Mechanisms of Morphological Development and Physiological Responses Regulated by Light Spectrum in Changchuan No. 3 Pepper Seedlings
Abstract
1. Introduction
2. Results
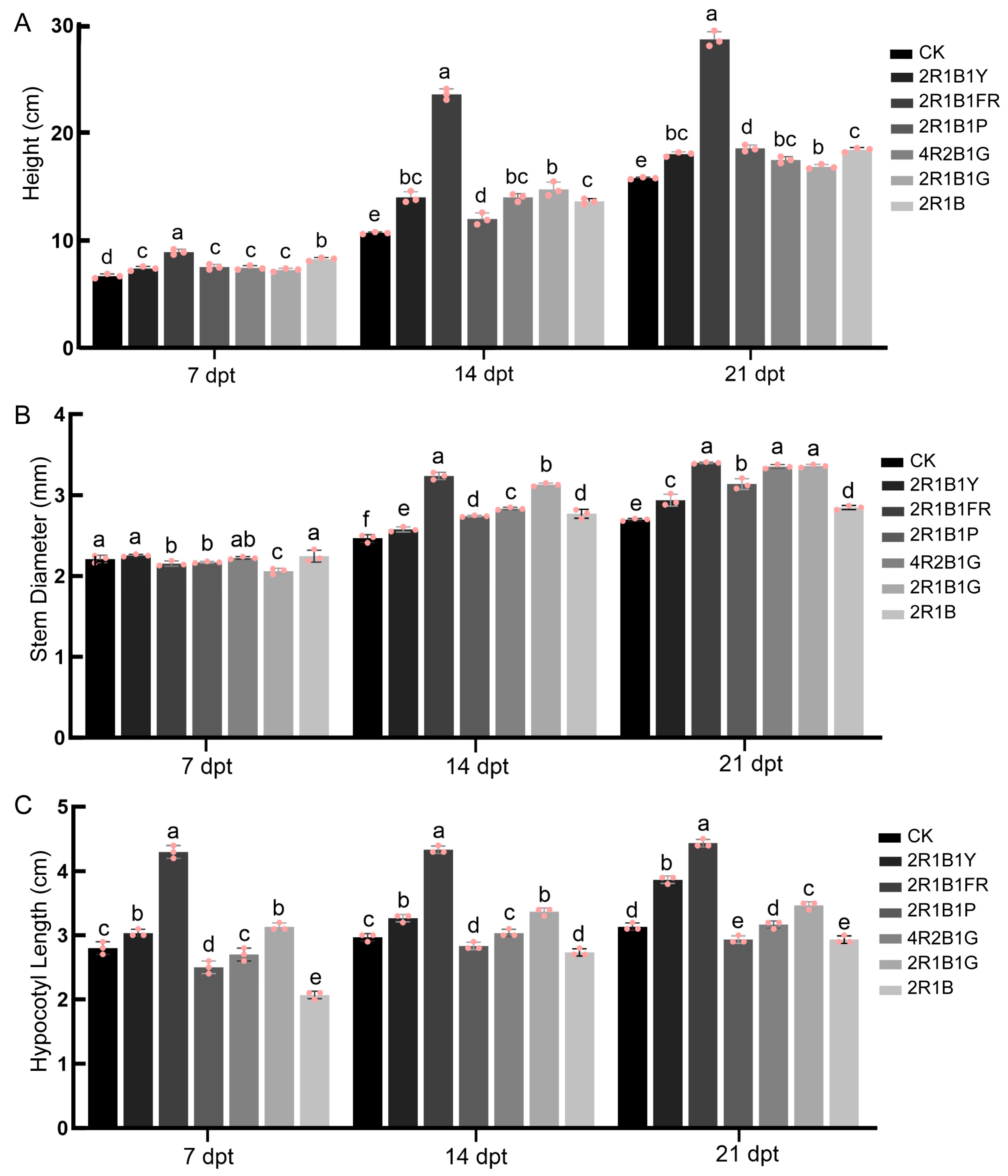
2.1. Morphological Parameters
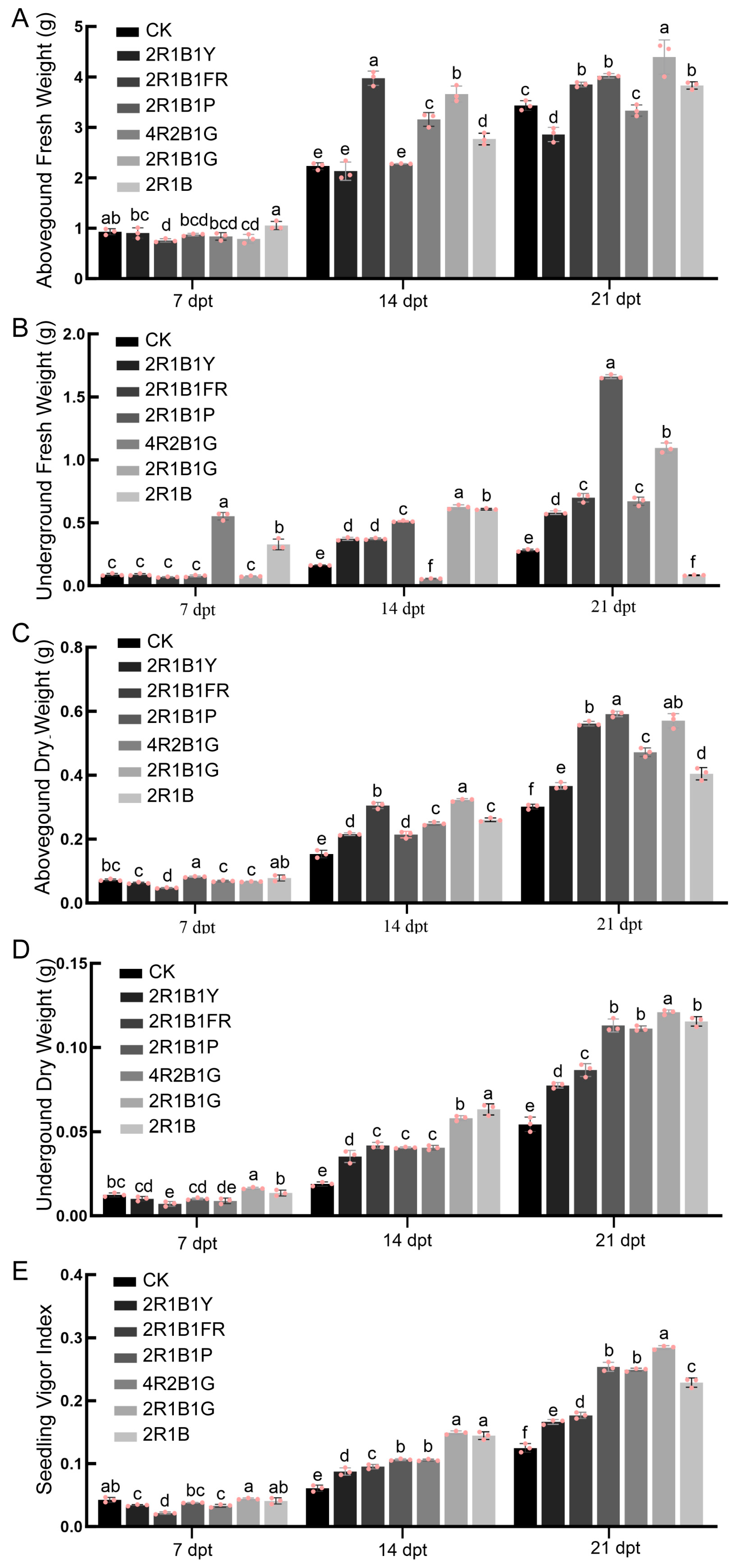
2.2. Biomass and Vigor Indicators
2.3. Metabolites and Stress Indicators
2.3.1. Soluble Protein Content
2.3.2. Soluble Sugar Content
2.3.3. Malondialdehyde (MDA) Content
2.4. Photosynthetic Pigment Indicators
3. Discussion
3.1. Spectral Regulation of Morphogenesis and Biomass Partitioning
3.2. Remodeling of the Photosynthetic Apparatus and Photoprotection
3.3. Modulation of Oxidative Stress and Metabolite Dynamics
3.4. Implications for Optimized Nursery Production
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Morphological Measurements
4.3. Biomass and Biochemical Measurements
4.4. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teixeira, R.T. Distinct Responses to Light in Plants. Plants 2020, 9, 894. [Google Scholar] [CrossRef]
- Fankhauser, C.; Staiger, D. Photoreceptors in Arabidopsis thaliana: Light perception, signal transduction and entrainment of the endogenous clock. Planta 2002, 216, 1–16. [Google Scholar] [CrossRef]
- Munyanont, M.; Lu, N.; Ruangsangaram, T.; Takagaki, M. Effect of Far-Red Light and Nutrient Solution Formulas on Calendula Production in a Plant Factory. Biology 2025, 14, 716. [Google Scholar] [CrossRef]
- Kitazaki, K.; Fukushima, A.; Nakabayashi, R.; Okazaki, Y.; Kobayashi, M.; Mori, T.; Nishizawa, T.; Reyes-Chin-Wo, S.; Michelmore, R.W.; Saito, K.; et al. Metabolic Reprogramming in Leaf Lettuce Grown Under Different Light Quality and Intensity Conditions Using Narrow-Band LEDs. Sci. Rep. 2018, 8, 7914. [Google Scholar] [CrossRef]
- Su, P.; Ding, S.; Wang, D.; Kan, W.; Yuan, M.; Chen, X.; Tang, C.; Hou, J.; Wu, L. Plant morphology, secondary metabolites and chlorophyll fluorescence of Artemisia argyi under different LED environments. Photosynth. Res. 2024, 159, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of Light-Emitting Diodes for Improving the Nutritional Quality and Bioactive Compound Levels of Some Crops and Medicinal Plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Schiff, J.A. Evolution of the control of pigment and plastid development in photosynthetic organisms. Bio Syst. 1981, 14, 123–147. [Google Scholar] [CrossRef]
- Rehman, M.; Ullah, S.; Bao, Y.; Wang, B.; Peng, D.; Liu, L. Light-emitting diodes: Whether an efficient source of light for indoor plants? Environ. Sci. Pollut. Res. Int. 2017, 24, 24743–24752. [Google Scholar] [CrossRef]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.; Guo, Y.; Liu, J.; Yu, W.; Guo, X. Comparative role of oxidative balance, alkaloid production, and metabolites in exposure to red-to-blue light in Catharanthus roseus. Plant Physiol. Biochem. 2025, 227, 110170. [Google Scholar] [CrossRef]
- Jishi, T.; Matsuda, R.; Fujiwara, K. Manipulation of Intraday Durations of Blue- and Red-Light Irradiation to Improve Cos Lettuce Growth. Front. Plant Sci. 2021, 12, 778205. [Google Scholar] [CrossRef]
- Samuoliene, G.; Virsile, A.; Miliauskiene, J.; Haimi, P.J.; Lauzike, K.; Brazaityte, A.; Duchovskis, P. The Physiological Response of Lettuce to Red and Blue Light Dynamics Over Different Photoperiods. Front. Plant Sci. 2020, 11, 610174. [Google Scholar] [CrossRef]
- Siuda, R.; Balcerowska, G.; Sadowski, C. Comparison of the usability of different spectral ranges within the near ultraviolet, visible and near infrared ranges (UV-VIS-NIR) region for the determination of the content of scab-damaged component in blended samples of ground wheat. Food Addit. Contam. 2006, 23, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Kristic, M.; Lisjak, M.; Tkalec Kojic, M.; Iljkic, D.; Jovic, J.; Kristek, S.; Markulj Kulundzic, A.; Antunovic, M. Antioxidative Response and Phenolic Content of Young Industrial Hemp Leaves at Different Light and Mycorrhiza. Plants 2024, 13, 840. [Google Scholar] [CrossRef]
- Wang, T.; Wei, H.; Zhou, C.; Gu, Y.; Li, R.; Chen, H.; Ma, W. Estimating cadmium concentration in the edible part of Capsicum annuum using hyperspectral models. Environ. Monit. Assess. 2017, 189, 548. [Google Scholar] [CrossRef]
- Gai, S.; Su, L.; Tang, C.; Xia, M.; Zhou, Z. The antagonistic effects of red and blue light radiation on leaf and stem development of pepper (Capsicum annuum L.) seedlings. Plant Sci. 2025, 351, 112338. [Google Scholar] [CrossRef]
- Gai, S.; Chen, Y.; Long, Y.; Luo, Y.; Yi, X.; Zhao, Z.; Li, X.; Zhou, Z. Effects of LED polarized and vortex light on growth and photosynthetic characteristics of pepper (Capsicum annuum L.). J. Plant Physiol. 2024, 303, 154360. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Marcelis, L.F.M.; Offringa, R.; Kohlen, W.; Heuvelink, E. Far-red light-enhanced apical dominance stimulates flower and fruit abortion in sweet pepper. Plant Physiol. 2024, 195, 924–939. [Google Scholar] [CrossRef]
- Kim, D.; Son, J.E. Adding Far-Red to Red, Blue Supplemental Light-Emitting Diode Interlighting Improved Sweet Pepper Yield but Attenuated Carotenoid Content. Front. Plant Sci. 2022, 13, 938199. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, G.; Shi, Q.; Yang, F.; Wei, M. Response of photomorphogenesis and photosynthetic properties of sweet pepper seedlings exposed to mixed red and blue light. Front. Plant Sci. 2022, 13, 984051. [Google Scholar] [CrossRef]
- Shin, J.; Runkle, E.S. Plant Morphology and a Phytochrome B Model Reveal That the Effects of Far-Red Light on Shade-Avoidance-Like Responses Persist Under High Light Intensity. Plant Cell Environ. 2025, 48, 5802–5818. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Oka, H.; Yoshimura, F.; Ishida, K.; Yamashino, T. Insight into the mechanism of end-of-day far-red light (EODFR)-induced shade avoidance responses in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2015, 79, 1987–1994. [Google Scholar] [CrossRef]
- Dubois, P.G.; Brutnell, T.P. Topology of a maize field: Distinguishing the influence of end-of-day far-red light and shade avoidance syndrome on plant height. Plant Signal. Behav. 2011, 6, 467–470. [Google Scholar] [CrossRef]
- Lee, H.; Depuydt, S.; Shin, K.; De Saeger, J.; Han, T.; Park, J. Interactive Effects of Blue Light and Water Turbulence on the Growth of the Green Macroalga Ulva australis (Chlorophyta). Plants 2024, 13, 266. [Google Scholar] [CrossRef]
- Alrajhi, A.A.; Alsahli, A.S.; Alhelal, I.M.; Rihan, H.Z.; Fuller, M.P.; Alsadon, A.A.; Ibrahim, A.A. The Effect of LED Light Spectra on the Growth, Yield and Nutritional Value of Red and Green Lettuce (Lactuca sativa). Plants 2023, 12, 463. [Google Scholar] [CrossRef]
- Trojak, M.; Skowron, E.; Sobala, T.; Kocurek, M.; Palyga, J. Effects of partial replacement of red by green light in the growth spectrum on photomorphogenesis and photosynthesis in tomato plants. Photosynth. Res. 2022, 151, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, C.; Krauss, R.W. The Effects of Light Intensity on the Growth Rates of Green Algae. Plant Physiol. 1958, 33, 109–113. [Google Scholar] [CrossRef]
- Sun, F.; Cheng, H.; Song, Z.; Yan, H.; Liu, H.; Xiao, X.; Zhang, Z.; Luo, M.; Wu, F.; Lu, J.; et al. Phytochrome-interacting factors play shared and distinct roles in regulating shade avoidance responses in Populus trees. Plant Cell Environ. 2024, 47, 2058–2073. [Google Scholar] [CrossRef]
- O’Rourke, J.A.; Vincent, S.A.; Williams, I.E.I.; Gascoyne, E.L.; Devlin, P.F. Phytochrome-mediated shade avoidance responses impact the structure and composition of the bacterial phyllosphere microbiome of Arabidopsis. Environ. Microbiome 2025, 20, 20. [Google Scholar] [CrossRef]
- Marco, F.; Batailler, B.; Thorpe, M.R.; Razan, F.; Le Hir, R.; Vilaine, F.; Bouchereau, A.; Martin-Magniette, M.L.; Eveillard, S.; Dinant, S. Involvement of SUT1 and SUT2 Sugar Transporters in the Impairment of Sugar Transport and Changes in Phloem Exudate Contents in Phytoplasma-Infected Plants. Int. J. Mol. Sci. 2021, 22, 745. [Google Scholar] [CrossRef] [PubMed]
- Unda, F.; Kim, H.; Hefer, C.; Ralph, J.; Mansfield, S.D. Altering carbon allocation in hybrid poplar (Populus alba x grandidentata) impacts cell wall growth and development. Plant Biotechnol. J. 2017, 15, 865–878. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Ren, M.; Zhang, F.; Zhang, Y.; Wang, Y.; Li, W.; Xie, Z.; Qi, K.; Zhang, S.; et al. Cryptochrome-mediated blue light regulates cell lignification via PbbHLH195 activation of the PbNSC in pear fruits. Mol. Hortic. 2025, 5, 27. [Google Scholar] [CrossRef]
- Chibani, K.; Gherli, H.; Fan, M. The role of blue light in plant stress responses: Modulation through photoreceptors and antioxidant mechanisms. Front. Plant Sci. 2025, 16, 1554281. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zeng, Z.; Yuan, M.; Li, H.; Guo, S.; Yang, Y.; Jiang, S.; Hawara, E.; Li, J.; Zhang, P.; et al. The blue-light receptor CRY1 serves as a switch to balance photosynthesis and plant defense. Cell Host Microbe 2025, 33, 137–150.e136. [Google Scholar] [CrossRef]
- Levin, G.; Yasmin, M.; Liveanu, V.; Burstein, C.; Hanna, R.; Kleifeld, O.; Simanowitz, M.C.; Meir, A.; Tadmor, Y.; Hirschberg, J.; et al. A desert Chlorella sp. that thrives at extreme high-light intensities using a unique photoinhibition protection mechanism. Plant J. 2023, 115, 510–528. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef]
- Bag, P.; Chukhutsina, V.; Zhang, Z.; Paul, S.; Ivanov, A.G.; Shutova, T.; Croce, R.; Holzwarth, A.R.; Jansson, S. Direct energy transfer from photosystem II to photosystem I confers winter sustainability in Scots Pine. Nat. Commun. 2020, 11, 6388. [Google Scholar] [CrossRef]
- Biswas, D.K.; Ma, B.L.; Xu, H.; Li, Y.; Jiang, G. Lutein-mediated photoprotection of photosynthetic machinery in Arabidopsis thaliana exposed to chronic low ultraviolet-B radiation. J. Plant Physiol. 2020, 248, 153160. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Liu, X.; Xue, J.; Ren, X.; Zhai, Y.; Zhang, X. The red/blue light ratios from light-emitting diodes affect growth and flower quality of Hippeastrum hybridum ‘Red Lion’. Front. Plant Sci. 2022, 13, 1048770. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Xu, Z.G.; Dong, R.Q.; Chang, S.X.; Wang, L.Z.; Khalil-Ur-Rehman, M.; Tao, J.M. An RNA-Seq Analysis of Grape Plantlets Grown in vitro Reveals Different Responses to Blue, Green, Red LED Light, and White Fluorescent Light. Front. Plant Sci. 2017, 8, 78. [Google Scholar] [CrossRef]
- Tassoni, A.; Durante, L.; Ferri, M. Combined elicitation of methyl-jasmonate and red light on stilbene and anthocyanin biosynthesis. J. Plant Physiol. 2012, 169, 775–781. [Google Scholar] [CrossRef]
- Chen, R.; Chen, Y.; Lin, K.; Ding, Y.; Liu, W.; Wang, S. Growth, Quality, and Nitrogen Metabolism of Medicago sativa Under Continuous Light from Red-Blue-Green LEDs Responded Better to High Nitrogen Concentrations than Under Red-Blue LEDs. Int. J. Mol. Sci. 2024, 25, 13116. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Dame, G.; Gloeckner, G.; Beck, C.F. Knock-out of a putative transporter results in altered blue-light signalling in Chlamydomonas. Plant J. 2002, 31, 577–587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoagland, D.R. Optimum Nutrient Solutions for Plants. Science 1920, 52, 562–564. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigment of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Kaur, A.; Zhang, L.; Maness, N.O.; Ferguson, L.; Graham, C.J.; Sun, Y.; Panta, S.; Pokhrel, N.; Yang, M.; Moss, J.Q. Dormant carbohydrate reserves enhance pecan tree spring freeze tolerance: Controlled environment observations. Front. Plant Sci. 2024, 15, 1393305. [Google Scholar] [CrossRef]
- Wang, F.H.; Wang, B.B.; Gao, J.; Yang, X.J.; Jia, Y.B.; Tian, S.Y.; Li, X.; Zhang, N.; Zhang, X.C.; Wei, Y.M.; et al. Determination of cyclic adenosine phosphate and protein content in dormant chlamydospore and nondormant chlamydospore of Arthrobotrys flagrans. J. Basic Microbiol. 2024, 64, e2400008. [Google Scholar] [CrossRef]
- Altaf, M.A.; Hao, Y.; He, C.; Mumtaz, M.A.; Shu, H.; Fu, H.; Wang, Z. Physiological and Biochemical Responses of Pepper (Capsicum annuum L.) Seedlings to Nickel Toxicity. Front. Plant Sci. 2022, 13, 950392. [Google Scholar] [CrossRef] [PubMed]




| Treatment | LED Composite Light Quality Ratio | Light Intensity | Photoperiod |
|---|---|---|---|
| CK | LED-white light | 150 μmol m−2s−1 | 12 h light/12 h dark |
| 2R1B1Y | Red/blue/yellow = 2:1:1 | 150 μmol m−2s−1 | 12 h light/12 h dark |
| 2R1B1FR | Red/blue/far-red = 2:1:1 | 150 μmol m−2s−1 | 12 h light/12 h dark |
| 2R1B1P | Red/blue/purple = 2:1:1 | 150 μmol m−2s−1 | 12 h light/12 h dark |
| 4R2B1G | Red/blue/green = 4:2:1 | 150 μmol m−2s−1 | 12 h light/12 h dark |
| 2R1B1G | Red/blue/green = 2:1:1 | 150 μmol m−2s−1 | 12 h light/12 h dark |
| 2R1B | Red/blue = 2:1 | 150 μmol m−2s−1 | 12 h light/12 h dark |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Huang, Z.; Zhao, S.; Chen, Z.; Xu, B.; Huang, Q.; Wang, Y.; Wu, Y.; Guo, Y.; Chen, H.; et al. Mechanisms of Morphological Development and Physiological Responses Regulated by Light Spectrum in Changchuan No. 3 Pepper Seedlings. Horticulturae 2025, 11, 1161. https://doi.org/10.3390/horticulturae11101161
Zhu W, Huang Z, Zhao S, Chen Z, Xu B, Huang Q, Wang Y, Wu Y, Guo Y, Chen H, et al. Mechanisms of Morphological Development and Physiological Responses Regulated by Light Spectrum in Changchuan No. 3 Pepper Seedlings. Horticulturae. 2025; 11(10):1161. https://doi.org/10.3390/horticulturae11101161
Chicago/Turabian StyleZhu, Wanli, Zhi Huang, Shiting Zhao, Zhi Chen, Bo Xu, Qiang Huang, Yuna Wang, Yu Wu, Yuanzhen Guo, Hailing Chen, and et al. 2025. "Mechanisms of Morphological Development and Physiological Responses Regulated by Light Spectrum in Changchuan No. 3 Pepper Seedlings" Horticulturae 11, no. 10: 1161. https://doi.org/10.3390/horticulturae11101161
APA StyleZhu, W., Huang, Z., Zhao, S., Chen, Z., Xu, B., Huang, Q., Wang, Y., Wu, Y., Guo, Y., Chen, H., & Shi, L. (2025). Mechanisms of Morphological Development and Physiological Responses Regulated by Light Spectrum in Changchuan No. 3 Pepper Seedlings. Horticulturae, 11(10), 1161. https://doi.org/10.3390/horticulturae11101161






