A Preliminary Study on the Resistance Mechanism of Pleurotus ostreatus to Mitigate the Impact of Insecticides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Effect of Insecticides on Enzyme Activities Associated with P. ostreatus
2.4. Transcriptome Sequencing Analysis
2.5. Enrichment Analysis of Differentially Expressed Genes (DEGs) in P. ostreatus
2.6. qRT-PCR Verification
3. Results
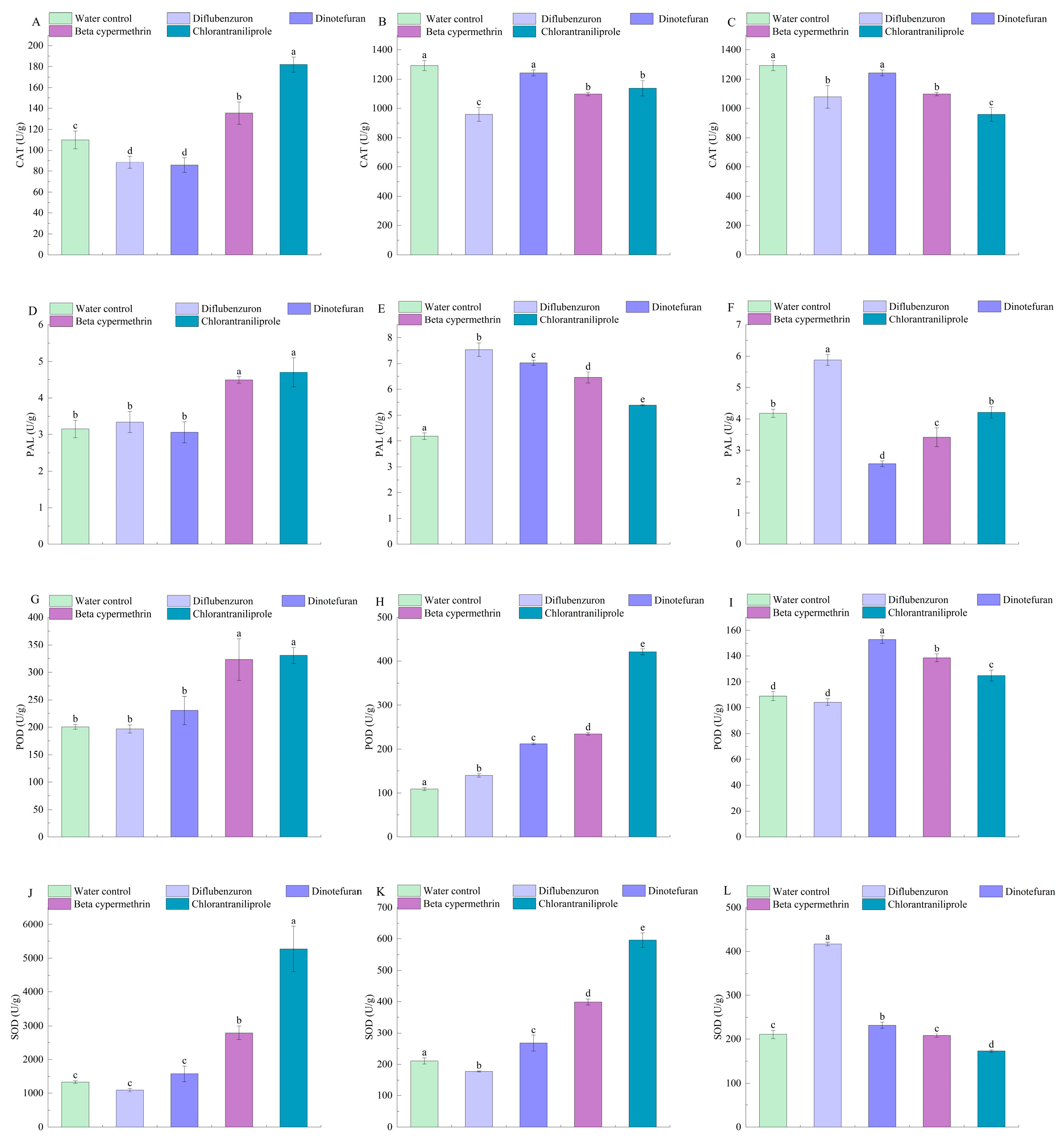
3.1. Effect of Insecticides on Enzyme Activity of P. ostreatus
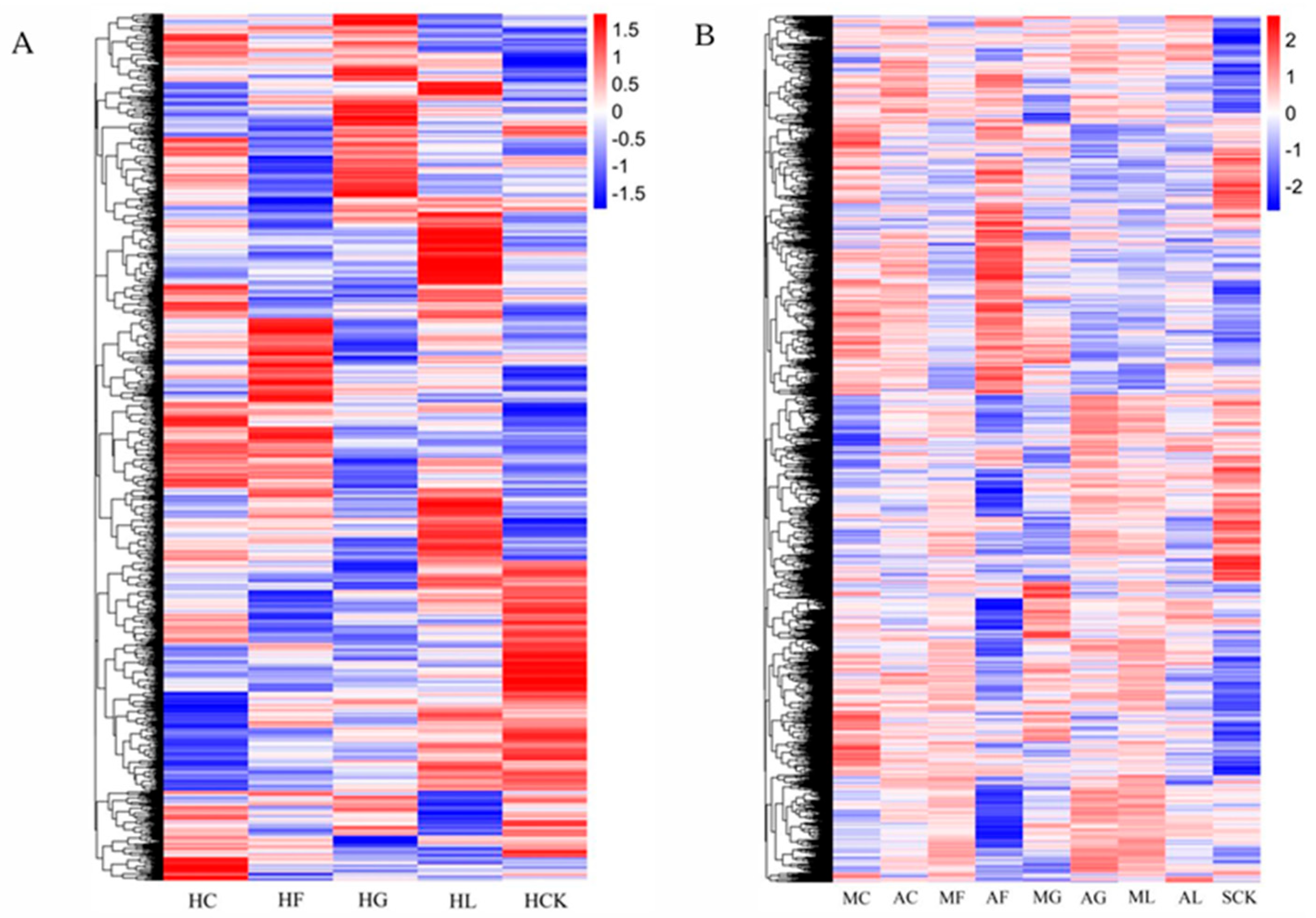
3.2. Quality Analysis of Transcriptome Sequencing Data
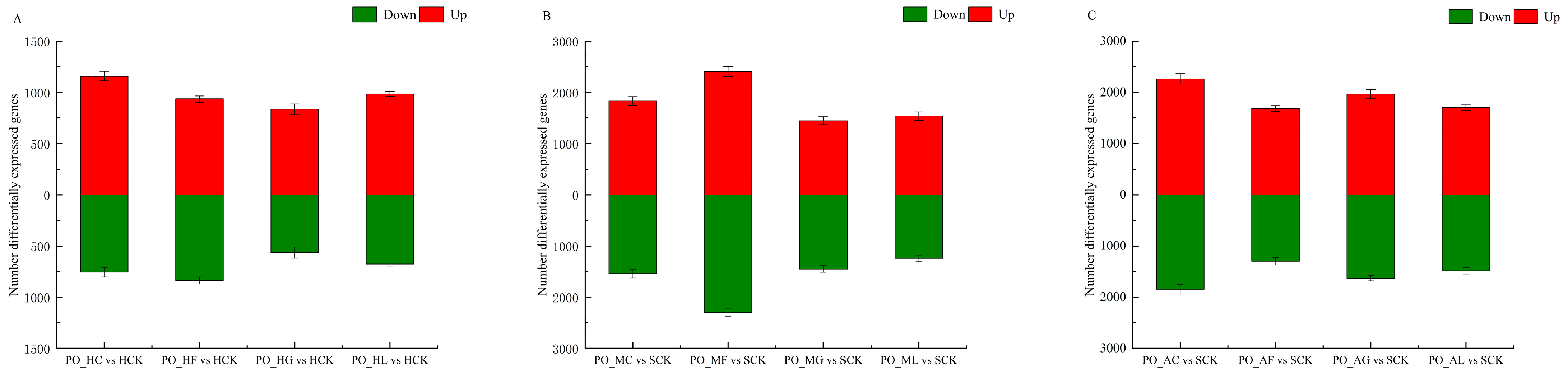
Statistical Results of DEGs
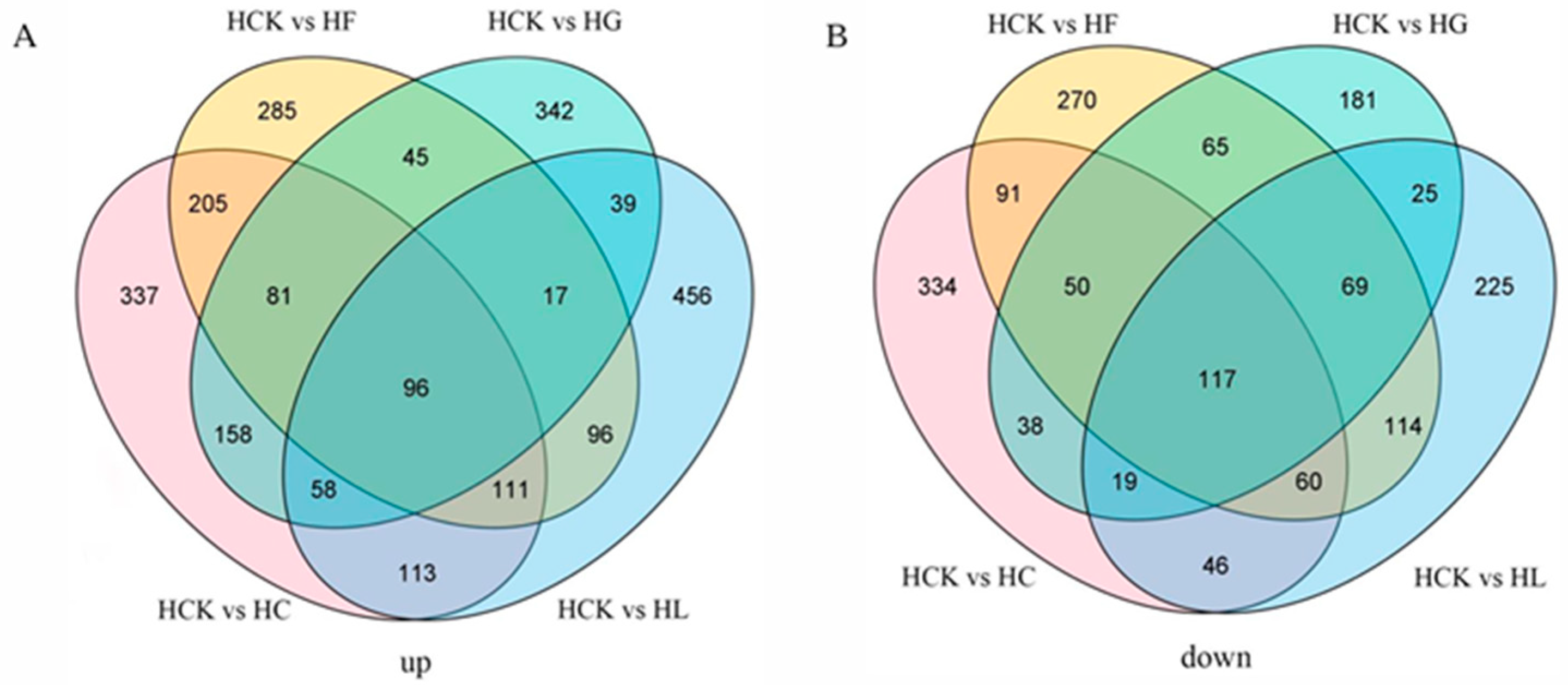
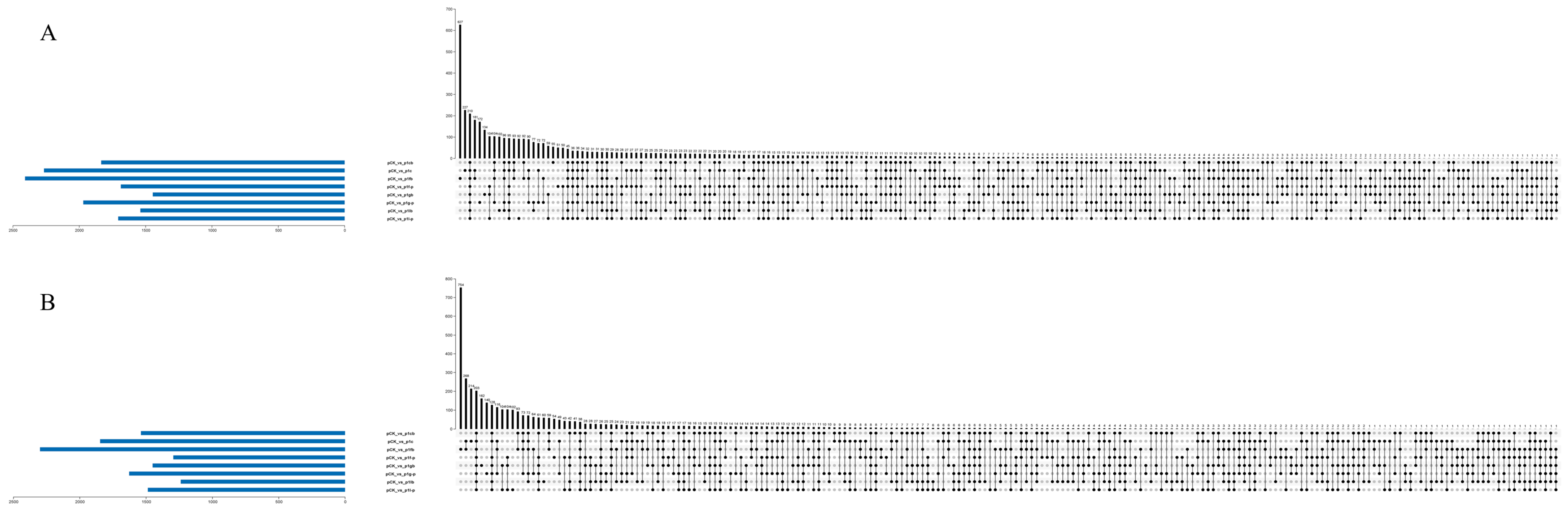
3.3. Venn Analysis Results
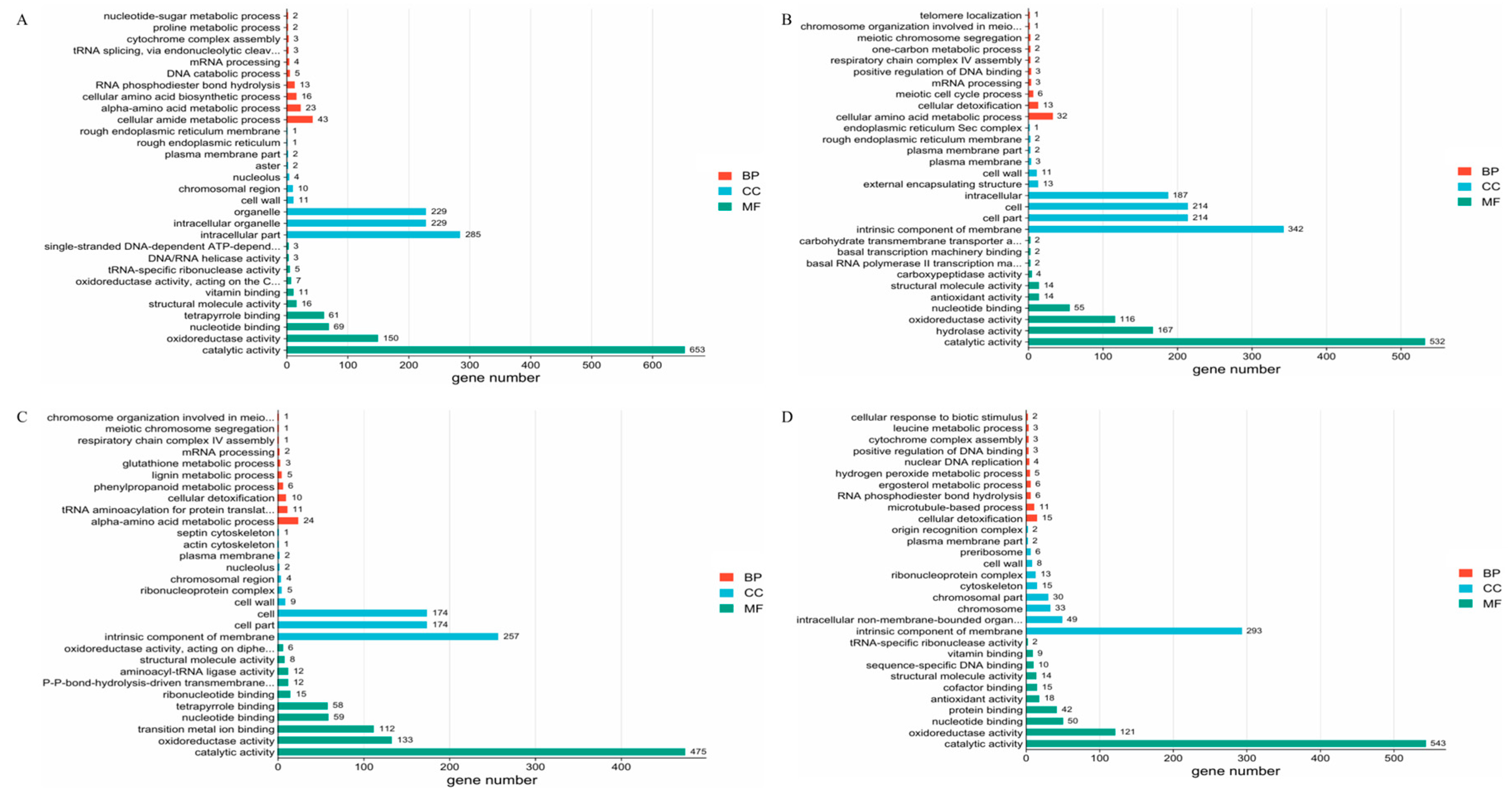
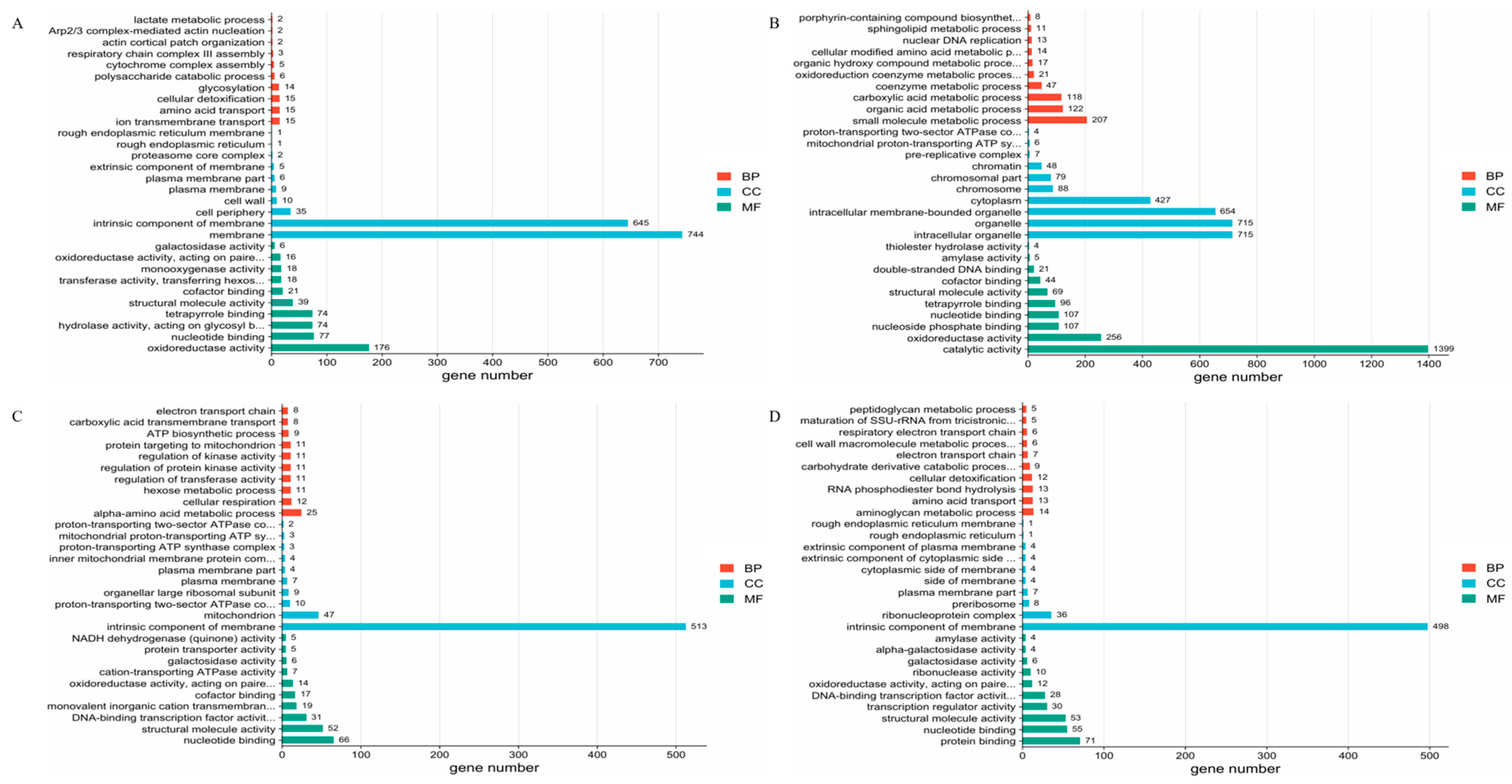
3.4. Functional Enrichment Analysis of DEG
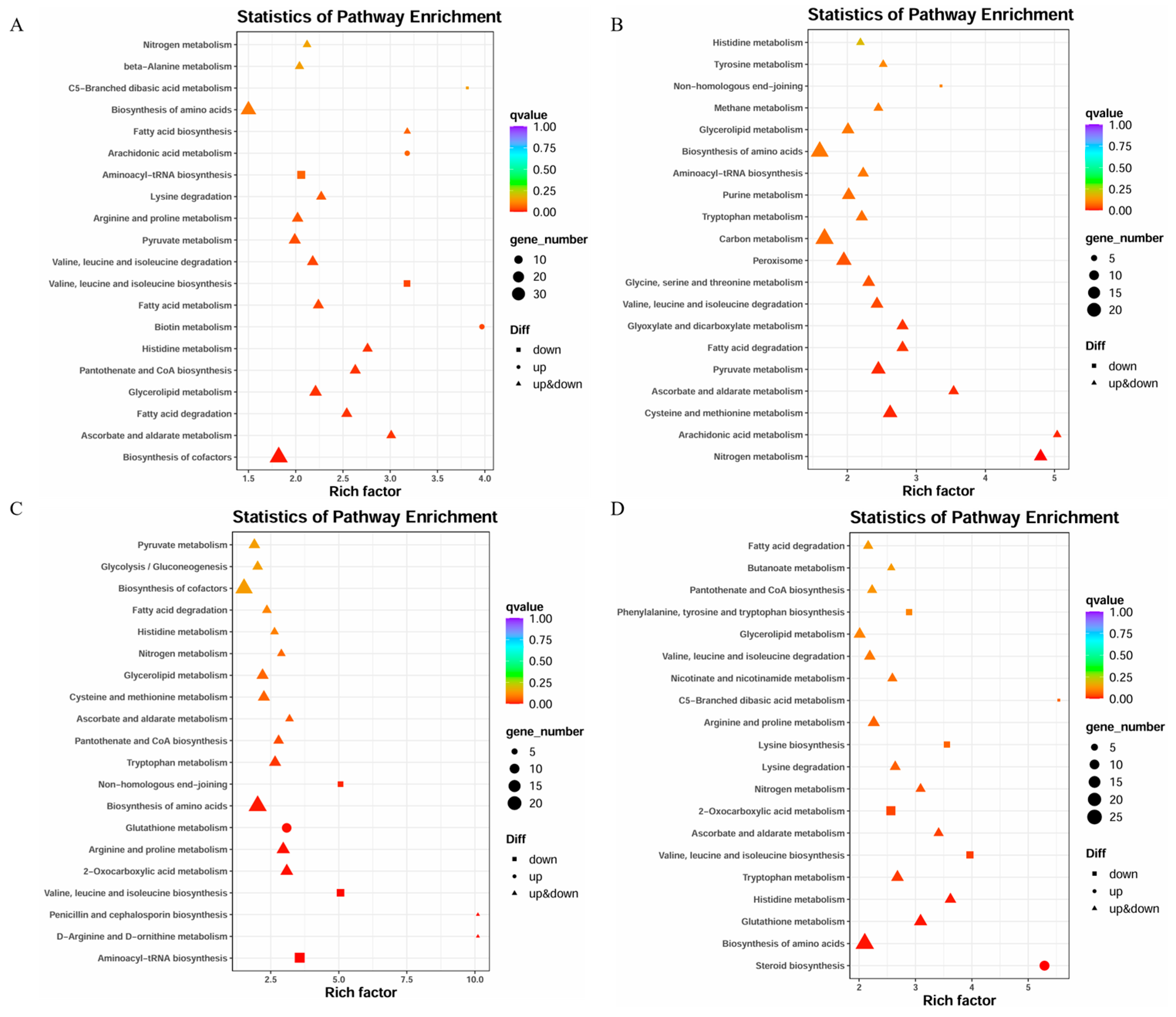
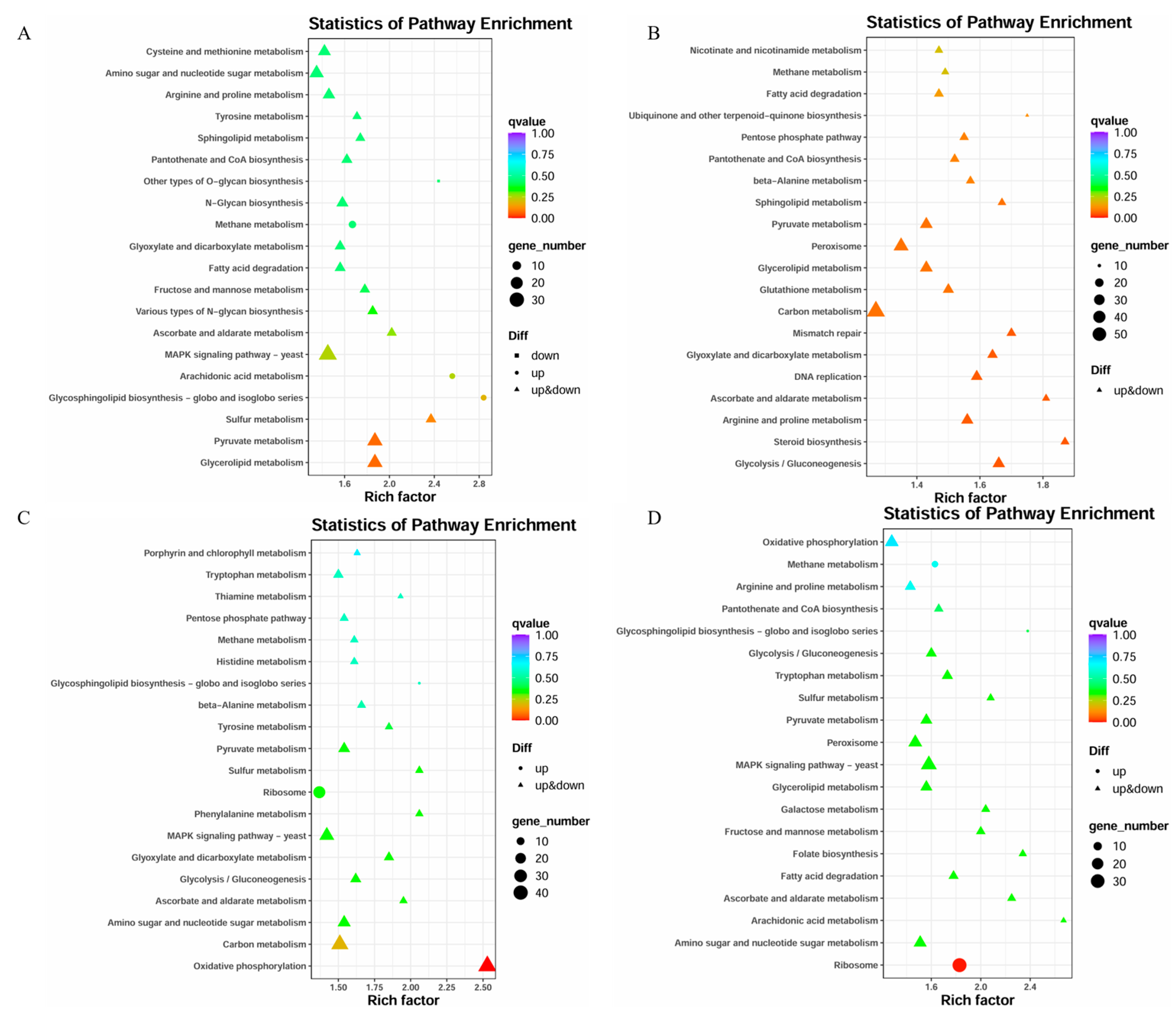
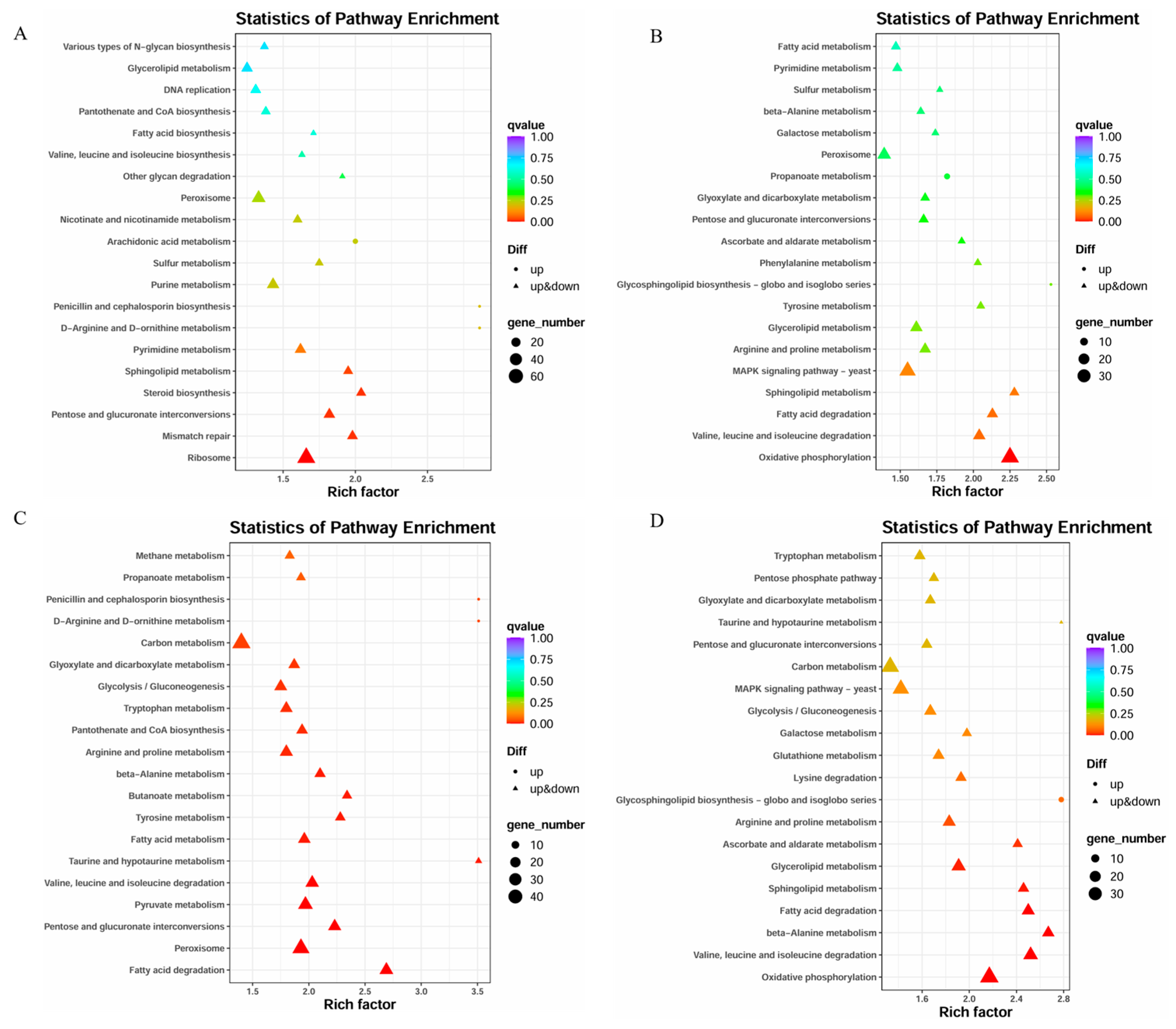
3.5. KEGG Pathway Enrichment Analysis
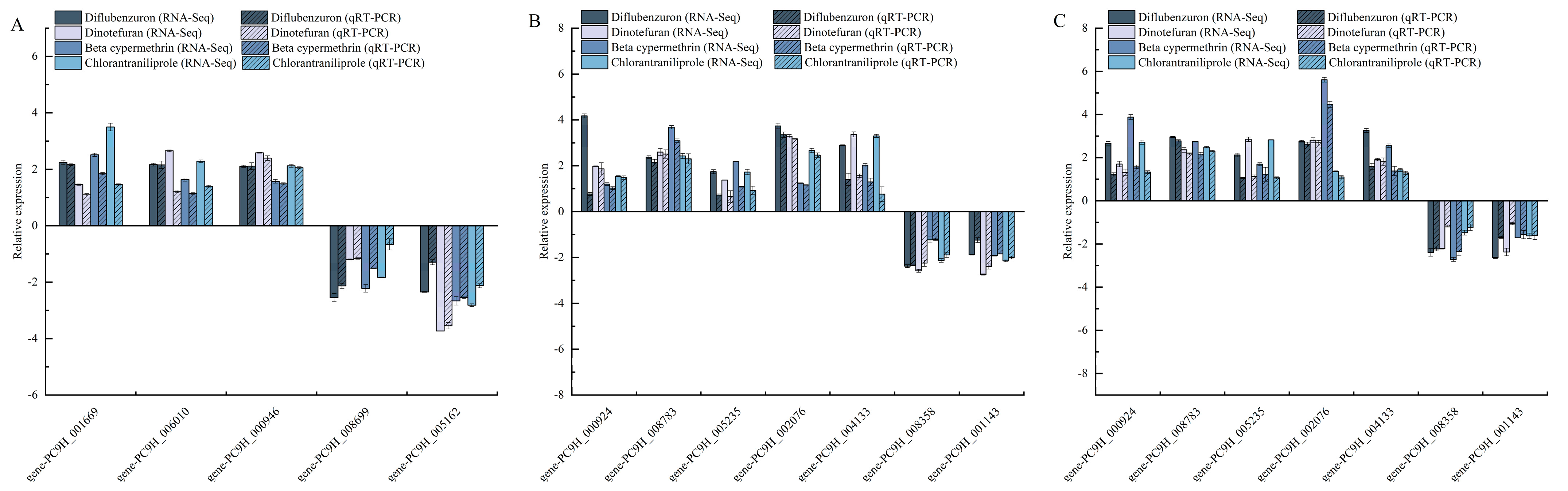
3.6. qRT-PCR Validation of Transcriptomic Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Todorov, S.K.; Tomasikova, F.; Hansen, M.; Shetty, R.; Jansen, C.L.; Jacobsen, C.; Hobley, T.J.; Lametsch, R.; Berthelsen, C.H.B. Using pre-fermented sugar beet pulp as a growth medium to produce Pleurotus ostreatus mycelium for meat alternatives. Int. J. Food Microbiol. 2024, 425, 110872. [Google Scholar] [CrossRef]
- Bhatia, A.; Jarial, R.S.; Jarial, K. Evaluation of Different Grain Substrates for Spawn Production and Yield Performance of Blue Oyster Mushroom [Hypsizygus ulmarius (Bull.: Fr.) Redhead] through Bio-Conversion of Agri/Industrial Wastes. Indian J. Ecol. 2022, 49, 2389–2394. [Google Scholar]
- Oyebamiji, G.H.; Jonathan, G.S.; Akinyemi, D.S.; Popoola, K.K.O. Fungal and Insect Pests of the Edible Mushroom Pleurotus ostreatus. Not. Sci. Biol. 2018, 10, 379–386. [Google Scholar] [CrossRef]
- Ma, L.; Gu, L.T.; Qu, S.X.; Lin, J.S.; Hou, L.J.; Li, H.P.; Jiang, N.; Xu, P. Research progress on pest control of edible fungi. J. Environ. Entomol. 2024, 46, 332–340. [Google Scholar]
- Li, H.P.; Liu, J.J.; Hou, Z.Q.; Luo, X.; Lin, J.S.; Jiang, N.; Hou, L.J.; Ma, L.; Li, C.X.; Qu, S.X. Activation of mycelial defense mechanisms in the oyster mushroom Pleurotus ostreatus induced by Tyrophagus putrescentiae. Food Res. Int. 2022, 160, 111708. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.S.; Liu, S.C.; Li, R.X.; Li, Z.B.; Piao, J.Z.; Zhou, R.J. Calcium enhanced the resistance against Phoma arachidicola by improving cell membrane stability and regulating reactive oxygen species metabolism in peanut. BMC Plant Biol. 2024, 24, 501. [Google Scholar] [CrossRef]
- Akbar, M.U.; Aqeel, M.; Shah, M.S.; Jeelani, G.; Iqbal, N.; Latif, A.; Elnour, R.O.; Hashem, M.; Alzoubi, O.M.; Habeeb, T.; et al. Molecular regulation of antioxidants and secondary metabolites act in conjunction to defend plants against pathogenic infection. S. Afr. J. Bot. 2023, 161, 247–257. [Google Scholar] [CrossRef]
- Wei, J.; Xu, C.; Li, K.; He, H.; Xu, Q. Progress on superoxide dismutase and plant stress resistance. Plant Physiol. J. 2020, 56, 2571–2584. [Google Scholar] [CrossRef]
- Haider, F.U.; Shakoor, N.; Zulfiqar, U.; Ahmed, S.; Tariq, S.; Li, X. The synergistic effects of phenylalanine and biochar to ameliorate cadmium (Cd) stress and restoring the morpho-physiological traits of maize (Zea mays) in Cd-contaminated agricultural soil. Ecotoxicol. Environ. Saf. 2025, 302, 118673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Qiu, Q.; Hou, L.J.; Xu, P.; Jiang, N.; Lin, J.S.; Li, H.P.; Qu, S.X.; Ma, L.; Wang, W.X.; et al. Insecticides have been shown to affect the growth of P. ostreatus, but the specific regulatory mechanisms remain unclear. Acta Agric. Zhejiangensis 2025, 37, 1733–1742. [Google Scholar]
- Christine, V.; M, M.E. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.L.; Xu, C.T.; Qi, M.; Zhou, Y.Y.; Li, Z.H.; Mi, C.X.; Zou, Y.J. Transcriptome analysis of the medicinal mushroom Sanghuangporus vaninii in response to white light stress. Gene 2024, 930, 148825. [Google Scholar] [CrossRef]
- Liu, Z.N.; Li, M.H.; Yan, P.; Zhu, Z.; Liao, L.Y.; Chen, Q.; Luo, Y.; Li, H.W.; Li, J.; Wang, Q.X.; et al. Transcriptome analysis of the effects of Hericium erinaceus polysaccharide on the lymphocyte homing in Muscovy duck reovirus-infected ducklings. Int. J. Biol. Macromol. 2019, 140, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, R.C.; Miao, R.Y.; Lin, J.B.; Cao, L.P.; Ni, Y.Q.; Li, W.S.; Zhao, X. Combined transcriptomics and metabolomics analysis reveals the molecular mechanism of heat tolerance of Le023M, a mutant in Lentinulaedodes. Heliyon 2023, 9, e18360. [Google Scholar] [CrossRef]
- Voisin, A.S.; Rehberger, K.; Fasel, M.; Beauvais, R.; Segner, H.; Werner, I. Physiological and transcriptomic responses in brown trout, Salmo trutta, to multiple stressors: Pesticide mixtures, elevated water temperature and the proliferative kidney disease. Sci. Total Environ. 2025, 986, 179727. [Google Scholar] [CrossRef]
- Cao, H.; Yuan, J.; Wan, Y.; Tang, Y.; Zheng, X.; Wang, J.; Qian, K.; Feng, J.; Chen, S.; Zhang, Y.; et al. Transcriptome analysis of insecticide resistance mechanisms in field populations of the bean flower thrips, Megalurothrips usitatus (Bagnall). Ecotoxicol. Environ. Saf. 2025, 298, 118316. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Z.; Hu, Y.; Li, G.; Yao, X.; Cao, L. Nutritional value, elemental bioaccumulation and antioxidant activity of fruiting bodies and mycelial cultures of an unrecorded wild Lactarius hatsudake from Nanyue mountainous region in China. Food Res. Int. 2023, 173, 113358. [Google Scholar] [CrossRef]
- Dedousi, M.; Melanouri, E.M.; Karayannis, D.; Kaminarides, E.I.; Diamantopoulou, P. Utilization of spent substrates and waste products of mushroom cultivation to produce new crops of Pleurotus ostreatus, Pleurotus eryngii and Agaricus bisporus. Carbon Resour. Convers. 2024, 7, 100196. [Google Scholar] [CrossRef]
- Yang, R.R.; Wei, Y.N.; Peng, H.; Wang, T.; Yun, J.M.; Yin, Y.; Bi, Y. Role of benzothiadiazole in regulating reactive oxygen species and inhibiting cap opening in Agaricus bisporus during storage. Postharvest Biol. Technol. 2025, 222, 113372. [Google Scholar] [CrossRef]
- Feng, L.; Jiang, X.; Hiroaki, K.; Wang, X.Y.; Guo, Y.Y.; Li, L.; Liu, H.P.; Wang, Y.F.; Wang, J. Characterization of bioactive films loaded with melatonin and regulation of postharvest ROS scavenging and ascorbate-glutathione cycle in Agaricus bisporus. Postharvest Biol. Technol. 2022, 194, 112107. [Google Scholar] [CrossRef]
- Yao, P.; Shi, L.W.; Fan, X.R.; Zhang, Y.X.; Yue, T.L.; Guo, H. Research progress on Absorption and Transformation Mechanism and Biological Function of Selenium-enriched Edible Fungi. Sci. Technol. Food Ind. 2024, 45, 439–450. [Google Scholar]
- Liu, J.H.; Zhang, X.X.; Zhang, Z.L.; Zheng, P.H.; Li, J.T.; Xian, J.A.; Lu, Y.P. Research Progress on the Toxicological Effects of Neonicotinoid Pesticides on Non-Target Aquatic Animals. Acta Ecotoxicol. Sin. 2025, 20, 238–252. [Google Scholar]
- Zhang, Z.Y.; Ma, L.; Qu, S.X.; Jiang, N.; Li, H.P.; Hou, L.J.; Lin, J.S.; Xu, P.; Wang, W.X.; Li, F.H. Evaluation of the safety of four insecticides on mycelium and substrate of Pleurotus ostreatus. China Gua Cai 2024, 37, 109–115. [Google Scholar]
- Fan, X.M.; Qin, L.; Wang, J.X.; Zhan, F.D.; He, Y.M.; Zu, Y.Q. A Review of Glutathione Metabolism and Cadmium Tolerance in Plants. West. For. Sci. 2019, 48, 50–56. [Google Scholar]
- Lee, J.E.; Lee, H.; Baek, E.; Choi, B.; Yun, H.S.; Yoo, Y.K.; Lee, Y.S.; Song, G.J.; Cho, K.S. The role of glial and neuronal Eph/ephrin signaling in Drosophila mushroom body development and sleep and circadian behavior. Biochem. Biophys. Res. Commun. 2024, 720, 150072. [Google Scholar] [CrossRef]
- Fu, Y.P.; Liang, Y.; Dai, Y.T.; Yang, C.T.; Duan, M.Z.; Zhang, Z.; Hu, S.N.; Zhang, Z.W.; Li, Y. De Novo Sequencing and Transcriptome Analysis of Pleurotus eryngii subsp. tuoliensis (Bailinggu) Mycelia in Response to Cold Stimulation. Molecules 2016, 21, 560. [Google Scholar]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-Fungus interaction: Review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef]
- Ham, H.; Kim, S.; Ha, K. Relationship between dietary protein and amino acid intake and handgrip strength in Korean adults: Data from the 2014–2019 Korea National Health and Nutrition Examination Survey. Nutr. J. 2025, 24, 61. [Google Scholar] [CrossRef] [PubMed]

| Test Agent | 25% Diflubenzuron (WP) | 20% Dinotefuran (SC) | 20% Chlorantraniliprole (SC) | 10% Beta-Cypermethrin (SC) | Distilled Water | |
|---|---|---|---|---|---|---|
| Experimental Treatment | ||||||
| Mycelium collection | HC | HF | HL | HG | HCK | |
| Fruiting body collection—Mixing | MC | MF | ML | MG | MCK | |
| Fruiting body collection—Spraying | AC | AF | AL | AG | ACK | |
| Gene ID | Primer Sequence (5′-3′) F | Primer Sequence (5′-3′) R |
|---|---|---|
| PC9H_000946 | ACATGCCTCTGACTGTCGTG | GCACCACCGTCCTTCTTGTA |
| PC9H_001669 | GCTGGGAGCAGTACACCTTT | TTCCCACCACGGTTAAGCTC |
| PC9H_006010 | TCAAGCGTGCTACCGATGTT | TTCCCACCACGGTTAAGCTC |
| PC9H_005162 | TCGAAAAGATCCCCACACCG | TTCACGAGAACTACCGCACC |
| PC9H_008699 | GCAGACAAGATCACCGGACA | TCCAAGGGATGATTTGGCCC |
| PC9H_000924 | ATCCGTCGTTGGCAGAGTTT | TTCCGTGGTTTCATCGAGGG |
| PC9H_008783 | GGCACGGTTTTCATGCTGTT | GGTCCAAAGGTCCCTCACAG |
| PC9H_005235 | TAGTCGCCTATTCCAACGCC | CTCTGATACCAGGGCTGTGC |
| PC9H_002076 | AAGAGATCTTTGGGCCGGTG | GCATGAGCTACTCGCAAAGC |
| PC9H_004133 | CAGCGTCGAAAGAAGACCCT | CTTCTTGAGCCATGCCCTGA |
| PC9H_008358 | TCCTGGGGCCATGATGAGTA | CGGTGCCATGGGTAGAAACT |
| PC9H_001143 | TCGGCGGTTTATCTACGCTC | CATGCCTTTGTTTGGTGGGG |
| actin | TCCGTCTGGATTGGTGGTTC | AAGCACTCTGCGACTCCATC |
| Gene ID | Name | Length (bp) | Function | GenBank ID |
|---|---|---|---|---|
| PC9H_000946 | SAM2 | 1484 | Cysteine and methionine metabolism | PV690236 |
| PC9H_001669 | ADH1_1 | 1687 | Glycolysis/gluconeogenesis | PV690235 |
| PC9H_006010 | SAH1 | 1843 | Cysteine and methionine metabolism | PV690234 |
| PC9H_005162 | LEU1 | 2685 | Valine, leucine, and isoleucine biosynthesis | PV690238 |
| PC9H_008699 | ALD5_3 | 2033 | Glycolysis/gluconeogenesis | PV690237 |
| PC9H_000924 | TRP1 | 1483 | Tryptophan metabolism | PV690239 |
| PC9H_008783 | GAL1 | 2042 | Galactose metabolism | PV690240 |
| PC9H_005235 | FAD1 | 981 | Fatty acid degradation | PV690243 |
| PC9H_002076 | GLY1 | 2226 | Glycolysis/gluconeogenesis | PV690242 |
| PC9H_004133 | GPMT1 | 911 | Glycerophospholipid metabolism | PV690245 |
| PC9H_008358 | ALD1 | 1219 | Ascorbate and aldarate metabolism | PV690244 |
| PC9H_001143 | CDC25 | 1372 | MAPK signaling pathway—yeast | PV690241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Qiu, Q.; Hou, L.; Xu, P.; Jiang, N.; Lin, J.; Qu, S.; Li, H.; Li, F.; Wang, W.; et al. A Preliminary Study on the Resistance Mechanism of Pleurotus ostreatus to Mitigate the Impact of Insecticides. Horticulturae 2025, 11, 1180. https://doi.org/10.3390/horticulturae11101180
Zhang Z, Qiu Q, Hou L, Xu P, Jiang N, Lin J, Qu S, Li H, Li F, Wang W, et al. A Preliminary Study on the Resistance Mechanism of Pleurotus ostreatus to Mitigate the Impact of Insecticides. Horticulturae. 2025; 11(10):1180. https://doi.org/10.3390/horticulturae11101180
Chicago/Turabian StyleZhang, Zhiying, Qin Qiu, Lijuan Hou, Ping Xu, Ning Jiang, Jinsheng Lin, Shaoxuan Qu, Huiping Li, Fuhou Li, Weixia Wang, and et al. 2025. "A Preliminary Study on the Resistance Mechanism of Pleurotus ostreatus to Mitigate the Impact of Insecticides" Horticulturae 11, no. 10: 1180. https://doi.org/10.3390/horticulturae11101180
APA StyleZhang, Z., Qiu, Q., Hou, L., Xu, P., Jiang, N., Lin, J., Qu, S., Li, H., Li, F., Wang, W., Ma, L., & Yuan, W. (2025). A Preliminary Study on the Resistance Mechanism of Pleurotus ostreatus to Mitigate the Impact of Insecticides. Horticulturae, 11(10), 1180. https://doi.org/10.3390/horticulturae11101180






