Abstract
Apple and sour cherry pomace, by-products resulting from fruit processing for juice production, were analysed using high-performance liquid chromatography (HPLC) to identify and quantify individual phenolic compounds. In order to determine the most efficient method of extracting these phenolic compounds from pomace, different concentrations of ethanol were used as the organic solvent. The following phenolic compounds were analysed: gallic acid, neochlorogenic acid, (+)-catechin, (−)-epicatechin, chlorogenic acid, vanillic acid, caffeic acid, ferulic acid, sinapic acid, salicylic acid, ellagic acid, rutin, and myricetin. The amounts of these compounds varied depending on the concentration of ethanol used in the extraction process. Neochlorogenic acid, a potent antioxidant, was quantified in apple and sour cherry pomace extracts, showing significant variation with solvent concentration. In apple pomace, the highest amount was found in ethanol 100% (46.44 mg 100 g−1 DW), followed by ethanol 75% (32.09 mg 100 g−1 DW) and ethanol 50% (7.66 mg 100 g−1 DW). In sour cherry pomace, the highest amount was also extracted into ethanol 100% (45.20 mg 100 g−1 DW) and the lowest in ethanol 50% (29.12 mg 100 g−1 DW). Catechin was detected exclusively in cherry pomace, with a maximum yield observed in 75% ethanol (137.86 mg 100 g−1 DW), which was significantly higher compared to the other phenolic compounds analysed. The obtained results demonstrate the importance of apple and sour cherry pomace as valuable by-products, with the potential to be used in the production of functional foods.
1. Introduction
Phenolic compounds are natural substances found in plants [1], and their presence varies depending on species, cultivar, growing conditions, maturation stage, as well as storage and processing conditions [2]. Compounds containing one or more aromatic rings linked to one or more hydroxyl groups are known as phenolic compounds and are among the most common secondary metabolites in plants, with over 8000 identified structures [3]. Significant advances in the fields of chemistry, biology, and agriculture have enabled better utilization of agricultural resources and by-products from various industrial processes, whether as biofuels, biosolvents, animal feed [4], or functional foods due to their beneficial composition for the body [5]. Fruit pomace is the primary by-product of the juice processing industry, accounting for approximately 30% of the unprocessed fruit [6]. Annually, significant quantities of pomace are produced globally, and its disposal can have negative environmental impacts [5,7,8]. Fruit pomace is a rich source of bioactive compounds that are valuable for human health. Studies show that only a small fraction, between 3% and 10%, of these compounds are present in the juice, with the remainder concentrated in the pulp and especially in the fruit skins [7]. Additionally, pomace can serve as an important source of compounds with antioxidant activity, offering potential applications in the food industry (dietary fibres, colorants, thickening agents), pharmaceutical industry (antioxidant properties, source of vitamins and minerals), and cosmetic industry (antimicrobial properties), among others [9,10].
Lyu et al. [11] indicated that apple pomace, due to its content of fibres and natural polyphenols, can enhance the nutritional value of confectionery products, yogurt, jams, and meat products, while also acting as a natural stabilizer and improving their texture. Thus, by-products from apples and cherries, rich in bioactive compounds, emerge as a valuable resource for the food industry, offering significant potential for the development of functional products. This direction could lead to improvements in overall health and the prevention and management of lifestyle-related conditions such as obesity, diabetes, hyperglycaemia, hypertension, and cardiovascular diseases. By diversifying food options and highlighting available alternatives, consumers may be encouraged to make long-term health-conscious choices, thus opening new opportunities for the food industry to reassess how the population engages with their food.
Therefore, developing a sustainable food system involves understanding the chemical composition of raw materials and diversifying the methods for extracting phenolic compounds, as their solubility and separation properties are affected by structural differences, with the structure of a compound significantly influencing its polarity, conjugation, and interaction with the sample matrix [3]. High molecular weight phenols are often insoluble due to their structural composition, and the stability of phenolic compounds varies due to their uneven distribution in plants (some phenolic compounds are stable, while others are prone to oxidation, thermolabile, or volatile) [3]. Due to their instability, extracting these compounds poses a challenge, with biological activity being affected by both extraction process parameters and external factors such as the presence of oxygen and light [12].
The present study investigated the impact of ethanol concentration on the phenolic compound concentration in apple and cherry pomace. Various ethanol concentrations in the solvent were employed for phenolic extraction, evaluating the effects of these variations on the yield and efficiency of the extraction process. Thus, this study aimed to determine the impact of different ethanol concentrations on the quantity and composition of extracted phenolic compounds, highlighting the importance of this parameter in optimizing methods for isolating bioactive substances from apple and cherry pomace.
2. Materials and Methods
2.1. Biological Material
The by-products (skin and flesh of the fruit) from apples of the ‘Golden Delicious’, ‘Florina’, and ‘Jonathan’ varieties, and sour cherries of the variety ‘Nana’, were collected from a company specialized in juice production. Obtained from cold pressing of the fruits, the by-products were packed in polyethylene bags and frozen at −18 °C until use. The subsequent process involved drying the by-products in an industrial dehydrator at a controlled temperature between 50 °C and 60 °C until their moisture content reached 10%. After drying, the resulting material was ground into a fine powder and stored at 22 °C (±3 °C) in hermetically sealed glass containers.
2.2. Chemical Products and Reagents
The chemicals and reagents used were methanol, ethanol, water (LC-MS Grade, LiChrosolv® Merck-Sigma-Aldrich, Darmstadt, Germany), acetic acid, and standards of gallic acid, neochlorogenic acid, (+)-catechin hydrate, chlorogenic acid, vanillic acid, caffeic acid, (−)-epicatechin, ferulic acid, sinapic acid, salicylic acid, ellagic acid, rutin, and myricetin (Merck-Sigma-Aldrich, Darmstadt, Germany).
2.3. Methods of Analysis
2.3.1. Sample Preparation
1 g of dry sample of apple pomace or sour cherry was weighed in centrifuge tubes with a high-precision analytical balance (Radwag AS 220.R2, RADWAG Headquarters, Radom, Poland), over which 10 mL of organic solvent was added (different concentrations as follows: 100% ethanol, 75% ethanol, and 50% ethanol). Each sample was vortexed for 3 min (RX3 Vortex Mixer, VELP SCIENTIFICA, Usmate, Italy), then ultrasonicated in an ultrasonic bath with a heater (Fungilab, Barcelona, Spain) at a temperature maintained at 30 °C for 30 min, followed by centrifugation (Eppendorf Centrifuge 5430 R, Hamburg, Germany) for 30 min at 6000 rpm. Samples were then stored for 24 h in a refrigerator at 4 °C (±2 °C). Before HPLC analysis, the protocol was repeated, namely vortexing for 3 min, ultrasonication at a temperature maintained at 30 °C for 30 min, and centrifugation for 30 min at 6000 rpm. The resulting supernatant was filtered with Whatman no. 4 filter paper, then passed through a 0.45 µ syringe filter (Merck, Darmstadt, Germany) into the vial for UHPLC analysis.
2.3.2. HPLC Analysis
Analysis of phenolic compounds was performed on an ultra-high performance liquid chromatograph (UltiMate 3000 XRS Liquid Chromatograph, Thermo Scientific, Waltham, MA, USA) combined with a Dionex UltiMate 3000 XRS Autosampler, an XRS pump, and an RS Diode UV–VIS matrix detector, in the order of appearance on the chromatogram: gallic acid, neochlorogenic acid, (+)-catechin hydrate, chlorogenic acid, vanillic acid, caffeic acid, (−)-epicatechin, ferulic acid, sinapic acid, salicylic acid, ellagic acid, rutin, and myricetin, using the method described by Stoenescu et al. [13]. All standards were purchased from Sigma (HPLC grade, Sigma Aldrich, Germany). Stock solutions of each individual phenolic compound were prepared by dissolving the compounds separately in methanol, and were stored in amber bottles at −15 °C. Different concentrations (25, 50, 75, and 100 ppm) of standard solutions were used to establish the calibration curve.
The mobile phase consisted of 1% aqueous acetic acid solution (B) and 100% MeOH (C). The samples were eluted with the following gradient: 90% B and 10% C from 0 to 6 min, 84% B and 16% C from 7 to 25 min, 72% B and 28% C from 26 to 37 min, 65% B and 35% C from 38 to 47 min, 50% B and 50% C from 48 to 64 min, and 90% B and 10% C from 65 to 70 min, to restore the initial conditions before injection of a new sample. The flow rate was 0.8 mL/min, with an injection volume of 5 µL. The column used was a Hypersil Gold (150 × 4.6) (Thermo Scientific, Waltham, MA, USA) with the temperature maintained at 25 °C. The detection of phenolic compounds was performed by UV absorption at λ = 278 nm. Each compound was identified based on its retention time and by comparison with standards under the same conditions. All results were expressed in mg 100 g−1 DW and represented the mean and standard deviation of three consecutive determinations.
2.4. Statistical Analysis
Statistical analysis was performed with IBM SPSS Statistics 26 software. One-way ANOVA and Duncan multiple range tests at p < 0.05 were used (different superscript letters indicate statistically significant differences between solvent concentrations).
3. Results
The results obtained regarding the content of phenolic compounds in apple and sour cherry pomace are presented in Table 1 and Table 2. The statistical analysis of the data revealed significant differences between the concentrations of solvent used and the phenolic compounds (p < 0.05), highlighted by the Duncan’s multiple range test. The identified phenolic compounds belong to different chemical classes, thus explaining the observed variations (Figure 1 and Figure 2). The type of compound was correlated with the concentration of the extraction solvent, influencing the extraction efficiency. The extraction of phenolic compounds from plant materials depends on their solubility in the solvent used and the extraction method applied [3,14,15]. From the class of phenolic acids, the subclass of hydroxybenzoic acids and gallic, ellagic, and vanillic acids were identified in apple and sour cherry pomace, and salicylic acid was found only in sour cherry pomace.

Table 1.
Identified phenolic compounds in dried apple pomace.

Table 2.
Identified phenolic compounds in dried sour cherry pomace.
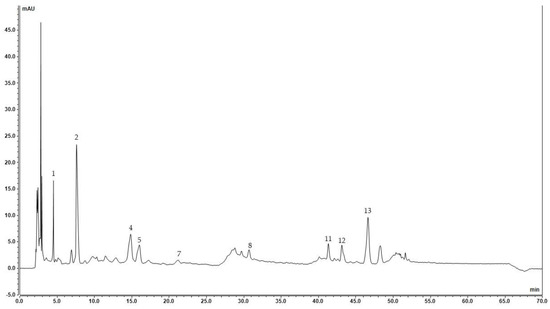
Figure 1.
Chromatogram of 75% Ethanol apple pomace (1, gallic acid; 2, neochlorogenic acid; 4, chlorogenic acid; 5, vanillic acid; 7, (−)-epicatechin; 8, ferulic acid; 11, ellagic acid; 12, rutin; and 13, myricetin).
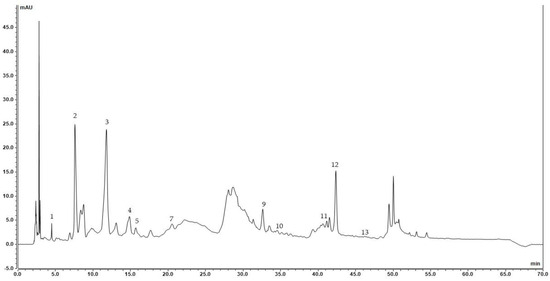
Figure 2.
Chromatogram of 50% Ethanol sour cherry pomace (1, gallic acid; 2, neochlorogenic acid; 3, (+)-catechin; 4, chlorogenic acid; 5, vanillic acid; 7, (−)-epicatechin; 9, sinapic acid; 10, salicylic acid; 11, ellagic acid; 12, rutin; and 13, myricetin).
UHPLC analysis of gallic acid (GA) showed its presence in all analysed extracts. In apple pomace, the highest content of gallic acid (mg/100 g DW) was extracted with 75% ethanol (2.68), followed by 100% ethanol (1.42) and 50% ethanol (1.25). The DUNCAN test indicated statistically significant differences between the concentrations of the solvent used (p < 0.05). In sour cherry pomace, the gallic acid content, although lower, was also influenced by the solvent concentration: ethanol 75% (0.36), ethanol 100% (0.17), and ethanol 50% (0.07). It turns out that choosing the appropriate solvent and concentration is important for the extraction of gallic acid, a fact confirmed by Singh et al. [16] in pomegranate.
Ellagic acid (EA) was quantified in both types of pomace. In apple pomace, the highest content was identified in 75% ethanol (6.90) and the lowest in 100% ethanol (3.48), with significant differences between concentrations. In sour cherry pomace, ellagic acid content varied significantly between solvent concentrations, with the highest content being identified in 75% ethanol (1.79).
For gallic acid and ellagic acid, the concentration of 75% ethanol gave the highest values. Conversely, for vanillic acid, the 50% ethanol concentration was the most effective, both in apple (8.01) and sour cherry (0.95) pomace. For salicylic acid, the highest amount was quantified in ethanol 75% (1.74), followed by ethanol 50% (1.11) in the case of sour cherry pomace. Conversely, this compound was not identified in apple pomace.
From the subclass of hydroxycinnamic acids, neochlorogenic (NCHA), chlorogenic (CHA), caffeic (CFA), ferulic (FE), and sinapic (SA) acids were identified and quantified (Table 1 and Table 2). Lyu et al. [11] confirmed chlorogenic, caffeic, and ferulic acids as being among the main phenolic acids in apple pomace.
Neochlorogenic acid, a powerful antioxidant, was quantified in all types of extracts for both types of pomace, with significant differences depending on the concentration of the solvent used. In apple pomace, the highest amount was found in 100% ethanol (46.44), followed by 75% ethanol (32.09) and 50% ethanol (7.66). In sour cherry pomace, the highest amount was also extracted in 100% ethanol (45.20), and the lowest in ethanol 50% (29.12).
Chlorogenic acid was identified in the concentrations of 75% and 50% ethanol in both types of pomace, with the highest values in the 50% ethanol extracts: 5.73 mg/100 g DW in apple pomace, and 3.55 mg/100 g DW in sour cherry pomace. The amount of chlorogenic acid was similar to that identified by Zardo et al. [17] in apple pomace (45.5 mg/kg DW in ethanol) and by Bai et al. [18] using 70% ethanol (62.8 mg/kg DW) as the extraction solvent.
Caffeic acid was identified only in apple pomace, using a concentration of 50% ethanol (4.21 mg/100 g DW). Ferulic acid (FA) was identified in apple pomace at concentrations of 75% ethanol (0.42 mg/100 g DW) and 50% ethanol (0.66 mg/100 g DW), with the differences being insignificant. Leyva-Corral et al. [19] confirmed the presence of chlorogenic acid (41.55 mg/100 g) and caffeic acid (7.09 mg/100 g) in apple pomace. Sinapic acid (SA) was identified only in sour cherry pomace, with insignificant differences between the extracts: 8.21–8.35 mg/100 g DW in 75% and 50% ethanol, respectively. Lower concentrations of ethanol allowed better extraction of sinapic, chlorogenic, and ferulic acid.
From the class of flavonoids, several compounds were identified and quantified in the study, including (+)-catechin (C), (−)-epicatechin (EC), myricetin (MYR), and rutin (RUT). Catechin was found in significantly higher concentrations in sour cherry pomace compared to the other phenolic compounds analysed. The highest concentration of catechin was recorded in the extract obtained with 75% ethanol (137.86 mg 100 g−1 DW), followed by 50% ethanol (72.27 mg 100 g−1 DW) and 100% ethanol (44.63 mg 100 g−1 DW). The study showed that the use of an aqueous solvent combined with ethanol was effective in extracting catechin from sour cherry pomace, confirming the findings of the study conducted by Xia and Ahmmed [20] on the aqueous extract of Prunus insititia. Epicatechin was identified only in the extracts obtained with 75% and 50% ethanol, showing significant differences between the two types of pomace analysed. In the case of apple pomace, the epicatechin content varied between 1.13 and 3.37 mg 100 g−1 DW for the extracts with 75% and 50% ethanol, while in the case of sour cherry pomace, the values varied between 3.90 and 20.26 mg 100 g−1 DW for the same extraction conditions.
In the study, myricetin (MYR) was identified and quantified in significantly higher concentrations in apple pomace. The highest content was recorded in the extract with 75% ethanol (26.41 mg 100 g−1 DW), followed by the extract with 50% ethanol (23.24 mg 100 g−1 DW), while the lowest content was observed in the extract with 100% ethanol (14.28 mg 100 g−1 DW). In contrast, the amounts of myricetin in sour cherry pomace were significantly lower, ranging from 1.50 mg 100 g−1 DW (100% ethanolic extract) to 1.69 mg 100 g−1 DW (75% ethanolic extract). Rutin (RUT) was identified in all analysed extracts, both in apple and sour cherry pomace. The amounts were considerably higher in sour cherry pomace, ranging between 26.67 mg 100 g−1 DW (100% ethanolic extract) and 44.26 mg 100 g−1 DW (75% ethanolic extract). Conversely, in apple pomace, the amounts were much lower, oscillating between 0.78 mg 100 g−1 DW (100% ethanolic extract) and 3.95 mg 100 g−1 DW (75% ethanolic extract). For both types of pomace, 75% ethanol extracts showed the highest concentrations. Previous studies have confirmed the presence of gallic acid, chlorogenic acid, catechin, and rutin in apple pomace, and the current results are consistent with these findings [6]. Additionally, Yılmaz et al. [21] highlighted the predominant polyphenols in sour cherry pomace, highlighting the consistency with the data obtained in this research.
4. Discussion
It is known that the content of phenolic compounds in pomace extracts largely depends on the type of solvent used, its concentration, the extraction temperature, and other influencing factors in the sample preparation stage [1]. Solvents with low viscosity, low density, and high diffusivity have the ability to efficiently disperse into the pores of plant matter to extract bioactive compounds [22]. Adding water to the solvent can improve the extraction capacity compared to using absolute solvents (100%) [23]. The polarity of the extraction solvent also plays a crucial role in the efficiency of the extraction process of bioactive compounds [24,25]. Thus, using a larger amount of water or organic solvent can facilitate the extraction of phenolic acids and flavonoids [26,27]. Previous studies, such as one by Zhu et al. [28], demonstrated that higher concentrations of ethanol led to better results in the extraction of sinapic acid and p-coumaric acid, while for gallic acid, chlorogenic acid, vanillic acid, syringic acid, and catechin, the optimal results were obtained at a concentration of 40% ethanol. These findings are consistent with the results obtained in the present study, where gallic acid, ellagic acid, salicylic acid, catechin, rutin, and myricetin were effectively extracted in ethanol-based solvents of 75% concentration. Regarding the effects of ethanol concentration, the study by Kobus et al. [4] showed that a concentration of 60% ethanol was more effective in the extraction of polyphenols from apple pomace (variety ‘Jonagold’) compared to 96% ethanol. In our experiment, the use of an aqueous solvent in a higher proportion (50% ethanol) led to a more efficient extraction of vanillic, chlorogenic, sinapic, caffeic, epicatechin, and ferulic acid. According to Reis et al. [29], water is considered an excellent option for the extraction of phenolic compounds due to its safety, accessibility, and low cost. They recommend using a mixture of water and organic solvents up to 40% to minimize costs while benefiting from a moderately polar environment that favours the efficient extraction of polyphenols [30].
Previous studies, such as the one by Bucić-Kojić et al. [31] on the extraction of phenolic compounds from grape seeds, confirmed that the use of 50% ethanol as the extraction solvent was more effective than the use of 70% or 96% ethanol, both for total and individual phenolic compounds. For example, for gallic acid and catechin, our study identified a higher concentration using 75% ethanol compared to using 100% or 50% ethanol. A study by Do et al. [23] pointed out that for the extraction of phenolic compounds, a concentration of 75% methanol or ethanol is more effective than 100% solvent, a conclusion also confirmed by the present study for a wide variety of phenolic compounds such as gallic acid, ellagic acid, salicylic acid, catechin, myricetin, and rutin. These findings emphasize the importance of the appropriate choice of solvent and its concentration in optimising the extraction process for phenolic compounds, with a direct impact on the extraction yield and quality. The concentration of 100% ethanol was found to be the most effective for extracting neochlorogenic acid from apple and cherry pomace, followed by the 75% and 50% ethanol concentrations. This highlights that the efficiency of neochlorogenic acid extraction decreases with a reduction in the organic solvent percentage, and the presence of water in the solvent diminishes the extraction yield. According to Mokrani and Madani [32], a single solvent cannot simultaneously extract all classes of phenolic compounds from a sample. They observed that ethanol is effective in the extraction of flavonoids and their glycosides, results that are consistent with those obtained in this study. However, methanol is recommended for the extraction of phenolic acids and catechin. These findings emphasize the importance of the correct choice of solvent and its concentration depending on the type and classes of phenolic compounds we wish to extract from plant materials. All these conclusions are due to the polarity of the solvent used for the extraction and the solubility of the phenols. The solubility of polyphenols mainly depends on their hydroxyl groups, molecular size, and hydrocarbon length [15]. The use of organic solvents mixed in different proportions with water contributes to the creation of a moderately polar environment, favourable for the extraction of phenolic compounds [14].
5. Conclusions
In conclusion, the results obtained in this work confirm that apple and sour cherry pomace are potential sources of bioactive compounds, especially neochlorogenic acid, chlorogenic acid, myricetin, and ellagic acid, in apple pomace, and neochlorogenic acid, catechin, and rutin in sour cherry pomace. Furthermore, the influence of the solvent concentration on the identification and quantification process of these compounds of interest was highlighted.
The use of aqueous ethanolic extracts is preferable due to safety, economic efficiency, and applicability in the food industry. In the future, further studies are needed to explore different methods of sample preparation and processing, the optimisation of extraction times and temperatures, and the reduction of organic solvent usage to identify the minimum effective level for extraction of compounds of interest. These steps will contribute to maximizing the value and sustainable use of by-products resulting from fruit processing for juice production.
Author Contributions
Conceptualization, S.C. and M.B.M.; methodology, A.-M.S.; software, M.B.M.; validation, S.C., M.B.M. and A.-M.S.; investigation, M.B.M.; writing—original draft preparation, S.C. and M.B.M.; writing—review and editing, S.C. and M.B.M.; supervision, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, M.; Chen, X.; Deng, J.; Ouyang, D.; Wang, D.; Liang, Y.; Chen, Y.; Sun, Y. Effect of thermal processing on free and bound phenolic compounds and antioxidant activities of hawthorn. Food Chem. 2020, 332, 127429. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2022, 60, 1388–1416. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Kobus, Z.; Wilczynski, K.; Nadulski, R.; Rydzak, L.; Guz, T. Effect of solvent polarity on the efficiency of ultrasound-assisted extraction of polyphenols from apple pomace. In IX International Scientific Symposium; University of Life Sciences: Lublin, Poland, 2017. [Google Scholar] [CrossRef]
- Chaouch, M.A.; Benvenuti, S. The Role of Fruit By-Products as Bioactive Compounds for Intestinal Health. Foods 2020, 9, 1716. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Parastouei, K.; Khodaiyan, F. Simultaneous extraction optimization and characterization of pectin and phenolics from sour cherry pomace. Int. J. Biol. Macromol. 2020, 158, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Schulz, P.; Rizvi, S.S. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Paliotta, M.; Centioni, L.; Mencarelli, F.; Carbone, K. The effect of genotype and drying condition on the bioactive compounds of sour cherry pomace. Eur. Food Res. Technol. 2018, 244, 635–645. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Stoenescu, A.-M.; Trandafir, I.; Cosmulescu, S. Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]
- Phuong, N.N.M.; Le, T.T.; Dang, M.Q.; Van Camp, J.; Raes, K. Selection of extraction conditions of phenolic compounds from rambutan (Nephelium Lappaceum L.) peel. Food Bioprod. Process. 2020, 122, 222–229. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Solid-state fermentation of apple pomace using Phanerocheate chrysosporium–Liberation and extraction of phenolic antioxidants. Food Chem. 2011, 126, 1071–1080. [Google Scholar] [CrossRef]
- Singh, M.; Jha, A.; Kumar, A.; Hettiarachchy, N.; Rai, A.K.; Sharma, D. Influence of the solvents on the extraction of major phenolic compounds (punicalagin, ellagic acid and gallic acid) and their antioxidant activities in pomegranate aril. J. Food Sci. Technol. 2014, 51, 2070–2077. [Google Scholar] [CrossRef]
- Zardo, D.M.; Alberti, A.; Zielinski, A.A.F.; Prestes, A.A.; Esmerino, L.A.; Nogueira, A. Influence of solvents in the extraction of phenolic compounds with antibacterial activity from apple pomace. Sep. Sci. Technol. 2020, 56, 903–911. [Google Scholar] [CrossRef]
- Bai, X.; Yue, T.; Yuan, Y.; Zhang, H. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef]
- Leyva-Corral, J.; Quintero-Ramos, A.; Camacho-Dávila, A.; de Jesús Zazueta-Morales, J.; Aguilar-Palazuelos, E.; Ruiz-Gutiérrez, M.G.; Meléndez-Pizarro, C.O.; de Jesús Ruiz-Anchondo, T. Polyphenolic compound stability and antioxidant capacity of apple pomace in an extruded cereal. LWT Food Sci. Technol. 2016, 65, 228–236. [Google Scholar] [CrossRef]
- Xia, P.; Ahmmed, M.K. Exploring efficient extraction methods: Bioactive compounds and antioxidant properties from New Zealand damson plums. Food Biosci. 2023, 55, 103057. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Ersus Bilek, S.; Uygun, Ö.; Bircan, C. Sour cherry by-products: Compositions, functional properties and recovery potentials—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3549–3563. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Satapathy, A.K.; Behera, S.K.; Yadav, A.; Laxmi Mahour, L.N.; Yelamaggad, C.V.; Sandhya, K.L.; Sahoo, B. Tuning the fluorescence behavior of liquid crystal molecules containing Schiff-base: Effect of solvent polarity. J. Lumin. 2019, 210, 371–375. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Wang, C.H.; Zhang, Y.S.; Yang, Y.; Shang, Y.F.; Niu, X.L.; Sun, L.Y.; Ma, Y.L.; Wei, Z.J. Insight into solvent effects on phenolic content and antioxidant activity of bamboo leaves extracts by HPLC analysis. J. Food Meas. Charact. 2018, 12, 2240–2246. [Google Scholar] [CrossRef]
- Muhamad, N.; Muhmed, S.A.; Yusoff, M.M.; Gimbun, J. Influence of solvent polarity and conditions on extraction of antioxidant, flavonoids and phenolic content from Averrhoa bilimbi. J. Food Eng. 2014, 4, 255–260. [Google Scholar] [CrossRef]
- Zhu, K.; Ma, J.; Cong, J.; Zhang, T.; Lei, H.; Xu, H.; Luo, Z.; Li, M. The road to reuse of walnut by-products: A comprehensive review of bioactive compounds, extraction and identification methods, biomedical and industrial applications. Trends Food Sci. Technol. 2024, 143, 104264. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem. 2012, 135, 1991–1998. [Google Scholar] [CrossRef]
- Rasheed, A.; Cobham, E.; Zeighmami, M.; Ong, S. Extraction of phenolic compounds from pineapple fruit. In Proceedings of the 2nd International Symposium on Processing & Drying of Foods, Fruits & Vegetables, Kuala Lumpur, Malaysia, 8–19 June 2012. [Google Scholar]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).