Abstract
Soluble inorganic pyrophosphatase (s-PPase), a pyrophosphate hydrolase, is crucial for various physiological processes including plant growth and development, metabolic functions, and responses to abiotic stresses. However, research on s-PPase in woody plants is limited. To investigate the potential role of soluble inorganic pyrophosphatase in Eucommia ulmoides Oliver (E. ulmoides) in drought stress, the E. ulmoides soluble inorganic pyrophosphatase 5 (EuSIP5) cDNA sequence was amplified via RT-PCR. A bioinformatic analysis suggested that EuSIP5 may be an unstable amphipathic protein predominantly localized in the cytoplasm. In E. ulmoides, the highest expression of the EuSIP5 gene was detected in the leaves and pericarp of male plants from April to October, and in the leaves in July and September. Under drought conditions, the expression of EuSIP5 in E. ulmoides leaves was significantly greater than that in the control. An overexpression vector containing EuSIP5 was constructed and introduced into Nicotiana tabacum L. cv. Xanthi (N. tabacum L.). Compared with that in wild-type (WT) plants, wilting in N. tabacum L. EuSIP5-overexpressing (OE) plants was delayed by 4 days under drought stress. Additionally, the expression levels of the drought-related genes DET2, CYP85A1, P5CS, ERF1, F-box, and NCED1 were elevated in the leaves of transgenic N. tabacum L. Moreover, the activities of the protective enzymes peroxidase, superoxide dismutase, and catalase were significantly greater, whereas the malondialdehyde content was lower in the transgenic plants than in the WT plants. These findings suggest that the introduction of the EuSIP5 gene into N. tabacum L. enhances drought-related gene expression, increases antioxidant capacity, and reduces oxidative stress damage, thereby improving drought resistance.
1. Introduction
Drought stress is a major abiotic factor that impacts plant growth worldwide, with substantial negative effects on plant growth, development, and reproduction. Under drought conditions, water uptake in plants is restricted, resulting in decreased intracellular water, damage to cell membrane structures, and decreased cellular activity [1]. Additionally, drought induces alterations in osmoregulatory substances, which disrupt the intracellular ion balance and signaling, further affecting plant growth, development, and reproduction [2]. The enhancement of plant tolerance to drought stress is crucial for agricultural productivity and ecosystem sustainability. By identifying and utilizing drought-resistance genes in plant breeding, scientists can develop crop varieties with improved drought tolerance, thus increasing crop yield and quality under drought conditions [3].
Guizhou Province, situated on the eastern side of the Yunnan–Guizhou Plateau in southwestern China, experiences a monsoon climate that is variable and susceptible to both droughts and floods. Despite receiving substantial rainfall, the region frequently faces droughts due to engineered water shortages in karst landscapes. These droughts are widespread and prolonged, leading to decreased food production, frequent secondary disasters, and ecological degradation. Consequently, crops must exhibit drought tolerance to endure these conditions [4].
Eucommia ulmoides Oliver (E. ulmoides) is a rare and endangered plant in China. It has medicinal and edible value [5,6]. Its leaves can be used for tea, and its bark can be used as a medicinal material, as it has antihypertensive effects, reduces blood sugar, reduces blood lipids, and has anticancer and other health effects [7,8,9]. E. ulmoides is distributed mainly in Guizhou, Sichuan, Chongqing, Yunnan, Shanxi, Hunan, and Hubei Provinces. It can be successfully introduced into arid and semiarid areas because of its strong vitality and adaptability. Many functional genes, such as genes related to resistance to adversity, insects, and disease, have been cloned and studied in the E. ulmoides genome [10,11,12,13]. By studying stress-related genes in E. ulmoides, scientists have investigated the expression patterns of E. ulmoides genes in harsh environments and used these functional genes to improve the resistance of other crops to stress [14,15].
In recent decades, attention has been given to the adverse response functions of the inorganic pyrophosphatase (PPase) gene family. PPases represent a group of hydrolytic enzymes present in plants, animals, and microorganisms, and these enzymes are crucial for several physiological processes, including plant growth and development, sugar metabolism, and responses to salt and drought stress. PPases can hydrolyze the inorganic pyrophosphate (PPi) generated during plant growth and metabolism into two inorganic orthophosphates (Pi). If PPi is not quickly removed, its excessive accumulation can lead to cytotoxicity and cell death [16]. The hydrolysis of PPi by PPases not only supplies energy to plant cells but also contributes to the maintenance of intracellular PPi homeostasis [17]. The role of PPases in plant responses to abiotic stresses may be significant.
Current research has categorized PPases into two broad groups: enzymes prevalent in plant cells, which are further classified into two main families: membrane-integral pyrophosphatase (H+-PPase) and soluble pyrophosphatase (s-PPase) [18]. Soluble pyrophosphatases are further subdivided into three subtypes: s-PPase I, s-PPase II, and s-PPase III. In plants, s-PPase classification is limited to s-PPase I [19]. Chen et al. [20] cloned seven soluble inorganic pyrophosphatase genes, encoding ZmPPases, from maize (Zea mays L.). These genes exhibited upregulation or downregulation under salt and drought stress, respectively, suggesting a role of ZmPPases in plant responses to these stresses. The expression of the soluble inorganic pyrophosphatase genes HbSIP1/2/3 in the Brazilian rubber tree [Hevea brasiliensis (Willd. ex A. Juss.) Müll. Arg]. This gene was found to be modulated in response to treatments such as ethylene (ET), jasmonic acid (JA), high temperature, and low temperature, indicating the potential involvement of these genes in the plant response to environmental stresses [21]. George et al. reported that silencing the s-PPase I gene in N. tabacum L. led to PPi accumulation; reduced chlorophyll, carotenoid, and starch contents; and decreased abscisic acid (ABA) levels, affecting stomatal closure and thereby decreasing the drought resistance of N. tabacum L. [22].
The AP2/ERF (ethylene transcription factor) family represents a widespread group of mega-family transcription factors in plants that play crucial roles in regulating plant responses to drought stress through interactions with downstream target genes [23,24,25,26]. NCED (9-cis-epoxycarotenoid dioxygenase) is a key rate-limiting enzyme in the ABA biosynthesis of plants. Drought stress prompts a rapid increase in the synthesis of ABA in plants. As an important phytohormone, ABA plays a crucial role in the response of plants to drought stress [27,28,29,30]. P5CS (pyrroline-5-carboxylic acid synthase) contributes to plant drought tolerance primarily through its function as a key enzyme in the proline biosynthesis pathway, which increases the proline content in plant cells by catalyzing the conversion of glutamate to proline [31]. The protein encoded by the CYP85A gene is a critical enzyme in the biosynthetic pathway of the endogenous plant hormone oleuropein lactone (BR). Oleuropein lactones are essential phytohormones involved in plant growth, development, and response to environmental stresses [32]. Additionally, the protein encoded by the DET gene acts as a rate-limiting enzyme in the BR biosynthetic pathway. The impact of BRs on drought tolerance in plants is mediated through their influence on BR biosynthesis [33]. F-box proteins are important for plant drought tolerance, as they are integral components of the ubiquitin-26S proteasome system (UPS), which regulates plant adaptation to drought by recognizing and degrading specific target proteins [34,35].
Under drought conditions, the excessive production of reactive oxygen species (ROS) occurs in plants, surpassing the scavenging capacity of the plant’s antioxidant system and thereby inducing oxidative stress. The enzymes superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) act synergistically to address this stress. SOD transforms superoxide anions into hydrogen peroxide, while POD and CAT further decompose hydrogen peroxide to sustain the intracellular redox balance; mitigate drought-induced oxidative damage; and safeguard cell membranes, proteins, and nucleic acids, which are essential for plant adaptation to drought conditions. This process is crucial for plant survival in arid environments [36,37,38].
In this study, EuSIP5, a soluble inorganic pyrophosphatase gene from E. ulmoides, was identified and screened from a previously constructed whole-gene annotation library of E. ulmoides. A bioinformatics analysis was then conducted to predict the protein structure. The subsequent transformation of N. tabacum L. (Nicotiana tabacum cv. Xanthin) with an overexpression vector containing the EuSIP5 gene was carried out. Preliminary analyses were performed to determine whether EuSIP5 enhances drought tolerance. This involved measuring relative gene expression in N. tabacum L.-overexpressing plants, evaluating their protective enzyme activities, and conducting phenotypic observations. These findings may provide a theoretical foundation for research into soluble inorganic pyrophosphatase as a means of overcoming drought resistance in plants or crops.
2. Materials and Methods
2.1. Materials
N. tabacum L., utilized for genetic transformation and subcellular localization, was maintained by the Key Laboratory of Plant Resources Conservation and Germplasm Innovation in Mountainous Regions (Ministry of Education) at Guizhou University. E. ulmoides was cultivated at the laboratory base. Young bark, leaves, male flowers, and fruits were collected from 18-year-old mature E. ulmoides plants, both female and male, at this location.
The Escherichia coli (E. coli) strains DH5α, Agrobacterium tumefaciens (A. tumefaciens) GV3101, and the empty vector (pSH737) were preserved and supplied by our laboratory. The subcellular localization empty vector (pEGOEP35S-H-GFP) was obtained from Wuhan Aidijing Biotechnology (Wuhan, China).
2.2. Methods
2.2.1. Process of Gene Cloning and Subsequent Bioinformatics Analysis
The EuSIP5 protein-coding gene was identified from the E. ulmoides whole-genome annotation database constructed by this project team. Primers specific to the EuSIP5 gene were designed for amplification and sequencing by the NCBI (Table S1). The coding sequence was amplified using PCR and sequenced, yielding a EuSIP5 cDNA sequence of 552 bp in length.
The use of online websites and software to analyze the cloned sequences bioinformatically and predict the physical and chemical properties, functions, and phylogenetics of the proteins was performed. The physicochemical properties of the proteins were analyzed using the ‘protparam’ program on the online website ExPASy (https://web.expasy.org/protparam/, accessed on 13 August 2024). The hydrophilicity and hydrophobicity of the proteins were analyzed via ProtScale (https://web.expasy.org/protscale/, accessed on 13 August 2024). A structural analysis of the conserved domains was performed via the ‘CD-Search’ tool, which is hosted by the National Center for Biotechnology Information Bureau (NCBI) (https://www.ncbi.nlm.nih.gov/cdd/, accessed on 13 August 2024). The analysis of protein phosphorylation sites was conducted using the ‘NetPhos 3.1’ tool (http://www.cbs.dtu.dk/services/NetPhos/, accessed on 13 August 2024). The protein signal peptide sequence was predicted using the web-based tool ‘SignalP 5.0’ (http://www.cbs.dtu.dk/services/SignalP/, accessed on 13 August 2024). The protein transmembrane regions were predicted using the ‘TMHMM 2.0’ server version 2.0 (http://www.cbs.dtu.dk/services/TMHMM/, accessed on 13 August 2024). The protein 3D structures were predicted via SWISS-MODEL software (https://swissmodel.expasy.org, accessed on 13 August 2024). The amino acid sequences of EuSIP5 were compared with those of other plant s-PPases using the DNAMAN V6 software program. A phylogenetic tree was generated using MEGA 7 to depict the evolutionary relationships among soluble inorganic pyrophosphatase family genes. The soluble inorganic pyrophosphatase sequences from other species are provided in Table S2.
2.2.2. Expression Analysis of the EuSIP5 Gene in E. ulmoides
The materials were collected from 19-year-old E. ulmoides plants. In the same year, various organs (young stem bark, young leaves, flowers, and fruits) from both male and female plants were collected in different months (from April to October). The total RNA was extracted using the OMEGA Plant Total RNA Extraction Kit and was reverse transcribed into cDNA via the Sevier Reverse Transcription Kit (Wuhan Servicebio Technology Co., Ltd., Wuhan, China).
2.2.3. Vector Construction
The GUS reporter genes were selected as marker genes for vector construction. The target gene fragments were integrated into the pSH737 vector using EcoRI and XbaI as digestion sites. The EuSIP5 plant overexpression vector pSH737-35S-EuSIP5-Nos was constructed through a one-step cloning method using the laboratory-constructed Kan resistance screening pSH737 as the original vector. E. coli DH5α containing the pSH737 plasmid was cultured on LB solid media supplemented with 100 mg·L–1 Kan. Plasmid DNA was extracted from Kan-resistant positive E. coli single colonies, and the recombinant plasmid DNA was stored at −20 °C for future use. The previously obtained EuSIP5 gene target fragment and pSH737 plasmid were double digested, and the target fragment was gel-purified. The digested target fragment and pSH737 plasmid were then ligated. After successful sequencing, the target fragment was transferred to Agrobacterium GV3101 and stored at −80 °C.
Vector selection of GUS reporter genes as marker genes. The target gene fragments were integrated into the pSH737 vector using EcoRI and XbaI as digestion sites. The EuSIP5 plant overexpression vector pSH737-35S-EuSIP5-Nos was constructed via a one-step cloning method via the original vector containing the laboratory constructed Kanresistance screening pSH737. E. coli DH5α containing the pSH737 plasmid was inoculated into LB solid medium containing 100 mg·L−1 Kan, the plasmid DNA of the Kan-susceptible positive E. coli single colony was extracted, and the collected recombinant plasmid DNA was stored at −20 °C for backup use. The previously obtained EuSIP5 gene target fragment and pSH737 plasmid were double digested, the obtained target fragment was gelatinized, and the digested target fragment and digested pSH737 plasmid were ligated. After correct sequencing, the target fragment was transferred to Agrobacterium GV3101 and stored at −80 °C.
2.2.4. Subcellular Localization
Transient expression of the EuSIP5 fusion protein was achieved by injecting N. tabacum L. leaves. The constructed pEGOEP35S-H-EuSIP5-eGFP vector plasmid was subsequently electroporated into A. tumefaciens GV3101. After overnight incubation at 37 °C, A. tumefaciens colonies were collected from the solid Petri dishes using an inoculation loop. N. tabacum L. plants, 3–4 weeks old, were selected. The back of each leaf was gently pierced with a 1 mL defoaming syringe needle and injected from the lower epidermal surface using a syringe with the needle removed. The waterlogged areas are marked. The injected N. tabacum L. plants were incubated at 25 ± 2 °C under low light for 16–18 h over 2 days. After incubation, the labeled N. tabacum L. leaves were removed, prepared into slides, and examined under a laser confocal microscope for observation and photography.
2.2.5. Genetic Transformation and Identification of N. tabacum L.
Wild-type N. tabacum L. (WT) seedlings, which were cultivated for 3–4 weeks and reached the 6–10-leaf stage, were selected for genetic transformation via the leaf disc method. Leaves from the WT N. tabacum L. were excised into discs of about 1.0 cm2. A. tumefaciens strain GV3101, harboring the pSH737-35S-EuSIP5-NOS vector, was grown overnight in YEP liquid media at 28 °C with shaking (180 rpm). Following centrifugation and resuspension, the bacterial culture reached a final optical density (OD) of 0.5 at 600 nm. The leaf discs were immersed in the bacterial suspension for 5 min, excess bacteria were removed using sterile filter paper, and the discs were cocultivated in the dark for 2 days. The discs were subsequently placed on screening media supplemented with Kan antibiotic, and the media was subsequently changed every 2 weeks. Once the differentiated shoots reached 1–2 cm in length, they were transferred to rooting media to promote rooting and develop robust seedlings for further treatments (incubation conditions were 25 ± 2 °C, 16/8 h diurnal photoperiod). The transgenic plants were ultimately recovered through high-temperature and high-pressure inactivation and incineration.
Since the pSH737 overexpression vector employs a GUS reporter gene as a marker, preliminary validation was conducted using GUS chemical tissue staining. Leaves from the generation N. tabacum L. seedlings, which were subsequently grown on Kan media containing antibiotics, were immersed in GUS stain for 15 min under a 25 kPa vacuum before being transferred to a 37 °C incubator overnight incubation. The samples were subsequently decolorized with ethanol, and the GUS staining results were evaluated. DNA from the positively stained transgenic N. tabacum L. lines was extracted using the TIANGEN Plant Genomic DNA Kit (TIANGEN Biotech (Beijing) Co., Ltd., Beijing, China). Following extraction, 1 μL of the DNA template was subjected to PCR with the specific primers EuSIP5-F and EuSIP5-R to confirm the integration of the EuSIP5 gene into OE N. tabacum L.
2.2.6. Quantitative Fluorescence Real-Time PCR (qRT-PCR)
Specific qRT-PCR primers were designed via Primer Premier 5 software. Total plant RNA was extracted following the OMEGA kit instructions, and RNA was reverse transcribed to cDNA using the Sevier Reverse Transcription Kit. The optimal thermal cycling conditions for qRT-PCR were established as follows: predenaturation at 95 °C for 5 min, followed by cycling. The cycling protocol included 40 cycles at 95 °C (30 s) and 58 °C (30 s), with fluorescent signals collected at each annealing and extension phase (58 °C). Upon completion of all the qRT-PCR cycles, a melting curve was generated with a temperature gradient of 65–95 °C (0.1 °C/s) to confirm the specificity of the product.
2.2.7. Drought Stress Treatment of Transgenic N. tabacum L.
To assess the response of EuSIP5 transgenic N. tabacum L. to drought stress, wild-type (WT) and transgenic (OE) plants of the same strain and growth conditions were subjected to simulated drought conditions by withholding water for 10 days. Sampling was conducted at 0, 2, 4, 6, and 8 days. During the normal growth period in the climate chamber, the plants were watered every 3 days. The third day after the last watering was designated 0 days of drought treatment, with subsequent sampling occurring every 2 days for observation and photography. In the artificial climate chamber, the same watering schedule was applied, and the third day post-watering was considered 0 days of drought treatment. Samples were collected every 2 days, observed, and photographed. After quick freezing in liquid nitrogen, the samples were stored at −80 °C. RNA was extracted, reverse transcribed into cDNA, and analyzed using qRT-PCR. The NtEF1α gene of N. tabacum L. served as an internal reference gene. Primer characterization was evaluated with reference to Hellemans et al. (Table S3), and relative expression was calculated using the 2−ΔΔCt method [39]. When the amplification efficiency of the genes were similar and nearly 100% and within 5% of each other, the method, 2−ΔΔCt was used.
2.2.8. Expression Analysis of Genes Related to Drought Resistance in Transgenic N. tabacum L.
The expression of drought tolerance-related genes in transgenic N. tabacum L. was evaluated using qRT-PCR, with NtEF1α serving as the internal reference gene. The drought tolerance-associated genes in N. tabacum L. included NtNCED1 (NM_001325669.1), NtERF1 (D38123.1), NtP5CS (GBEA01096483.1), NtCYP85A1 (DQ649022.1), NtDET2 (GBEA01057209.1), and NtF-Box (GBEA01054484.1), and specific qRT-PCR primers were designed using Primer Premier 5 software (Table S1). For the expression analysis, transgenic and wild-type tobacco leaves that had been rooted and grown for 3–4 weeks and were at the 6–10-leaf stage were selected.
2.2.9. Determination of Protective Enzyme Activity
Three wild-type (WT) plants and three overexpressing (OE) plants of the same strain were collected under identical growth conditions. The same leaves were subjected to drought conditions for 0, 2, 4, 6, or 8 days. The activities of catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD), as well as the content of malondialdehyde (MDA), were measured following the provided instructions, with the WT plants serving as the control group. The CAT enzyme activity assay kit and MDA content assay kit were obtained from Servicebio (Wuhan Servicebio Technology Co., Ltd., Wuhan, China), the SOD enzyme activity assay kit from Nanjing Jianjian Online Shopping Mall (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and the POD enzyme activity assay kit from Solarbio (Wuhan Servicebio Technology Co., Ltd., Beijing, China). Each experiment was conducted with three biological replicates and three analytical replicates.
2.2.10. Statistical Analysis
All the data are presented as the means ± standard errors from three biological replicates and their respective technical replicates. Statistical analyses were conducted using two-way ANOVA, with multiple comparisons performed using Dunnett’s test (for drought-related gene expression in N. tabacum L.) and Tukey’s test (for relative expression of the EuSIP5 gene in E. ulmoides). The Shapiro-Wilk test was used to assess normality, and Levene’s test was used to check for homogeneity of variance. Significance is indicated by asterisks (*) and letters; an asterisk (*) indicates a significant difference, whereas “ns” indicates no significant difference. In the letter method, different letters denote significant differences, whereas the same letter indicates no significant difference. Analyses were carried out using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA), and all figures were generated using GraphPad Prism 9.0.
3. Results
3.1. Cloning and Analysis of the EuSIP5 Gene
The primers used were specifically designed on the basis of the cDNA sequence of the EuSIP5 gene, with E. ulmoides cDNA serving as the template for PCR amplification. Analysis using 1% agarose gel electrophoresis revealed a prominent band within the range of 500 bp to 750 bp. Sequencing yielded a fragment of 552 bp, which corresponded to the sequence comparison data in the database (Figure S1).
The sequencing results indicated that the cDNA sequence from which the gene was cloned was 552 bp in length. Bioinformatic analysis revealed that the theoretical relative molecular mass (MW) of the EuSIP5 protein was 20.651 kDa, with an isoelectric point (pI) of 6.44. The protein was found to contain 26 acidic amino acid residues (Asp + Glu) and 24 basic amino acid residues (Arg + Lys). The instability index was 41.51, the aliphatic index was 85.82, and the grand average of hydropathicity (GRAVY) was −0.334, suggesting that the protein is an unstable amphiphilic protein. The CELLO prediction system server was used to predict the subcellular localization of the protein in the cytoplasmic and nuclear membranes. Analysis of the conserved structural domains revealed a conserved domain named “PLN02373”, previously described as a soluble inorganic pyrophosphatase in the pyrophosphatase superfamily (Figure 1a). Comparison with homologous proteins from other species revealed that EuSIP5 shares 88.10% similarity with these proteins (Figure 1b). Phylogenetic analysis of the amino acid sequence of EuSIP5 revealed that it is most closely related to soluble inorganic pyrophosphatases in Camellia lanceoleosa and Cornus florida (Figure 1c).
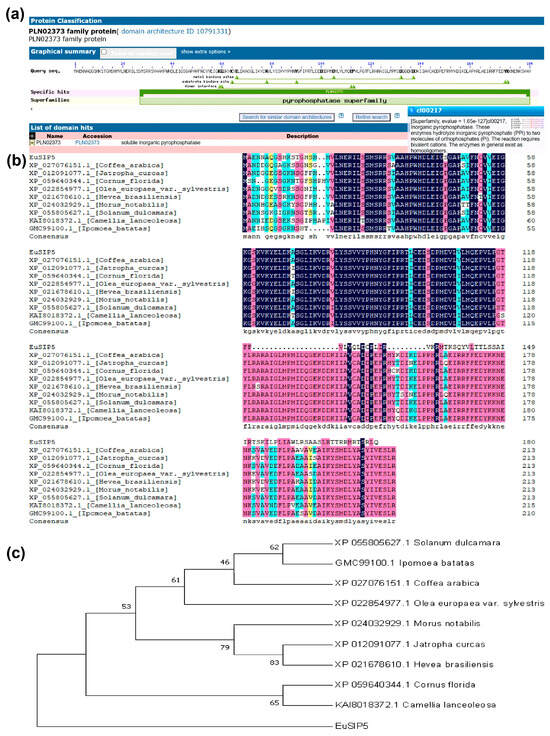
Figure 1.
Prediction of conserved structural domains of EuSIP5, multiple sequence alignment of EuSIP5 with other species and construction of the EuSIP5 phylogenetic tree. (a) Conserved domain analysis of EuSIP5 revealed that it is a soluble inorganic pyrophosphatase of the pyrophosphatase superfamily. (b) EuSIP5 has 88.10% similarity to homologous proteins of other species. (c) The results revealed that the soluble inorganic pyrophosphatase EuSIP5 in E. ulmoides was most closely related to the soluble inorganic pyrophosphatases in Camellia lanceoleosa and Cornus florida. Colored according to sequence homology: black = 100%, pink ≥ 75%, blue ≥ 50%, yellow ≥ 33%.
3.2. Spatiotemporal Expression Characteristics of the EuSIP5 Gene
To examine the expression characteristics of the EuSIP5 gene in male and female strains of E. ulmoides, qRT-PCR was used to analyze gene expression in various tissues collected at different times. The EuSIP5 gene exhibited variable expression across all tissues of both male and female E. ulmoides plants, with tissue-specific patterns observed. The highest expression was detected in male plant leaves, followed by male stem bark, female plant leaves, female stem bark, female fruit, female roots, male roots, female flowers, and male flowers. These findings suggest that the gene functions predominantly in nutrient-producing organs (Figure 2a). The overall expression level of the EuSIP5 gene in both female and male plant leaves was greater than that in female and male stems from April to October. In July, female plant leaves presented the highest expression, which was 9.63 times greater than that in female stems. In male plant leaves, the highest expression occurred in September, which was being 12.51 times greater than that in male stems during the same month (Figure 2c,d). The expression of the EuSIP5 gene displayed significant spatial and temporal specificity, particularly during periods and in parts of the plant with high metabolic activity, indicating a close association with nutritional growth and metabolic processes. Compared with the initial level, under natural drought conditions for 15 days, the expression of the EuSIP5 gene significantly increased (p < 0.05), with a 1.41-fold increase, demonstrating that the gene responds to drought stress (Figure 2b).
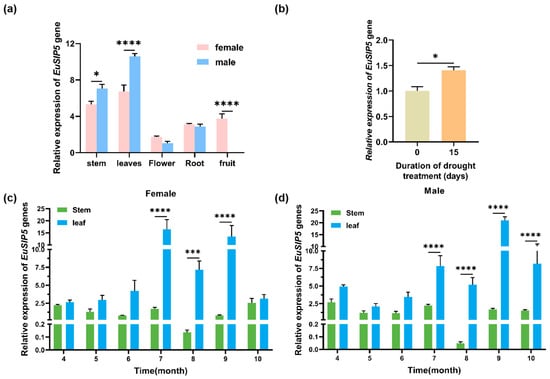
Figure 2.
Relative expression of the EuSIP5 gene in different organs of E. ulmoides and in plants of different sexes. (a) Expression patterns of the EuSIP5 gene in different organs of E. ulmoides; (b) Expression of the EuSIP5 gene under drought treatment; (c) Comparison of the relative expression of the EuSIP5 gene in different organs of female E. ulmoides in different months; (d) Comparison of the relative expression of the EuSIP5 gene in different organs of male E. ulmoides in different months. The error bars indicate the standard error (SE); * p < 0.05, *** p < 0.001, **** p < 0.0001.
3.3. Subcellular Localization Analysis of EuSIP5
In Arabidopsis thaliana, six variants of soluble inorganic pyrophosphatase (AtPPA1-AtPPA6) have been characterized ntified [40,41]. Localization studies using green fluorescent protein (GFP) as a marker revealed that AtPPA1-AtPPA5 are found mainly in the cytoplasm, whereas AtPPA6 is located in chloroplasts [42]. Subcellular localization experiments with GFP-tagged PbrPPA1, PbrPPA2, PbrPPA5, PbrPPA9, and PbrPPA10 revealed that PbrPPA1, PbrPPA2, and PbrPPA5 were located in the cytoplasm and associated with the nuclear membrane, whereas PbrPPA9-GFP and PbrPPA10-GFP were found in chloroplasts [43].
EcoRI and HindIII were utilized as cleavage sites for double-digestion verification, and the resulting vector was designated pEGOEP35S-H-EuSIP5-eGFP following successful verification (Figure S2a). EuSIP5 protein fluorescence was observed in the cytoplasm of cells using laser confocal microscopy, indicating that the soluble inorganic pyrophosphatase EuSIP5 may function within the cytoplasm (Figure 3).
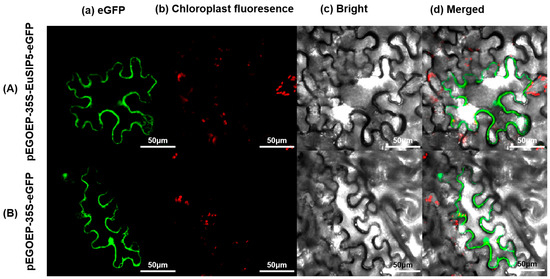
Figure 3.
Subcellular localization of EuSIP5 in N. tabacum L. cells. (A) Localization map of pEGOEP-35S-EuSIP5-eGFP in N. tabacum L. cells; (B) localization map of pEGOEP-35S-eGFP in N. tabacum L. cells; (a) Green fluorescence from labeled proteins; (b) chloroplast self-illumination; (c) bright field; (d) superposition field. Scale bar: 50 μm.
3.4. Genetically Modified N. tabacum L. and Identification of Positive Transgenic Plants
The overexpression vector for the EuSIP5 gene was constructed and designated pSH737-35S-EuSIP5-Nos following correct sequencing (Figure S2b). Agrobacterium containing the pSH737-35S-EuSIP5-Nos vector was introduced into N. tabacum L. Leaves of the Agrobacterium-infected N. tabacum L. plants developed into small green shoots after 4 weeks. These shoots were then removed, placed on rooting media, and subsequently transplanted into the soil once the shoots had grown and the root system was sufficiently developed (Figure S3a–d). Transgenic N. tabacum L. plants were thus obtained.
GUS histochemistry revealed that the leaves of the WT N. tabacum L. plants did not exhibit blue staining, whereas the leaves of the OE plants were stained blue (Figure S3e), confirming the transfer of pSH737-35S-EuSIP5 into N. tabacum L. PCR analysis revealed that the transgenic N. tabacum L. plants produced a gene-specific band of 552 bp, whereas the WT N. tabacum L. plants did not, confirming the integration of the EuSIP5 gene into the N. tabacum L. genome (Figure S3f). Among the transgenic lines, OE3, OE5, and OE10, which presented the best growth and highest expression, were selected for further experiments.
3.5. Effect of the Trans-EuSIP5 Gene on Drought Tolerance in N. tabacum L.
The expression of the EuSIP5 gene and drought-related genes in N. tabacum L. was evaluated in WT and OE plants from 0 to 8 days under simulated natural drought conditions using qRT-PCR. The results revealed that the expression of the DET2 gene in OE plants initially decreased but then increased, with significantly lower expression than that in WT plants after 2 days of drought treatment. The expression subsequently increased continuously, reaching a level that was 6.63 greater higher than that in the WT plants at 8 days (Figure 4a). The expression of the CYP85A1 gene in OE plants remained stable from 0 to 2 days, then increased and subsequently decreased. The peak expression occurred at 4 days, which was 5.73-fold greater than that of the WT plants (Figure 4b). The expression of the P5CS, ERF1, and F-box genes in OE plants was greater than that in WT plants after treatment, with the F-box gene exhibiting the highest expression at 6 days, which was 1.75 times greater than that in WT plants (Figure 4c). The P5CS and ERF1 genes reached their peak expression at 4 days, being which was 2.82 and 5.90 times higher greater than that in the WT plants, respectively (Figure 4d,e). The NCED1 gene exhibiting was highest expression at 8 days, 1.19 times greater than that in WT plants (Figure 4f).
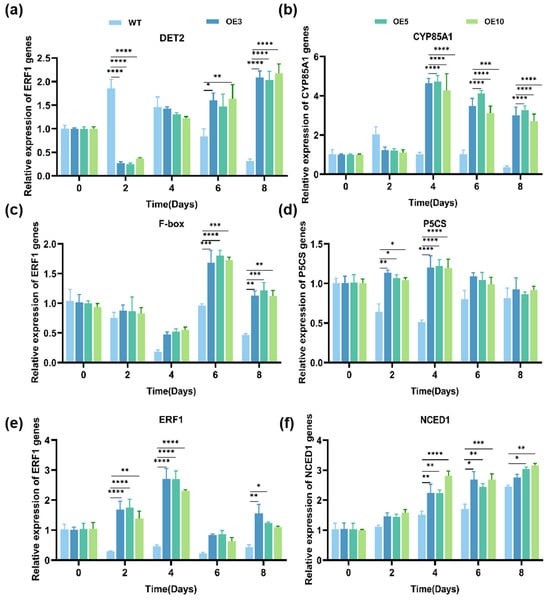
Figure 4.
Expression level analysis of genes related to N. tabacum L. drought resistance. (a) Relative expression of the NtDET2 gene in N. tabacum L.; (b) Relative expression of the NtCYP85A1 gene in N. tabacum L.; (c) Relative expression of the NtF-box gene in N. tabacum L.; (d) Relative expression of the NtP5CS gene in N. tabacum L.; (e) Relative expression of the NtERF1 gene in N. tabacum L.; (f) Relative expression of the NtNCED1 gene in N. tabacum L.; WT: wild-type plants; OE: transgenic plants; error bars indicate standard error (SE); * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Phenotypic observations indicated that wilting and stem collapse began on day 4 of natural drought in WT N. tabacum L., whereas in OE N. tabacum L., wilting of the leaf tips started on day 8 of treatment, with overall symptoms being less severe (Figure 5).
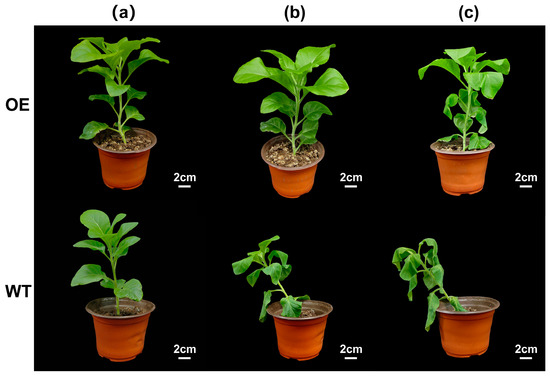
Figure 5.
Phenotypic comparison of N. tabacum L. under drought treatment (0–8 d). (a) After drought treatment for 0 days, the WT and OE N. tabacum L. plants were in good condition. (b) Wild-type N. tabacum L. plants were subjected to drought treatment for 4 days. (c) After drought treatment for 8 days, the transgenic N. tabacum L. plants began to wilt. WT: wild-type plants; OE: transgenic plants; scale bar: 2 cm.
3.6. Effect of Trans-EuSIP5 on the Activity of N. tabacum L. Protective Enzymes
The activities of protective enzymes in WT and OE N. tabacum L. plants were measured after 0 to 8 days of simulated natural drought treatment. The activities of POD, SOD, and CAT enzymes were greater in OE plants than in WT plants. The POD activity peaked at 1732.65 U·g−1 on day 4, which was 2.00 times greater than that in WT plants (Figure 6a). The SOD enzyme activity reached its maximum at 960.663 U·g−1 after 8 days of treatment, which was 1.13 times greater than the in the WT plants (Figure 6b). The highest CAT activity was observed on day 6, with a value of 49.4174 μmol·g−1 FW, which was 1.60 times greater than that in the WT controls (Figure 6c). The MDA content in OE N. tabacum L. plants was lower than that in WT plants after treatment, with a significant decrease to 2.77 μmol·L−1 after 6 days, representing a 19.36-fold reduction compared to with that in WT plants (Figure 6d).
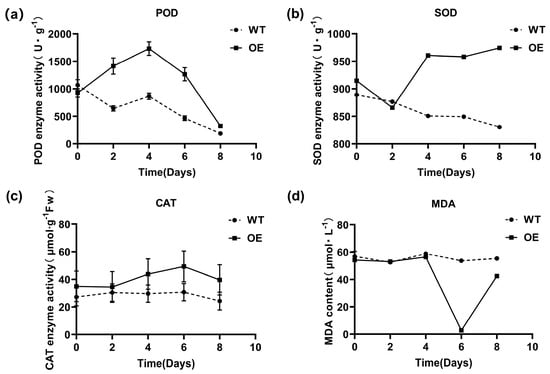
Figure 6.
Analysis of N. tabacum L. protective enzyme viability. (a) POD enzyme activity; (b) SOD enzyme activity; (c) CAT enzyme activity; (d) MDA content. The error bars indicate the standard error (SE).
4. Discussion
Globally, drought is the leading cause of reduced agricultural output, with annual losses due to drought representing 53% of the total, surpassing the combined impact of other natural disasters [44]. This study aimed to explore the potential of the EuSIP5 gene, a soluble inorganic pyrophosphatase from E. ulmoides, in enhancing plant drought tolerance. Cloning, bioinformatics analysis, overexpression vector construction, and expression of the EuSIP5 gene in N. tabacum L. revealed that overexpression of the EuSIP5 gene increased the drought tolerance of N. tabacum L.
The secondary structure of the protein encoded by the ZmPPase gene, a soluble inorganic pyrophosphatase from maize, is largely characterized by α-helices and β-folds, which facilitate its catalytic activity. The protein’s molecular weight varies from 22.8 to 25.6 kDa, with an isoelectric point ranging from 4.84 to 6.24 [20]. In comparison, the present study revealed that the EuSIP5 protein features three distinct secondary structures: α-helices, extended linkages, and irregular coils. The EuSIP5 protein has a molecular mass of 20,108.47 Da and an isoelectric point of 9.79. This variation might be due to evolutionary differences in s-PPase genes among species. Domain analysis revealed a soluble inorganic pyrophosphatase domain within the EuSIP5 protein. Sequence comparison and phylogenetic analysis confirmed strong similarity to homologous proteins and the presence of the inorganic pyrophosphatase structural motif. Additionally, subcellular localization studies indicated that EuSIP5 is localized in the cytoplasm, which aligns with the observations of Gutiérrez-Luna, Navarro de la Sancha, Valencia-Turcotte, Vázquez-Santana and Rodríguez-Sotres [42]. These findings suggest that the EuSIP5 gene encodes a soluble inorganic pyrophosphatase in E. ulmoides.
In a study of the EuERD16 gene in perennial adult E. ulmoides, Li Bo reported that its expression was greater in viviparous leaves collected in March and May and in fruits from July [45]. The EuEFD16 gene was consistently upregulated in tissues that exhibited rapid division and growth during the early stages of development. This study examined the spatiotemporal expression patterns of the EuSIP5 gene in E. ulmoides. These results suggest that EuSIP5 plays a role in the growth and development of E. ulmoides. Moreover, the expression of this gene is tissue-specific, with the highest levels found in the leaves of male plants. Additionally, variations in EuSIP5 gene expression across different months suggest a potential influence of environmental factors. The rapid growth and development of E. ulmoides from July to September were linked to increased plant respiration and photosynthesis, along with a significant increase in EuSIP5 gene expression in nutrient-producing organs. Conversely, high temperatures in August led to reduced respiration and transpiration, which was associated with decreased expression of the EuSIP5 gene.
Genetic transformation of N. tabacum L. was utilized to overexpress the EuSIP5 gene in N. tabacum L. Phenotypic comparison of the plants under drought stress demonstrated that the OE plants presented significantly greater growth and development than the WT plants. Additionally, the drought tolerance of in the transgenic N. tabacum L. was notably improved, as indicated by a reduction in wilting rates. Analysis of the expression changes in drought-related genes of N. tabacum L., including DET2, CYP85A1, P5CS, ERF1, F-box NCED1 genes, suggested that the EuSIP5 gene might enhance drought responsiveness in N. tabacum L. by modulating the expression of drought-responsive genes, leading to increased antioxidant enzyme activity and decreased MDA content.
When drought inhibits the normal growth and metabolism of plants, the content of the membrane lipid peroxidation product MDA in plant tissues increases significantly [46]. This leads to the suppression of plant photosynthesis, which activates a protective enzyme system, including protective enzymes such as SOD. The POD and CAT enzymes contribute to the protection of cell membranes. Among these, SOD is considered the central component of the protective system and is positively correlated with the antioxidant stress capacity of plants [47,48]. SOD mitigates membrane lipid peroxidation damage through dismutation reactions using superoxide anion (O2−) as a substrate [49], whereas POD scavenges harmful free radicals and degrades toxic substances within cells [50]. CAT effectively decomposes excess H2O2 in tissues [51]. The coordinated action of various antioxidant enzymes maintains low intracellular levels of reactive oxygen species (ROS), thereby protecting plant cells from or mitigating damage caused by oxidative stress [38]. Measurement of protective enzyme activities revealed that the activities of POD, SOD, and CAT enzymes were greater in the OE plants than in the WT plants, whereas the MDA content was lower. These findings suggest that the overexpression of EuSIP5 enhances resistance to lipid oxidation and reduces oxidative stress damage in N. tabacum L., thus improving the drought tolerance of the plants.
In conclusion, this study offers new insights into the potential of genetic engineering technology to increase drought tolerance in plants and identifies promising genetic resources for the development of drought-resistant crop varieties in the future. This advancement could contribute to the sustainability of agricultural production and ecosystems. However, additional research is needed to clarify the specific role of the EuSIP5 gene in the mechanisms underlying drought tolerance in plants.
5. Conclusions
In this study, the EuSIP5 gene was successfully cloned, and it was identified as a potentially unstable amphiphilic protein localized in the cytoplasm. Analysis of the spatiotemporal and tissue-specific expression of the EuSIP5 gene across various tissues and developmental stages of E. ulmoides revealed its close association with plant nutrient growth and metabolic activities. Furthermore, the introduction of the EuSIP5 gene into N. tabacum L. was shown to increase its drought resistance.
To investigate the role of the EuSIP5 gene in enhancing drought tolerance in E. ulmoides, gene cloning, bioinformatics analysis, overexpression vector construction, and expression in N. tabacum L. were performed. These results demonstrated that the overexpression of the EuSIP5 gene increased drought tolerance in N. tabacum L.
The secondary structure, molecular weight, and isoelectric point of the EuSIP5 protein differ from those of analogous proteins in other species. Subcellular localization analysis revealed that EuSIP5 is located in the cytoplasm. Spatiotemporal expression analysis of the EuSIP5 gene in E. ulmoides revealed its involvement in plant growth and development, tissue-specific expression, and potential sensitivity to environmental factors. Transgenic N. tabacum L. overexpressing the EuSIP5 gene presented increased growth and development, improved drought tolerance, altered expression of drought-related genes, increased antioxidant enzyme activities, and reduced MDA content.
For future research, a more detailed investigation into the specific role of the EuSIP5 gene in the mechanisms of drought tolerance in plants is warranted. This study advances the understanding of how genetic engineering technology can be utilized to improve drought tolerance in plants and contribute to the development of drought-resistant crop varieties, supporting the sustainable development of agricultural production and ecosystems.
Supplementary Materials
The following supporting information is available for download at https://www.mdpi.com/article/10.3390/horticulturae10091010/s1, Table S1: List of primers used for the experiments; Table S2: Soluble inorganic pyrophosphatase sequences retrieved from NCBI for different plant species; Table S3: Characteristics of the used primers; Figure S1: Target gene band test; Figure S2: Carrier construction maps; Figure S3: Genetic transformation process in N. tabacum L., GUS staining, and PCR identification.
Author Contributions
D.Z. and C.L. conceived and designed the experiments; Y.L. and X.C. performed the experiments and analyzed the data; Y.L. wrote the Manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 31870285), Guizhou Academy of Agricultural Sciences Talent Special Project (No. 2023-02), and by Talent Base for Germplasm Resources Utilization and Innovation of Characteristic Plant in Guizhou (RCJD2018-14).
Data Availability Statement
The data that support the fundings of this study are available from the corresponding author on reasonable request.
Acknowledgments
We thank all the colleagues that helped with the development of different parts of this manuscript and Key Laboratory of Mountain Plant Resources Protection and Germplasm Innovation of the Ministry of Education and Guizhou Plant Conservation Technology Center, Guizhou Key Laboratory for providing equipment and technical support for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance; John Wiley & Sons: New York, NY, USA, 2013; pp. 209–250. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.X.; Shi, J.W.; Li, R.; An, Y.C.; Yang, B.L. Effects of extreme drought on plant species in Karst area of Guizhou Province, Southwest China. Chin. J. Appl. Ecol. 2011, 22, 1127–1134. [Google Scholar] [CrossRef]
- Ren, N.; Gong, W.W.; Zhao, Y.C.; Zhao, D.G.; Xu, Y.W. Innovation in sweet rice wine with high antioxidant activity: Eucommia ulmoides leaf sweet rice wine. Front. Nutr. 2023, 9, 1108843. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Huang, X.; Zhao, Y.; Zhao, D. Cloning and Function Identification of a Phytoene Desaturase Gene from Eucommia ulmoides. Phyton 2023, 92, 1377–1389. [Google Scholar] [CrossRef]
- Park, S.A.; Choi, M.S.; Kim, M.J.; Jung, U.J.; Kim, H.J.; Park, K.K.; Noh, H.J.; Park, H.M.; Park, Y.B.; Lee, J.S.; et al. Hypoglycemic and hypolipidemic action of Du-zhong (Eucommia ulmoides Oliver) leaves water extract in C57BL/KsJ-db/db mice. J. Ethnopharmacol. 2006, 107, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Chiba, A.; Murakami, M.; Sekihashi, K.; Tanaka, M.; Takahoko, M.; Moribayashi, S.; Kudou, C.; Hara, Y.; Nakazawa, Y.; et al. Antimutagenicity of Tochu tea (an aqueous extract of Eucommia ulmoides leaves): 2. Suppressing effect of Tochu tea on the urine mutagenicity after ingestion of raw fish and cooked beef. Mutat. Res. Genet. Toxicol. 1996, 371, 203–214. [Google Scholar] [CrossRef]
- Yen, G.C.; Hsieh, C.L. Reactive Oxygen Species Scavenging Activity of Du-zhong (Eucommia ulmoides Oliv.) and Its Active Compounds. J. Agric. Food Chem. 2000, 48, 3431–3436. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, Y.C.; Ran, X.; Guo, L.X.; Zhao, D.G. Overexpression of a New Chitinase Gene EuCHIT2 Enhances Resistance to Erysiphe cichoracearum DC. in Tobacco Plants. Int. J. Mol. Sci. 2017, 18, 2361. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, B.; Zhao, Y.C.; Zhao, D.G. Cloning and characterization of the DIR1 promoter from Eucommia ulmoides Oliv and its response to hormonal and abiotic stress. Plant Cell Tissue Organ Cult. 2021, 146, 313–322. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, D.G. Cloning, Characterization, and Functional Analysis of EuTIL1, a Gene-Encoding Temperature-Induced Lipocalin in Eucommia ulmoides Oliv. Horticulturae 2023, 9, 950. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Liu, Y.Q.; Dong, X.; Liu, J.J.; Zhao, D.G. Identification of a novel laccase gene EuLAC1 and its potential resistance against Botrytis cinerea. Transgenic Res. 2022, 31, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Dong, X.; Huang, X.Z.; Zhao, D.G.; Zhao, Y.C.; Peng, L. Combined analysis of the transcriptome and proteome of Eucommia ulmoides Oliv. (Duzhong) in response to Fusarium oxysporum. Front. Chem. 2022, 10, 1053227. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Li, Y.; Zhao, Y.; Zhao, D. Overexpression of β-1,4-Glucanase Gene EuEG1 Improves Micrografting of Eucommia ulmoides. Phyton 2023, 92, 3063–3075. [Google Scholar] [CrossRef]
- Gloria, S.B.; Agustín, H.; Guillermo, L.L.; José Román, P.C.; Plácido, N.; Aurelio, S. Inorganic Pyrophosphatase Defects Lead to Cell Cycle Arrest and Autophagic Cell Death through NAD+ Depletion in Fermenting Yeast*. J. Biol. Chem. 2013, 288, 13082–13092. [Google Scholar] [CrossRef]
- Li, X.Y.; Zeng, R.Z.; Xiao, X.Z. Advances on the Research of Pyrophosphatase in Plants. Life Sci. Res. 2004, 8, 83–87. [Google Scholar] [CrossRef]
- Xian, J.H.; Zhang, M.P.; Sun, C.Y.; Wang, Y.F.; Wang, K.Y.; Chen, J.; Zhao, M.Z.; Wang, Y. Research Progress of Soluble Pyrophosphatase. Genom. Appl. Biol. 2019, 38, 4030–4035. [Google Scholar]
- Huang, H.; Patskovsky, Y.; Toro, R.; Farelli, J.D.; Pandya, C.; Almo, S.C.; Allen, K.N.; Dunaway-Mariano, D. Divergence of structure and function in the haloacid dehalogenase enzyme superfamily: Bacteroides thetaiotaomicron BT2127 is an inorganic pyrophosphatase. Biochemistry 2011, 50, 8937–8949. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.R.; Wang, X.L.; Fang, T.; Zhen, S.H.; Lu, J.W.; Zhang, J.; Fu, J.J. Cloning and Expression Analysis of Soluble Inorganic Pyrophosphatase Family Genes in Maize (Zea mays L.). J. Plant Genet. Resour. 2021, 22, 455–465. [Google Scholar] [CrossRef]
- Zhu, J.H.; Xu, J.; Yu, X.H.; Chang, W.J.; Zhang, Z.L. Prokaryotic Expression for Three Soluble Inorganic Pyrophosphatase Genes from Hevea brasiliensis. Chin. J. Trop. Crops 2013, 34, 41–45. [Google Scholar]
- George, G.M.; van der Merwe, M.J.; Nunes-Nesi, A.; Bauer, R.; Fernie, A.R.; Kossmann, J.; Lloyd, J.R. Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana. Plant Physiol. 2010, 154, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Song, Q.; Wei, H.; Wang, Y.; Lin, M.; Sun, K.; Zhang, Y.; Yang, J.; Li, C.; Luo, K.J.N.P. The AP2/ERF transcription factor PtoERF15 confers drought tolerance via JA-mediated signaling in Populus. New Phytol. 2023, 240, 1848–1867. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, L.; Jiang, W. Understanding AP2/ERF Transcription Factor Responses and Tolerance to Various Abiotic Stresses in Plants: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 893. [Google Scholar] [CrossRef]
- Wang, H.; Ni, D.; Shen, J.; Deng, S.; Xuan, H.; Wang, C.; Xu, J.; Zhou, L.; Guo, N.; Zhao, J.J.F.i.P.S. Genome-wide identification of the AP2/ERF gene family and functional analysis of GmAP2/ERF144 for drought tolerance in soybean. Front. Plant Sci. 2022, 13, 848766. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, M.; Zhang, S.; Song, T.; Zhang, M.; Zhou, H.; Wang, Y.; Xiang, J.; Zhang, X. Transcriptomic Identification of Wheat AP2/ERF Transcription Factors and Functional Characterization of TaERF-6-3A in Response to Drought and Salinity Stresses. Int. J. Mol. Sci. 2022, 23, 3272. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, X.; Chen, L.; Zhao, G.; Li, S.; Zhou, H.; Dai, Y.; Sun, N.; Xie, Y.; Gao, J.; et al. Genome-wide identification and expression analysis of the NCED family in cotton (Gossypium hirsutum L.). PLoS ONE 2021, 16, e0246021. [Google Scholar] [CrossRef]
- Avico, E.H.; Acevedo, R.M.; Duarte, M.J.; Rodrigues Salvador, A.; Nunes-Nesi, A.; Ruiz, O.A.; Sansberro, P.A. Integrating Transcriptional, Metabolic, and Physiological Responses to Drought Stress in Ilex paraguariensis Roots. Plants 2023, 12, 2404. [Google Scholar] [CrossRef]
- Lee, S.-U.; Mun, B.-G.; Bae, E.-K.; Kim, J.-Y.; Kim, H.-H.; Shahid, M.; Choi, Y.-I.; Hussain, A.; Yun, B.-W. Drought Stress-Mediated Transcriptome Profile Reveals NCED as a Key Player Modulating Drought Tolerance in Populus davidiana. Front. Plant Sci. 2021, 12, 755539. [Google Scholar] [CrossRef]
- Chen, A.; Li, J.; Wang, H.; Zhao, P. Identification and Expression Profile of NCED Genes in Arachis hypogaea L. during Drought Stress. Int. J. Mol. Sci. 2024, 25, 5564. [Google Scholar] [CrossRef]
- Yang, D.; Ni, R.; Yang, S.; Pu, Y.; Qian, M.; Yang, Y.; Yang, Y. Functional Characterization of the Stipa purpurea P5CS Gene under Drought Stress Conditions. Int. J. Mol. Sci. 2021, 22, 9599. [Google Scholar] [CrossRef]
- Duan, F.M.; Ding, J.; Lee, D.S.; Lu, X.L.; Feng, Y.Q.; Song, W.W. Overexpression of SoCYP85A1, a spinach cytochrome p450 gene in transgenic tobacco enhances root development and drought stress tolerance. Front. Plant Sci. 2017, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.F.; Ma, Y.Z.; Lu, H.D.; An, W.K.; Zhang, F.C. Effects of Spraying Brassinolide on Expression of Steroid 5-Alpha Reductase Gene (Gh DET2) in Cotton under Drought Stress. Genom. Appl. Biol. 2018, 37, 859–866. [Google Scholar] [CrossRef]
- An, J.; Li, Q.X.; Yang, J.J.; Zhang, G.Q.; Zhao, Z.X.; Wu, Y.Z.; Wang, Y.; Wang, W. Wheat F-box protein TaFBA1 positively regulates plant drought tolerance but negatively regulates stomatal closure. Front. Plant Sci. 2019, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.H.; Zhao, Y.; Zhao, Y.; Feng, C.; Zhang, Y.H.; Wang, F.W.; Li, X.W.; Gao, H.T.; Liu, W.C.; Jing, Y. Soybean F-box-like protein GmFBL144 interacts with small heat shock protein and negatively regulates plant drought stress tolerance. Front. Plant Sci. 2022, 13, 823529. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Thakur, K.; Garg, N. Oxidative Stress and Antioxidant Enzymes in Cereals Under Abiotic Stress. In Sustainable Remedies for Abiotic Stress in Cereals; Abdel Latef, A.A.H., Ed.; Springer Nature: Singapore, 2022; pp. 51–82. [Google Scholar]
- Türkan, İ.; Bor, M.; Özdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Navarro-De la Sancha, E.; Coello-Coutiño, M.P.; Valencia-Turcotte, L.G.; Hernández-Domínguez, E.E.; Trejo-Yepes, G.; Rodríguez-Sotres, R. Characterization of two soluble inorganic pyrophosphatases from Arabidopsis thaliana. Plant Sci. 2007, 172, 796–807. [Google Scholar] [CrossRef]
- Schulze, S.; Mant, A.; Kossmann, J.; Lloyd, J.R. Identification of an Arabidopsis inorganic pyrophosphatase capable of being imported into chloroplasts. FEBS Lett. 2004, 565, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Luna, F.M.; Navarro de la Sancha, E.; Valencia-Turcotte, L.G.; Vázquez-Santana, S.; Rodríguez-Sotres, R. Evidence for a non-overlapping subcellular localization of the family I isoforms of soluble inorganic pyrophosphatase in Arabidopsis thaliana. Plant Sci. 2016, 253, 229–242. [Google Scholar] [CrossRef]
- Tang, C.; Qiao, X.; Zhu, X.X.; Khan, W.; Wu, J.; Zhang, S.L. Expression and evolutionary analysis of soluble inorganic pyrophosphatase gene family in pear and four other Rosaceae species. Plant Syst. Evol. 2020, 306, 46. [Google Scholar] [CrossRef]
- Wang, J.; Kang, L.Y.; Liu, Z.B.; Lv, J.H.; Liu, Y.H.; Zou, X.X. Research Progress on the Impact of Drought on Plant. Hunan Agric. Sci. 2017, 7, 123–126+130. [Google Scholar] [CrossRef]
- Li, B.; Zeng, Q.; Zhao, D.; Zhao, D.G. Cloning and Function Analysis of EuERD16 Gene in Eucommia ulmoides. Genom. Appl. Biol. 2023, 42, 373–383. [Google Scholar] [CrossRef]
- Chen, S.Y. Membrane lipid peroxidation and plant adversity stresses. Bull. Bot. 1989, 212–215. [Google Scholar]
- Li, M.Q. Physiological and Molecular Regulatory Mechanisms of Soybean in Response to Drought. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2023. [Google Scholar]
- Wang, Q.M. Effects of Drought Stress on Protective Enzymes Activities and Membrane Lipid Peroxidation in Leaves of Soybean Seedlings. J. Agro-Environ. Sci. 2006, 918–921. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Zheng, A.Z.; Liu, C.P.; Shen, Z.G. Effect of Cadmium on MDA Content, POD and SOD Activities of Brassica Pekinensis and Brassica Chinensis. Hubei Agric. Sci. 2005, 1, 67–69. [Google Scholar]
- Song, X.G.; She, X.P. The Generation and the Role of Hydrogen Peroxide in Plant. J. Lianyungang Teach. Coll. 2010, 27, 99–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).