Genome-Wide Identification of the ABC Gene Family in Rosaceae and Its Evolution and Expression in Response to Valsa Canker
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome-Wide Identification of ABC Gene Families Across 20 Plant Species
2.2. Phylogenetic Analyses
2.3. Expression Profiling of Apple ABC Transporters in Different Tissues
2.4. Analysis of the ABC Family Protein Interaction Network in Pyrus betulaefolia
2.5. GO and KEGG Enrichment Analysis
2.6. Expression Profile of ABC Under Valsa pyri Stress
2.7. Weighted Correlation Network Analysis (WGCNA)
2.8. Statistical Analysis
3. Results
3.1. Genome-Wide Identification and Phylogenetic Analysis of ATP-Binding Cassette (ABC) Transporters in 20 Plant Species
3.2. Expansion Rate Analysis
3.3. Rapid Amplification of the ABC Family Driven by Repetitive Events
3.4. Tissue-Specific Expression of ABC Genes in Apples
3.5. Analysis of Chromosomal Position and Evolutionary Selection Pressure of PbeABC Genes
3.6. GO and KEGG Enrichment Analysis of PbeABC Proteins
3.7. Protein Interaction Network Analysis of the PbeABC Family
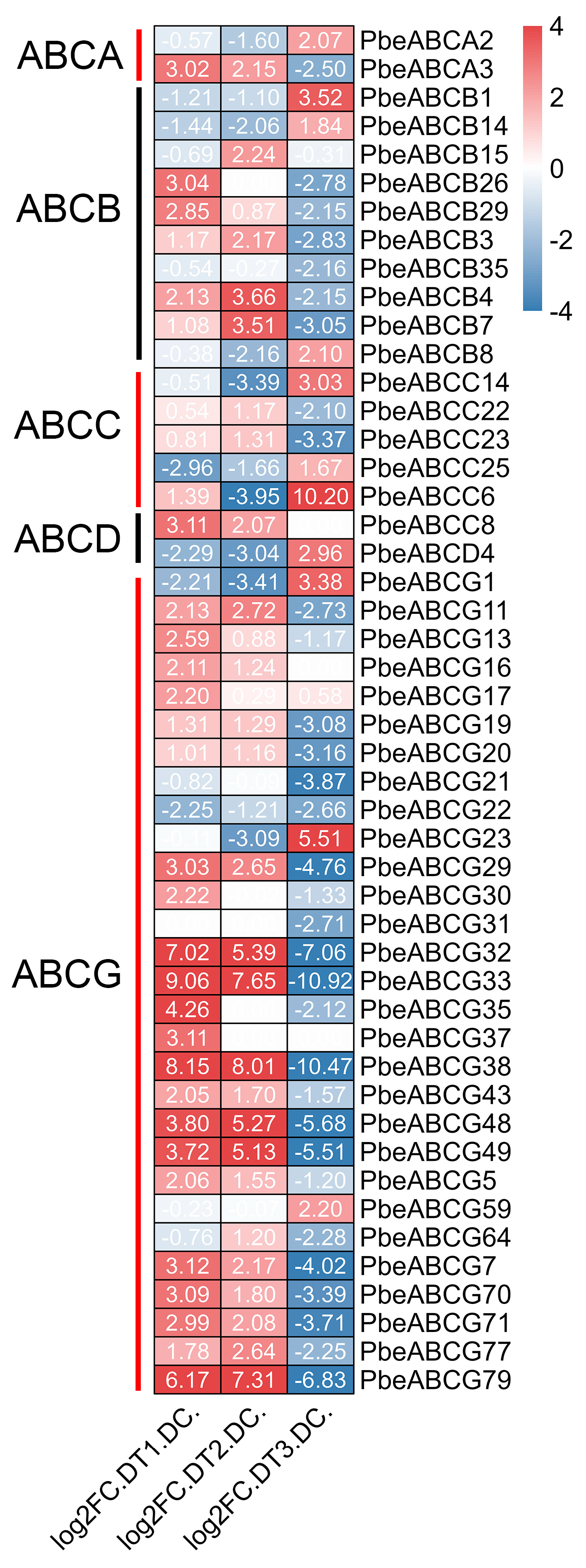
3.8. Expression Patterns of PbeABC Under Biotic Stress
3.9. The Response of the ABC Gene to Vp Is Crucial
4. Discussion
4.1. Evolutionary Insights and Subgroup Specialization
4.2. Gene Duplication and Evolutionary Processes
4.3. Expression Patterns and Their Physiological Implications
4.4. Stress Resistance and Biotic Interactions
4.5. Future Directions for Functional Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soundararajan, P.; Won, S.Y.; Kim, J.S. Insight on Rosaceae family with genome sequencing and functional genomics perspective. BioMed Res. Int. 2019, 2019, 7519687. [Google Scholar] [CrossRef] [PubMed]
- Shulaev, V.; Korban, S.S.; Sosinski, B.; Abbott, A.G.; Aldwinckle, H.S.; Folta, K.M.; Veilleux, R.E. Multiple models for Rosaceae genomics. Plant Physiol. 2008, 147, 985–1003. [Google Scholar] [CrossRef]
- Sardella, D.; Muscat, A.; Brincat, J.P.; Gatt, R.; Decelis, S.; Valdramidis, V. A comprehensive review of the pear fungal diseases. Int. J. Fruit. Sci. 2016, 16, 351–377. [Google Scholar] [CrossRef]
- Mizuno, A.; Tsukamoto, T.; Shimizu, Y.; Ooya, H.; Matsuura, T.; Saito, N.; Azegami, K. Occurrence of bacterial black shoot disease of European pear in Yamagata Prefecture. J. Gen. Plant Pathol. 2010, 76, 43–51. [Google Scholar] [CrossRef]
- Feng, H.; Wang, C.; He, Y.; Tang, L.; Han, P.; Liang, J.; Huang, L. Apple Valsa canker: Insights into pathogenesis and disease control. Phytopathol. Res. 2023, 5, 45. [Google Scholar] [CrossRef]
- Yuan, H.; Shi, B.; Wang, Z.; Qin, G.; Hou, H.; Tu, H.; Wang, L. Exploration of the biocontrol activity of Bacillus atrophaeus strain HF1 against pear Valsa canker caused by Valsa pyri. Int. J. Mol. Sci. 2023, 24, 15477. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, L. Canker and die-back of apples associated with Valsa ambiens. J. Pomol. Hortic. Sci. 1933, 11, 205–213. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, H.; Li, Z.; Ke, X.; Dou, D.; Gao, X.; Huang, L. Genome sequence of Valsa canker pathogens uncovers a potential adaptation of colonization of woody bark. New Phytol. 2015, 208, 1202–1216. [Google Scholar] [CrossRef]
- Chen, C.; Li, B.H.; Dong, X.L.; Wang, C.X.; Lian, S.; Liang, W.X.; Chen, C.; Li, B.H.; Dong, X.L.; Wang, C.X.; et al. Effects of temperature, humidity, and wound age on Valsa mali infection of apple shoot pruning wounds. Plant Dis. 2016, 100, 2394–2401. [Google Scholar] [CrossRef]
- Higgins, C.F.; Linton, K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004, 11, 918–926. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Srikant, S.; Gaudet, R. Mechanics and pharmacology of substrate selection and transport by eukaryotic ABC exporters. Nat. Struct. Mol. Biol. 2019, 26, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, R.; Davies, T.E.; Coleman, J.O.; Rea, P.A. The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J. Biol. Chem. 2001, 276, 30231–30244. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Zhang, Z.; Fang, Q.; Du, B.; Gong, X. Structural basis of substrate recognition and translocation by human ABCA4. Nat. Commun. 2021, 12, 3853. [Google Scholar] [CrossRef] [PubMed]
- Dassa, E.; Bouige, P. The ABC of ABCs: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001, 152, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Choi, J.; Rabbee, M.F.; Baek, K.H. In silico genome-wide analysis of the ATP-binding cassette transporter gene family in soybean (Glycine max L.) and their expression profiling. BioMed Res. Int. 2019, 2019, 8150523. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.W.; Holm, I.; Graille, M.; Dehoux, P.; Rzhetsky, A.; Wincker, P.; Brey, P.T. Identification of the Anopheles gambiae ATP-binding cassette transporter superfamily genes. Mol. Cells 2003, 15, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Zhao, Z.; Xu, X.; Li, C.; Wu, J.; Zhang, S. Identification and expression analysis of ATP-binding cassette (ABC) transporters revealed its role in regulating stress response in pear (Pyrus bretchneideri). BMC Genom. 2024, 25, 169. [Google Scholar] [CrossRef]
- Do, T.H.T.; Martinoia, E.; Lee, Y. Functions of ABC transporters in plant growth and development. Curr. Opin. Plant Biol. 2018, 41, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Nan, T.; Jiao, H.; Yue, S.; Huang, L.; Yuan, Y. Genome-wide analysis of Citrus medica ABC transporters reveals the regulation of fruit development by CmABCB19 and CmABCC10. Plant Physiol. Biochem. 2024, 215, 109027. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Li, Y.; Zhang, Y. A comparative genome-wide analysis of the ABC transporter gene family among three Gossypium species. Crop Sci. 2021, 61, 2489–2509. [Google Scholar] [CrossRef]
- Ma, F.; Liu, M.; Yan, P.; He, S.; Hu, J.; Zhang, X.; Luo, X. Multi-genome evolutionary study of the ABC1 gene family and identification of the pleiotropic effects of OsABC1-13 in rice development. Crop J. 2024, 12, 1022–1030. [Google Scholar] [CrossRef]
- Dhara, A.; Raichaudhuri, A. ABCG transporter proteins with beneficial activity on plants. Phytochemistry 2021, 184, 112663. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef] [PubMed]
- Panikashvili, D.; Shi, J.X.; Schreiber, L.; Aharoni, A. The Arabidopsis ABCG13 transporter is required for flower cuticle secretion and patterning of the petal epidermis. New Phytol. 2011, 190, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Biała, W.; Banasiak, J.; Jarzyniak, K.; Pawela, A.; Jasiński, M. Medicago truncatula ABCG10 is a transporter of 4-coumarate and liquiritigenin in the medicarpin biosynthetic pathway. J. Exp. Bot. 2017, 68, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Ma, Q.; Du, J.; Yu, M.; Li, F.; Luan, J.; Li, H. Preliminary classification of the ABC transporter family in Betula halophila and expression patterns in response to exogenous phytohormones and abiotic stresses. Forests 2019, 10, 722. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.U.; Lee, M.; Kim, Y.Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef]
- Wang, G.; Gu, J.; Long, D.; Zhang, X.; Zhao, C.; Zhang, H.; Ji, W. Genome-wide identification of wheat ABC gene family and expression in response to fungal stress treatment. Plant Biotechnol. Rep. 2024, 18, 401–413. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Man, J.; Wen, L.; Tan, S.Q.; Liu, S.L.; Li, Y.H.; Wei, Y.M. ATP-binding cassette transporter TaABCG2 contributes to Fusarium head blight resistance by mediating salicylic acid transport in wheat. Mol. Plant Pathol. 2024, 25, e70013. [Google Scholar] [CrossRef]
- Zhang, H.; Jing, W.; Zheng, J.; Jin, Y.; Wu, D.; Cao, C.; Zhang, W. The ATP-binding cassette transporter OsPDR1 regulates plant growth and pathogen resistance by affecting jasmonates biosynthesis in rice. Plant Sci. 2020, 298, 110582. [Google Scholar] [CrossRef] [PubMed]
- Burra, D.D.; Lenman, M.; Levander, F.; Resjö, S.; Andreasson, E. Comparative membrane-associated proteomics of three different immune reactions in potato. Int. J. Mol. Sci. 2018, 19, 538. [Google Scholar] [CrossRef]
- Aryal, B.; Xia, J.; Hu, Z.; Stumpe, M.; Tsering, T.; Liu, J.; Geisler, M.M. An LRR receptor kinase controls ABC transporter substrate preferences during plant growth-defense decisions. Curr. Biol. 2023, 33, 2008–2023.e8. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bateman, A. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.; Bateman, A. PFAM: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Paterson, A.H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef]
- Celton, J.; Gaillard, S.; Bruneau, M.; Pelletier, S.; Aubourg, S.; Martin-Magniette, M.; Renou, J. Widespread anti-sense transcription in apple is correlated with si RNA production and indicates a large potential for transcriptional and/or post-transcriptional control. New Phytol. 2014, 203, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Von Mering, C. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Shitan, N.; Sato, S.; Nakamura, Y.; Tabata, S.; Yazaki, K. Genome-wide analysis of ATP-binding cassette (ABC) proteins in a model legume plant, Lotus japonicus: Comparison with Arabidopsis ABC protein family. DNA Res. 2006, 13, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ortiz, C.; Dutta, S.K.; Natarajan, P.; Peña-Garcia, Y.; Abburi, V.; Saminathan, T.; Nimmakayala, P.; Reddy, U.K. Genome-wide identification and gene expression pattern of ABC transporter gene family in Capsicum spp. PLoS ONE 2019, 14, e0215901. [Google Scholar] [CrossRef]
- Gupta, B.B.; Selter, L.L.; Baranwal, V.K. Updated inventory, evolutionary and expression analyses of G (PDR) type ABC transporter genes of rice. Plant Physiol. Biochem. 2019, 142, 429–439. [Google Scholar] [CrossRef]
- Badouin, H.; Gouzy, J.; Grassa, C.J.; Murat, F.; Staton, S.E.; Cottret, L.; Lelandais-Brière, C.; Owens, G.L.; Carrère, S.; Mayjonade, B. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature 2017, 546, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wang, H.; Guo, C.; Zhang, N.; Zeng, L.; Chen, Y.; Ma, H.; Qi, J. Widespread whole genome duplications contribute to genome complexity and species diversity in Angiosperms. Mol. Plant 2018, 11, 414–428. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, Z.; Zeng, B.; Liu, X. Genome-wide analysis of the ABC gene family in almond and functional predictions during flower development, freezing stress, and salt stress. BMC Plant Biol. 2024, 24, 12. [Google Scholar] [CrossRef]
- Yang, X.; Cui, X.; Chang, J.; Wang, J.; Wang, Y.; Liu, H.; Zhou, Y. Variations in Protein and Gene Expression Involved in the Pathways of Carbohydrate, Abscisic Acid, and ATP-Binding Cassette Transporter in Soybean Roots under Drought Stress. Agronomy 2024, 14, 843. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Zhang, P.; Wang, R.; Sun, L.; Wan, J.; Xu, J. ABCF3 regulates the expression of aquaporin genes and endoplasmic reticulum stress-related genes in Arabidopsis. Theor. Exp. Plant Physiol. 2018, 30, 215–222. [Google Scholar] [CrossRef]
- Moon, S.; Jung, K.H. Genome-wide expression analysis of rice ABC transporter family across spatio-temporal samples and in response to abiotic stresses. J. Plant Physiol. 2014, 171, 1276–1288. [Google Scholar]
- Khare, D.; Choi, H.; Huh, S.U.; Bassin, B.; Kim, J.; Martinoia, E.; Lee, Y. Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin. Proc. Natl. Acad. Sci. USA 2017, 114, E5712–E5720. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Li, C.; Jiang, X.; Gai, Y. Transcriptomic Insights into Functions of LkABCG36 and LkABCG40 in Nicotiana tabacum. Plants 2023, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, S.; Sun, Q.; Yang, L.; Zhu, Y.; Yuan, Y.; Hua, J. A role of cytokinin transporter in Arabidopsis immunity. Mol. Plant-Microbe Interact. 2017, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, E.; Mao, X.; Chen, Z.; Xu, T.; Zuo, L.; Zuo, C. Evolutionary and functional analysis reveals the crucial roles of receptor-like proteins in resistance to Valsa canker in Rosaceae. J. Exp. Bot. 2023, 74, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yu, H.; Tian, D.; Sun, E.; Zuo, L.; Jiang, D.; Zuo, C.; Fan, R. Phylogeny, Expression Profiling, and Coexpression Networks Reveals the Critical Roles of Nucleotide-BindingLeucine-Rich Repeats on Valsa Canker Resistance. Horticulturae 2023, 9, 345. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Yu, H.; Hu, H.; Dou, Z.; Zuo, C.; Niu, J. Genome-Wide Identification of the ABC Gene Family in Rosaceae and Its Evolution and Expression in Response to Valsa Canker. Horticulturae 2025, 11, 1. https://doi.org/10.3390/horticulturae11010001
Du C, Yu H, Hu H, Dou Z, Zuo C, Niu J. Genome-Wide Identification of the ABC Gene Family in Rosaceae and Its Evolution and Expression in Response to Valsa Canker. Horticulturae. 2025; 11(1):1. https://doi.org/10.3390/horticulturae11010001
Chicago/Turabian StyleDu, Chenglong, Hongqiang Yu, Huanhuan Hu, Zhiqi Dou, Cunwu Zuo, and Junqiang Niu. 2025. "Genome-Wide Identification of the ABC Gene Family in Rosaceae and Its Evolution and Expression in Response to Valsa Canker" Horticulturae 11, no. 1: 1. https://doi.org/10.3390/horticulturae11010001
APA StyleDu, C., Yu, H., Hu, H., Dou, Z., Zuo, C., & Niu, J. (2025). Genome-Wide Identification of the ABC Gene Family in Rosaceae and Its Evolution and Expression in Response to Valsa Canker. Horticulturae, 11(1), 1. https://doi.org/10.3390/horticulturae11010001





