Study on the Establishment of Efficient Leaf Regeneration System in ‘Yuluxiang’ Pear
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Condition
2.2. Drug Information
2.3. The Screened of Plant Growth Regulators on the Leaf Regeneration of ‘Yuluxiang’ In Vitro
2.4. The Screened of Organic Matters on the Leaf Regeneration of ‘Yuluxiang’ In Vitro
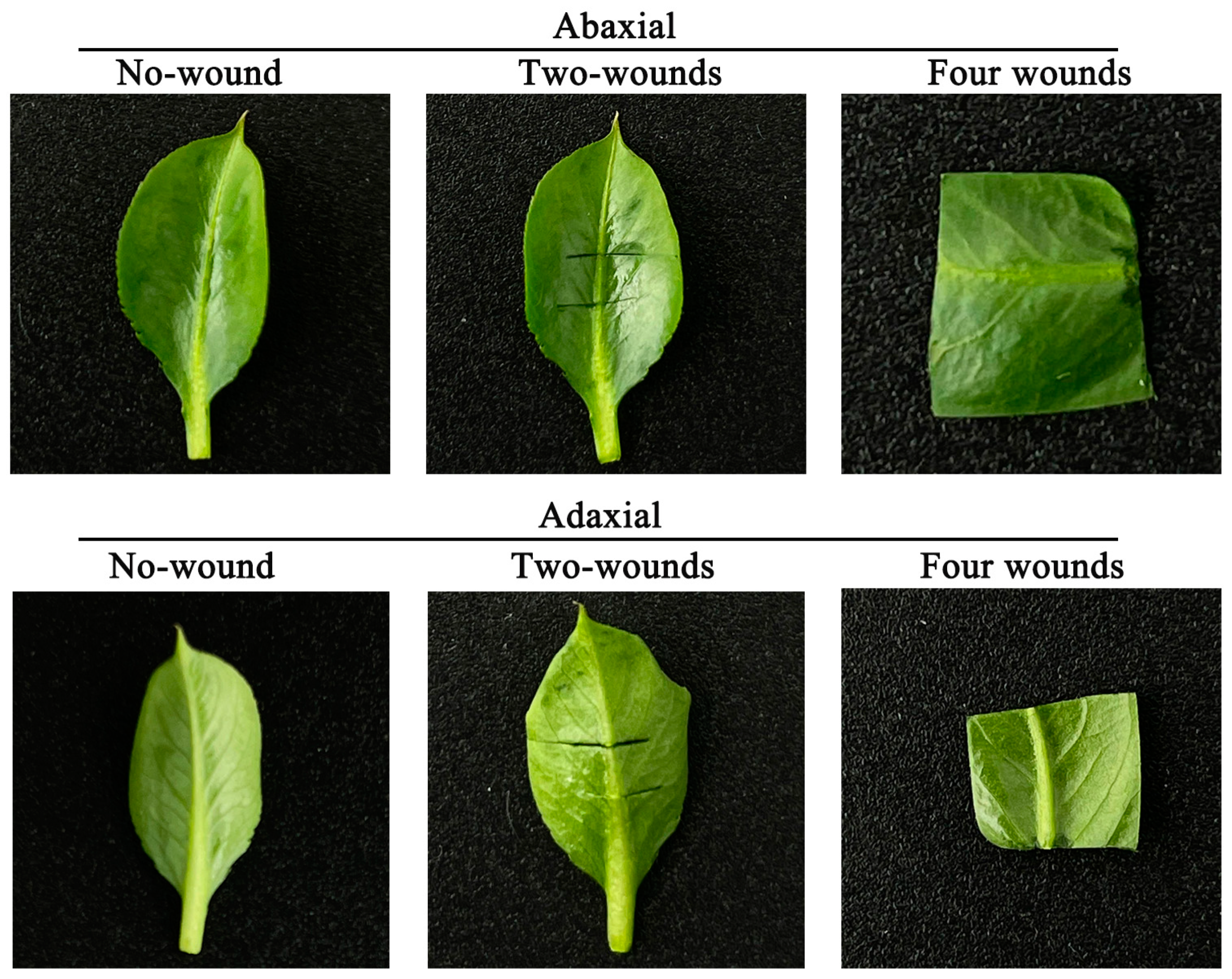
2.5. The Sccreened of Leaf Wounding and Positioning Method, Dark Culture Time, Plantlet Ages and Lines on the ‘Yuluxiang’ Leaf Regeneration In Vitro
2.6. The Screened of Cultured Containers on the Leaf Regeneration of ‘Yuluxiang’ In Vitro
2.7. The Screened ‘Yuluxiang’ Leaf Regeneration System Applied to Other Pear Genotypes
2.8. Observation and Statistics of Leaf Regeneration Mode in the Screened ’Yuluxiang’ Leaf Regeneration System
2.9. Statistical Analysis
3. Results
3.1. Effects of Plant Growth Regulators on the ‘Yuluxiang’ Leaf Regeneration In Vitro
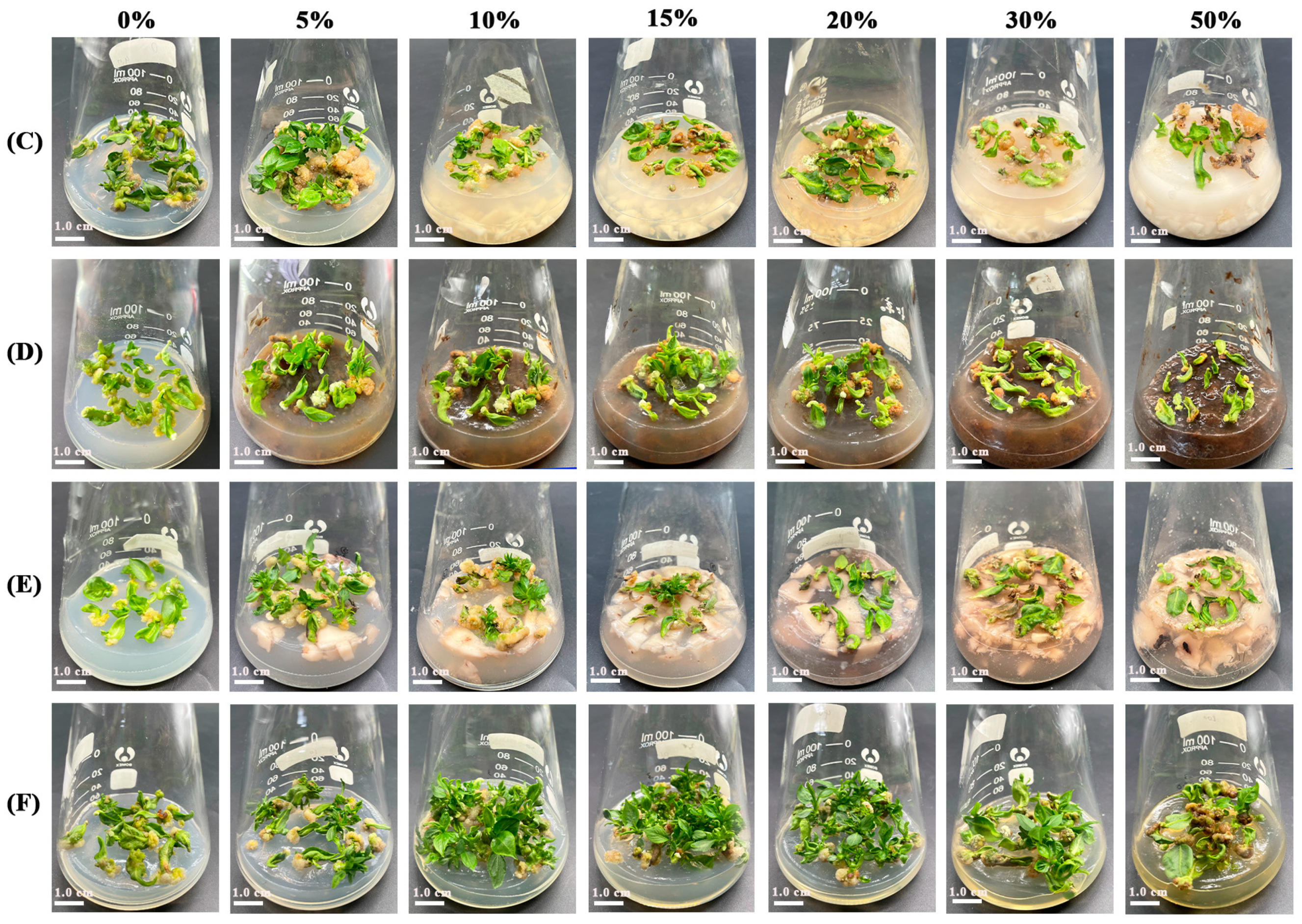
3.2. Effects of Organic Matters on the ‘Yuluxiang’ Leaf Regeneration In Vitro
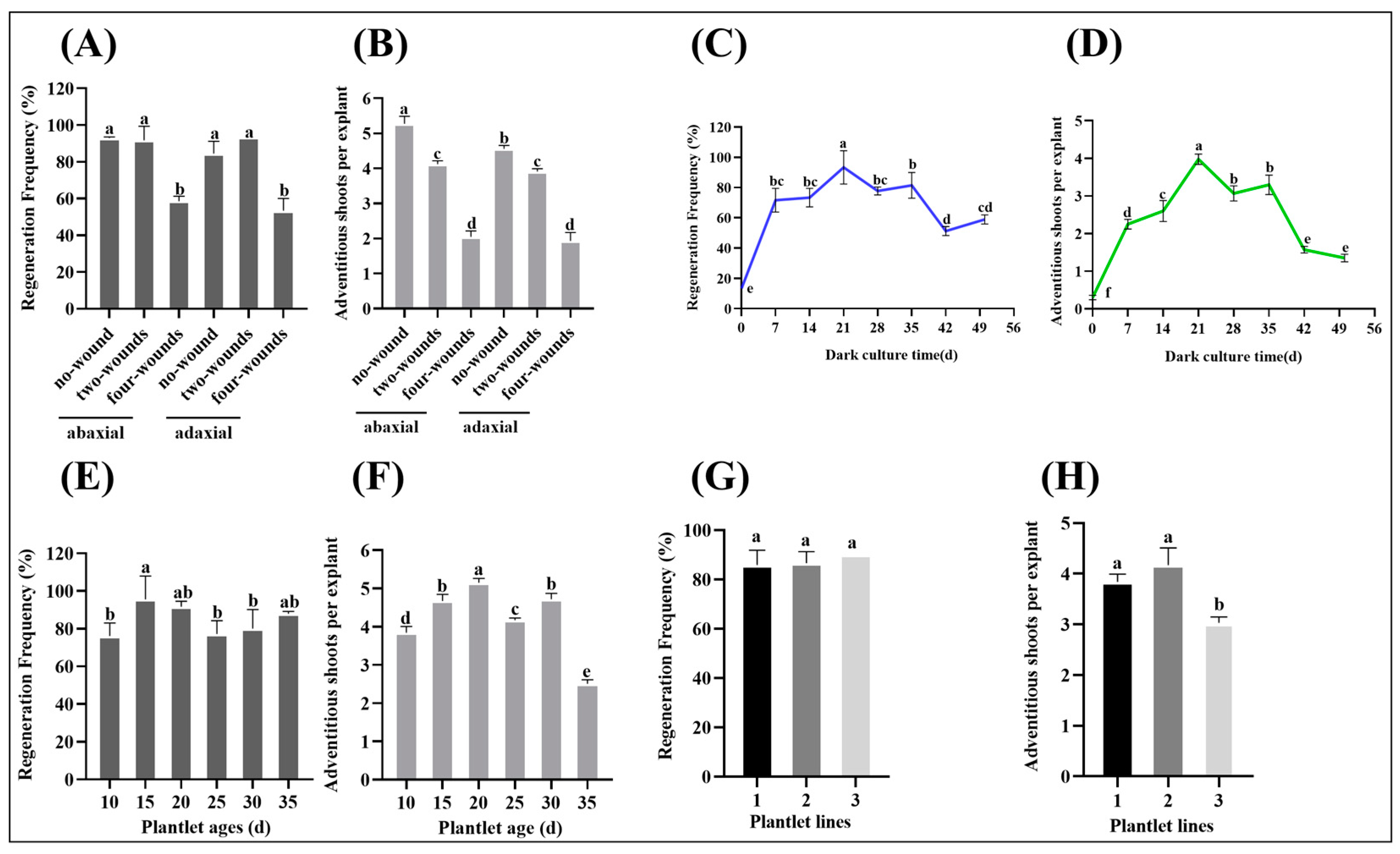
3.3. Effects of Leaf Wounding and Positioning Method, Dark Culture Time, Plantlet Ages and Lines on the ‘Yuluxiang’ Leaf Regeneration In Vitro
3.4. Effects of Culture Containers on the ‘Yuluxiang’ Leaf Regeneration In Vitro
3.5. The Adaptability of Screened ‘Yuluxiang’ Leaf Regeneration System to Other Pear Genotypes
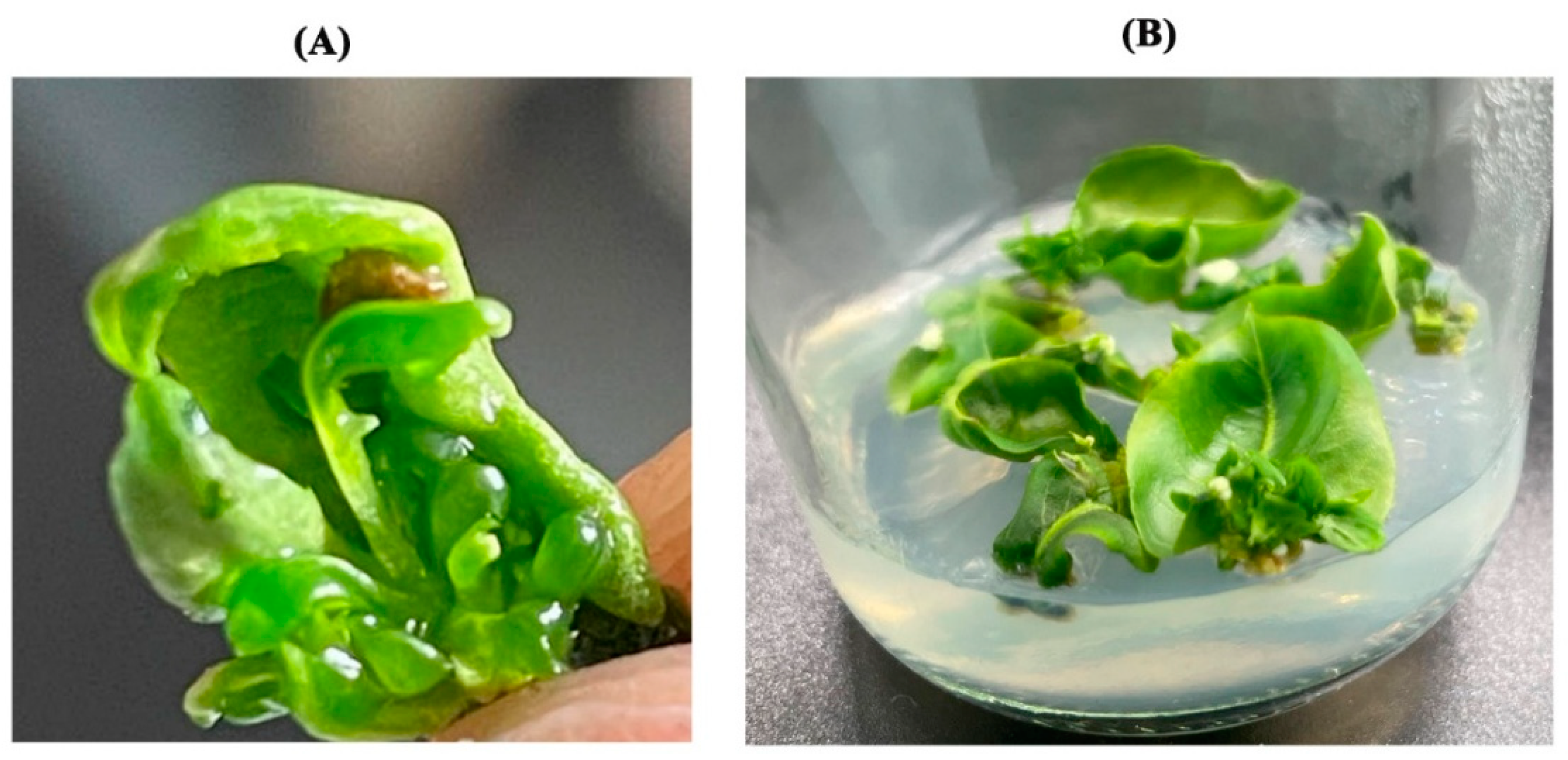
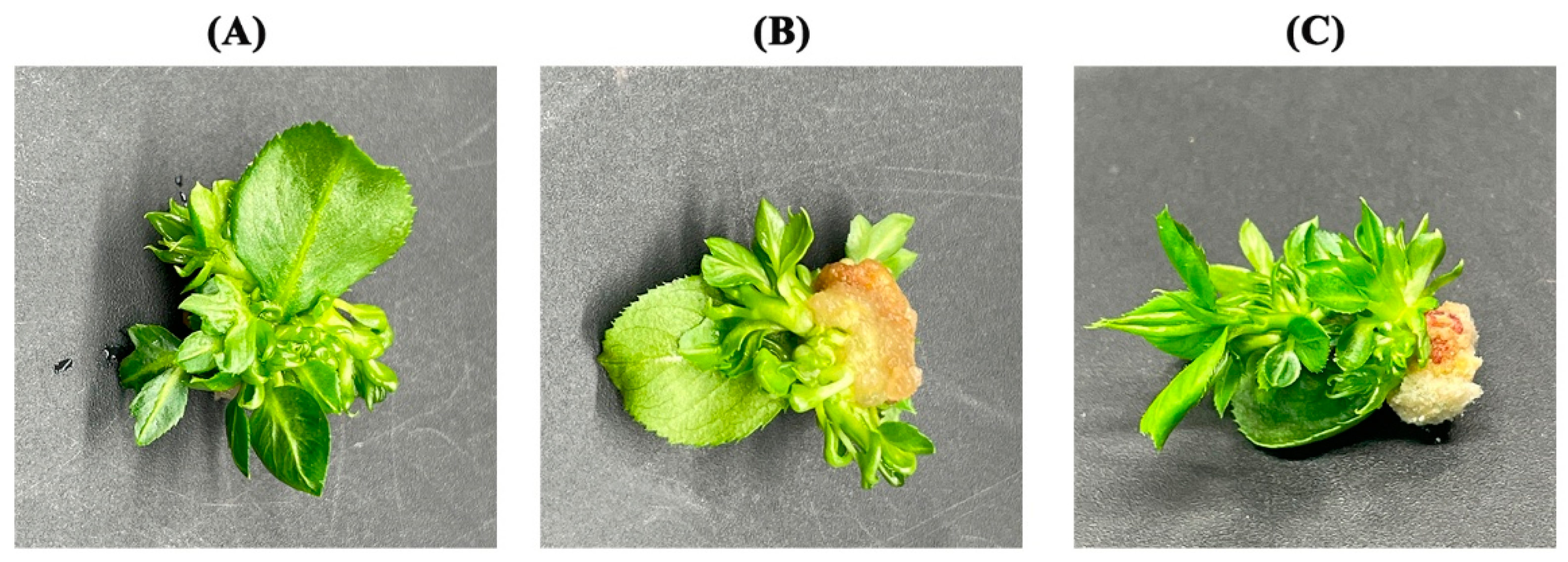
3.6. Leaf Regeneration Mode in the Screened ’Yuluxiang’ Leaf Regeneration System
4. Discussion
4.1. 6-BA and NAA Were the Suitable Plant Growth Regulators for the ‘Yuluxiang’ Leaf Regeneration In Vitro
4.2. Natural Organic Matters Significantly Promoted the ‘Yuluxiang’ Pear Leaf Regeneration
4.3. No-Wound Was the Optimal Wounding Method for the ‘Yuluxiang’ Leaf Regeneration
4.4. The Plantlet Lines Had a Significant Effect on the ‘Yuluxiang’ Leaf Regeneration
4.5. Culture Container Had a Significant Effect on the ‘Yuluxiang’ Leaf Regeneration
4.6. Applicability of the Leaf Regeneration System of ‘Yuluxiang’ to Other Genotypes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Wang, Z.; Shi, Z.; Zhang, S.; Ming, R.; Zhu, S.; Khan, M.A.; Tao, S.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Xu, J.; Korban, S.S.; Fei, Z.; Tao, S.; Ming, R.; Tai, S.; Khan, A.M.; Postman, J.D. Diversification and independent domestication of Asian and European pears. Genome Biol. 2018, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Caboni, E.D.; D’Angeli, S.; Chiappetta, A.; Innocenti, A.M.; Damiano, C. Adventitious shoot regeneration from vegetative shoot apices in pear and putative role of cytokinin accumulation in the morphogenetic process. Plant Cell Tissue Organ Cult. 2002, 70, 199–206. [Google Scholar] [CrossRef]
- Hackett, W.P.; Murray, J.R. Maturation and rejuvenation in woody plants. Hort Rev. 1985, 195–204. [Google Scholar] [CrossRef]
- Yang, X.H.; Liang, Z.; Lu, C.M. Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol. 2005, 138, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tomes, S.; Gleave, A.P.; Hall, W.; Luo, Z.; Xu, J.; Yao, J. Significant improvement of apple (Malus domestica Borkh.) transgenic plant production by pre-transformation with a baby boom transcription factor. Hortic. Res. 2022, 9, 3174–3187. [Google Scholar] [CrossRef]
- Xie, C.H.; Liu, J. Plant Cell Engineering, 2nd ed.; Higher Education Press: Beijing, China, 2011; pp. 258–268. [Google Scholar]
- Bell, R.L.; Scorza, R.; Lomberk, D. Adventitious shoot regeneration of pear (Pyrus spp.) genotypes. Plant Cell Tissue Organ Cult. 2012, 108, 229–236. [Google Scholar] [CrossRef]
- Chevreau, E.; Mourgues, F.; Neveu, M.; Chevalier, M. Effect of gelling agents and antibiotics on adventitious bud regeneration from in vitro leaves of pear. Vitr. Cell. Dev. Biol. Plant. 1997, 33, 173–179. [Google Scholar] [CrossRef]
- Leblay, C.; Chevreau, E.; Raboin, L.M. Adventitious shoot regeneration from in vitro leaves of several pear cultivars (Pyrus communis L.). Plant Cell Tissue Organ Cult. 1991, 25, 99–105. [Google Scholar] [CrossRef]
- Maren, N.A.; Duan, H.; Da, K.; Yencho, G.C.; Ranney, T.G.; Liu, W. Genotype-independent plant transformation. Hortic. Res. 2023, 9, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.W. Regeneration of pear plants from shoot meristem-tips. Plant Sci. Lett. 1979, 16, 337–342. [Google Scholar] [CrossRef]
- Zhu, W.B. Transform gene PuNHA into Malus Robusta Rehd. and Pyrus Betulaefolia Bge; Southwest University: Chongqing, China, 2008. [Google Scholar]
- Zhong, Y.; Feng, J.R.; Fan, X.M.; Ren, H.X.; Zhang, X.K.; Xu, Z.Y. Establishment of regeneration system of leaves in-vitro of Korla Fragrant pear. Xinjiang Agric. Sci. 2018, 55, 829–836. [Google Scholar]
- Ricci, A.; Mezzetti, B.; Navacchi, O.; Sabbadini, S. In vitro shoot regeneration from leaves of Pyrus communis L. rootstock and cultivars. Plant Biotechnol. Rep. 2023, 17, 341–352. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.; Wang, C.; Wang, X.; Li, J.; Wang, R. Construction of high efficiency regeneration and transformation systems of Pyrus ussuriensis maxim. Plant Cell Tissue Organ Cult. 2017, 131, 139–150. [Google Scholar] [CrossRef]
- Guo, H.; Li, X.; Zhang, J. A new variety of high-quality medium ripe red pear ‘Yuluxiang’ (tentative name). Shanxi Fruits 2001, 1, 3–4. [Google Scholar]
- Xie, P.; Yu, L.; Wang, H.; Lin, L.; Niu, Z. Comprehensive analysis on the fruit quality of ‘Yuluxiang’ pear in different production areas. J. Fruit Sci. 2023, 40, 2371–2380. [Google Scholar]
- Yu, W.; Wang, W.; Zhang, X.; Yan, W.; Sun, X.; Jia, X. Effects of exogenous melatonin on fruit quality and physiological characteristics during room temperature storage in ‘Yuluxiang’ pear. J. Fruit Sci. 2023, 40, 1583–1591. [Google Scholar]
- Jia, X.; Wang, W.; Jiang, Y.; Wang, Z.; Du, Y.; Tong, W. Effects of harvest maturity on fruit quality and storage life of ‘Yuluxiang’ pears. J. Fruit Sci. 2016, 33, 594–603. [Google Scholar]
- Chen, X. Establishment of Regeneration System of Leaves and Study on Genetic Transformation of ‘Yuluxiang’; Hebei Agricultural University: Baoding, China, 2021. [Google Scholar]
- Liu, C.Q.; Tang, H.R. An overview of progress on shoot regeneration from pear leaves and genetic transformation. J. Fruit Sci. 2003, 20, 374–378. [Google Scholar]
- Sun, H.Y.; Tao, J.H.; Xin, L.; Sun, Q.R. Effects of TDZ and BA on adventitious bud regeneration from leaf explants of pear cultivars ‘Jinhua’ and ‘Yali’. Shandong Agric. Sci. 2016, 48, 26–28,39. [Google Scholar]
- Caboni, E.; Tonelli, M.; Lauri, P.; D’Angeli, S.; Damino, C. In vitro shoot regeneration from leaves of wild pear. Plant Cell Tissue Organ Cult. 1999, 59, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Poudyal, B.K.; Zhang, Y.X.; Jiao, Z.; Qi, J. Adventitious shoot regeneration from the leaves of in vitro grown ‘Zhongli 1’ pear (Pyrus spp.). Front. Agric. China 2009, 3, 60–66. [Google Scholar] [CrossRef]
- Fu, Z.F.; Meng, H.G.; Zhang, C.H.; Wang, Y.J. Primary establishment of plant regeneration system of pear scion cultivar ‘Dangshansu’. North. Hortic. 2011, 14, 98–101. [Google Scholar]
- Available online: https://m.maigoo.com/goomai/300322.html (accessed on 5 January 2025).
- Overbeek, J.V.; Conklin, M.E.; Blakeslee, A.F. Factors in coconut milk essential for growth and development of very young datura embryos. Science 1941, 94, 350–351. [Google Scholar] [CrossRef]
- Guo, C.L.; Chen, L.G.; He, X.H.; Syed, A.T. Plant regeneration from immature embryos of Ginkgo biloba. Acta Hortic. Sin. 2005, 32, 105–107. [Google Scholar]
- Chen, P.H.; Chen, S.B.; Chen, L.F.; Xu, L.P.; Wang, H.B.; Chen, Y.Q.; Chen, R.K. Effect of coconut water on sugarcane propagation in vitro. Chin. J. Trop. Crops. 2009, 30, 1818–1823. [Google Scholar]
- Li, H.M. Advances in studies of tissue culture and rapid multiplication of Orchids. Agric. Res. Appl. 2014, 27, 53–56. [Google Scholar]
- Banno, K.; Tamura, F.; Tanabe, K. Plant regeneration from leaf segments cultures in vitro in Japanese pear. In Proceedings of the X XIII ISHS Congress, Florence, Italy, 27 August–1 September 1990. [Google Scholar]
- Khalifah, R.A. Indolyl-3-acetic acid from the developing banana. Nature 1966, 212, 1471–1472. [Google Scholar] [CrossRef]
- Khalifah, R.A. Gibberellin-like substances from the developing banana fruit. Plant Physiol. 1966, 41, 771–773. [Google Scholar] [CrossRef]
- Ubalua, A.O.; Ikpeama, A.I.; Okeagu, O.D. Effect of different concentrations of orange juice for in vitro regeneration and multiplication of Cocoyam (Taro). Am. J. Plant Sci. 2015, 6, 2569–2575. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.F. Plant Tissue Culture, 2nd ed.; China Agriculture Press: Beijing, China, 2013; pp. 19,26,60–61. [Google Scholar]
- Yan, H.X.; Huang, C.Y.; Zhang, Z.B.; Cui, X.Q.; Deng, J.L.; Guan, S.K.; Bu, Z.Y. Tissue culture and plant regeneration of leaves of Primulina guigangensis. Chin. J. Trop. Crops 2019, 40, 98–106. [Google Scholar]
- Liu, Y.S.; Wang, H.Y.; Zhao, Y.J.; Jin, Y.B.; Li, C.; Ma, F.W. Establishment of an efficient regeneration and genetic transformation system for Malus prunifolia Borkh. ‘Fupingqiuzi’. J. Integr. Agric. 2022, 21, 2615–2627. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, N.; Luo, C.; Zhang, Q.; Sun, P.; Fu, J.; Li, S.; Li, Z. Shoot organogenesis and regeneration from leaf seedlings of Diospyros oleifera Cheng. Plants 2023, 12, 3507. [Google Scholar] [CrossRef]
- Javadi, S.; Kermani, M.J.; Irian, S.; Majd, A. Indirect regeneration from in vitro grown leaves of three pear cultivars and determination of ploidy level in regenerated shoots by flow cytometry. Sci. Hortic. 2013, 164, 455–460. [Google Scholar] [CrossRef]
- Yang, G.Y. Studies on tissue culture propagation and leaf regeneration of different pear rootstock seedlings (Pyrus betulifolia Bunge.). Chin. Acad. Agric. Sci. 2022. [Google Scholar] [CrossRef]
- Xu, L.F.; Ma, F.W.; Wang, Z.Z.; Ren, X.L.; Cao, X.Y. Leaf culture and plantlet regeneration of pear (Pyrus). Acta Hortic. Sin. 2002, 29, 367–368. [Google Scholar]




| Treatment | The Concentration of Plant Growth Regulators | Regeneration Frequency (%) | Adventitious Shoots per Explant | |||||
|---|---|---|---|---|---|---|---|---|
| 6-BA (mg/L) | TDZ (mg/L) | IBA (mg/L) | NAA (mg/L) | |||||
| 1 | 1.5 | - | 0.2 | - | 43.3 ± 3.3 | cde | 1.0 ± 0.1 | cd |
| 2 | 2.0 | - | 0.2 | - | 56.7 ± 3.3 | ab | 1.4 ± 0.2 | b |
| 3 | 3.0 | - | 0.2 | - | 51.7 ± 7.6 | bc | 1.5 ± 0.1 | b |
| 4 | 5.0 | - | 0.2 | - | 36.7 ± 2.9 | ef | 0.7 ± 0.1 | ef |
| 5 | - | 0.2 | 0.2 | - | 27.8 ± 6.9 | f | 0.5 ± 0.1 | fg |
| 6 | - | 0.5 | 0.2 | - | 37.0 ± 3.4 | ef | 0.9 ± 0.2 | cd |
| 7 | - | 1.0 | 0.2 | - | 50.6 ± 2.7 | bcd | 0.9 ± 0.1 | cd |
| 8 | - | 2.0 | 0.2 | - | 30.0 ± 0.0 | f | 0.5 ± 0.2 | g |
| 9 | - | 3.0 | 0.2 | - | 45.5 ± 1.5 | cde | 0.8 ± 0.1 | de |
| 10 | - | 4.0 | 0.2 | - | 18.9 ± 5.1 | g | 0.4 ± 0.1 | g |
| 11 | - | 5.0 | 0.2 | - | 30.4 ± 8.3 | f | 0.7 ± 0.0 | ef |
| 12 | 1.5 | - | - | 0.2 | 47.1 ± 2.8 | cd | 1.0 ± 0.1 | c |
| 13 | 1.5 | - | - | 0.4 | 65.0 ± 8.7 | a | 1.6 ± 0.0 | a |
| 14 | 1.5 | - | - | 0.6 | 45.0 ± 5.0 | cde | 0.7 ± 0.0 | ef |
| 15 | 1.5 | - | 0.4 | - | 51.7 ± 2.9 | bc | 1.4 ± 0.0 | b |
| 16 | 1.5 | - | 0.6 | - | 43.3 ± 2.9 | de | 0.8 ± 0.1 | de |
| Total Leaves Number | Leaf Number of Direct Organogenesis | Frequency of Regeneration of Direct Organogenesis (%) | Leaf Number of Indirect Organogenesis | Frequency of Regeneration of Indirect Organogenesis (%) | Leaf Number of Both Direct and Indirect Organogenesis | Frequency of Regeneration of Both Direct and Indirect Organogenesis (%) |
|---|---|---|---|---|---|---|
| 200 | 136 | 68.00% | 141 | 70.50% | 78 | 39.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Zhang, W.; Wei, Y.; Zhang, Y. Study on the Establishment of Efficient Leaf Regeneration System in ‘Yuluxiang’ Pear. Horticulturae 2025, 11, 77. https://doi.org/10.3390/horticulturae11010077
Cheng X, Zhang W, Wei Y, Zhang Y. Study on the Establishment of Efficient Leaf Regeneration System in ‘Yuluxiang’ Pear. Horticulturae. 2025; 11(1):77. https://doi.org/10.3390/horticulturae11010077
Chicago/Turabian StyleCheng, Xiaohua, Weilong Zhang, Yarui Wei, and Yuxing Zhang. 2025. "Study on the Establishment of Efficient Leaf Regeneration System in ‘Yuluxiang’ Pear" Horticulturae 11, no. 1: 77. https://doi.org/10.3390/horticulturae11010077
APA StyleCheng, X., Zhang, W., Wei, Y., & Zhang, Y. (2025). Study on the Establishment of Efficient Leaf Regeneration System in ‘Yuluxiang’ Pear. Horticulturae, 11(1), 77. https://doi.org/10.3390/horticulturae11010077






