Transcriptome and Metabolome Analyses Reveal Response Mechanisms to Alternaria brassicicola-Induced Black Spot Disease in Diverse Chinese Cabbage Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Treatment
2.2. Methods for Identification of Plant Disease Resistance
2.3. Trypan Blue Staining
2.4. Reactive Oxygen Species Staining
2.5. RNA-Seq Experiments
2.6. Transcriptomic Data Analysis
2.7. Validation of Candidate Genes via Reverse Transcription Quantitative PCR (RT-qPCR)
2.8. Metabolites Extraction and LC-MS/MS Analysis
2.9. Metabolomic Data Analysis
3. Results
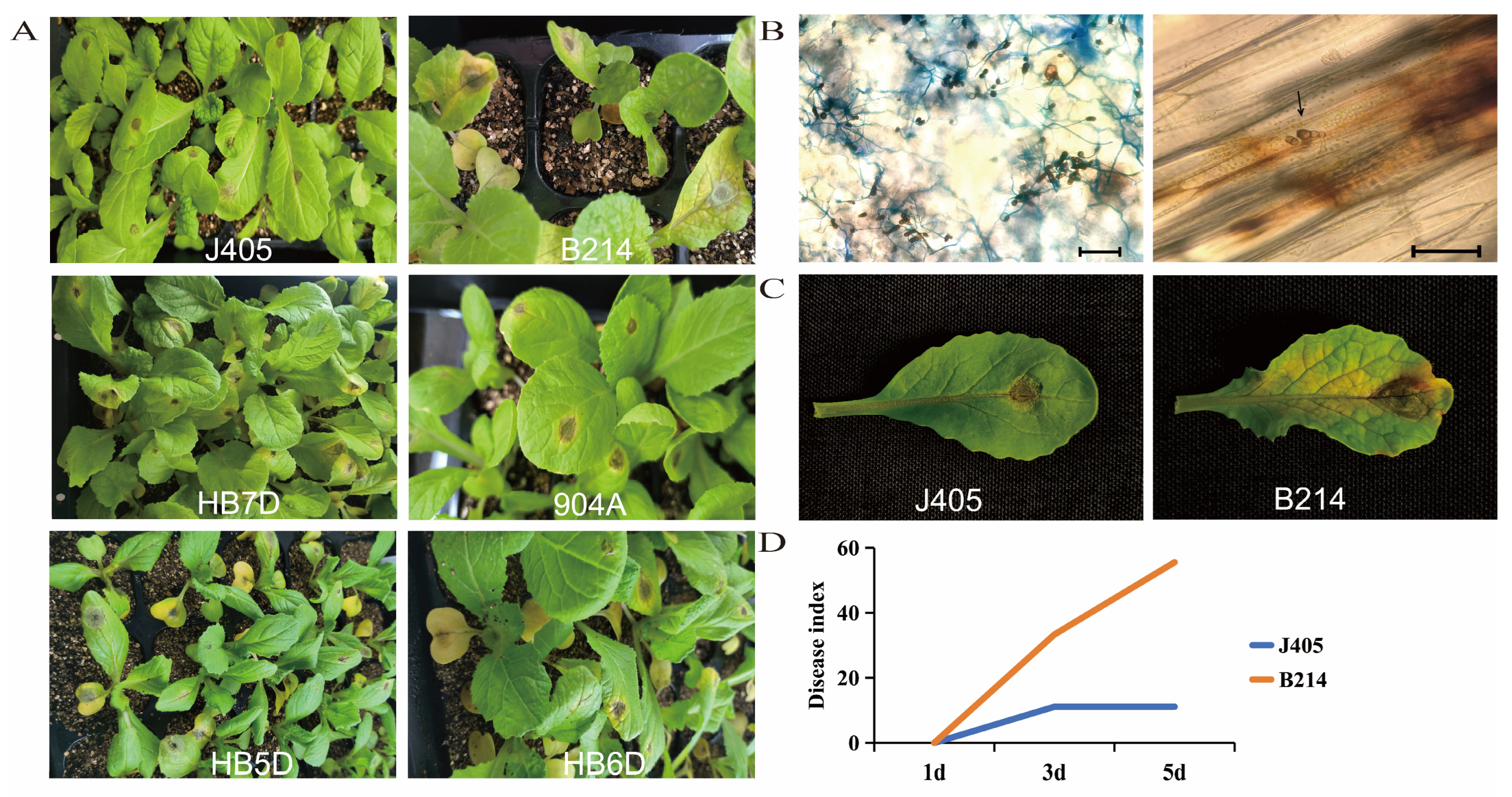
3.1. Black Spot Disease Severity across Diverse Chinese Cabbage Genotypes
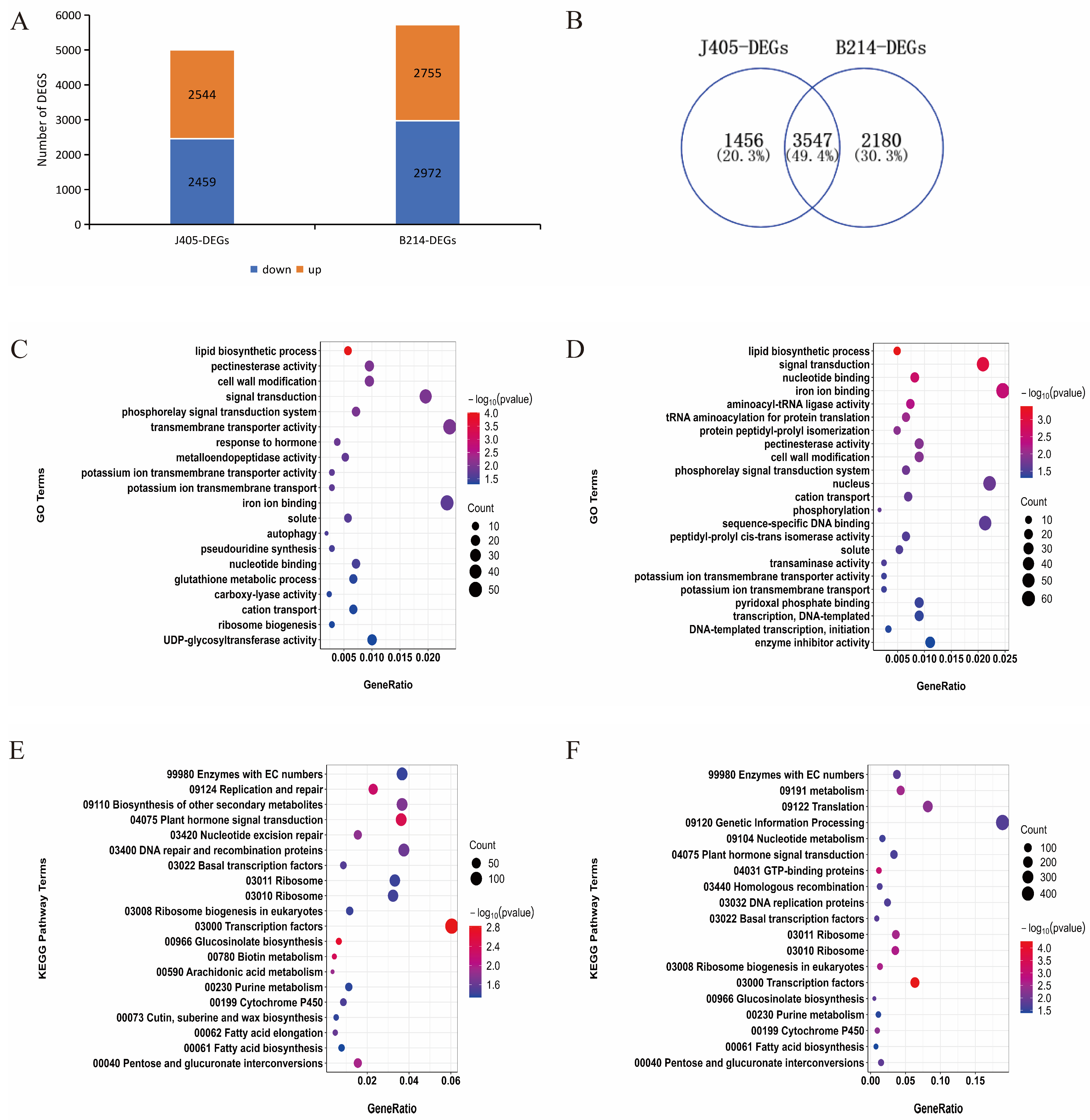
3.2. DEGs in Chinese Cabbage Genotypes J405 and B214 in Response to Black Spot Disease
3.3. Response of Genes Related to Hormone Signaling Pathways in Chinese Cabbage to Infection by A. brassicicola
3.4. Response of Genes Associated with Disease Resistance Pathways in Chinese Cabbage to Infection by A. brassicicola
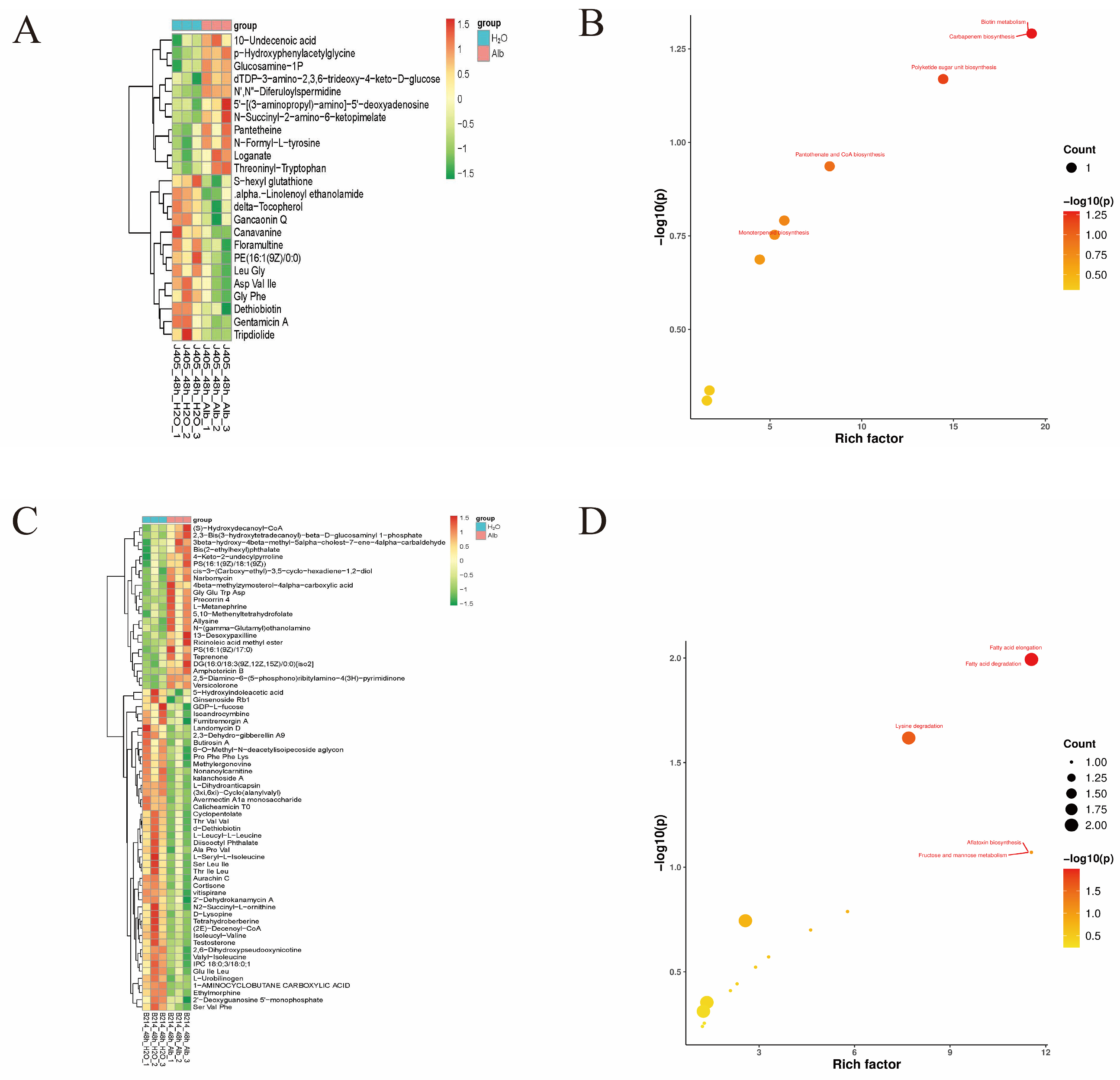
3.5. DAMs in Chinese Cabbage Genotypes J405 and B214 in Response to Black Spot Disease
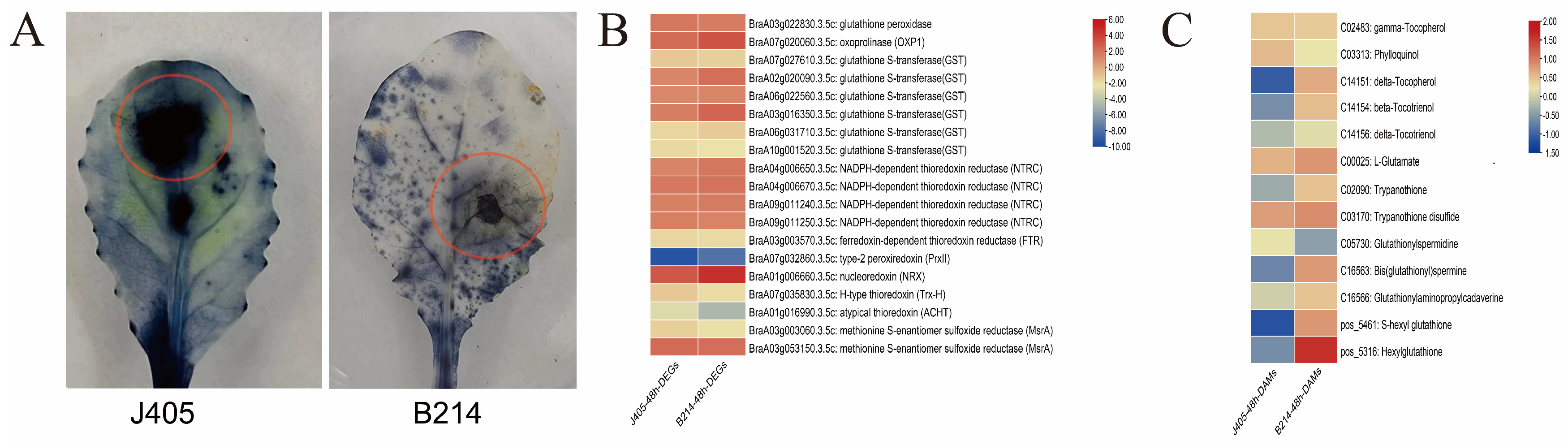
3.6. Redox Substances Are Essential in Disease Resistance and Defense of Chinese Cabbage
3.7. Various Secondary Metabolites Play Important Roles in Disease Resistance and Defense of Chinese Cabbage
3.8. Association Analysis of Key Genes and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.H.; Lee, G.A.; Subramanian, P.; Hahn, B.S. Quantification and diversity analyses of major glucosinolates in conserved Chinese cabbage (Brassica rapa L. ssp. pekinensis) Germplasms. Foods 2023, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.A.; Jung, Y.H.; Lim, S.H.; Park, S.U.; Kim, J.K. Metabolic profiling in Chinese cabbage (Brassica rapa L. subsp. pekinensis) cultivars reveals that glucosinolate content is correlated with carotenoid content. J. Agric. Food Chem. 2016, 64, 4426–4434. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Y.N.; Nan, N.; Lu, B.H.; Xia, W.Y.; Wu, X.Y. Alternaria brassicicola causes a leaf spot on isatis indigotica in China. Plant Dis. 2014, 98, 1431. [Google Scholar] [CrossRef] [PubMed]
- Friesen, T.L.; Faris, J.D.; Solomon, P.S.; Oliver, R.P. Host-specific toxins: Effectors of necrotrophic pathogenicity. Cell. Microbiol. 2008, 10, 1421–1428. [Google Scholar] [CrossRef]
- Chen, A.; Mao, X.; Sun, Q.; Wei, Z.; Li, J.; You, Y.; Zhao, J.; Jiang, G.; Wu, Y.; Wang, L.; et al. Alternaria mycotoxins: An overview of toxicity, metabolism, and analysis in food. J. Agric. Food Chem. 2021, 69, 7817–7830. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 17, 745–758. [Google Scholar] [CrossRef]
- Lorang, J.M.; Sweat, T.A.; Wolpert, T.J. Plant disease susceptibility conferred by a “resistance” gene. Proc. Natl. Acad. Sci. USA 2007, 104, 14861–14866. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Greenberg, J.T.; Vinatzer, B.A. Identifying type III effectors of plant pathogens and analyzing their interaction with plant cells. Curr. Opin. Microbiol. 2003, 6, 20–28. [Google Scholar] [CrossRef]
- Nimchuk, Z.; Eulgem, T.; Holt, B.F.; Dangl, J.L. Recognition and response in the plant immune system. Annu. Rev. Genet. 2003, 37, 579–609. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. Hypersensitive response-related death. Plant Mol. Biol. 2000, 44, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Usadel, B.; Nagel, A.; Thimm, O.; Redestig, H.; Blaesing, O.E.; Palacios-Rojas, N.; Selbig, J.; Hannemann, J.; Piques, M.C.; Steinhauser, D.; et al. Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol. 2005, 138, 1195–1204. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Boccard, J.; Rutledge Douglas, N.A. Consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Corwin, J.A.; Copeland, D.; Feusier, J.; Eshbaugh, R.; Chen, F.; Atwell, S.; Kliebenstein, D.J. Plastic transcriptomes stabilize immunity to pathogen diversity: The jasmonic acid and salicylic acid networks within the Arabidopsis/Botrytis Pathosystem. Plant Cell. 2017, 29, 2727–2752. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 25, 170. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, S.; Yang, D.; Fan, Z. Revealing shared and distinct genes responding to JA and SA signaling in Arabidopsis by meta-analysis. Front. Plant Sci. 2020, 26, 908. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 18, 1349. [Google Scholar] [CrossRef]
- Samac, D.A.; Hironaka, C.M.; Yallaly, P.E.; Shah, D.M. Isolation and characterization of the genes encoding basic and acidic chitinase in Arabidopsis thaliana. Plant Physiol. 1990, 93, 907–914. [Google Scholar] [CrossRef]
- Anfang, M.; Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Li, M.; He, P.; Zhang, Y. Loss-of-function of Arabidopsis receptor-like kinase BIR1 activates cell death and defense responses mediated by BAK1 and SOBIR1. New Phytol. 2016, 212, 637–645. [Google Scholar] [CrossRef]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Igari, K.; Endo, S.; Hibara, K.; Aida, M.; Sakakibara, H.; Kawasaki, T.; Tasaka, M. Constitutive activation of a CC-NB-LRR protein alters morphogenesis through the cytokinin pathway in Arabidopsis. Plant J. 2008, 55, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Fichman, Y.; Mittler, R. Integration of electric, calcium, reactive oxygen species and hydraulic signals during rapid systemic signaling in plants. Plant J. 2021, 107, 7–20. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Gao, H.; Yu, C.; Liu, R.; Li, X.; Huang, H.; Wang, X.; Zhang, C.; Jiang, N.; Li, X.; Cheng, S.; et al. The glutathione S-transferase PtGSTF1 improves biomass production and salt tolerance through regulating xylem cell proliferation, ion homeostasis and reactive oxygen species scavenging in poplar. Int. J. Mol. Sci. 2022, 23, 11288. [Google Scholar] [CrossRef]
- Peláez-Vico, M.Á.; Fichman, Y.; Zandalinas, S.I.; Van Breusegem, F.; Karpiński, S.M.; Mittler, R. ROS and redox regulation of cell-to-cell and systemic signaling in plants during stress. Free Radic. Biol. Med. 2022, 193, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Dudareva, N. Plant specialized metabolism. Curr. Biol. 2023, 33, R473–R478. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Fatima, M.; Sharif, Y.; Zafar, M.H.; Ali, H.; Khan, K.A. Role of primary metabolites in plant defense against pathogens. Microb. Pathog. 2019, 137, 103728. [Google Scholar] [CrossRef]
| KOID | GeneID | Annotation | J405-48h-DEGs | B214-48h-DEGs |
|---|---|---|---|---|
| K13463 | BraA05g006060.3.5C | COI-1, coronatine-insensitive protein 1, JA | −1.33 | −2.37 |
| K13464 | BraA07g030900.3.5C | JAZ, jasmonate ZIM domain-containing protein, JA | 2.90 | 4.30 |
| K14431 | BraA10g029510.3.5C | TGA, transcription factor TGA, SA | −2.41 | −2.26 |
| K14508 | BraA01g016900.3.5C | NPR1, regulatory protein NPR1, SA | 2.06 | 3.07 |
| K13449 | BraA03g012320.3.5C | PR1, pathogenesis-related protein 1, SA | - | −3.42 |
| K14509 | BraA08g035470.3.5C | ETR, ethylene receptor, ET | - | −1.28 |
| K14514 | BraA07g029620.3.5C | EIN3, ethylene-insensitive protein 3, ET | - | 1.71 |
| K14516 | BraA03g016550.3.5C | ERF1, ethylene-responsive transcription factor 1, ET | - | −2.78 |
| K14510 | BraA10g032440.3.5C | CTR1, serine/threonine-protein kinase CTR1, ET | −2.53 | −2.10 |
| K14512 | BraA05g003850.3.5C | MPK6, mitogen-activated protein kinase 6, ET | - | −3.62 |
| K20547 | BraA03g035780.3.5C | CHIB, basic endochitinase B, ET | 1.84 | 2.42 |
| K13946 | BraA02g025050.3.5C | AUX1, auxin influx carrier, Auxin | 3.96 | 4.46 |
| K14484 | BraA10g002540.3.5C | IAA, auxin-responsive protein IAA, Auxin | −2.59 | −3.16 |
| K14486 | BraA04g025570.3.5C | ARF, auxin response factor, Auxin | −3.11 | −5.07 |
| K14487 | BraA03g020320.3.5C | GH3, auxin responsive GH3 gene family, Auxin | −2.44 | −4.19 |
| K14488 | BraA01g003530.3.5C | SAUR, SAUR family protein, Auxin | −3.36 | −4.53 |
| K14485 | BraA04g000640.3.5C | TIR1, transport inhibitor response 1, Auxin | 1.90 | 2.10 |
| K14490 | BraA07g026540.3.5C | AHP, histidine-containing phosphotransfer protein, cytokinin | 1.61 | 1.50 |
| K14491 | BraA03g038160.3.5C | ARR-B, two-component response regulator ARR-B family, cytokinin | −2.33 | −2.92 |
| K14492 | BraA06g007350.3.5C | ARR-A, two-component response regulator ARR-A family, cytokinin | −4.00 | −4.31 |
| K14489 | BraA03g042410.3.5C | AHK2_3_4, Arabidopsis histidine kinase 2/3/4, cytokinin | 1.47 | 2.28 |
| K14494 | BraA02g017510.3.5C | DELLA, DELLA protein, Gibberellin | −4.83 | −2.91 |
| K16189 | BraA01g008670.3.5C | PIF4, phytochrome-interacting factor 4 TF, Gibberellin | −1.73 | −3.66 |
| K12126 | BraA03g024450.3.5C | PIF3, phytochrome-interacting factor 3 TF, Gibberellin | 1.17 | 2.67 |
| K14432 | BraA07g023280.3.5C | ABF, ABA responsive element binding factor, ABA | - | −1.04 |
| K14496 | BraA08g013180.3.5C | PYL, abscisic acid receptor PYR/PYL family, ABA | −4.55 | −5.10 |
| K14497 | BraA10g006150.3.5C | PP2C, protein phosphatase 2C, ABA | 4.53 | 6.48 |
| K14498 | BraA03g003810.3.5C | SNRK2, serine/threonine-protein kinase SRK2, ABA | −1.88 | −1.54 |
| K14503 | BraA01g002020.3.5C | BZR1_2, brassinosteroid resistant 1/2, BR | −2.58 | −1.18 |
| K14500 | BraA03g044040.3.5C | BSK, BR-signaling kinase, BR | 2.69 | 3.09 |
| K14505 | BraA01g004150.3.5C | CYCD3, cyclin D3, plant, BR | 2.96 | 3.47 |
| K13415 | BraA01g000440.3.5C | BRI1, protein brassinosteroid insensitive 1, BR | −2.36 | −1.31 |
| K13416 | BraA03g058830.3.5C | BAK1, brassinosteroid insensitive 1-associated receptor kinase 1, BR | - | 5.87 |
| KOID | GeneID | Annotation | J405-48h-DEGs | B214-48h-DEGs |
|---|---|---|---|---|
| K05391 | BraA01g028830.3.5C | CNGC, cyclic nucleotide gated channel | 5.30 | - |
| K02183 | BraA07g020630.3.5C | CALM, calmodulin | 3.98 | 3.16 |
| K13448 | BraA07g024690.3.5C | CML, calcium-binding protein | 3.93 | 3.32 |
| K13412 | BraA02g044280.3.5C | CPK, calcium-dependent protein kinase | 2.53 | 3.76 |
| K13447 | BraA02g040970.3.5C | RBOH, respiratory burst oxidase | 1.92 | - |
| K16224 | BraA08g034860.3.5C | FRK1, senescence-induced receptor-like serine/threonine-protein kinase | 1.13 | 1.54 |
| K13449 | BraA06g003510.3.5C | PR1, pathogenesis-related protein 1 | −3.57 | −3.85 |
| K20547 | BraA03g035780.3.5C | CHIB, basic endochitinase B | 1.84 | 2.42 |
| K13436 | BraA05g013950.3.5C | PTI1, pto-interacting protein 1 | −3.95 | −3.74 |
| K13457 | BraA10g002960.3.5C | RPM1, disease resistance protein | 2.92 | 2.24 |
| K13458 | BraA02g016130.3.5C | RAR1, disease resistance protein | 1.18 | - |
| K12795 | BraA01g014280.3.5C | SUGT1, suppressor of the G2 allele of SKP1 | −3.08 | −3.20 |
| K09487 | BraA08g020900.3.5C | HSP90B, heat shock protein 90 kDa beta | 1.21 | 1.38 |
| K13459 | BraA08g031690.3.5C | RPS2, disease resistance protein | 1.53 | 1.21 |
| K13430 | BraA09g003180.3.5C | PBS1, serine/threonine-protein kinase | - | −1.09 |
| K13460 | BraA06g009450.3.5C | RPS5, disease resistance protein | 1.82 | - |
| K18873 | BraA06g005150.3.5C | PIK1, pathogen-induced protein kinase | −4.44 | −2.74 |
| K16226 | BraA03g028120.3.5C | RPS4, disease resistance protein | −1.43 | −1.24 |
| K01373 | BraA03g047330.3.5C | CTSF, cathepsin F | 1.54 | - |
| K15397 | BraA03g057320.3.5C | KCS, 3-ketoacyl-CoA synthase | −2.09 | −2.03 |
| K13423 | BraA02g002290.3.5C | WRKY25, WRKY transcription factor 25 | 3.08 | 2.72 |
| K02358 | BraA03g050050.3.5C | Tuf, elongation factor Tu | 2.21 | 2.71 |
| K04368 | BraA03g012900.3.5C | MAP2K1, mitogen-activated protein kinase 1 | 2.28 | 2.03 |
| K13414 | BraA03g026820.3.5C | MEKK1, mitogen-activated protein kinase 1 | −2.58 | −2.52 |
| K14512 | BraA03g023140.3.5C | MPK6, mitogen-activated protein kinase 6 | −1.51 | −2.47 |
| K18835 | BraA03g013060.3.5C | WRKY2, WRKY transcription factor 2 | 1.18 | - |
| K13416 | BraA03g058830.3.5C | BAK1, brassinosteroid insensitive 1-associated receptor kinase 1 | - | 5.87 |
| K18834 | BraA03g042400.3.5C | WRKY1, WRKY transcription factor 1 | - | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Wang, C.; Zhang, H.; Fan, W.; Liu, X.; Huang, Z.; Wang, Y.; Zhang, B. Transcriptome and Metabolome Analyses Reveal Response Mechanisms to Alternaria brassicicola-Induced Black Spot Disease in Diverse Chinese Cabbage Genotypes. Horticulturae 2024, 10, 1001. https://doi.org/10.3390/horticulturae10091001
Yan W, Wang C, Zhang H, Fan W, Liu X, Huang Z, Wang Y, Zhang B. Transcriptome and Metabolome Analyses Reveal Response Mechanisms to Alternaria brassicicola-Induced Black Spot Disease in Diverse Chinese Cabbage Genotypes. Horticulturae. 2024; 10(9):1001. https://doi.org/10.3390/horticulturae10091001
Chicago/Turabian StyleYan, Wenyuan, Chaonan Wang, Hong Zhang, Weiqiang Fan, Xiaohui Liu, Zhiyin Huang, Yong Wang, and Bin Zhang. 2024. "Transcriptome and Metabolome Analyses Reveal Response Mechanisms to Alternaria brassicicola-Induced Black Spot Disease in Diverse Chinese Cabbage Genotypes" Horticulturae 10, no. 9: 1001. https://doi.org/10.3390/horticulturae10091001
APA StyleYan, W., Wang, C., Zhang, H., Fan, W., Liu, X., Huang, Z., Wang, Y., & Zhang, B. (2024). Transcriptome and Metabolome Analyses Reveal Response Mechanisms to Alternaria brassicicola-Induced Black Spot Disease in Diverse Chinese Cabbage Genotypes. Horticulturae, 10(9), 1001. https://doi.org/10.3390/horticulturae10091001






