Deciphering the Effects of Different Calcium Sources on the Plant Growth, Yield, Quality, and Postharvest Quality Parameters of ‘Tomato’
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Treatments and Sowing Methodology
2.3. Construction of Plastic Tunnel and Beds
2.4. Postharvest Storage of Fruits
2.5. Data Collection
2.5.1. Preharvest Quality Parameters
Plant Height, Root Length, and Shoot Length (cm)
No. of Leaves per Plant, Leaf Chlorophyll Content (SPAD Value), and Leaf Area (cm2/Plant)
Number of Flowers, Number of Fruits, and Fruit Yield per Plant (g)
Shoot Fresh and Dry Weight; Root Fresh and Dry Weight (g)
Total Soluble Solids (°Brix); Titratable Acidity (%) and Ripening Index
DPPH Scavenging Activity (%); Ascorbic Acid Contents (mg100 g−1) and Ascorbate Peroxidase (mmoles−1kg−1)
Beta-Carotene (µg g−1) and Phenolic Contents (mg g−1 FW)
Catalase, Peroxidase, Superoxide Dismutase (mmole s−1 kg−1), and Lycopene Contents (mg 100 g−1)
Sensory Evaluation (Score)
2.5.2. Postharvest Quality Parameters
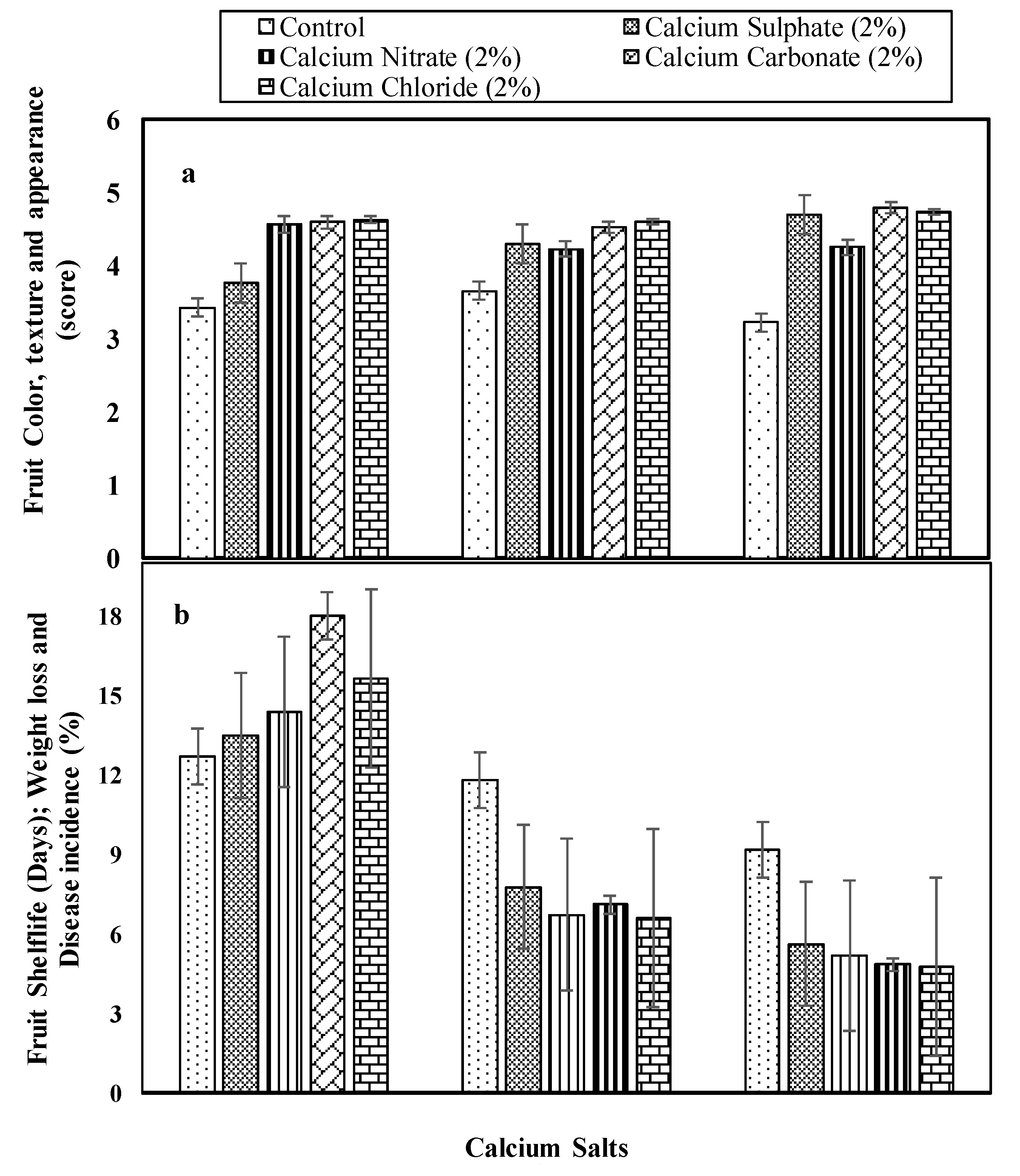
Fruit Shelf Life (Days), Weight Loss, and Disease Incidence (%)
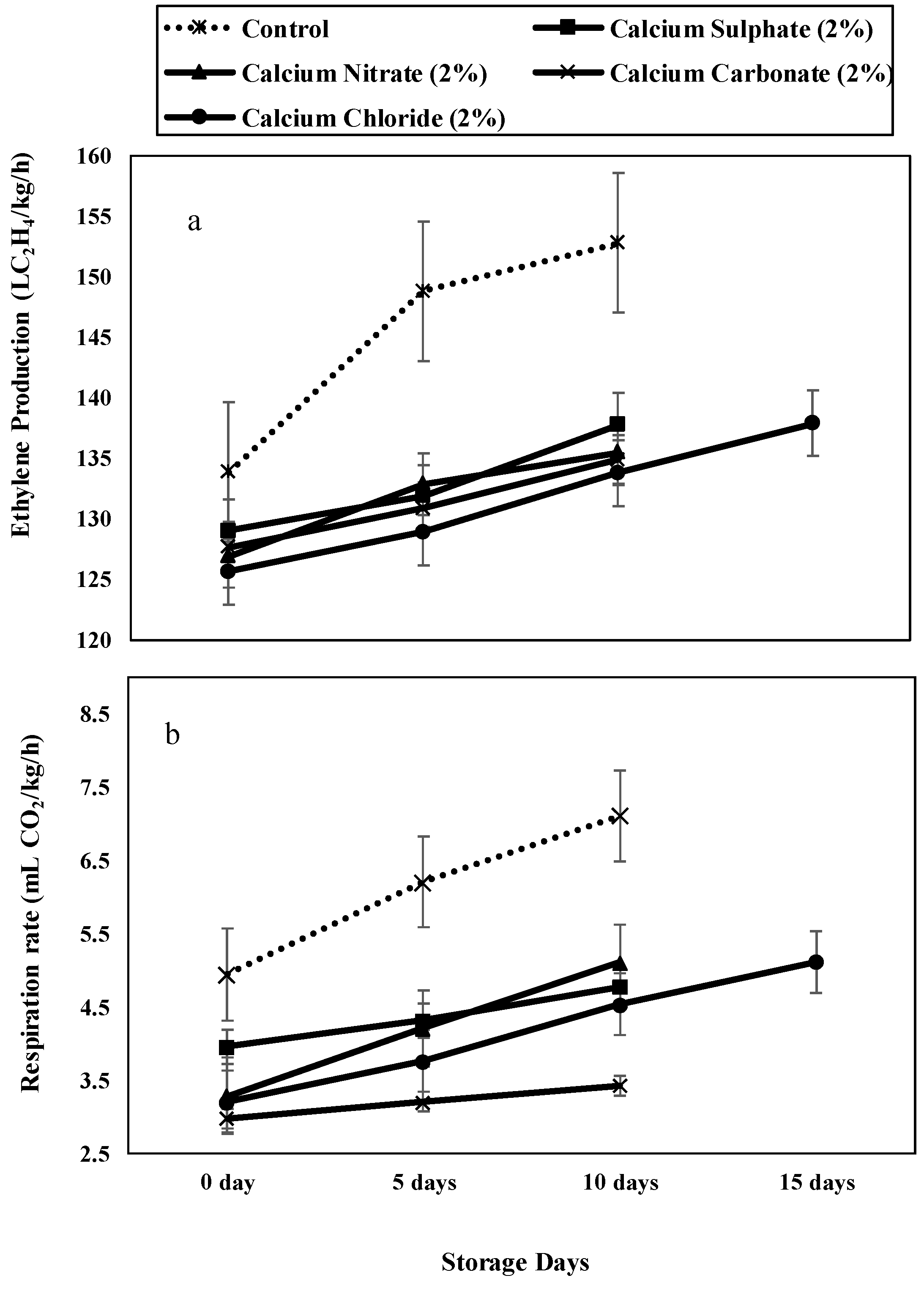
Ethylene Production (µLC2H4/kg/h) and Respiration Rate (mL of CO2/kg/h)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preharvest Quality and Yield Parameters
3.1.1. Plant Height, Root Length, and Shoot Length (cm)
3.1.2. No. of Flowers, No. of Fruits, and Fruit Yield per Plant (kg)
| Treatments | Plant Height (cm) | Root Length (cm) | Shoot Length (cm) | No. of Leaves | No. of Fruits | No. of Flowers | Leaf Chlorophyll Contents (SPAD) | Leaf Area (cm2/Plant) | Fruit Yield per Plant (kg) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 60.87 E | 15.07 D | 45.8 D | 177.0 D | 23.88 E | 32.0 D | 44.11 B | 28.98 C | 1.28 E | 55.63 D | 12.66 E | 10.76 E | 0.95 B |
| Calcium Sulphate (2%) | 77.51 D | 18.5 B | 59.01 C | 220.0 A | 28.11 C | 32.99 C | 48.93 A | 34.76 A | 2.16 B | 66.787 B | 13.16 D | 14.12 D | 2.1 A |
| Calcium Nitrate (2%) | 80.09 C | 15.16 D | 64.93 B | 215.0 B | 26.66 D | 28.66 E | 47.9 AB | 31.26 B | 1.56 D | 61.58 C | 17.66 C | 14.67 B | 2.30 A |
| Calcium Carbonate (2%) | 83.23 B | 23.16 A | 60.07 C | 212.0 C | 29.44 B | 36.33 B | 47.5 AB | 31.98 B | 1.95C | 68.68 AB | 20.83 A | 17.98 C | 2.34 A |
| Calcium Chloride (2%) | 85.27 A | 16.4 C | 68.87 A | 221.7 A | 34.51 A | 42.66 A | 48.8 A | 34.81 A | 2.36 A | 69.16 A | 18.0 B | 18.43 A | 2.01 A |
3.1.3. No. of Leaves per Plant, Leaf Chlorophyll Content (SPAD), and Leaf Area (cm2/Plant)
3.1.4. Shoot Fresh Weight, Root Fresh Weight, Shoot Dry Weight, and Root Dry Weight (g)
3.1.5. Total Soluble Solids (°Brix); Titratable Acidity (%) and Ripening Index
3.1.6. Antioxidant Activities and Enzymes
DPPH Scavenging Activity (%); Ascorbic Acid Contents (g kg−1) and Ascorbate Peroxidase (mmol s−1kg−1)
Beta-Carotene (µg g−1) and Phenolic Contents (mg g−1 FW)
Catalase, Peroxidase, Superoxide Dismutase (mmole s−1kg−1), and Lycopene (mg 100 g−1)
3.2. Postharvest Storage and Quality Parameters
3.2.1. Sensory Evaluation (Score)
3.2.2. Fruit Shelf Life (Days), Weight Loss, and Disease Incidence (%)
3.2.3. Ethylene Production (µLC2H4/kg/h) and Respiration Rate (mL of CO2/kg/h)
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kimura, S.; Sinha, N. Tomato (Solanum lycopersicum): A Model Fruit-Bearing Crop. Cold Spring Harb. Protoc. 2008, 11, pdb-emo105. [Google Scholar] [CrossRef] [PubMed]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard, G.R.; Bigot, S.; Martinez, J.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 01554. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Zhang, Z.; Carriquiry, A.L.; Gunn, J.P.; Kuklina, E.V.; Saydah, S.H.; Yang, Q.; Moshfegh, A.J. Sodium and Potassium Intakes among US Adults: NHANES 2003–2008. Am. J. Clin. Nutr. 2012, 96, 647–657. [Google Scholar] [CrossRef] [PubMed]
- FAO. Production: Crops and Livestock Products. In FAOSTAT; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 January 2024).
- Munhuewyi, K. Postharvest Losses and Changes in Quality of Vegetables from Retail to Consumer: A Case Study of Tomato, Cabbage and Carrot. Ph.D. Dissertation, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Rehman, M.; Khan, N.; Jan, I. Postharvest Losses in Tomato Crop (A Case Study of Peshawar Valley). Sarhad J. Agric. 2021, 23, 1279–1284. [Google Scholar]
- Mazumder, M.N.; Azizah, M.; Phebe, D.; Puter, E.M.W.; Azhar, M. Preharvest Foliar Spray of Calcium Chloride on Growth, Yield, Quality, and Shelflife Extension of Different Lowland Tomato Varieties in Malaysia. Horticulturae 2021, 7, 466. [Google Scholar] [CrossRef]
- Pervaiz, U.; Salam, A.; Jan, D.; Khan, A.; Iqbal, M. Adoption Constraints of Improved Technologies Regarding Tomato Cultivation in District Mardan, KP. Sarhad J. Agric. 2018, 34, 428–434. [Google Scholar] [CrossRef]
- Weerasinghe, P. Best Practices of Integrated Plant Nutrition System in Sri Lanka. In Best Practices of Integrated Plant Nutrition System in SAARC Countries; Fatema, N.J., Tayan, R.J., Eds.; SAARC Agriculture Centre: Dhaka, Bangladesh, 2014; pp. 143–168. [Google Scholar]
- Chauhan, S.; Deependra, Y.; Kumar, S.; Kumar, R.; Kumar, A. Effect of Calcium on the Growth and Yield of Tomato (Solanum lycopersicum L.). Biol. Forum. 2023, 15, 1162–1166. [Google Scholar]
- Hao, X.; Papadopoulos, A.P. Effects of Calcium and Magnesium on Plant Growth, Biomass Partitioning, and Fruit Yield of Winter Greenhouse Tomato. HortScience 2004, 39, 512–515. [Google Scholar] [CrossRef]
- Shafeek, M.R.; Helmy, Y.I.; El-Tohamy, W.A.; El-Abagy, H.M. Changes in Growth, Yield and Fruit Quality of Cucumber (Cucumis sativus L.) in Response to Foliar Application of Calcium and Potassium Nitrate under Plastic House Conditions. Res. J. Agric. Biol. Sci. 2013, 9, 114–118. [Google Scholar]
- Olaniyi, J.O.; Ajibola, A. Effects of Inorganic and Organic Fertilizers Application on the Growth, Fruit Yield and Quality of Tomato (Lycopersicon lycopersicum). J. Appl. Biosci. 2008, 8, 236–242. [Google Scholar]
- Tuna, A.L. Influence of Foliarly Applied Different Triazole Compounds on Growth, Nutrition, and Antioxidant Enzyme Activities in Tomato (Solanum lycopersicum L.) under Salt Stress. Aust. J. Crop Sci. 2014, 8, 71–79. [Google Scholar]
- EU. Regulation (EC) No. 1272/2008 of the European Parliament and of the Council on Classification, Labelling and PACKAGING of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. 2008. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC089390 (accessed on 3 September 2024).
- Hortwitz, W. Official and Tentative Methods of Analysis, 9th ed.; Association of Official Agricultural Chemists: Washington, DC, USA, 1960; pp. 314–320. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Ali, S.; Javed, H.U.; Rehman, R.N.U.; Sabir, I.A.; Naeem, M.S.; Siddiqui, M.Z. Foliar Application of Some Macro and Micro Nutrients Improves Tomato Growth, Flowering and Yield. Int. J. Biosci. 2013, 3, 280–287. [Google Scholar]
- Metkar, S.; Saptarshi, S.; Kadam, A. Studies on Extraction, Isolation and Applications of Lycopene. Indo Am. J. Pharm. Res. 2014, 3, 234–242. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Contents and Other Oxidation Substances in Plant Tissue Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Liu, D.; Zou, J.; Meng, Q.; Zou, J.; Jiang, W. Uptake and Accumulation and Oxidative Stress in Garlic (Allium sativum L.) under Lead Phytotoxicity. Ecotoxicology 2009, 18, 134–143. [Google Scholar] [CrossRef]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Sensory Evaluation Practices, 4th ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2012; p. 448. [Google Scholar]
- Zhu, S.H.; Zhou, J. Effect of Nitric Oxide on Ethylene Production in Strawberry Fruit During Storage. Food Chem. 2007, 100, 1517–1522. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics: A Biological Approach, 3rd ed.; McGraw Hill Book Co. Inc.: New York, NY, USA, 1997. [Google Scholar]
- Abrol, G.S.; Thakur, K.S.; Pal, R.; Punetha, S. Role of Calcium in Maintenance of Postharvest Quality of Horticultural Crops. Int. J. Econ. Plants. 2017, 4, 88–93. [Google Scholar]
- Kamal, B.A. Physiological Studies on Nutrition Status and Productivity of Olive Tree under New Land Conditions. Ph.D. Thesis, Zagazig University, Zagazig, Egypt, 2000. [Google Scholar]
- Haleema, B.; Rab, A.; Hussain, S.A.; Sajid, M.; Arif, M.; Shah, S.T. Influence of Calcium Concentrations and Sources on the Fruit Quality of Tomato (Lycopersicon esculentum Mill) at Different Storage Conditions. Fresenius Environ. Bull. 2020, 29, 1866–1877. [Google Scholar]
- Tomar, S.; Rajiv; Beniwal, D.; Sourabh. Effect of Transplanting Dates and Mulching on Growth and Yield of Tomato (Solanum lycopersicum L.). Veg. Sci. 2019, 46, 39–43. [Google Scholar] [CrossRef]
- Bhattarai, D.R.; Gautam, D.R. Effect of Harvesting Method and Calcium on Postharvest Physiology of Tomato. Nepal Agric. Res. J. 2006, 7, 37–41. [Google Scholar] [CrossRef]
- Islam, M.M.; Jahan, K.; Sen, A.; Urmi, T.A.; Haque, M.M.; Ali, H.M.; Siddiqui, M.H.; Murata, Y. Exogenous Application of Calcium Ameliorates Salinity Stress Tolerance of Tomato (Solanum lycopersicum L.) and Enhances Fruit Quality. Antioxidants 2023, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Experiment. Bot. 2007, 59, 173–175. [Google Scholar] [CrossRef]
- Safari, Z.S.; Ding, P.; Zahidi, N.M.; Atif, A.; Wafa, S.; Aziz, A.; Yusoff, S.F. Maintenance of Defence Enzyme Activities in Tomato Fruit During Storage by Chitosan and Vanillin Coating. Int. J. Appl. Sci. Res. 2021, 4, 177–188. [Google Scholar]
- Patane, C.; Pellegrino, A.; Silvestro, I.D. Effects of calcium carbonate application on physiology, yield and quality of field-grown tomatoes in a semi-arid Mediterranean climate. Crop Pasture Sci. 2018, 69, 411–418. [Google Scholar] [CrossRef]
- Kushwaha, R.; Singh, M.; Singh, V.; Puranik, V.; Kaur, D. Variation of Bioactive Compounds and Antioxidant Activity During Ripening of Tomato Cultivars. J. Food Agric. Res. 2021, 1, 15–29. [Google Scholar]
- Santhosh, S.; Chitdeshwari, T.; Chinnasamy, K. Calcium Influences Biochemical and Antioxidant Enzymatic Activities in Tomato Fruits During Storage. J. Appl. Biol. Biotechnol. 2023, 11, 158–164. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending Shelf Life and Maintaining Quality of Tomato Fruit by Calcium Chloride, Hydrogen Peroxide, Chitosan, and Ozonated Water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Navjot, S.; Gurcharan, S. Studies on Storage Behaviour of Peach cv. Earli Grande. Int. J. Agric. Sci. 2006, 2, 541–543. [Google Scholar]
- Manganaris, G.A.; Vasilakakis, M.; Diamantidis; MignaniIlaria, G. Effect of In-Season Calcium Application on Cell Wall Physicochemical Properties of Nectarine Fruit (Prunus persica var. nectarine Ait. Maxim) After Harvest or Cold Storage. J. Sci. Food Agric. 2006, 86, 2597–2602. [Google Scholar] [CrossRef]
- Kumar, N.; Kaur, P.; Devgan, K.; Attkan, A.K. Shelf Life Prolongation of Cherry Tomato Using Magnesium Hydroxide Reinforced Bio-Nanocomposite and Conventional Plastic Films. J. Food Process. Preserv. 2020, 44, e14379. [Google Scholar] [CrossRef]
- Demes, R.; Satheesh, N.; Fanta, S.W. Effect of Different Concentrations of Gibberellic Acid and Calcium Chloride Dipping on Quality and Shelf-Life of Kochoro Variety Tomato. Philipp. J. Sci. 2021, 150, 335–349. [Google Scholar] [CrossRef]
| Treatments | TSS (%) | TA (%) | Ripening Index |
|---|---|---|---|
| Control | 6.1 B | 0.58 B | 10.65 AB |
| Calcium Sulphate (2%) | 7.5 A | 0.65 A | 11.54 A |
| Calcium Nitrate (2%) | 6.0 B | 0.63 A | 9.55 B |
| Calcium Carbonate (2%) | 6.3 B | 0.66 A | 9.60 B |
| Calcium Chloride (2%) | 5.5 C | 0.54 B | 10.18 B |
| Treatments | DPPH-Scavenging Activity (%) | Ascorbic Acid Contents (mg/100 g) | Ascorbate Peroxidase (mmole/s/kg) | Catalase (mmole/s/kg) | Peroxidase (mmole/s/kg) | Superoxide Dismutase (mmole/s/kg) | Lycopene (mg/100 g) | Total Phenolic Contents (mg/g FW) | β Carotene (µg/g) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 45.99 C | 237.65 C | 255.15 D | 16.49 D | 4.71 D | 5.53 C | 9.98 D | 1.31 C | 0.37 B |
| Calcium Sulphate (2%) | 55.62 B | 252.65 AB | 277.58 B | 17.30 C | 4.29 C | 5.65 C | 13.78 C | 2.39 B | 0.41 B |
| Calcium Nitrate (2%) | 58.13 AB | 253.91 AB | 261.27 C | 17.44 C | 6.04 B | 6.17 B | 14.87 B | 2.37 B | 0.47 A |
| Calcium Carbonate (2%) | 59.0 AB | 251.41 B | 261.38 C | 18.09 B | 5.97 B | 6.27 B | 14.98 B | 2.45 A | 0.39 B |
| Calcium Chloride (2%) | 61.25 A | 259.78 C | 290.75 A | 18.71 A | 7.82 A | 7.13 A | 16.09 A | 2.44 A | 0.48 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, S.T.-A.; Anjum, M.A.; Shah, M.N.; Hassan, A.U.; Parveen, M.; Danish, S.; Alharbi, S.A.; Alfarraj, S. Deciphering the Effects of Different Calcium Sources on the Plant Growth, Yield, Quality, and Postharvest Quality Parameters of ‘Tomato’. Horticulturae 2024, 10, 1003. https://doi.org/10.3390/horticulturae10091003
Haider ST-A, Anjum MA, Shah MN, Hassan AU, Parveen M, Danish S, Alharbi SA, Alfarraj S. Deciphering the Effects of Different Calcium Sources on the Plant Growth, Yield, Quality, and Postharvest Quality Parameters of ‘Tomato’. Horticulturae. 2024; 10(9):1003. https://doi.org/10.3390/horticulturae10091003
Chicago/Turabian StyleHaider, Sakeena Tul-Ain, Muhammad Akbar Anjum, Muhammad Nadeem Shah, Adeeb Ul Hassan, Maqsooda Parveen, Subhan Danish, Sulaiman Ali Alharbi, and Saleh Alfarraj. 2024. "Deciphering the Effects of Different Calcium Sources on the Plant Growth, Yield, Quality, and Postharvest Quality Parameters of ‘Tomato’" Horticulturae 10, no. 9: 1003. https://doi.org/10.3390/horticulturae10091003
APA StyleHaider, S. T.-A., Anjum, M. A., Shah, M. N., Hassan, A. U., Parveen, M., Danish, S., Alharbi, S. A., & Alfarraj, S. (2024). Deciphering the Effects of Different Calcium Sources on the Plant Growth, Yield, Quality, and Postharvest Quality Parameters of ‘Tomato’. Horticulturae, 10(9), 1003. https://doi.org/10.3390/horticulturae10091003








