Optimizing Micropropagation of Tanacetum balsamita L.: A Machine Learning Approach to Compare Semisolid Media and Temporary Immersion System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Media, and Culture Conditions
2.2. Morpho-Physiological Parameters
2.2.1. Plant Growth and Stomatal Characteristics
2.2.2. Determination of Chlorophyll
2.2.3. Total Polyphenolic Content and Antiradical Activity
2.3. Acclimatization
2.4. Experimental Design and Statistical Analysis
2.5. Modeling Procedure
3. Results and Discussion
3.1. Plant Growth Parameters
3.2. Stomatal Analysis
3.3. Chlorophyll Content
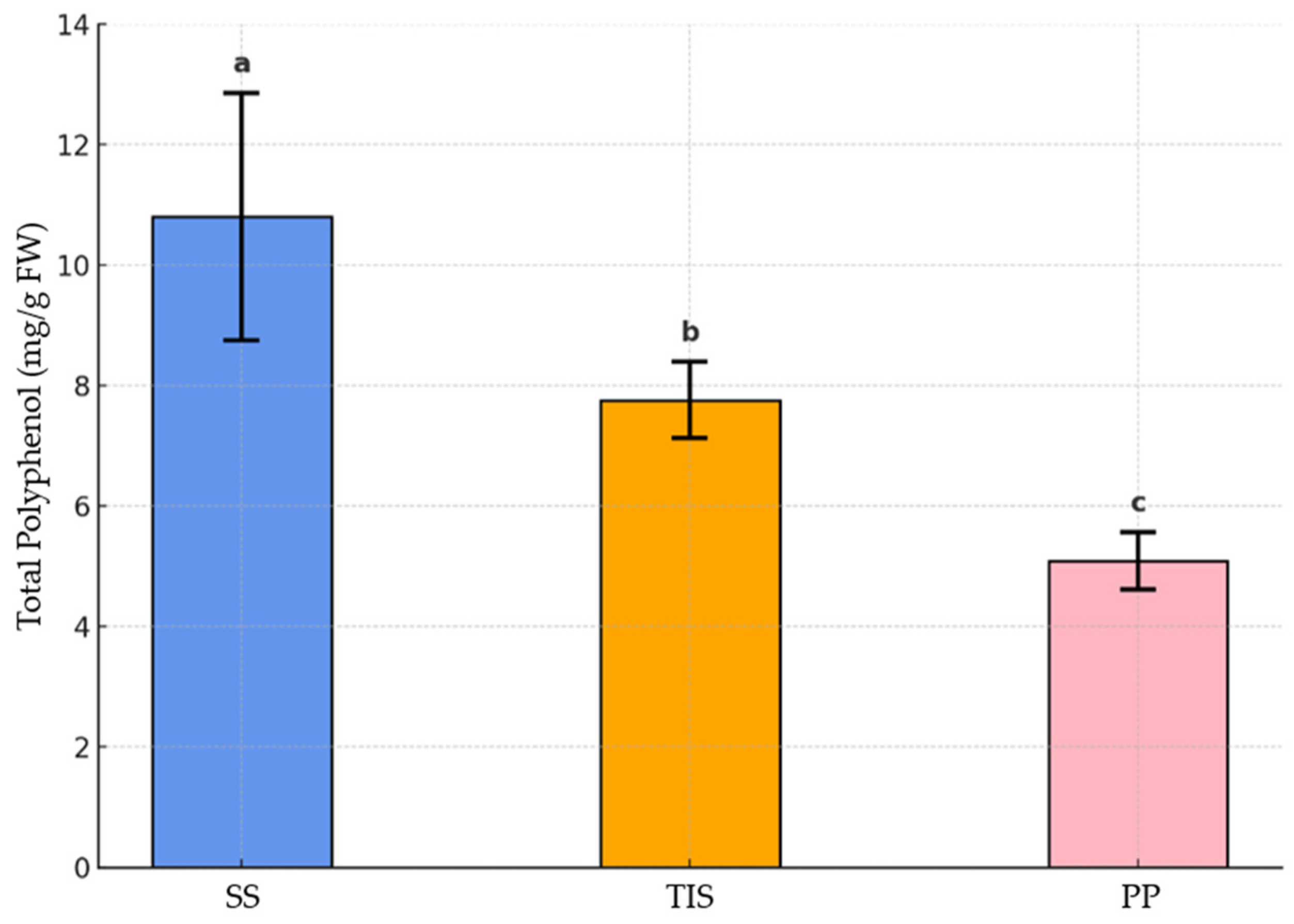
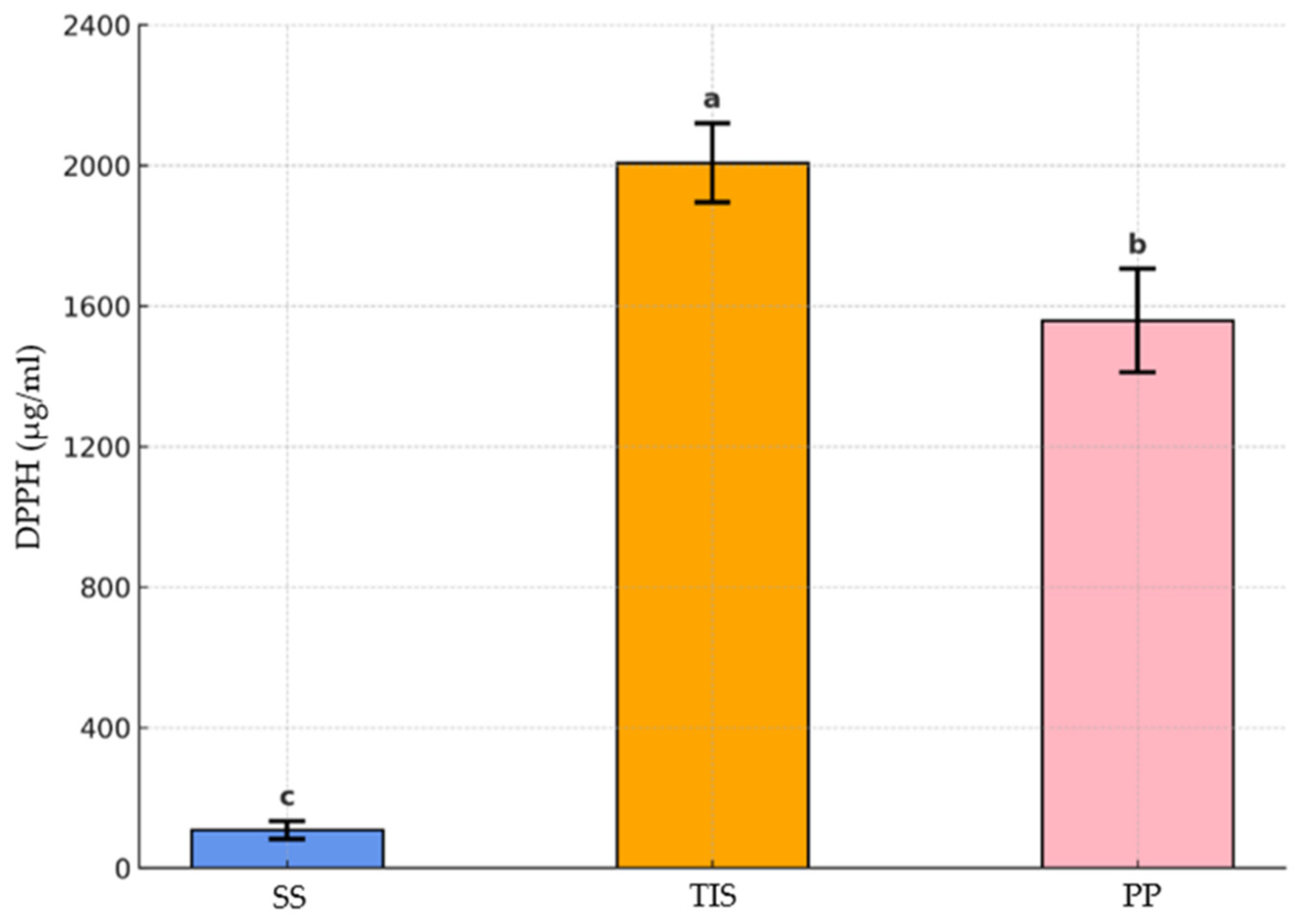
3.4. Total Polyphenolic Content and Antiradical Activity
3.5. Acclimatization
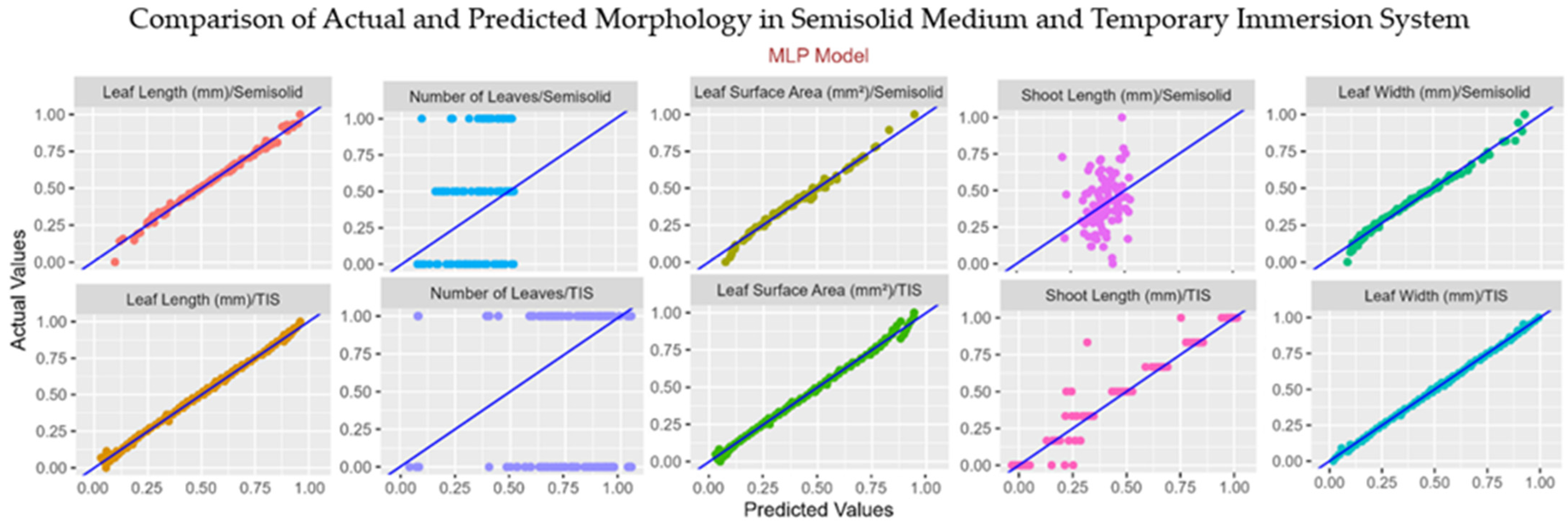
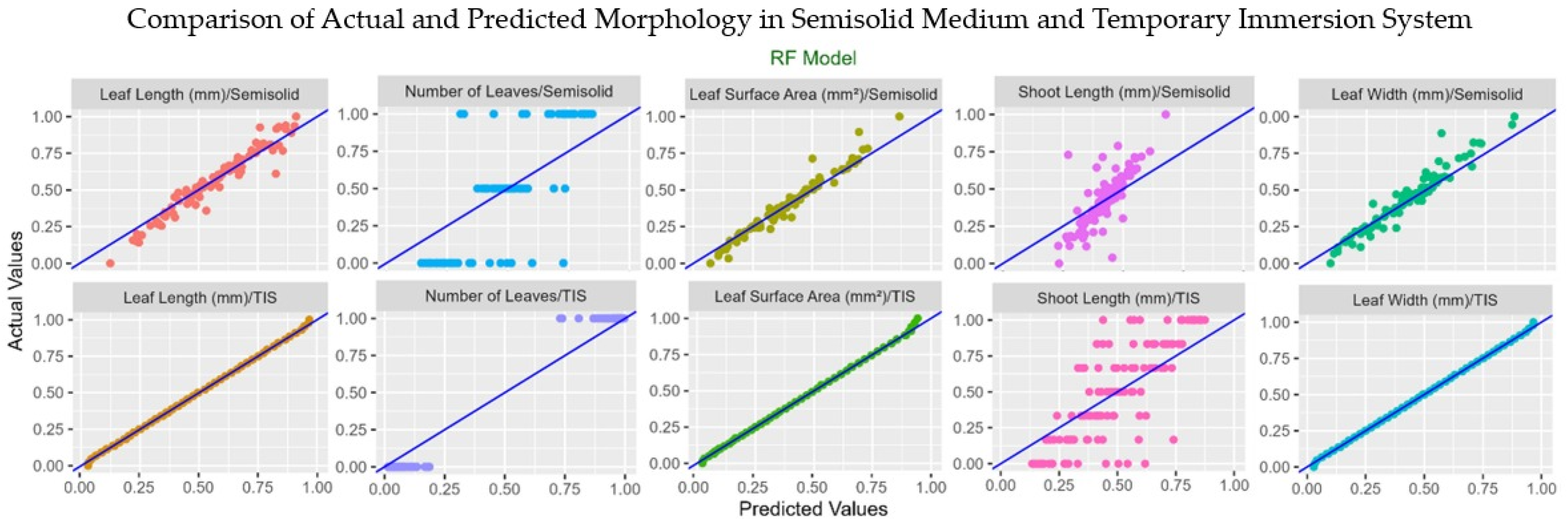
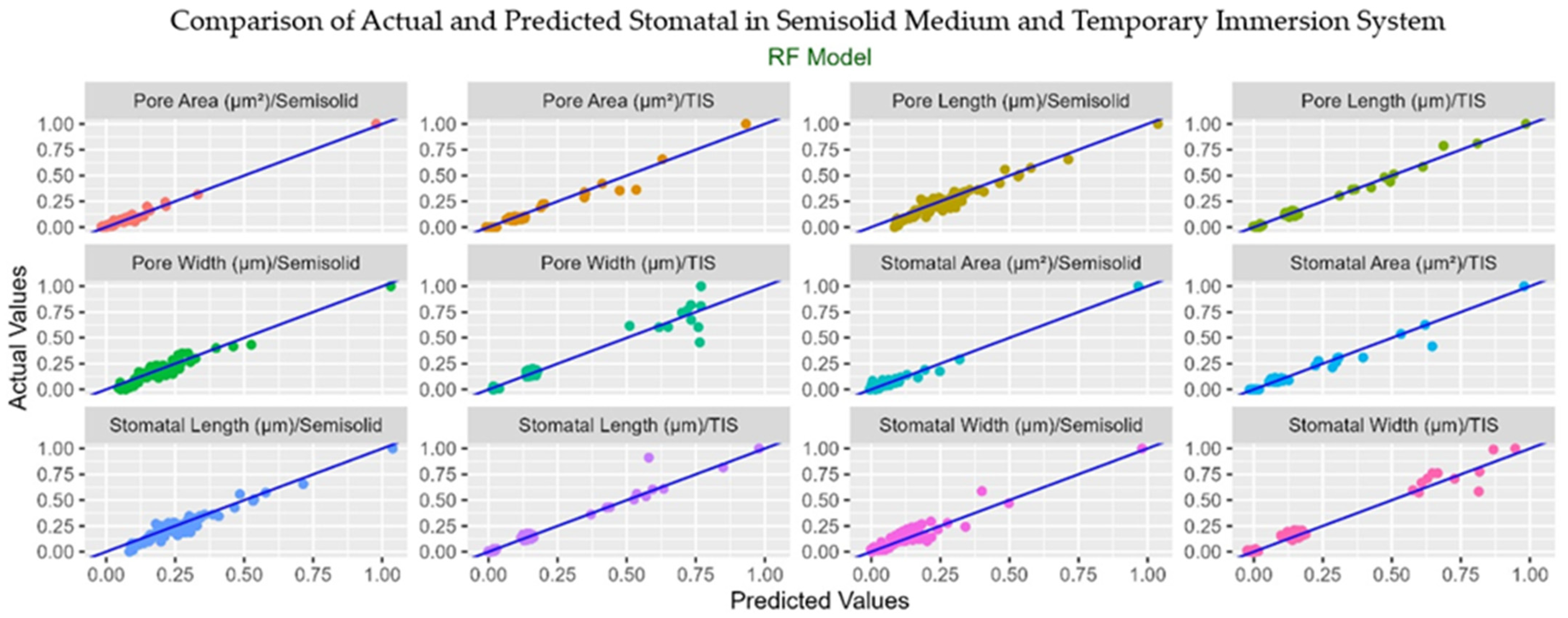
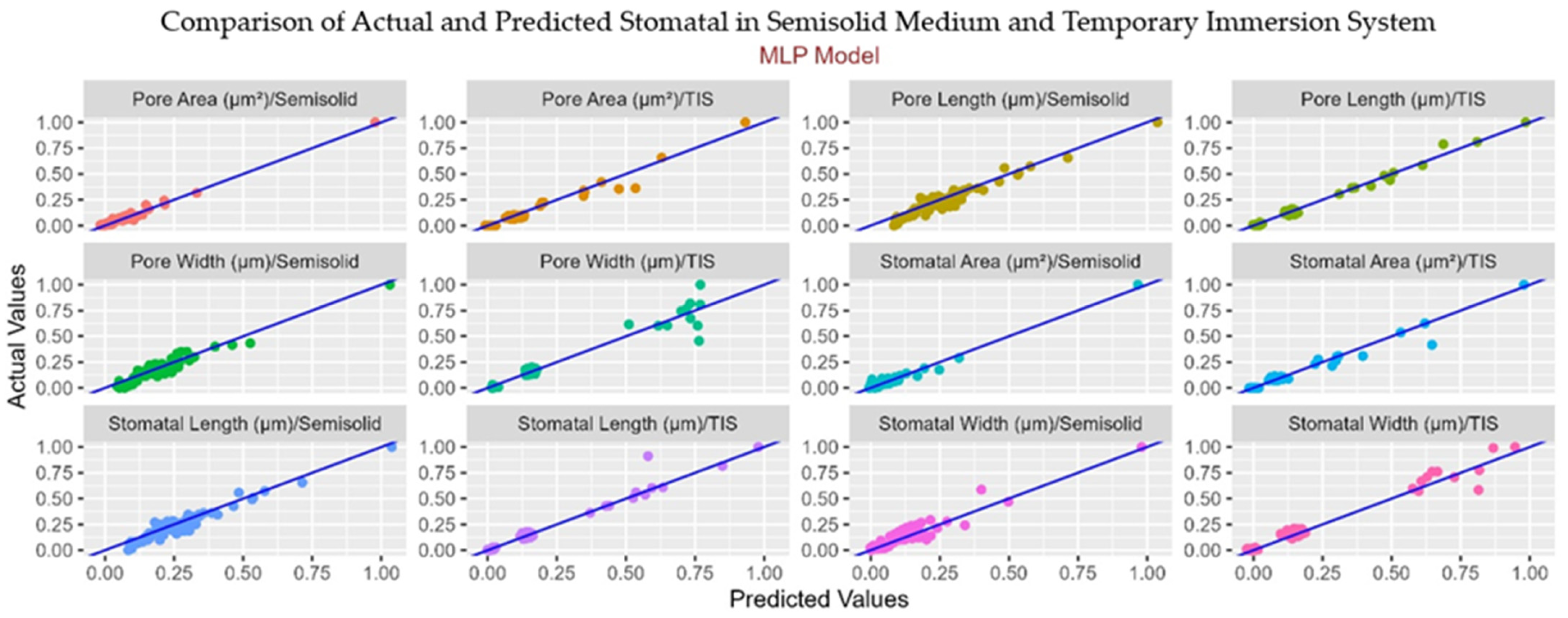
3.6. Machine Learning Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ihtyarova, M.; Angelova, V. Heavy metal accumulation and chemical composition of essential oils of tansy (Tanacetum vulgare L.) cultivated on heavy metal-contaminated soils. Sci. Pap. Ser. E. L. Reclam. Earth Obs. Surv. Environ. Eng. 2023, XII, 320–329. [Google Scholar]
- Bylaite, E.; Venskutonis, R.; Roozen, J.P.; Posthumus, M.A. Composition of essential oil of costmary [Balsamita major (L.) Desf.] at different growth phases. J. Agric. Food Chem. 2000, 48, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Hassanpouraghdam, M.; Tabatabaie, S.; Nazemiyeh, H.; Vojodi, L.; Aazami, M.; Shoja, A.M.; Applied, D. Chrysanthemum balsamita (L.) Baill.: A forgotten medicinal plant. Facta Univ. Ser. Med. Biol. 2008, 15, 119–124. [Google Scholar]
- Marculescu, A.; Sand, D.; Barbu, C.H.; Bobit, D.; Hanganu, D. Possibilities of influencing the biosynthesis and accumulation of the active principles in Chrysanthemum balsamita L. species. Rom. Biotechnol. Lett. 2001, 7, 577–584. [Google Scholar]
- Hassanpouraghdam, M.B. Flowerhead volatile oil composition of soilless culture-grown Chrysanthemum balsamita L. Nat. Prod. Res. 2009, 23, 672–677. [Google Scholar]
- Jaimand, K.; Rezaee, M.B. Chemical constituents of essential oils from Tanacetum balsamita L. ssp. balsamitoides (Schultz-Bip.) Grierson. from Iran. J. Essent. Oil Res. 2005, 17, 565–566. [Google Scholar] [CrossRef]
- Khatib, S.; Faraloni, C.; Bouissane, L. Tanacetum balsamita L.: Botany, traditional uses, phytochemical profiling, and biological activities. Drugs Drug Candidates 2025, 4, 10. [Google Scholar] [CrossRef]
- Venskutonis, P.R. Costmary (Chrysanthemum balsamita) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 365–375. [Google Scholar]
- Marculescu, A.; Hanganu, D.; Kinga, O.N. Qualitative and quantitative determination of the caffeic acid and chlorogenic acid from three chemovarieties of Chrysanthemum balsamita L. Rom. Biotechnol. Lett. 2001, 6, 477–484. [Google Scholar]
- Ghiorghita, G.; Ionica, D.E.; Toma, I.; Nicuta, D. Observations on the in vitro and ex vitro behaviour of Chrysanthemum balsamita L. Species. Analele Stiint. Univ. Alexandru Ioan Cuza Iasi Sec. II a Genet. Biol. Mol. 2004, 5, 232–239. [Google Scholar]
- Shoja, A.M.; Hassanpouraghdam, M.B.; Khosrowshahli, M.; Movafeghi, A. Callogenesis capability and calli somaclonal variation of costmary (Tanacetum balsamita L.). Rom. Biotechnol. Lett. 2010, 15, 5120–5124. [Google Scholar]
- Faraloni, C.; Bonetti, A.; Leva, A.R. Long–term micropropagation of Balsamita major as a promising in vitro strategy for producing phenolic compounds with antioxidant capacity. Plant Biosyst. 2020, 155, 336–343. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary immersion system for production of biomass and bioactive compounds from medicinal plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Watt, M.P. The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechnol. 2012, 11, 14025–14035. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Rezaei, H.; Mirzaie-Asl, A.; Abdollahi, M.R.; Tohidfar, M. Enhancing petunia tissue culture efficiency with machine learning: A pathway to improved callogenesis. PLoS ONE 2023, 18, e0293754. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, Ö.; Sekerci, A.D.; Isak, M.A.; Bulut, F.; Izgü, T.; Tütüncü, M.; Dönmez, D. Optimizing micropropagation and rooting protocols for diverse lavender genotypes: A synergistic approach integrating machine learning techniques. Horticulturae 2024, 10, 52. [Google Scholar] [CrossRef]
- Özcan, E.; Ali, S.A.; Aasim, M.; Atar, H.H. Precision in vitro propagation by integrating response surface methodology and machine learning for Glossostigma elatinoides (Benth) Hook. F. Vitr. Cell. Dev. Biol.-Plant 2025, 61, 827–841. [Google Scholar] [CrossRef]
- Tarraf, W.; İzgü, T.; Şimşek, Ö.; Cicco, N.; Benelli, C. An innovative method by temporary immersion system (TIS), Integrated with machine learning analysis. Horticulturae 2024, 10, 454. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gatti, E.; Sgarbi, E.; Ozudogru, E.A.; Lambardi, M. The effect of PlantformTM bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture system on gelled medium, and assessment of the microenvironment influence on leaf structure. Plant Biosyst. 2017, 151, 1129–1136. [Google Scholar] [CrossRef]
- Mancilla-álvarez, E.; Pérez-Sato, J.A.; Núñez-Pastrana, R.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Comparison of different semi-automated bioreactors for in vitro propagation of taro (Colocasia esculenta L. schott). Plants 2021, 10, 1010. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls Carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Luthria, D.L.; Mukhopadhyay, S.; Kwansa, A.L. A systematic approach for extraction of phenolic compounds using parsley (Petroselinum crispum) flakes as a model substrate. J. Sci. Food Agric. 2006, 86, 1350–1358. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- de Mendiburu, F. Statistical Procedures for Agricultural Research. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 9 September 2025).
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Şimşek, Ö.; Dönmez, D.; Sarıdaş, M.A.; Acar, E.; Kaçar, Y.A.; Kargı, S.P.; İzgü, T. In vitro and ex vitro propagation of Turkish myrtles through conventional and plantform bioreactor systems. PeerJ 2023, 11, e16061. [Google Scholar] [CrossRef]
- Isak, M.A.; Bozkurt, T.; Tütüncü, M.; Dönmez, D.; İzgü, T.; Şimşek, Ö. Leveraging machine learning to unravel the impact of cadmium stress on goji berry micropropagation. PLoS ONE 2024, 19, e0305111. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, D.H.; Lee, K.E. Concordance correlation coefficients for multivariate measurements. J. Korean Stat. Soc. 2025, 54, 685–717. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A. In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (PlantformTM). 3 Biotech 2018, 8, 317. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Inglese, P.; Barone, E.; Sottile, F. In vitro regeneration of Capparis spinosa L. by using a temporary immersion system. Plants 2019, 8, 177. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Sottile, F. Temporary immersion system as an innovative approach for in vitro propagation of Sorbus domestica L. Horticulturae 2024, 10, 164. [Google Scholar] [CrossRef]
- Elazab, D.; Capuana, M.; Ozudogru, E.A.; Anichini, M.; Lambardi, M. Use of liquid culture with the electis bioreactor for faster recovery of blackberry (Rubus fruticosus L.) shoots from conservation at 4 °C. Horticulturae 2023, 9, 680. [Google Scholar] [CrossRef]
- Thanonkeo, S.; Kitwetcharoen, H.; Thanonkeo, P.; Klanrit, P. Temporary immersion bioreactor (TIB) system for large-scale micropropagation of Musa sp. cv Kluai Numwa Pakchong 50. Horticulturae 2024, 10, 1030. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kwon, S.H.; Murthy, H.N.; Yun, S.W.; Pyo, S.S.; Park, S.Y. Temporary immersion bioreactor system as an efficient method for mass production of in vitro plants in horticulture and medicinal plants. Agronomy 2022, 12, 364. [Google Scholar] [CrossRef]
- Aka Kaçar, Y.; Biçen, B.; Şimşek, Ö.; Dönmez, D.; Erol, M.H. Evaluation and comparison of a new type of temporary immersion system (TIS) bioreactors for myrtle (Myrtus communis L.). Appl. Ecol. Environ. Res. 2020, 18, 1611–1620. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Bello-Bello, J.J. SETISTM bioreactor increases in vitro multiplication and shoot length in vanilla (Vanilla planifolia Jacks. Ex Andrews). Acta Physiol. Plant. 2021, 43, 52. [Google Scholar] [CrossRef]
- Ramos-Castellá, A.; Iglesias-Andreu, L.G.; Bello-Bello, J.; Lee-Espinosa, H. Improved propagation of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Vitr. Cell. Dev. Biol.-Plant 2014, 50, 576–581. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. Vitr. Cell. Dev. Biol.-Plant 2016, 52, 154–160. [Google Scholar] [CrossRef]
- Rosales, C.; Brenes, J.; Salas, K.; Arce-Solano, S.; Abdelnour-Esquivel, A. Micropropagación de Stevia rebaudiana en sistemas de inmersión temporal para incursionar en la producción hortícola. Rev. Chapingo Ser. Hortic. 2018, 24, 69–84. [Google Scholar] [CrossRef]
- Sacco, E.; Mascarello, C.; Pamato, M.; Musso, V.; Ruffoni, B. Evaluation of Temporary Immersion System for in vitro propagation of Stevia rebaudiana Bertoni. Acta Hortic. 2015, 1083, 327–333. [Google Scholar] [CrossRef]
- Melviana, A.C.; Esyanti, R.R.; Mel, M.; Setyobudi, R.H. Biomass enhancement of Stevia rebaudiana bertoni shoot culture in temporary immersion system (TIS) RITA® bioreactor optimized in two different immersion periods. E3S Web Conf. 2021, 226, 00007. [Google Scholar] [CrossRef]
- Clapa, D.; Radomir, A.M.; Peticila, A.G.; Hârța, M. Evaluation of biomass production of Stevia Rebaudiana Bertoni using classical in vitro culture and temporary immersion bioreactor system. Sci. Pap. Ser. B Hortic. 2023, 67, 558–565. [Google Scholar]
- Vilariño, S.; Florido, M.d.C.; García, J.L.; Cantos, M. Effects of culture system and substrate composition on micropropagated plantlets of two varieties of Stevia rebaudiana Bert. Physiologia 2023, 3, 74–85. [Google Scholar] [CrossRef]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid medium by temporary immersion bioreactor in comparison with solid culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.J.; Han, J.E.; Murthy, H.N.; Song, H.Y.; Lee, S.Y.; Park, S.Y. A comparison of semi-solid, liquid, and temporary immersion bioreactor systems for effective plant regeneration of Gerbera jamesonii “Shy Pink”. Horticulturae 2024, 10, 836. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees-Struct. Funct. 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Zobayeda, S.M.A. Ventilation in micropropagation. In Photoautotrophic (Sugar-Free Medium) Micropropagation as a New Micropropagation and Transplant Production System; Kozai, T., Afreen, F., Zobayed, S.M.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 147–186. [Google Scholar]
- Deccetti, S.F.C.; Soares, A.M.; Paiva, R.; de Castro, E.M. Effect of the culture environment on stomatal features, epidermal cells and water loss of micropropagated Annona glabra L. plants. Sci. Hortic. 2008, 117, 341–344. [Google Scholar] [CrossRef]
- Mancilla-Álvarez, E.; Spinoso-Castillo, J.L.; Schettino-Salomón, S.S.; Bello-Bello, J.J. Temporary immersion systems induce photomixotrophism during in vitro propagation of agave Tobalá. 3 Biotech 2024, 14, 74. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Pérez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). Vitr. Cell. Dev. Biol.-Plant 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Martínez-Estrada, E.; Islas-Luna, B.; Pérez-Sato, J.A.; Bello-Bello, J.J. Temporary immersion improves in vitro multiplication and acclimatization of Anthurium andreanum Lind. Sci. Hortic. 2019, 249, 185–191. [Google Scholar] [CrossRef]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Cochard, H.; Coll, L.; Le Roux, X.; Améglio, T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol. 2002, 128, 282–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, F.; Chen, Z.H.; Shabala, S. Hypoxia Sensing in Plants: On a Quest for Ion Channels as Putative Oxygen Sensors. Plant Cell Physiol. 2017, 58, 1126–1142. [Google Scholar] [CrossRef]
- Schulze, E.D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Water deficiency (drought). In Plant Ecology; Schulze, E.-D., Beck, E., Buchmann, N., Clemens, S., Müller-Hohenstein, K., Scherer-Lorenzen, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 165–202. [Google Scholar]
- Thi, L.T.; Park, Y.G.; Jeong, B.R. Growth and development of carnation ‘Dreambyul’ plantlets in a temporary immersion system and comparisons with conventional solid culture methods. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 539–548. [Google Scholar] [CrossRef]
- Arano-Avalos, S.; Gómez-Merino, F.C.; Mancilla-Álvarez, E.; Sánchez-Páez, R.; Bello-Bello, J.J. An efficient protocol for commercial micropropagation of malanga (Colocasia esculenta L. Schott) using temporary immersion. Sci. Hortic. 2020, 261, 108998. [Google Scholar] [CrossRef]
- Mousavi, M. Improve bougainvillea shoot growth in vitro using a modified temporary immersion system (TIS). Int. J. Rev. Life Sci. 2015, 5, 325–330. [Google Scholar]
- Matuszkiewicz, M.; Koter, M.D.; Filipecki, M. Limited ventilation causes stress and changes in Arabidopsis morphological, physiological and molecular phenotype during in vitro growth. Plant Physiol. Biochem. 2019, 135, 554–562. [Google Scholar] [CrossRef]
- Damiano, C.; Arias, P.; La Starza, S.R.; Frattarelli, A. Temporary immersion system for temperate fruit trees. Acta Hortic. 2007, 748, 87–90. [Google Scholar] [CrossRef]
- Haq, I.; Dahot, U.M. Effect of immersion systems on chlorophyll contents in micro-propagating banana. Afr. J. Biotechnol. 2007, 6, 1095–1101. [Google Scholar]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red clover). Biomed Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef]
- Achar, S.S.; Pethakamsetty, L.; Vinjamuri, S. Phytochemical analysis and hypoglycemic potential of Exacum bicolor Roxb. in diet induced obese mice. Indian J. Pharm. Sci. 2025, 87, 44–50. [Google Scholar] [CrossRef]
- Gonçalves, S.; Martins, N.; Romano, A. Physiological traits and oxidative stress markers during acclimatization of micropropagated plants from two endangered Plantago species: P. algarbiensis Samp. and P. almogravensis Franco. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 249–255. [Google Scholar] [CrossRef]
- Monja-Mio, K.M.; Olvera-Casanova, D.; Herrera-Herrera, G.; Herrera-Alamillo, M.Á.; Sánchez-Teyer, F.L.; Robert, M.L. Improving of rooting and ex vitro acclimatization phase of Agave tequilana by temporary immersion system (BioMINTTM). Vitr. Cell. Dev. Biol.-Plant 2020, 56, 662–669. [Google Scholar] [CrossRef]
- Bozkurt, T.; İnan, S.; Dündar, İ.; Isak, M.A.; Şimşek, Ö. Optimizing the In Vitro Propagation of Tea Plants: A Comparative Analysis of Machine Learning Models. Horticulturae 2024, 10, 721. [Google Scholar] [CrossRef]
- Jafari, M.; Daneshvar, M.H.; Jafari, S.; Hesami, M. Machine Learning-Assisted In Vitro Rooting Optimization in Passiflora caerulea. Forests 2022, 13, 2020. [Google Scholar] [CrossRef]
- Okumuş, O.; Şimşek, Ö.; Isak, M.A.; Şahin, N.K.; Aydin, A.; Eren, B.; Demirel, F.; Kahramanoğulları, C.T.; Uzun, S.; Yaman, M. Integrating Machine Learning and In Vitro Screening to Evaluate Drought and Temperature Stress Responses for Vicia Species. Processes 2025, 13, 1845. [Google Scholar] [CrossRef]
- Koçak, M.; Yılmaz, M.C.; Kuzğun, C.; Sirke, S.T.; Yildiz, M. Synthetic seed production in Crataegus monogyna L. and prediction of regeneration of synthetic seeds with machine learning algorithms. Plant Cell. Tissue Organ Cult. 2025, 161, 22. [Google Scholar] [CrossRef]
- Meimand, M.J.M.; Ruffoni, B.; Mascarello, C.; Shamshiri, M.H.; Malekzadeh, K. Micropropagation of Pistacia lentiscus L.—Optimization of the surface sterilization protocol and forced ventilation in Temporary Immersion Culture. Fruit Grow. Res. 2020, 36, 75–82. [Google Scholar] [CrossRef]
- Carvalho, L.S.O.; Ozudogru, E.A.; Lambardi, M.; Paiva, L.V. Temporary immersion system for micropropagation of tree species: A bibliographic and systematic review. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 269–277. [Google Scholar] [CrossRef]
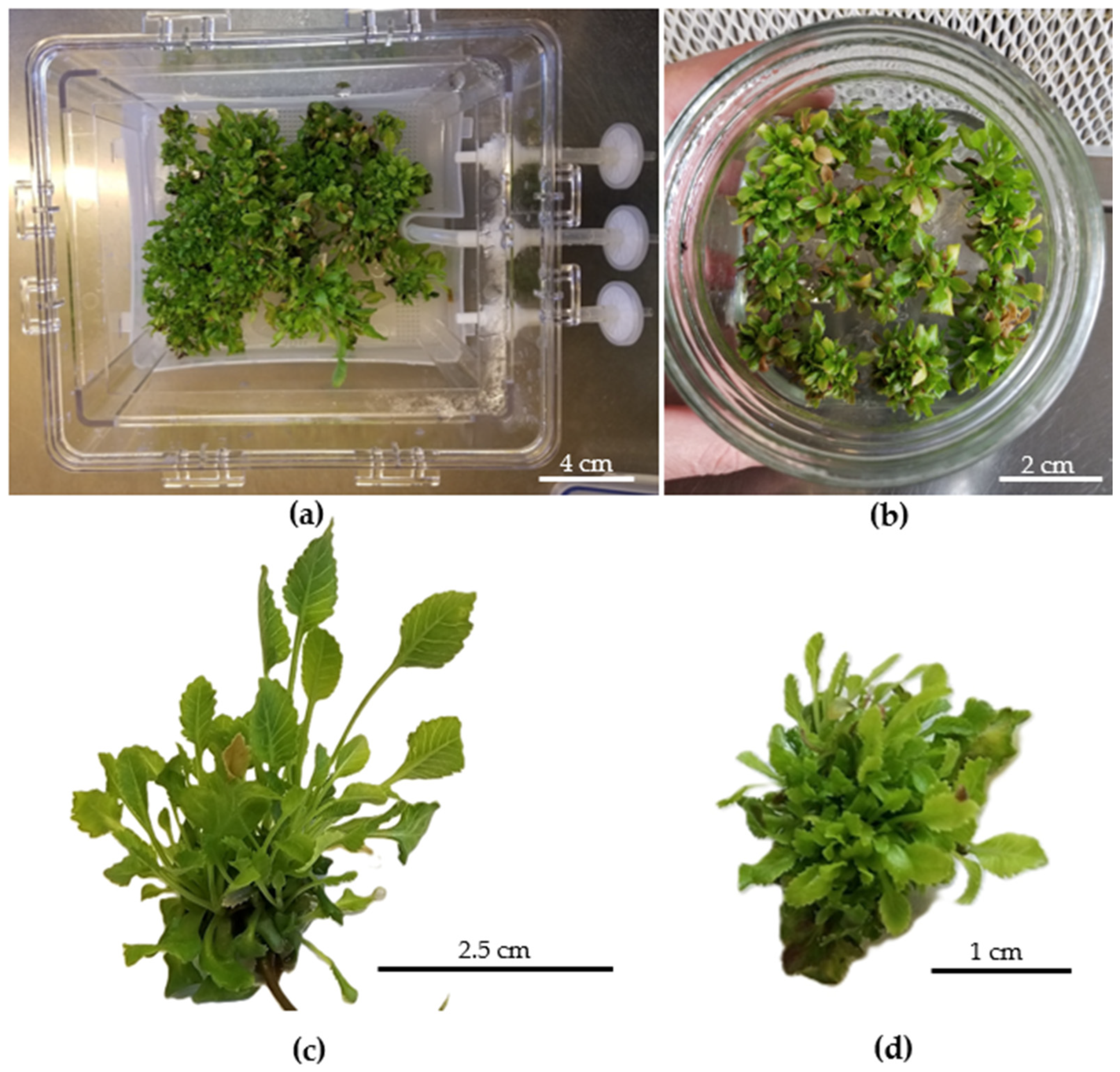
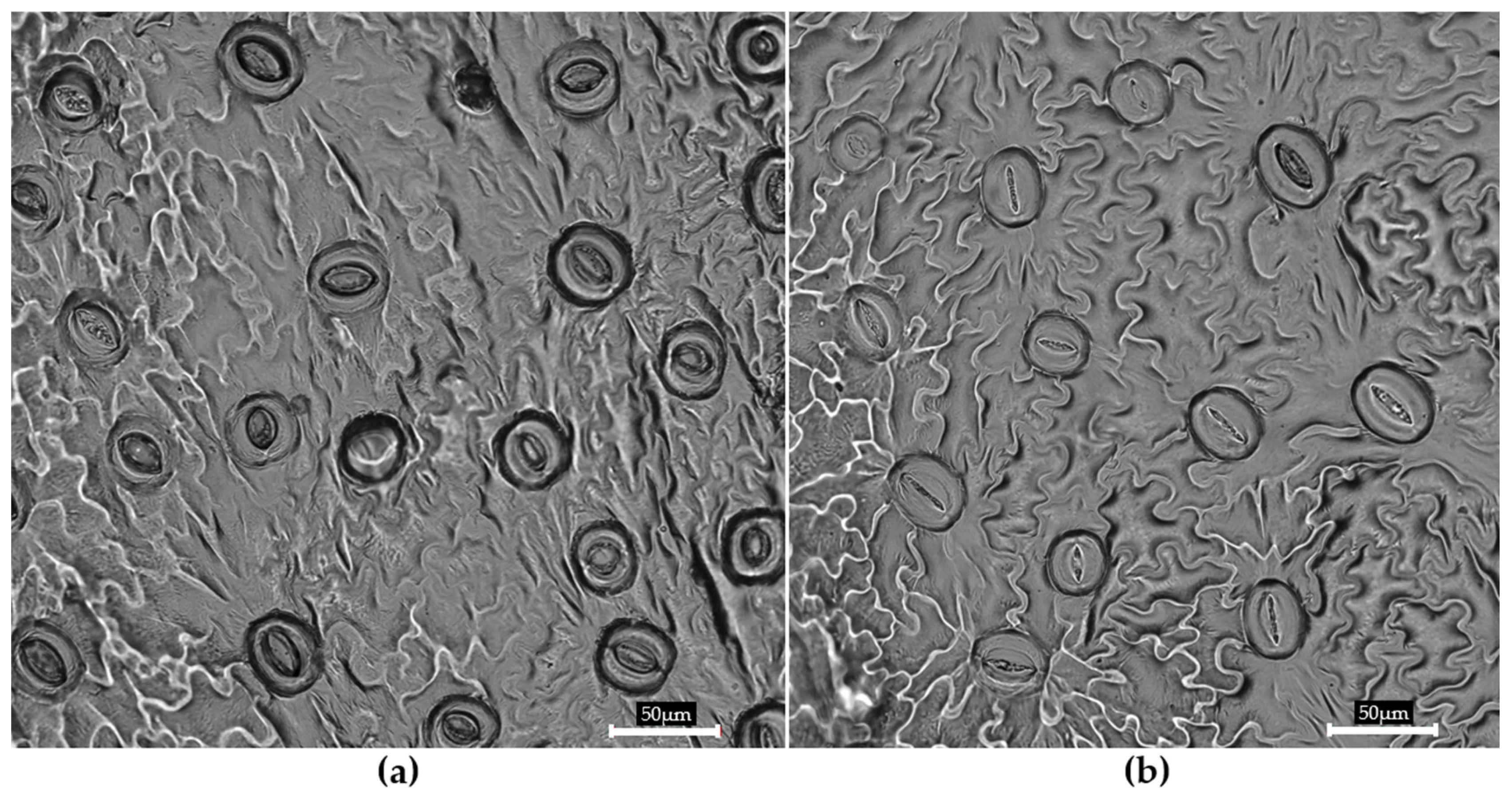


| Culture System | 30 Days of Culture | 60 Days of Culture | ||
|---|---|---|---|---|
| Initial/Final Weight (g) | RGR | Initial/Final Weight (g) | RGR | |
| TIS | 2.82/15.01 | 5.5 | 15.01/30.03 | 3.2 |
| SS medium | 2.76/7.19 | 3.9 | 7.19/15.26 | 2.8 |
| Parameters | TIS (Mean ± SE) | Semisolid (Mean ± SE) | p-Value |
|---|---|---|---|
| Leaf Length (mm) | 8.16 ± 0.26 a | 4.95 ± 0.02 b | 0.0001 *** |
| Leaf Width (mm) | 2.98 ± 0.13 | 3.01 ± 0.03 | 0.9249 ns |
| Leaf Area (mm2) | 19.64 ± 1.47 | 14.86 ± 0.17 | 0.1235 ns |
| Number of Leaves | 2.57 ± 0.00 b | 3.97 ± 0.06 a | 0.0000 *** |
| Shoot Length (mm) | 48.00 ± 0.00 a | 11.87 ± 0.12 b | 0.0001 *** |
| Parameters | TIS | Semisolid | p-Value |
|---|---|---|---|
| Stomatal Area (µm2) | 215.00 ± 4.00 a | 125.00 ± 3.50 b | 0.00001 *** |
| Stomatal Length (µm) | 29.00 ± 0.70 a | 20.00 ± 0.60 b | 0.00001 *** |
| Stomatal Width (µm) | 15.20 ± 0.35 a | 10.10 ± 0.30 b | 0.00001 *** |
| Pore Length (µm) | 9.10 ± 0.25 a | 6.10 ± 0.20 b | 0.00002 *** |
| Pore Width (µm) | 4.20 ± 0.15 a | 2.30 ± 0.10 b | 0.00002 *** |
| Pore Area (µm2) | 34.50 ± 0.38 a | 30.10 ± 0.35 b | 0.00003 *** |
| Trait | TIS (Mean ± SD) | SS (Mean ± SD) | p-Value |
|---|---|---|---|
| Open Stomata (%) | 15 ± 2 b | 30 ± 2 a | 0.0016 ** |
| Closed Stomata (%) | 85 ± 2 a | 70 ± 2 b | 0.0016 ** |
| Stomatal Density (mm2) | 250 ± 5 a | 187 ± 5 b | 0.0001 *** |
| Parameters | Evaluation Metrics | SS | TIS | ||
|---|---|---|---|---|---|
| MLP | RF | MLP | RF | ||
| Leaf Length | R2 | 0.99 | 0.85 | 0.99 | 0.95 |
| RMSE | 0.02 | 0.10 | 0.02 | 0.06 | |
| CCC | 0.99 | 0.86 | 0.99 | 0.99 | |
| MAE | 0.02 | 0.08 | 0.01 | 0.05 | |
| Leaf Width | R2 | 0.98 | 0.80 | 0.99 | 0.96 |
| RMSE | 0.03 | 0.11 | 0.01 | 0.07 | |
| CCC | 0.99 | 0.96 | 0.99 | 0.99 | |
| MAE | 0.02 | 0.08 | 0.01 | 0.05 | |
| Leaf Area | R2 | 0.97 | 0.92 | 0.98 | 0.95 |
| RMSE | 0.04 | 0.07 | 0.02 | 0.07 | |
| CCC | 0.98 | 0.90 | 0.99 | 0.98 | |
| MAE | 0.02 | 0.05 | 0.01 | 0.05 | |
| Number of Leaves | R2 | 0.49 | 0.30 | 0.50 | 0.96 |
| RMSE | 0.44 | 0.37 | 0.71 | 0.51 | |
| CCC | 0.43 | 0.27 | 0.57 | 0.98 | |
| MAE | 0.39 | 0.32 | 0.68 | 0.36 | |
| Shoot Length | R2 | 0.42 | 0.36 | 0.85 | 0.30 |
| RMSE | 0.19 | 0.17 | 0.12 | 0.19 | |
| CCC | 0.35 | 0.26 | 0.93 | 0.29 | |
| MAE | 0.15 | 0.15 | 0.08 | 0.17 | |
| Parameters | Evaluation Metrics | SS | TIS | ||
|---|---|---|---|---|---|
| MLP | RF | MLP | RF | ||
| Stomatal Area | R2 | 0.78 | 0.65 | 0.89 | 0.93 |
| RMSE | 0.05 | 0.05 | 0.05 | 0.04 | |
| CCC | 0.91 | 0.76 | 0.90 | 0.98 | |
| MAE | 0.03 | 0.02 | 0.03 | 0.02 | |
| Stomatal Length | R2 | 0.82 | 0.78 | 0.96 | 0.96 |
| RMSE | 0.06 | 0.06 | 0.03 | 0.06 | |
| CCC | 0.84 | 0.93 | 0.92 | 0.91 | |
| MAE | 0.05 | 0.04 | 0.02 | 0.03 | |
| Stomatal Width | R2 | 0.61 | 0.62 | 0.97 | 0.97 |
| RMSE | 0.06 | 0.05 | 0.03 | 0.04 | |
| CCC | 0.86 | 0.70 | 0.95 | 0.97 | |
| MAE | 0.04 | 0.04 | 0.02 | 0.02 | |
| Pore Length | R2 | 0.82 | 0.74 | 0.97 | 0.96 |
| RMSE | 0.06 | 0.06 | 0.02 | 0.06 | |
| CCC | 0.83 | 0.85 | 0.98 | 0.93 | |
| MAE | 0.05 | 0.04 | 0.01 | 0.03 | |
| Pore Width | R2 | 0.71 | 0.65 | 0.96 | 0.95 |
| RMSE | 0.08 | 0.07 | 0.04 | 0.05 | |
| CCC | 0.89 | 0.86 | 0.93 | 0.93 | |
| MAE | 0.05 | 0.05 | 0.02 | 0.03 | |
| Pore Area (µm2) | R2 | 0.84 | 0.74 | 0.92 | 0.95 |
| RMSE | 0.04 | 0.05 | 0.04 | 0.05 | |
| CCC | 0.97 | 0.90 | 0.92 | 0.97 | |
| MAE | 0.02 | 0.02 | 0.02 | 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benelli, C.; Faraloni, C.; İzgü, T.; Şimşek, Ö.; Tarraf, W. Optimizing Micropropagation of Tanacetum balsamita L.: A Machine Learning Approach to Compare Semisolid Media and Temporary Immersion System. Horticulturae 2025, 11, 1173. https://doi.org/10.3390/horticulturae11101173
Benelli C, Faraloni C, İzgü T, Şimşek Ö, Tarraf W. Optimizing Micropropagation of Tanacetum balsamita L.: A Machine Learning Approach to Compare Semisolid Media and Temporary Immersion System. Horticulturae. 2025; 11(10):1173. https://doi.org/10.3390/horticulturae11101173
Chicago/Turabian StyleBenelli, Carla, Cecilia Faraloni, Tolga İzgü, Özhan Şimşek, and Waed Tarraf. 2025. "Optimizing Micropropagation of Tanacetum balsamita L.: A Machine Learning Approach to Compare Semisolid Media and Temporary Immersion System" Horticulturae 11, no. 10: 1173. https://doi.org/10.3390/horticulturae11101173
APA StyleBenelli, C., Faraloni, C., İzgü, T., Şimşek, Ö., & Tarraf, W. (2025). Optimizing Micropropagation of Tanacetum balsamita L.: A Machine Learning Approach to Compare Semisolid Media and Temporary Immersion System. Horticulturae, 11(10), 1173. https://doi.org/10.3390/horticulturae11101173









