight into micromorphology and phytochemistry of Lavandula angustifolia Mill. from Italy. S. Afr. J. Bot. 2023, 153, 83–93. [Google Scholar] [CrossRef]




| Stem | Leaf | Involucral Bract | Interfloral Scales | Tubular Floret | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adax | Abax | Adax | Abax | Adax | Abax | Ovary | Corolla | ||
| non-glandular trichomes | ++ | ++ | +++ | − | + | − | + | − | − |
| biseriate glandular trichomes | ± | +++ | +++ | − | ++ | ++ | ++ | − | ++ |
| secretory ducts | ++ | ++ | ++ | ++ | − | − | |||
| Stainings | Target-Compounds | Biseriate Glands | Secretory Ducts |
|---|---|---|---|
| Fluoral Yellow-088 | Total lipids | ++ | ++ |
| Nile Red | Neutral lipids | ++ | ++ |
| Nadi reagent | Terpenoids | ++ | ++ |
| PAS reagent | Total polysaccharides | − | − |
| Ruthenium Red | Acid polysaccharides | − | − |
| Alcian Blue | Muco-polysaccharides | − | − |
| FeCl3 | Polyphenols | ++ | ++ |
| AlCl3 | Flavonoids | + | + |
| N. | Type | LRI | Compound | Relative Abundance (%) | |
|---|---|---|---|---|---|
| 2021 | 2022 | ||||
| 1 | MH | 890 | santolina triene | 0.97 | 1.25 |
| 2 | MH | 920 | 2,5,5-trimethyl-1,3,6-heptatriene | 0.18 | 0.21 |
| 3 | MH | 931 | α-pinene | 0.59 | 0.49 |
| 4 | MH | 949 | camphene | 3.45 | 3.42 |
| 5 | MH | 970 | sabinene | 1.58 | 0.77 |
| 6 | MH | 976 | β-pinene | 1.79 | 1.12 |
| 7 | MH | 990 | β-myrcene | 3.30 | 0.92 |
| 8 | MO | 1008 | 3,6-heptadien-2-ol,2,5,5-trimethyl | 1.60 | 1.99 |
| 9 | MH | 1033 | 3-carene | - | 0.08 |
| 10 | MO | 1034 | santolina alcohol | 0.64 | 0.55 |
| 11 | MH | 1038 | β-phellandrene | 1.06 | 1.00 |
| 12 | MO | 1079 | artemisia ketone | 52.74 | 55.67 |
| 13 | MO | 1092 | artemisia alcohol | 0.37 | 0.40 |
| 14 | MH | 1102 | p-cymenene | 0.18 | 0.34 |
| 15 | NH | 1105 | amyl isovalerate | 0.51 | 0.66 |
| 16 | MO | 1156 | camphor | 13.00 | 16.18 |
| 17 | MO | 1172 | lavandulol | 0.35 | 0.25 |
| 18 | MO | 1176 | pinocarvone | 0.25 | 0.16 |
| 19 | MO | 1183 | endo-borneol | 0.07 | 0.09 |
| 20 | MO | 1190 | 4-terpineol | 2.94 | 1.97 |
| 21 | MO | 1197 | p-cymen-8-ol | 0.81 | 0.69 |
| 22 | MO | 1199 | cryptone | 0.28 | 0.32 |
| 23 | MO | 1205 | myrtenol | 0.77 | 0.63 |
| 24 | MO | 1224 | carveol | 0.03 | 0.02 |
| 25 | MO | 1230 | methyl thymol | 0.49 | 0.48 |
| 26 | NH | 1234 | 2-butenoic acid, 3-methyl-, 3-methyl-2-butenyl ester | 0.10 | 0.07 |
| 27 | NH | 1238 | 3-methyl-2-butenyl tiglate | 0.11 | 0.16 |
| 28 | NH | 1250 | cuminaldehyde | 0.27 | 0.17 |
| 29 | MO | 1260 | piperitone | 0.02 | 0.03 |
| 30 | MO | 1284 | phellandral | 0.20 | 0.14 |
| 31 | NH | 1384 | benzyl valerate | 0.15 | 0.13 |
| 32 | SH | 1387 | β-elemene | 0.12 | 0.13 |
| 33 | SH | 1420 | β-caryophyllene | 0.02 | 0.01 |
| 34 | SH | 1448 | β-farnesene | 0.10 | 0.04 |
| 35 | SH | 1480 | α-curcumene | 3.04 | 1.79 |
| 36 | SO | 1539 | italicene ether | 0.28 | 0.17 |
| 37 | SO | 1561 | nerolidol | 0.65 | 0.68 |
| 38 | SO | 1587 | caryophyllene oxide | 0.15 | 0.19 |
| 39 | SO | 1658 | bisabolol oxide II | 0.29 | 0.27 |
| 40 | SO | 1691 | α-bisabolol | 4.05 | 4.49 |
| Oil Yields (%) | 0.42 | 0.48 | |||
| Monoterpene hydrocarbons (MH) | 15.06 | 10.48 | |||
| Oxygenated monoterpenes (MO) | 72.67 | 78.61 | |||
| Sesquiterpene hydrocarbons (SH) | 3.28 | 1.98 | |||
| Oxygenated sesquiterpenes (SO) | 5.43 | 5.80 | |||
| Aromatic esters (AE) | 1.13 | 1.19 | |||
| Total identified | 97.57 | 98.06 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliani, C.; Milani, F.; Falsini, S.; Spada, A.; Bruschi, P.; Papini, A.; Santagostini, L.; Bottoni, M.; Fico, G. Secretory Structures and Essential Oil Composition in Santolina chamaecyparissus L. Cultivated in Northern Italy. Horticulturae 2025, 11, 1184. https://doi.org/10.3390/horticulturae11101184
Giuliani C, Milani F, Falsini S, Spada A, Bruschi P, Papini A, Santagostini L, Bottoni M, Fico G. Secretory Structures and Essential Oil Composition in Santolina chamaecyparissus L. Cultivated in Northern Italy. Horticulturae. 2025; 11(10):1184. https://doi.org/10.3390/horticulturae11101184
Chicago/Turabian StyleGiuliani, Claudia, Fabrizia Milani, Sara Falsini, Alberto Spada, Piero Bruschi, Alessio Papini, Laura Santagostini, Martina Bottoni, and Gelsomina Fico. 2025. "Secretory Structures and Essential Oil Composition in Santolina chamaecyparissus L. Cultivated in Northern Italy" Horticulturae 11, no. 10: 1184. https://doi.org/10.3390/horticulturae11101184
APA StyleGiuliani, C., Milani, F., Falsini, S., Spada, A., Bruschi, P., Papini, A., Santagostini, L., Bottoni, M., & Fico, G. (2025). Secretory Structures and Essential Oil Composition in Santolina chamaecyparissus L. Cultivated in Northern Italy. Horticulturae, 11(10), 1184. https://doi.org/10.3390/horticulturae11101184








