Abstract
Studies on the genetic diversity of coffee trees are important, considering their role in the maximization of productivity and quality. However, the success of a breeding program depends on the existence of genetic variability in the population under study. Our study aimed to evaluate the genetic diversity of the morpho-agronomic and anatomical leaf traits of different cultivars of Coffea arabica grown in the Gorongosa mountain region, in the area of Gorongosa National Park, Mozambique. The experiment assessed nine coffee cultivars based on their morpho-agronomic and anatomical traits. The plagiotropic branch diameter, leaf dry mass, leaf mass per area, number of epidermal cells + stomata, and stomatal form indicated differences among the studied cultivars. Among the nine C. arabica cultivars grown in the mountainous region of Gorongosa National Park, Mozambique, low genetic variability in morpho-agronomic traits was detected, while the genetic variability in leaf anatomical traits was higher. The nine cultivars were segregated into two groups, one consisting of Catucaí Amarelo 2SL and Catuaí Vermelho IAC 81, and the second consisting of Catuaí Amarelo IAC 39, Catucaí Vermelho 19/8, Acauã, Catucaí 785-15, Costa Rica, Catimor 128, and Catuaí Vermelho IAC 44. The cultivar segregation into the two groups indicated that the morpho-anatomical traits can be considered during the selection stages in breeding programs.
1. Introduction
The genus Coffea is currently represented by 130 species according to the taxonomic classification [1], but only Coffea arabica L. (Arabica coffee) and C. canephora Pierre ex A. Froehner (Canephora coffee) are commercially important in the worldwide production and trade of coffee [2]. Species like C. liberica Bull. Ex Hiern and C. racemosa Lour. have almost negligible participation in the global coffee market. The C. arabica is an endemic species from Southwestern Ethiopia (provinces of Illubabor and Kaffa), Boma Plateau in Southeastern Sudan, and Marsabit in Northern Kenya. At those localities, wild C. arabica specimens may still be found in tropical rainforests [3,4,5,6]. This species is naturally found at high altitudes of 1600–2800 m a.s.l., characterized by average annual temperatures of 18 to 22 °C, and annual precipitations of 1600 to 2000 mm [7].
C. arabica species is an allotetraploid segmental type (2n = 4x = 44 chromosomes), constituted by two diploid genomes (2n = 2x = 22) inherited from wild ancestors of C. canephora and C. eugenioides Moore [8]. It is self-compatible, predominantly characterized by self-pollination. About 10% of cross-pollination usually occurs. Self-pollination ensures reproduction, but also contributes to the narrow genetic variability of the species [9]. Most C. arabica cultivars grown worldwide (groups of Catura, Catuaí, and Mundo Novo) were selected from two populations, commonly called Typica and Bourbon [10]. The Typica population descended from a single plant imported from Java to Amsterdam, while the Bourbon population originated from a few plants introduced from Yemen to Bourbon Island (now L’Ille de Réunion) in the XVIII century [11]. Despite the relatively narrow genetic base of these cultivars [12], the commercialized cultivars exhibit some genetic variability, arising from a series of mutations and both natural and artificial crossings [13].
Several methods are used to assess genetic diversity, including morphological, physicochemical, and molecular markers. DNA-based morphological markers [8,11,14] were primarily used in molecular coffee breeding [15,16]. A significant number of Brazilian C. arabica coffee cultivars are obtained through classical breeding, involving the introgression of genes from three other species, C. canephora, C. liberica, and C. racemosa. For resistance against coffee leaf rust (CLR) caused by the fungi Hemileia vastatrix Berkeley and Broome, genes of C. canephora are utilized, resulting in several resistant cultivars of C. arabica, including Icatu, Obatã, IAC 125 RN, Iapar 59, IPR 107, Catiguá, and Arara. The introgression of the SH3 gene from C. liberica into the cultivar Catuaí of C. arabica is the origin of the cultivar IAC Catuaí SH3, resistant to all races of the H. vastatrix identified so far in Brazil. Genes of C. racemosa contributed to the resistance of Arabica coffee to leaf miner, Leucoptera coffeella Guérin-Menéville, utilized in breeding of some Siriema cultivars [17].
C. arabica is an evergreen, short-day species, characterized by continuous growth. Its architecture is classified as a Roux model [18], marked by the existence of branch dimorphism. The main axis is orthotropic (erect), with opposite leaves in decussate phyllotaxy, while the lateral branches of the second to fifth order are plagiotropic [19]. Gas exchanges in the leaf, of CO2 for photosynthesis, and H2O for transpiration (controlling leaf temperature and leaf water balance), are primarily performed through stomata, which are found on the abaxial leaf surface in C. arabica [20]. In plants, leaf gas exchanges (photosynthesis, and transpiration) are determined by the degree of stomatal aperture [21], together with stomata size and density [20,21]. In C. arabica, net photosynthesis and stomatal aperture/conductance generally exhibit low values, even under favorable environmental conditions, as compared to many other C3 species [22]. In various species, an increase in stomatal density can promote an increase in stomatal conductance and photosynthetic rate under high-light conditions [23]. The stomatal opening and closing are driven by various external environmental and internal mechanisms and factors [24]. Generally, stomata open in response to increasing light intensity, low CO2 concentration, high temperatures, and low vapor pressure deficit (VPD), while closure is induced by low light, high CO2, and high VPD [23]. This pore connects the leaf’s inner layers with the environment [25]. Stomatal density and sensitivity to environmental inductions and variations is species and genotype dependent [21,26,27], as can be seen by the genus Coffea [28]. In Coffea spp., the net photosynthetic rate is usually about 5–8 μmol CO2 m−2 s−1, but the rate can reach values up to 18 μmol CO2 m−2 s−1, while stomatal conductance is usually about 100–200 mmol H2O m−2 s−1 but can attain values of 500 mmol H2O m−2 s−1 [29]. The mentioned elevated values of leaf gas exchanges are usually observed in highly productive C. canephora genotypes.
Mozambique has great edaphoclimatic diversity, with record production data of C. arabica, C. zanguebariae, and C. racemosa, the latter two species being considered native [1,30,31]. The Gorongosa mountain, part of the Gorongosa National Park (PNG) is located above 700 m a.s.l., in central Mozambique. This area is one of the world’s most biodiverse regions, not including any Coffea spp. [32,33]. In recent years, C. arabica crops have been introduced to this region, where they are cultivated in agroforestry systems (AFS) with native forest trees. The coffee AFS contribute to restoring the local degraded tropical forest and improving farmers’ livelihoods [2,31,34].
In this study, we proposed the hypothesis that among different cultivars of C. arabica, leaf anatomical traits would have higher genetic variability than morpho-agronomic traits due to their functional relation. To test this hypothesis, the study aimed to evaluate genetic diversity based on the morpho-agronomic and anatomical leaf traits of nine C. arabica cultivars cultivated in the Gorongosa mountain region, Mozambique.
2. Materials and Methods
2.1. Experimental Design and Plant Material
The experiment was conducted in the Gorongosa mountain region (longitude 34°04′05″ E; latitudes −18°47′99″ to −18°48′05″ S; altitude of ca. 960 m a.s.l), in the area of Gorongosa National Park (PNG), Gorongosa district, Sofala province, central Mozambique.
The field experiment was established in November 2018, when the coffee seedlings produced in the nursery were eight months old. The planting areas were historically occupied by rainforest vegetation but were transformed into savannas due to anthropogenic disturbances, mostly associated with agricultural slash-and-burn implementation. These areas should now be reverted to a state closer to the original forest, with the contribution of the coffee crop managed under agroforestry system, using native trees for shading. In total, nine C. arabica cultivars, predominantly of Brazilian breeding (Catuaí Vermelho IAC 44, Catucaí Amarelo 2SL, Catuaí Amarelo IAC 39, Catucaí 785-15, Catucaí Vermelho 19/8, Catuaí Vermelho IAC 81, Acauã, Catmor 128, and one cultivar named Costa Rica—Table 1), were planted in a randomized block design with four blocks and three replications.

Table 1.
Identification and life cycle length (considering fruit maturation) of the Coffea arabica L. cultivars cropped in the experimental area in Gorongosa mountain, Mozambique.
The coffee planting arrangement was 2 m between rows and 1.5 m between plants within the rows (3333 plants ha−1). Cultural management involved only manual weeding, and this was performed several times per year, without any application of fertilizers, insecticides, fungicides, or additional irrigation, respecting the PNG area of preservation, where the AFS were established.
The predominant vegetation in the region is dry Miombo forest, with dominant species including Brachystegia spiciformis, Brachystegia boehmii, and Julbernardia globiflora, interspersed with evergreen shrubs that appear in the deeper alluvial sand soils [35,36]. In the experimental area of coffee AFS, small trees (~1 m of height) of the previously mentioned species were planted, with the arrangement of 20 m × 6 m, totaling 83 trees ha−1. This AFS tree arrangement was projected to provide a level of ~30% shade to the coffee plants.
Regarding the climate and soil characteristics, according to the Köppen–Geiger classification [37], the mean annual temperature of the region is 20 °C, the mean annual rainfall is 1697 mm, and the climate ranges from tropical savanna wet (Aw) to subtropical winter-dry (Cw). The mountain surface is characterized by wavy relief and consists of eroded surfaces of granite and basalt complexes, producing sandy and clayey soils [32], where the soil of the experimental area was classified as clayey. Soil samples were collected at a soil depth of 0–20 cm for chemical analyses at the beginning of the experiment in 2021 (Table 2).

Table 2.
Global chemical characterization of the soil of the experimental area in Gorongosa mountain at the beginning of the experiment, evaluated under monoculture.
2.2. Morpho-Agronomic Traits of the Plants
The evaluations in the experiment were performed from June to July 2021, when the plants were approximately 32 months old. The following morphological and agronomical traits were evaluated: (1) plant height (PH, cm), measured on the orthotropic axis from the soil level to the point of insertion of the terminal bud; (2) orthotropic diameter (OD, cm), measured 1 cm from the soil level; (3) crown diameter (CD, cm) taking as a standard the two plagiotropic axes of the 2nd order of the longest length formed in the direction of the rows; (4) plagiotropic length (PL, cm), determined by measuring a plagiotropic branch (located in the middle third of the plant), from its insertion on the orthotropic branch to its terminal apex; (5) plagiotropic diameter (PD, cm), measured at the insertion of the 2nd order plagiotropic branch to orthotropic trunk; (6) mean internode length (IL, cm), estimated by dividing the plagiotropic branch length by the number of internodes; (7) number of 2nd order plagiotropic branches (NP), obtained by counting. All lengths and diameters were measured with a ruler and a caliper, respectively.
For leaf dry mass (LDM, mg) determination, 15 leaves per block were collected from the third or fourth metamer from the apex of 2nd order plagiotropic branches in the middle third of the plants. Those branches were oriented to the inter-row space and exposed to the sun. Leaf material was dried to a constant weight at 60 °C in a convection SH-142 oven (Shel Lab®, Columbus, OH, USA). The LDM and the estimated leaf area were used to calculate leaf mass per area (LMA, mg cm−2).
2.3. Leaf Anatomical Traits
The epidermal impression of coffee leaves was performed as described in Ramalho et al. [20]. A total of 3 leaves per cultivar were sampled from each of the 4 blocks (totaling 12 leaves per cultivar). Briefly, imprints from the abaxial leaf surface, at the point of maximal leaf width, from the margin to the main central leaf vein (and avoiding the veins) were made using colorless nail polish and adhesive transparent cellophane tape. The imprints were then placed on glass slides and examined and photographed under a Motic BA210 optical microscope, with a magnification of 400× and using a 50 μm scale, equipped with a Motic Cam 3® 3.0 MP and Motic Images Plus 2.0 software (Motic Incorporation Ltd., Hong Kong, China). Five fields of view within each sampled leaf were analyzed, resulting in 60 image fields per cultivar. Subsequently, the images were analyzed with the Anati Quanti software version 2.0 [38] counting and computing: (1) number of epidermal cells and stomata (EC, number of cells mm−2); (2) stomatal density (SD, number of stomata mm−2); (3) stomatal index (SI, %), as a percentage of stomata in the number of stomata plus the number of epidermal cells ([(stomata)/(total cells + stomata)] × 100); (4) stomatal form (SF), estimated as polar diameter (PD) divided by equatorial diameter (ED) [39,40].
2.4. Statistical Analysis
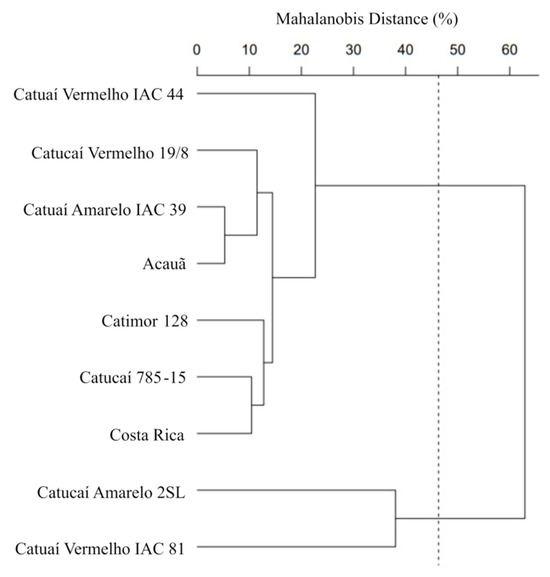
Data on coffee morpho-agronomic and leaf anatomic traits were analyzed using one-way ANOVA (95% confidence). Three replicates of each block were averaged, resulting in four blocks as repetitions. Means were grouped by the Scott–Knott test, always using 5% significance. The genetic dissimilarity was estimated by the coefficients of the Mahalanobis distance (D2) using the morpho-agronomic and anatomical leaf traits. The genetic dissimilarity among nine cultivars was represented by a hierarchical clustering method: the Unweighted Pair Group Method of Arithmetic (UPGMA). To establish a point of cut-off in the dendrogram and define the number of groups, the Mojena procedure [41] was used, based on the relative size of the merger levels (distances) in the dendrogram, always based on morpho-agronomic and anatomical leaf traits. Finally, the Pearson linear correlation analysis was performed to verify the correlations among the morpho-agronomic and anatomic traits. All statistical analyses were performed using the package ‘ExpDes’ in R programming language, version 4.3.0 [42], and Genes software—version 2023.45 [43].
3. Results
3.1. Morpho-Agronomic and Anatomical Dissimilarity between Genotypes and Genetic Contribution
Among the studied morpho-agronomic traits, variability between cultivars was found only for plagiotropic branch (PD), leaf dry mass (LDM), and leaf mass per area (LMA) (Table 2). The cultivars were segregated into two groups based on PD, with higher means observed in Catucaí 785-15 and Acauã, and the lowest in Catuaí Vermelho IAC 81. The LDM divided the cultivars into three groups, with greater values in Catimor 128, intermediate values in Costa Rica, and lower values in the rest of cultivars, with emphasis on the cultivars Catucaí Amarelo 2SL and Catuaí Vermelho IAC 44, which showed the lowest values. Regarding LMA, the cultivars formed two groups, with Catimor 128 and Catuaí Vermelho IAC 44 showing maximal and minimal values, respectively (Table 3).

Table 3.
Morpho-agronomic traits in C. arabica cultivars cropped in the experimental area in Gorongosa mountain, Mozambique).
Among the leaf anatomical traits, the cultivars showed significant differences in epidermal cells and stomata (EC) and stomatal form (SF) (Table 4, Figure 1). Based on EC, the studied cultivars were divided into two distinct groups, with the cultivar Catuaí Vermelho IAC 44 with the lowest mean isolated from the rest of the cultivars. Regarding SF, two groups were formed with the highest absolute values observed in the cultivars Catucaí 785-15, and Costa Rica, in contrast with the lowest values in cultivars Catuaí Amarelo IAC 39 and Acauã.

Table 4.
Leaf anatomical traits in nine C. arabica cultivars grown in Gorongosa National Park, Mozambique.
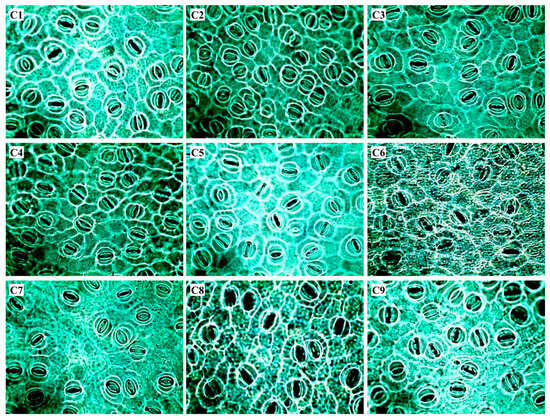
Figure 1.
Optical microscopy images of Coffea arabica leaf surfaces. C1: Catuaí Vermelho IAC 44; C2: Catucaí Amarelo 2SL; C3: Catuaí Amarelo IAC 39; C4: Catucaí 785-15; C5: Catucaí Vermelho 19/8; C6: Catuaí Vermelho IAC 81; C7: Acauã; C8: Catimor 128; C9: Costa Rica. Scale bar = 50 μm.
Delimitation of groups was performed considering 48% dissimilarity (Mojena, 1977) among the cultivars (Figure 2). The cutline was determined to be at the place where an abrupt change was detected in the dendrogram ramifications. Group 1 was formed from two cultivars (Catucaí Amarelo 2SL and Catuaí Vermelho IAC 81), which were isolated from the other seven cultivars that formed Group 2 (Costa Rica, Catuaí Amarelo IAC 39, Catucaí Vermelho 19/8, Catimor 128, Catucaí 785-15, Catuaí Vermelho IAC 44, and Acauã). The shortest genetic distance was observed between the cultivars Catuaí Amarelo IAC 39 and Acauã, whereas the greatest distance was between Catuaí Vermelho IAC 44, and Catuaí Vermelho IAC 81. The segregation into two groups reflected the genetic variability among the studied cultivars, as the method minimizes the intra-group distance and maximizes the inter-group distances.
3.2. Correlations among Morpho-Agronomic and Anatomical Leaf Traits in Nine Cultivars of C. arabica Cultivated in Gorongosa National Park, Mozambique
The Pearson’s correlations for the morpho-agronomic and anatomical leaf traits (Figure 3) revealed several significant binary relationships (both positive and negative). Among the variables, PH was very strongly correlated with CD, OD, NP, PL, and IL. Strong positive correlations were observed for CD × OD, CD × NP, CD × PD, CD × PL, PD × PL, PL × IL, LDM × LMA and EC × SD. These very strong and strong correlations between variables indicate that these traits were closely linked and may influence one another. Moderate positive correlations were also found for CD × SF, OD × NP, OD × PL, NP × PL, and EC × SF. Low positive correlations were observed between OD × IL, OD × LMD, NP × LMA, and PL × SF. Moderate and low correlations indicate less pronounced relationships among the variables studied, although they are still significant. A strong negative correlation was found between SD × SI, which may indicate an inverse relationship between the variables. Overall, these correlations provided insights into how much the different variables were related and they can help in the plant breeding and selection processes.

Figure 3.
Pearson’s correlation matrix between morpho-agronomic and anatomical leaf traits in nine cultivars of C. arabica cultivated in Gorongosa mountain, Mozambique. Red asterisks indicate significant correlations: · p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001. PH: plant height (cm); CD: crown diameter (cm); OD: orthotropic trunk diameter (cm); NP: number of plagiotropic branches; PD: plagiotropic branch diameter (cm); PL: plagiotropic branch length (cm); IL: internode length (cm); LDM: leaf dry mass (mg); LMA: leaf mass per area (mg cm−2); EC: number of epidermal cells + stomata (total cell number per mm2); SI: stomatal index (%); SD: stomatal density (number of stomata per mm2); SF: stomatal form (polar diameter/equatorial diameter).
4. Discussion
The high level of genetic and phenotypic similarity in C. arabica is considered a bottleneck, as the genetic improvement in and sustainability of any crop plant species are directly linked to its genetic diversity [6]. The results of this study confirm the low genetic variability among the studied cultivars of C. arabica introduced in Mozambique. Low genetic variability was more expressed in the morpho-agronomic traits than in the leaf anatomical traits. Such results indicate the need for advancements in genetic breeding programs aiming at expanding the genetic base of the species. In our study, the CV values ranged from 3.32% to 11.7%, depending on the evaluated variable. According to Gomes [44] these values are classified as low and medium, by using a classification for CV that considers the value low when inferior to 10%; medium, from 11 to 20%; high, from 21 to 30%; and very high, when it is greater than 30%. The classification of CV is inversely proportional to the experiment precision. The observed results were at acceptable levels, indicating good accuracy and experimental precision for the evaluated variables [45,46,47,48].
Among the morpho-agronomic traits, significant differences among the cultivars were observed only in the PD, LDM, and LMA. The LMA is considered one of the central variables among leaf traits, which is the ratio between leaf dry mass and leaf area [48], it being highly correlated with leaf processes such as the maximum photosynthetic rate [49,50,51,52] and activities of the entire plant [53]. However, the LMA is a good indicator of the position of this species along an axis based on resource acquisition (leaf economic spectrum) [49].
Significant differences among the nine cultivars were observed in the leaf anatomical traits EC and SF. The largest degree of stomatal opening (herein represented by SF) was observed in the cultivars Catuaí Vermelho IAC 44 and Costa Rica (Table 4). More open stomata allow increased conductance, potentially leading to increased rates of photosynthesis and transpiration. Under water-shortage conditions, water conservation takes priority over carbon gain [54,55]. When water supply is not limited, stomata operate by increasing CO2 intake and/or promoting leaf cooling (via increased transpiration) [56,57]. Variations in stomatal anatomical features, including stomatal pore width, influence leaf gas exchanges in diverse ways. These variations are associated with genetic factors and/or environmental conditions, such as evaporative demand [58,59], CO2 level [60,61], and light regime [62,63].
Stomatal traits that are beneficial under one climatic scenario might be less adapted for plant performance in another environment [64,65]. Leaf anatomical characteristics can reflect environmental responses and hormonal stimulation; thus, a high level of variation is expected between individuals belonging to the same species [66]. Among the anatomical variables, the number of epidermal cells and stomatal form showed statistical differences in studied C. arabica cultivars. Such variations in stomatal form could be considered as structural intra-special adaptation to environment, especially light conditions [53]. In contrast, there was an absence of SD and SI differences in the studied C. arabica cultivars, in line with the reports of other genotypes (IPR108 and Icatu) that also showed values close to 192 (SD) and 25 (SI) under a control temperature of 25 °C [20]. However, the values of both stomatal traits greatly decline in response to increased temperatures and elevated air CO2 conditions within the same genotype [21]. This suggests strong genetic homogeneity under controlled conditions but a robust response to environmental changes, which would have a strong impact on maximal stomatal conductance, determined by stomatal size, density, and aperture [20,67].
The relationship between the polar and equatorial diameters (PD/ED) is associated with guard cells and constitutes an important characteristic of stomatal responses to light conditions [68]. Higher PD/ED in the elliptical stomata are characteristic of more functional stomata, whereas lower PD/ED in the rounded shape are associated with stomata that do not have adequate functioning, and the type and condition of cultivation can modify these results. Elliptical stomata can lead to greater absorption of CO2, causing greater photosynthetic potential and reduced transpiration rate [38].
This study highlighted the low genetic morpho-agronomic variability among the main C. arabica cultivars introduced in Mozambique, while variability was higher when leaf anatomical traits were analyzed. Low genetic variability poses a significant challenge to the sustainability and genetic breeding of the species. Future studies could focus on exploring new genetic sources and developing breeding strategies that expand the genetic base of the species. Additionally, future research could investigate how leaf anatomical variations respond to different environmental conditions.
5. Conclusions
Among the nine cultivars of C. arabica cultivated in the mountain region of Gorongosa National Park, Mozambique, narrow genetic diversity was detected based on the assessed morpho-agronomic traits. The inclusion of leaf anatomic traits, particularly those related to stomata, can assist plant breeders in genetic discrimination of individuals. This information about the epidermic cell density and stomatal form is highly relevant for the selection of promising materials. These traits could potentially lead to increased efficiency in leaf gas exchanges, which could provide insights for the development of Coffea arabica cultivars adaptable to and tolerant of climate change.
Author Contributions
Conceptualization, N.J.A., A.F., J.C.R., A.I.R.-B., E.M.A. and F.L.P., methodology, N.J.A., A.F., J.C.R., A.I.R.-B., E.M.A. and F.L.P.; validation, all authors; formal analysis, N.J.A., A.F., J.C.R., M.R., A.I.R.-B., E.M.A. and F.L.P.; investigation, N.J.A., A.F., J.C.R., A.I.R.-B., E.M.A. and F.L.P.; resources, N.J.A., A.F., J.C.R., A.I.R.-B., E.M.A. and F.L.P.; data curation, N.J.A., A.F., M.R., J.C.R., A.I.R.-B., E.M.A. and F.L.P.; writing—original draft preparation, N.J.A., M.R., A.F., J.C.R., A.I.R.-B., E.M.A., L.O.E.S. and F.L.P.; writing—review and editing, all authors; visualization, all authors; supervision, A.F., J.C.R., A.I.R.-B., M.R., E.M.A. and F.L.P.; project administration, J.C.R., A.I.R.-B. and F.L.P. All authors have read and agreed to the published version of the manuscript.
Funding
Authors acknowledge the Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES) (Proc. 2022-WTZQP for FLP and Proc. 2022-M465D for MR), National Council for Scientific and Technological Development (CNPq, Brazil, Grant—Proc. 309535/2021–2 for FLP, to F.L.P.), and CAPES for awarded fellowship (Finance Code 001) to N.J.A. This work received also funding support from Fundação para a Ciência e a Tecnologia, I.P. (FCT), Portugal, associated to A.I.R.-B. and J.C.R. through research units CEF (UIDB/00239/2020, https://doi.org/10.54499/UIDB/00239/2020), and GeoBioTec, (UIDP/04035/2020, https://doi.org/10.54499/UIDB/04035/2020), as well as through the Associate Laboratory TERRA (LA/P/0092/2020, https://doi.org/10.54499/LA/P/0092/2020).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
Gorongosa National Park, Conilon Coffee Research Excellence Core, TriCafé.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northern Madagascar. Kew Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- Cassamo, C.T.; Mangueze, A.V.; Leitão, A.E.; Pais, I.P.; Moreira, R.; Campa, C.; Chiulele, R.; Reis, F.O.; Marques, I.; Scotti-Campos, P.; et al. Shade and altitude implications on the physical and chemical attributes of green coffee beans from Gorongosa Mountain, Mozambique. Agronomy 2022, 12, 2540. [Google Scholar] [CrossRef]
- Meyer, F.G. Notes on wild Coffea arabica from southwestern Ethiopia, with some historical considerations. Econ. Bot. 1965, 19, 136–151. [Google Scholar] [CrossRef]
- Mishra, M.K.; Tornincasa, P.; De-Nardi, B.; Asquini, E.; Dreos, R.; Terra, L.D.; Rathinavelu, R.; Rovelli, P.; Pallavicini, A.; Graziosi, G. Genome organization in coffee as revealed by EST PCR-RFLP, SNPs and SSR analysis. J. Crop Sci. Biotech. 2011, 14, 25–37. [Google Scholar] [CrossRef]
- Scalabrin, S.; Toniutti, L.; Di-Gaspero, G.; Scaglione, D.; Magris, G.; Vidotto, M.; Pinosio, S.; Cattonaro, F.; Magni, F.; Jurman, I.; et al. A single polyploidization event at the origin of the tetraploid genome of Coffea arabica is responsible for the extremely low genetic variation in wild and cultivated germplasm. Sci. Rep. 2020, 10, 4642. [Google Scholar] [CrossRef]
- Jingade, P.; Kumar, C.H.A.; Kumar, M.M. First report on genome size and ploidy determination of five indigenous coffee species using flow cytometry and stomatal analysis genetics & evolutionary biology—Original article. Braz. J. Bot. 2021, 44, 381–389. [Google Scholar]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- Lashermes, P.; Combes, M.C.; Roberto, J.; Trouslot, P.; D’Hont, A.; Antônio, F.; Carregador, A. Molecular characterization and origin of the Coffea arabica L. Mol. Genet. Genom. 1999, 261, 259–266. [Google Scholar] [CrossRef]
- Wright, S.I.; Kalisz, S.; Slotte, T. Evolutionary consequences of self-fertilization in plants. Proc. Royal Soc. B Biol. Sci. 2013, 280, 20130133. [Google Scholar] [CrossRef]
- Anthony, F.; Quiros, O.; Ropart, P.; Bertrand, B.; Lashermes, P. Detection by simple sequence repeat markers of introgression from Coffea canephora in Coffea arabica varieties. Plant Breed. 2002, 121, 542–544. [Google Scholar] [CrossRef]
- Anthony, F.; Combes, M.C.; Astorga, C.; Bertrand, B.; Graziosi, G.; Lashermes, P. The origin of cultivated Coffea arabica L. varieties revealed by AFLP and SSR markers. Theor. Appl. Genet. 2002, 104, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Charrier, A.; Berthaud, J. Botanical classification of coffee. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Eds.; Springer: Boston, MA, USA, 1985; pp. 13–47. [Google Scholar]
- Aguiar, A.T.E.; Guerreiro-Filho, O.; Maluf, M.P.; Gallo, P.B.; Fazuoli, L.C. Characterization of Coffea arabica cultivars by minimum descriptors. Bragantia 2004, 63, 179–192. [Google Scholar] [CrossRef]
- Labouisse, J.P.; Bellachew, B.; Bertrand, B.; Kotecha, S. Current status of coffee (Coffea arabica L.) genetic resources in Ethiopia: Implications for conservation. Genet. Res. Crop Evol. 2008, 55, 1079–1093. [Google Scholar] [CrossRef]
- Akpertey, A.; Anim-Kwapong, E.; Ofori, A. Assessment of genetic diversity in Robusta coffee using morphological characters. Int. J. Fruit Sci. 2019, 19, 276–299. [Google Scholar] [CrossRef]
- Silva, L.O.E.; Schmidt, R.; Almeida, R.N.; Feitoza, R.B.B.; Cunha, M.; Partelli, F.L. Morpho-agronomic and leaf anatomical traits in Coffea canephora genotypes. Ciência Rural. 2023, 53, e20220005. [Google Scholar] [CrossRef]
- Oliveiro, G.F. Origin and Diverse Nature of Interspecific hybrid Coffee Cultivars; Documentos IAC, 119; Instituto Agronômico: Campinas, Brazil, 2021; p. 19. [Google Scholar]
- Hallé, F.; Oldeman, R.A.A.; Tomlinson, P.B. Tropical Trees and Forests-An Architectural Analysis; Springer: Berlin, Germany, 1978; 441p. [Google Scholar]
- Rakocevic, M. Coffee plant architecture. In Coffee—A Glimpse into the Future, Advances in Botanical Research; DaMatta, F.M., Ramalho, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rodrigues, A.P.; Semedo, J.N.; Pais, I.P.; Martins, L.D.; Simoões-Costa, M.C.; Leitão, A.E.; Fortunato, A.S.; Batista-Santos, P.; Palos, I.M.; et al. Sustained photosynthetic performance of Coffea spp. under long-term enhanced [CO2]. PLoS ONE 2013, 8, e82712. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Silva, P.C.; Junior, W.Q.R.; Ramos, M.L.G.; Rocha, O.C.; Veiga, A.D.; Silva, N.H.; Brasileiro, L.O.; Santana, C.C.; Soares, G.F.; Malaquias, J.V.; et al. Physiological changes of Arabica coffee under different intensities and durations of water stress in the Brazilian Cerrado. Plants 2022, 11, 2198. [Google Scholar] [CrossRef]
- Schlüter, U.; Muschak, M.; Berger, D.; Altmann, T. Photosynthetic performance of an Arabidopsis mutant with high stomatal density (sdd1-1) under different light regimes. J. Exp. Bot. 2003, 54, 867–874. [Google Scholar] [CrossRef]
- Blatt, M.R. Cell signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 221–241. [Google Scholar] [CrossRef]
- Pautov, A.; Bauer, S.; Ivanova, O.; Ivanova, A.; Krylova, E. The mechanical advantage of subsidiary cells depends on the structure of the stomatal complex. Flora 2024, 311, 152457. [Google Scholar] [CrossRef]
- Lawson, T.; Oxborough, K.; Morison, J.I.L.; Baker, N.R. The photosynthesis response of guard cells to CO2, O2, light and drought stress in a variety of species is similar. J. Exp. Bot. 2003, 54, 1734–1752. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2009, 181, 13–34. [Google Scholar] [CrossRef]
- Bernado, W.P.; Rakocevic, M.; Santos, A.R.; Ruas, K.F.; Baroni, D.F.; Abraham, A.C.; Pireda, S.; Oliveira, D.S.; Cunha, M.; Ramalho, J.C.; et al. Biomass and leaf acclimations to ultraviolet solar radiation in juvenile plants of Coffea arabica and C. canephora. Plants 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Martins, S.C.V.; Ramalho, J.D.C. Ecophysiology of coffee growth and production in a context of climate changes. In Coffee—A Glimpse into the Future, Advances in Botanical Research; DaMatta, F.M., Ramalho, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Bridson, D.M.; Verdcourt, B. Coffea in Flora of Tropical East Africa. In Flora of Tropical East Africa; A. A. Balkema: Rotterdam, The Netherlands, 1988; pp. 703–723. [Google Scholar]
- Alberto, N.J.; Ramalho, J.C.; Barros, A.I.R.; Viana, A.P.; Krohling, C.A.; Moiane, S.S.; Alberto, Z.; Rodrigues, W.P.; Partelli, F.L. Diversity in Coffea arabica cultivars in the mountains of Gorongosa National Park, Mozambique, regarding bean and leaf nutrient accumulation and physical fruit traits. Agronomy 2023, 13, 1162. [Google Scholar] [CrossRef]
- Tinley, K.L. Framework of the Gorongosa Ecosystem. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 1977. [Google Scholar]
- Bouley, P.; Poulos, M.; Branco, R.; Carter, N.H. Post-war recovery of the African lions in response to large-scale ecosystem restoration. Biol. Conserv. 2018, 227, 233–242. [Google Scholar] [CrossRef]
- Cassamo, C.T.; Draper, D.; Romeiras, M.M.; Marques, I.; Chiulele, R.; Rodrigues, M.; Stalmans, M.; Partelli, F.L.; Ribeiro-Barros, A.; Ramalho, J.C. Impact of climate changes in the suitable areas for Coffea arabica L. production in Mozambique: Agroforestry as an alternative management system to strengthen crop sustainability. Agric. Ecosyst. Environ. 2023, 346, 108341. [Google Scholar] [CrossRef]
- Ryan, C.M.; Williams, M. How does fire intensity and frequency affect Miombo woodland tree populations and biomass? Ecol. Appl. 2011, 21, 48–60. [Google Scholar] [CrossRef]
- Daskin, J.H.; Stalmans, M.; Pringle, R.M. Ecological legacies of civil war: 35-year increase in savanna tree cover following wholesale large-mammal declines. J. Ecol. 2016, 104, 79–89. [Google Scholar] [CrossRef]
- Köppen, W.P. Das Geographische System der Klimate; Gebrüdcr Borntraeger: Berlin, Germany, 1936. [Google Scholar]
- Aguiar, T.V.; Sant’Anna-Santos, B.F.; Azevedo, A.A.; Ferreira, R.S. Anati Quanti: Quantitative analysis software for plant anatomy studies. Planta Daninha 2007, 25, 649–659. [Google Scholar] [CrossRef]
- Castro, E.M.; Pereira, F.J.; Paiva, R. Vegetable Histology: Structure and Function of Vegetative Organs; Ufla: Lavras, Brazil, 2009; 234p. [Google Scholar]
- Sack, L.; Buckley, T.N. The developmental basis of stomatal density and flux. Plant Physiol. 2016, 171, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Mojena, R. Hierarchical grouping methods and stopping rules: An evaluation. Comput. J. 1977, 20, 359–363. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (R Version 4.3.0). 2022. Available online: https://www.r-project.org/ (accessed on 5 June 2023).
- Cruz, C.D. Programa Genes—Ampliado e integrado aos aplicativos R. Matlab e Selegen. Acta Sci.Agron. 2016, 38, 547–552. [Google Scholar] [CrossRef]
- Gomes, F.P. Curso de Estatística Experimental; Nobel: São Paulo, Brazil, 1985; 467p. [Google Scholar]
- Ferrão, R.G.; Cruz, C.D.; Ferreira, A.; Cecon, P.R.; Ferrão, M.A.G.; Fonseca, A.F.F.; Carneiro, P.C.S.; Silva, M.F. Genetic parameters in Conilon coffee. Pesqui. Agropecu. Bras. 2008, 43, 61–69. [Google Scholar] [CrossRef]
- Fritsche-Neto, R.; Vieira, R.A.; Scapim, C.A.; Miranda, G.V.; Rezende, L.M. Updating the ranking of the coefficients of variation from maize experiments. Acta Sci. Agron. 2012, 34, 99–101. [Google Scholar] [CrossRef]
- Couto, M.F.; Peternelli, L.A.; Barbosa, M.H.P. Classification of the coefficients of variation for sugarcane crops. Ciênc Rural. 2013, 43, 957–961. [Google Scholar] [CrossRef]
- Witkowski, E.T.F.; Lamont, B.B. Leaf specific mass confounds leaf density and thickness. Ecology 1991, 88, 486–493. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Oren, R.; Schulze, E.D.; Matyssek, R.; Zimmermann, R. Estimation of photosynthetic rate and annual carbon gain in conifers based on specific leaf weight and leaf biomass. Ecologia 1986, 70, 178–193. [Google Scholar]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From the tropics to the tundra: Global convergence in plant functioning. Proc. Nat. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Marañon, T.; Zamora, R. Interactions of the effects of drought and shade on seedlings of four Quercus species: Physiological and structural responses of the leaves. New Phytol. 2006, 170, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F. The global spectrum of the leaf economy. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Pompelli, M.F.; Martins, S.C.V.; Celin, E.F.; Ventrella, M.C.; DaMatta, F.M. What is the influence of ordinary epidermal cells and stomata on the leaf plasticity of coffee plants grown under full-sun and shady conditions? Braz. J. Biol. 2010, 70, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Medrano, H. Drought-inhibition of Photosynthesis in C3 Plants: Stomatal and Non-stomatal Limitations Revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Resco, V.; Ewers, B.E.; Sun, W.; Huxman, T.E.; Weltzin, J.F.; Williams, D.G. Drought-induced hydraulic limitations constrain leaf gas exchange recovery after precipitation pulses in the C3 woody legume, Prosopis velutina. New Phytol. 2009, 181, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Radin, J.W.; Turcotte, E.L.; Percy, R.; Zeiger, E. High yields in advanced lines of Pima cotton are associated with higher stomatal conductance, reduced leaf area and lower leaf temperature. Physiol. Plant 1994, 92, 266–272. [Google Scholar] [CrossRef]
- Radin, J.W.; Lu, Z.; Percy, R.G.; Zeiger, E. Genetic variability for stomatal conductance in Pima cotton and its relation to improvements of heat adaptation. Proc. Nat. Acad. Sci. USA 1994, 91, 7217–7221. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; van Meeteren, U. Natural variation in stomatal response to closing stimuli among Arabidopsis thaliana accessions after exposure to low VPD as a tool to recognize the mechanism of disturbed stomatal functioning. J. Exp. Bot. 2014, 65, 201412. [Google Scholar] [CrossRef]
- Giday, H.; Fanourakis, D.; Kjaer, K.H.; Fomsgaard, I.S.; Ottosen, C.O. Threshold response of stomatal closing ability to leaf abscisic acid concentration during growth. J. Exp. Bot. 2014, 65, 4361–4370. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Berry, J.A.; Beerling, D.J.; Franks, P.J. Stomata: Key players in the earth system, past and present. Curr. Opin. Plant Biol. 2010, 13, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Pieruschka, R.; Huber, G.; Berry, J.A. Control of transpiration by radiation. Proc. Nat. Acad. Sci. USA 2010, 107, 13372–13377. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2011, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fanourakis, D.; Giday, H.; Milla, R.; Pieruschka, R.; Kjaer, K.; Bolger, M.; Vasilevski, A.; Nunes-Nesi, A.N.; Fiorani, F.; Ottosen, C.O. Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Ann. Bot. 2015, 115, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Torii, K.U. Hormonal and environmental signals guiding stomatal development. BMC Biol. 2018, 16, 21. [Google Scholar] [CrossRef]
- Woodward, F.I.; Kelly, C.K. The influence of CO2 concentration on stomatal density. New Phytol. 1995, 131, 311–327. [Google Scholar] [CrossRef]
- Khan, P.S.S.V.; Kozai, T.; Nguyen, Q.T.; Kubota, C.; Dhawan, V. Growth and net photosynthetic rates of Eucalyptus tereticornis Smith under photomixotrophic and various photoautotrophic micropropagation conditions. Plant Cell Tissue Organ Cult. 2002, 71, 141–146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).