Non-Structural Carbohydrate Composition of ‘Hass’ Avocado Fruit Is Affected by Maturity, Storage, and Ripening
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit
2.2. Treatments
2.3. Assessment Methodologies
2.4. Chemical Analysis
- Sample extraction
- Sample analysis
2.5. Statistical Analysis
3. Results
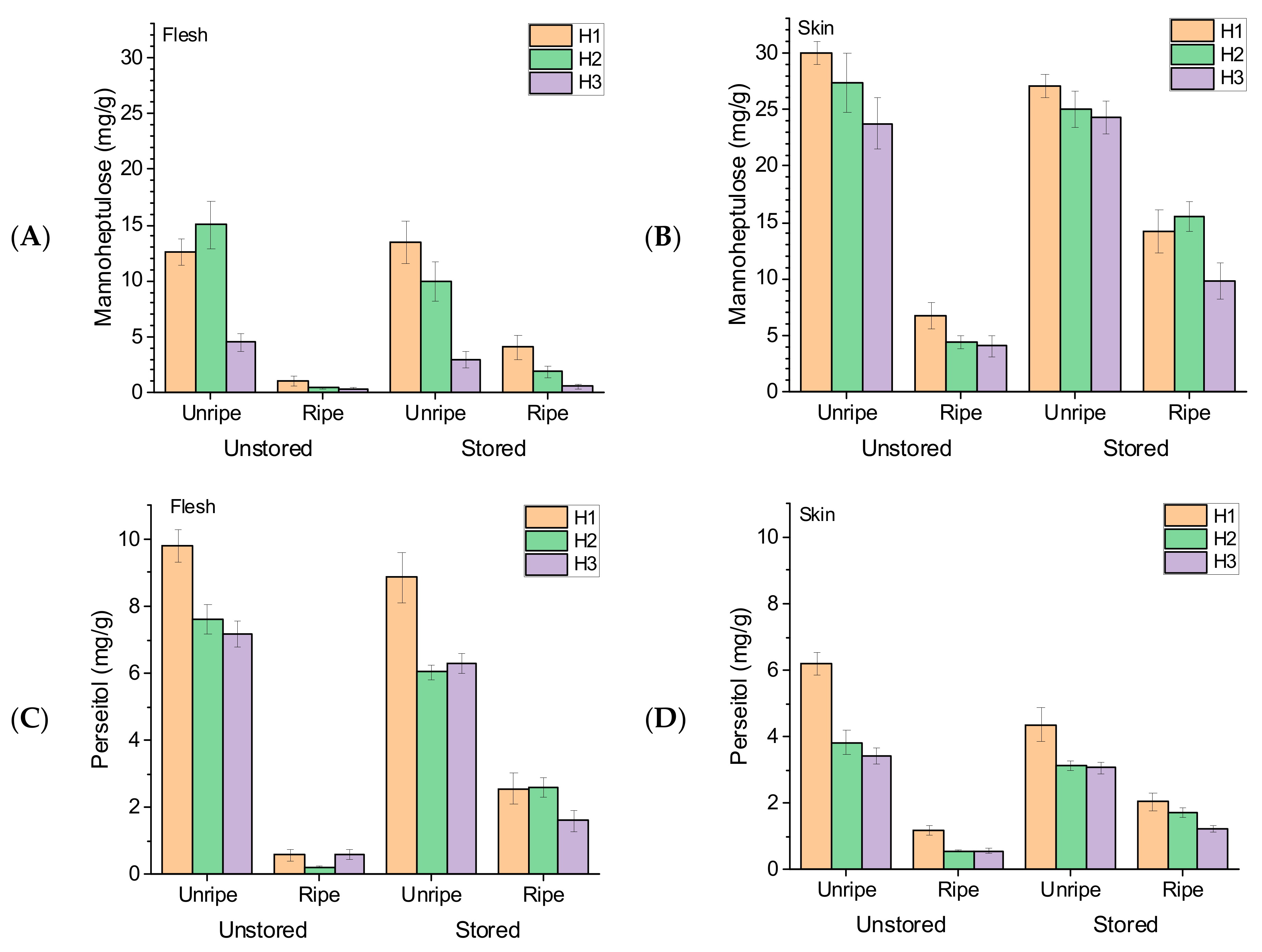
3.1. Fruit Mannoheptulose and Perseitol
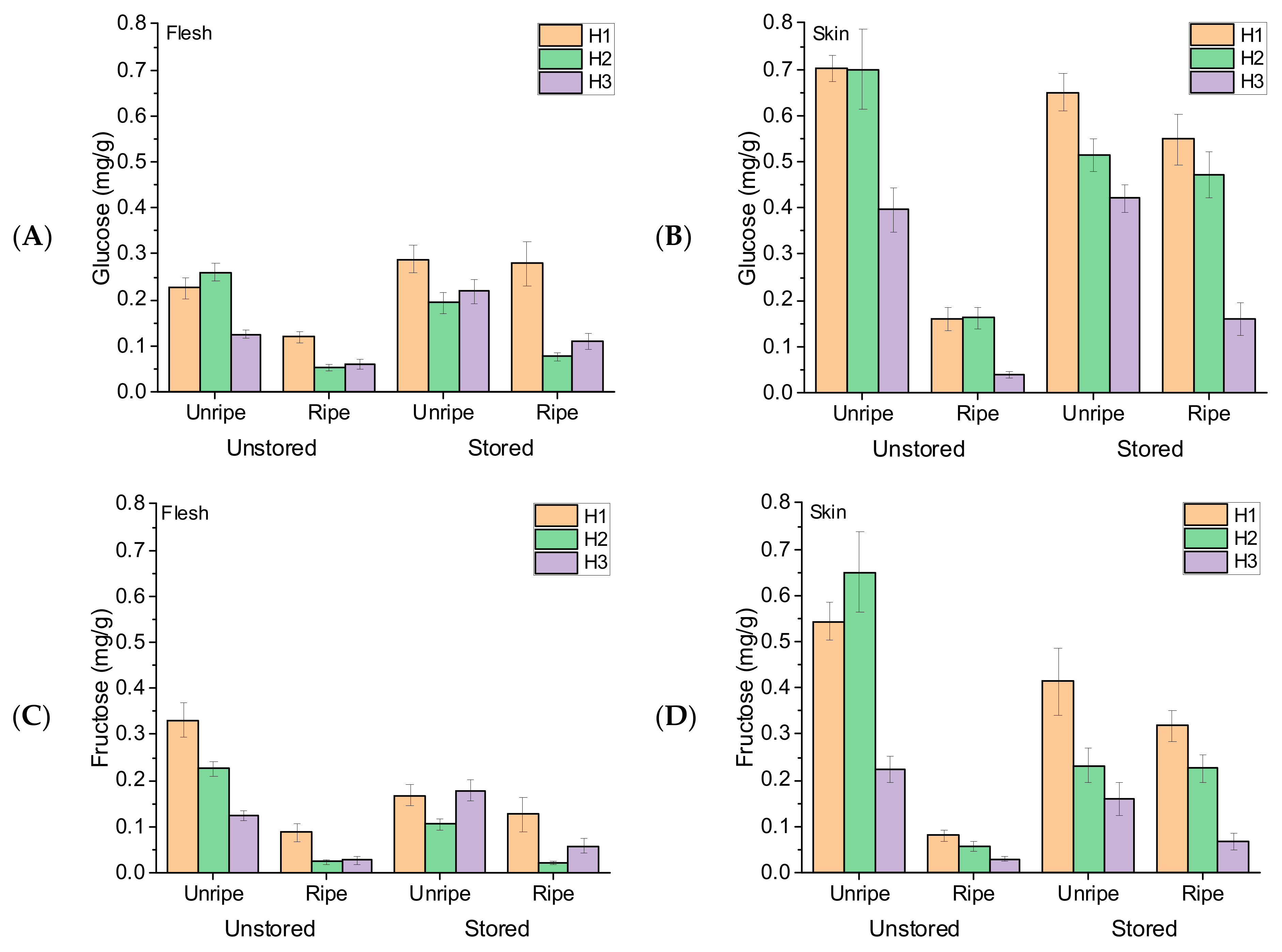
3.2. Fruit Glucose, Fructose, and Sucrose
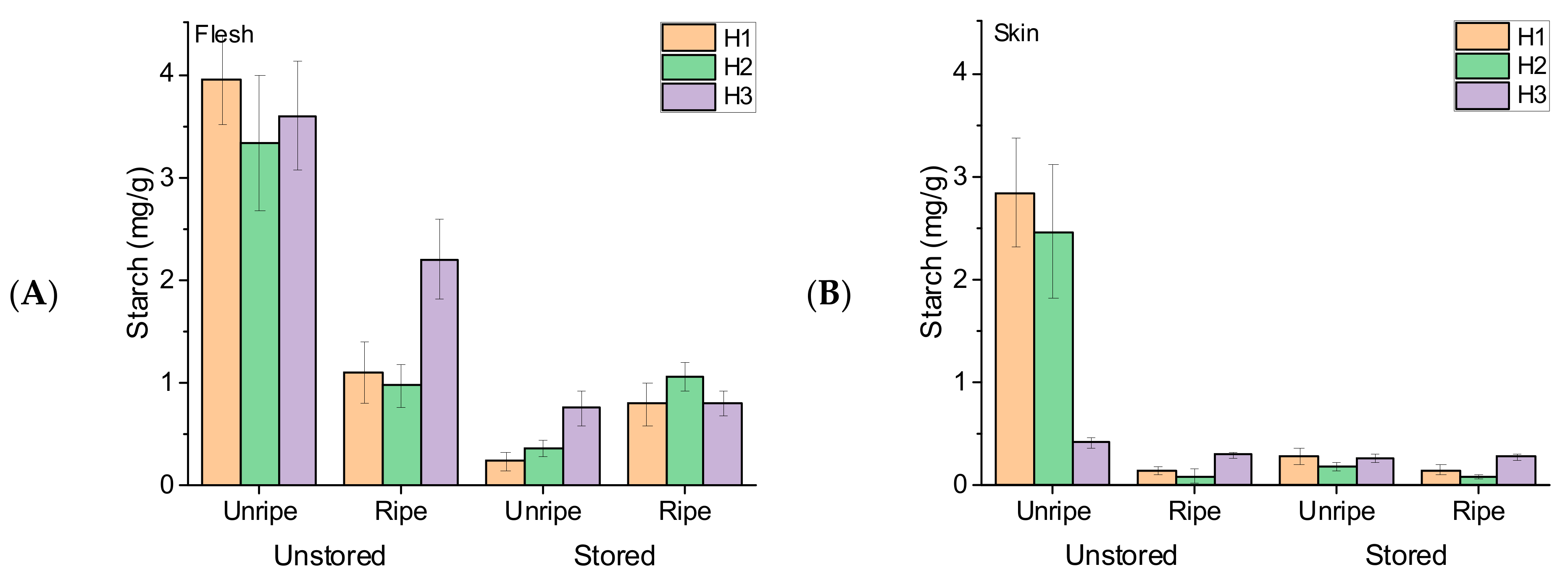
3.3. Fruit Starch
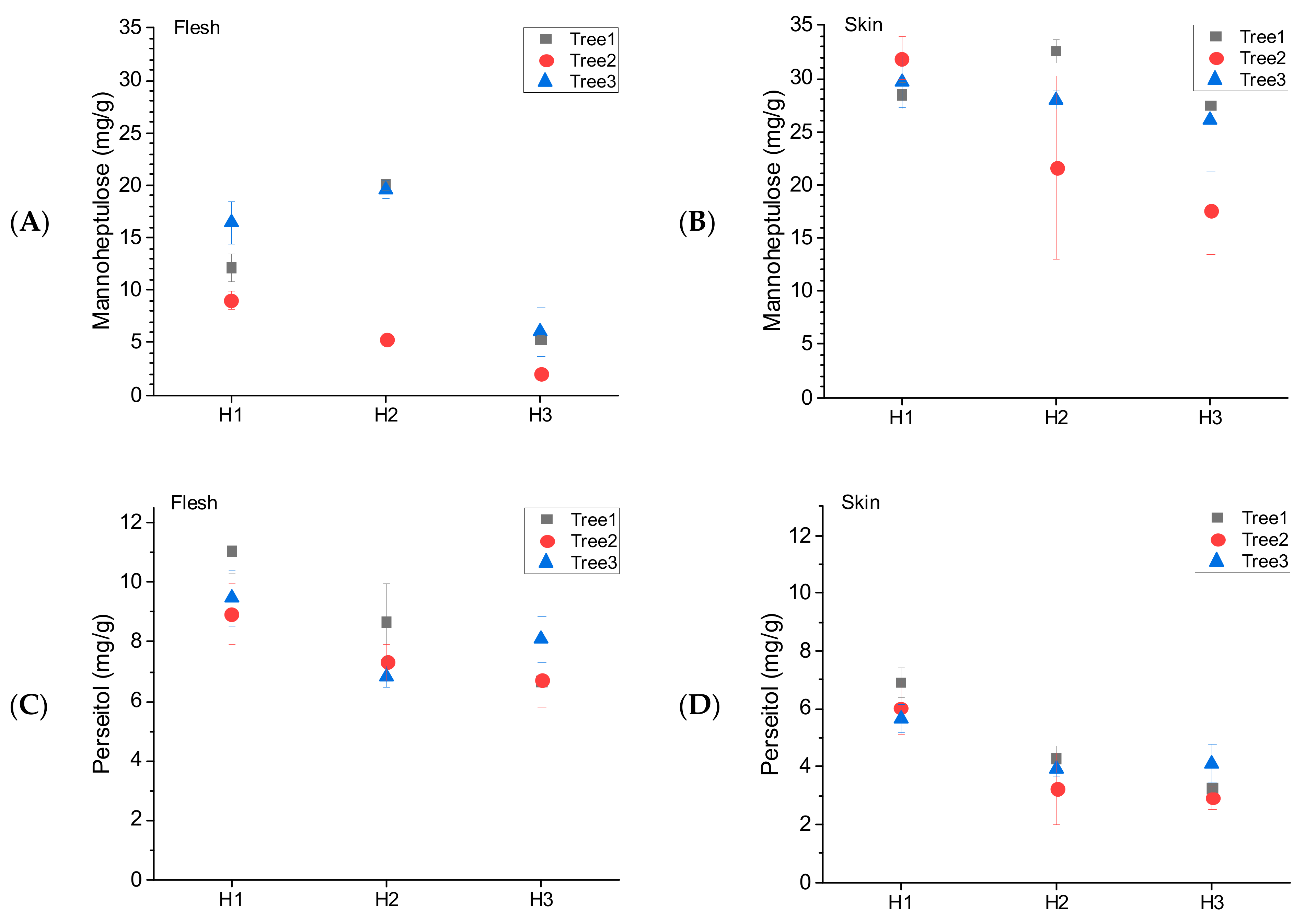
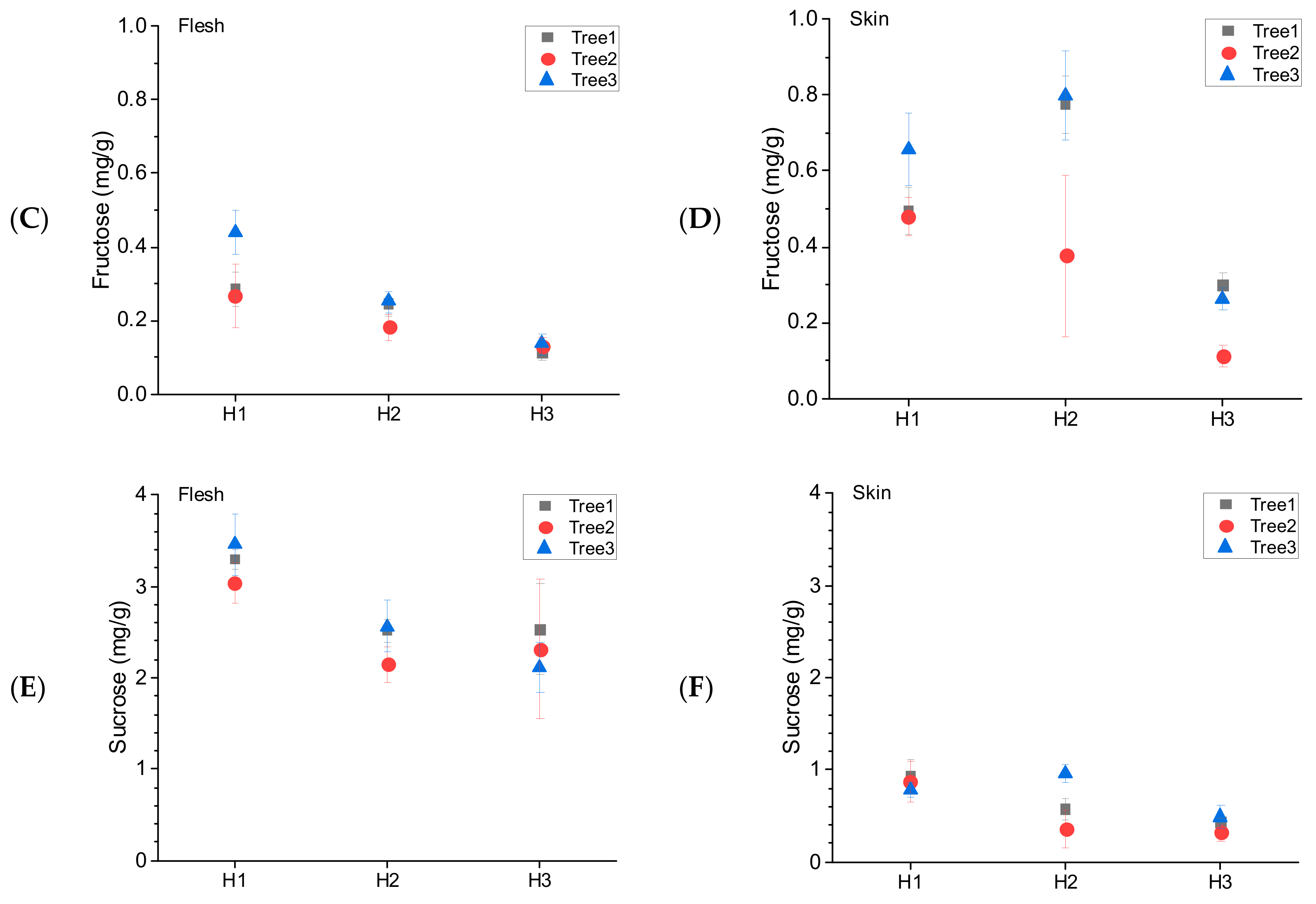
3.4. Tree-to-Tree Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Robinson, P.W.; Madore, M.A.; Witney, G.W.; Arpaia, M.L. ‘Hass’ avocado carbohydrate fluctuations. I Growth and phenology. J. Am. Soc. Hortic. Sci. 1999, 124, 671–675. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, P.W.; Madore, M.A.; Witney, G.W.; Arpaia, M.L. ‘Hass’ avocado carbohydrate fluctuations. II Fruit growth and ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 676–681. [Google Scholar] [CrossRef]
- Lee, S.K.; Young, R.E.; Schiffman, P.M.; Coggins, C.W. Maturity studies of avocado fruit based on picking dates and dry weight. J. Am. Soc. Hortic. Sci. 1983, 108, 390–394. [Google Scholar] [CrossRef]
- Cowan, A.K. Occurrence, metabolism, transport and function of seven-carbon sugars. Phytochem. Rev. 2017, 16, 137–157. [Google Scholar] [CrossRef]
- Liu, X.; Sievert, J.S.; Arpaia, M.L.; Madore, M.A. Postulated physiological roles of the seven-carbon sugars, mannoheptulose, and perseitol in avocado. J. Am. Soc. Hortic. Sci. 2002, 127, 108–114. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P.; Lovatt, C. The quest for the function of ‘Hass’ avocado carbohydrates: Clues from fruit and seed development as well as seed germination. Aust. J. Bot. 2012, 60, 79–86. [Google Scholar] [CrossRef][Green Version]
- Cowan, A.K.; Bornman, C.H. Metabolic control of avocado fruit growth: 3-hydroxy-3-methylglutaryl coenzyme a reductase, active oxygen species and the role of C7 sugars. S. Afr. J. Bot. 2004, 70, 75–82. [Google Scholar] [CrossRef]
- Bertling, I.; Bower, J.P. Sugars as energy sources—Is there a link to avocado fruit quality? S. Afr. Avocado Grow. Assoc. Yearb. 2005, 28, 24–27. [Google Scholar]
- Bertling, I.; Tesfay, S.Z.; Bower, J.P.; Mohammed Ahamed Ali, N.G. D-Mannoheptulose: Special carbohydrate in avocado: Presence postharvest and commercial importance. Acta Hortic. 2012, 934, 741–745. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P. Avocado orchard management: Effects of boron, and branch-girdling on special carbohydrates. Acta Hortic. 2012, 932, 491–497. [Google Scholar] [CrossRef]
- Blakey, R.J.; Bower, J.P.; Bertling, I. Protein and sugar trends during the avocado season. S. Afr. Avocado Grow. Assoc. Yearb. 2009, 32, 18–21. [Google Scholar]
- Blakey, R.J.; Tesfay, S.Z.; Bertling, I.; Bower, J.P. Changes in sugars, total protein, and oil in ‘Hass’ avocado (Persea americana Mill.) fruit during ripening. J. Hortic. Sci. Biotech. 2012, 87, 381–387. [Google Scholar] [CrossRef]
- Pesis, E.; Fuchs, Y.; Zuaberman, G. Starch content and amylase activity in avocado fruit pulp. J. Am. Soc. Hortic. Sci. 1978, 103, 673–676. [Google Scholar] [CrossRef]
- Kruger, F.J.; Volschenk, G.O.; Volschenk, E. Investigation into the potential use of avocado total soluble solids content for crop estimate and orchard management purposes. S. Afr. Avocado Grow. Assoc. Yearb. 2021, 44, 30–31. [Google Scholar]
- Kruger, F.J.; Volschenk, G.O.; Volschenk, L. Towards the development of a total soluble solids (refractometer) based maturity measurement and ripening prediction procedure for avocado fruit. S. Afr. Avocado Grow. Assoc. Yearb. 2018, 41, 115–117. [Google Scholar]
- Kruger, F.J.; Volschenk, G.O.; Volschenk, L. Further observations on the development of a total soluble solids (TSS) based maturity/ripening efficiency measurement tool for South African avocado fruit. S. Afr. Avocado Grow. Assoc. Yearb. 2019, 42, 82–84. [Google Scholar]
- Burdon, J.; Lallu, N.; Haynes, G.; Francis, K.; Boldingh, H.; Pak, H.A.; Fields, F.P.; Elmsly, T.A.; Smith, D.B.; Dixon, J.; et al. Preliminary studies of physiological and morphological indicators of potential poor quality in late season New Zealand ‘Hass’ avocados. In Proceedings of the VI World Avocado Congress, Viña del Mar, Chile, 12–16 November 2007. [Google Scholar]
- Burdon, J.; Lallu, N.; Haynes, G.; Pidakala, P.; Willcocks, P.; Billing, D.; McDermott, K.; Voyle, D.; Boldingh, H. Carbohydrate status of late season ‘Hass’ avocado fruit. N. Z. Avocado Grow. Assoc. Ann. Res. Rep. 2007, 7, 97–102. [Google Scholar]
- Tesfaye, T.; Gibril, M.; Sithole, B.; Ramjugernath, D.; Chavan, R.; Chunilall, V.; Gounden, N. Valorisation of avocado seeds: Extraction and characterisation of starch for textile applications. Clean Technol. Environ. Policy 2018, 20, 2135–2154. [Google Scholar] [CrossRef]
- Charles, A.C.; Dadmohammadi, Y.; Abbaspourrad, A. Food and cosmetic applications of the avocado seed: A review. Food Funct. 2022, 13, 6894–6901. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I.; Rodrigo, J. Pistil starch reserves at anthesis correlate with final flower fate in avocado (Persea americana). PLoS ONE 2013, 8, e78467. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; University of California Agriculture and Natural Resources Publication: Oakland, CA, USA, 2002. [Google Scholar]
- Hofman, P.J.; Fuchs, Y.; Milne, D.L. Harvesting, packing, postharvest technology, transport and processing. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CAB International: Wallingford, UK, 2002; pp. 363–401. [Google Scholar]
- Dixon, J. New Zealand Avocado Fruit Assessment Manual, 3rd ed.; Avocado Industry Council Limited: Tauranga, New Zealand, 2003. [Google Scholar]
- Burdon, J.; Lallu, N.; Haynes, G.; Pidakala, P.; Billing, D.; Houghton, P.; Voyle, D.; Boldingh, H. Fruit carbohydrate content and quality of New Zealand-grown ‘Hass’ avocado fruit. In Proceedings of the VII World Avocado Congress, Cairns, Australia, 5–9 September 2011; pp. 507–516. [Google Scholar]
- Magwaza, L.S.; Tesfay, S.Z. A review of destructive and non-destructive methods for determining avocado fruit maturity. Food Bioprocess Technol. 2015, 8, 1995–2011. [Google Scholar] [CrossRef]
- Pak, H.A. Pattern of disease development in late season fruit. N. Z. Avocado Grow. Assoc. Ann. Res. Rep. 2001, 1, 62–65. [Google Scholar]
- Thorp, G.T.; Anderson, P.; Camilleri, M. Avocado tree growth cycles—A quantitative model. In Proceedings of the World Avocado Congress III, Tel Aviv, Israel, 22–27 October 1995; pp. 76–79. [Google Scholar]
- Dixon, J.; Elmsly, T.A.; Dixon, E.M.; Mandemaker, A.J. ‘Hass’ avocado tree phenology 2004-2008 in the western Bay of Plenty. N. Z. Avocado Grow. Assoc. Ann. Res. Rep. 2007, 7, 21–30. [Google Scholar]
- Dixon, J.; Cotterell, C.; Hofstee, B.; Elmsly, T.A. ‘Hass’ avocado tree phenology 2004–2009 in the Western Bay of Plenty. N. Z. Avocado Grow. Assoc. Ann. Res. Rep. 2008, 8, 35–57. [Google Scholar]
- Rocha-Arroyo, J.L.; Salazar-Garcia, S.; Barcenas-Ortega, A.E.; Gonzalez-Duran, J.L.; Cossio-Vargas, L.E. Fenología del aguacate ‘Hass’ en Michoacán. Rev. Mex. De Cienc. Agric. 2010, 2, 303–316. [Google Scholar]
- Cameron, S.H.; Borst, G. Starch in the avocado tree. Proc. Am. Soc. Hortic. Sci. 1938, 436, 255–258. [Google Scholar]
- Kaiser, C.; Wolstenholme, B.N. Aspects of delayed harvest of ‘Hass’ avocado (Persea americana Mill.) fruit in a cool subtropical climate. II. Fruit size, yield, phenology and whole-tree starch cycling. J. Hort. Sci. 1994, 69, 447–457. [Google Scholar] [CrossRef]
- Wolstenholme, B.N. Alternate bearing in avocado: An overview. In Proceedings of the 4th Australian and New Zealand Avocado Growers’ Conference, Cairns, Australia, 21–24 July 2009; Available online: http://www.avocadosource.com (accessed on 15 July 2024).
- Scholefield, P.B.; Sedgely, M.; Alexander, D.M. Carbohydrate cycling in relation to shoot growth, floral initiation and development and yield in the avocado. Sci. Hortic. 1985, 25, 99–110. [Google Scholar] [CrossRef]



| Harvest | Dry Matter (%) | Skin Colour (0–100 Scale) | Firmness (N) | Time to Ripe (Days) |
|---|---|---|---|---|
| H1 | 28.9 (1.7) | 0 | 96.1 (3.1) | 12.8 (0.5) |
| H2 | 30.6 (1.1) | 0 | 105.9 (2.6) | 11.8 (0.6) |
| H3 | 36.9 (1.2) | 0 | 92.2 (2.8) | 13.9 (0.5) |
| Source of Variation | ANOVA p-Value | |||||
|---|---|---|---|---|---|---|
| Mannoheptulose | Perseitol | Glucose | Fructose | Sucrose | Starch | |
| Maturity (M) | <0.001 | <0.001 | <0.001 | <0.001 | 0.168 | 0.181 |
| Ripeness (R) | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 |
| Storage (S) | 0.796 | 0.145 | <0.001 | 0.029 | 0.168 | <0.001 |
| M.R | <0.001 | <0.001 | 0.005 | 0.457 | 0.058 | 0.588 |
| M.S | 0.055 | 0.686 | <0.001 | <0.001 | 0.951 | 0.299 |
| R.S | 0.008 | <0.001 | 0.064 | <0.001 | 0.782 | <0.001 |
| M.R.S | 0.260 | 0.166 | 0.048 | 0.001 | 0.006 | 0.083 |
| Source of Variation | Variance Ratio | |||||
| Mannoheptulose | Perseitol | Glucose | Fructose | Sucrose | Starch | |
| Maturity (M) | 29.86 | 19.52 | 22.34 | 20.28 | 1.81 | 1.73 |
| Ripeness (R) | 167.06 | 821.82 | 61.86 | 114.31 | 10.40 | 21.72 |
| Storage (S) | 0.07 | 2.15 | 17.21 | 4.85 | 1.92 | 96.14 |
| M.R | 15.84 | 11.83 | 5.62 | 0.79 | 2.92 | 0.53 |
| M.S | 2.97 | 0.38 | 9.16 | 7.87 | 0.05 | 1.22 |
| R.S | 7.15 | 44.76 | 3.48 | 16.62 | 0.08 | 48.34 |
| M.R.S | 1.36 | 1.82 | 3.11 | 7.05 | 5.34 | 2.54 |
| Source of Variation | ANOVA p-Value | |||||
|---|---|---|---|---|---|---|
| Mannoheptulose | Perseitol | Glucose | Fructose | Sucrose | Starch | |
| Maturity (M) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.006 |
| Ripeness (R) | <0.001 | <0.001 | <0.001 | <0.001 | 0.319 | <0.001 |
| Storage (S) | <0.001 | 0.722 | <0.001 | 0.209 | 0.718 | <0.001 |
| M.R | 0.744 | <0.001 | 0.913 | 0.021 | <0.001 | <0.001 |
| M.S | 0.603 | 0.074 | 0.197 | 0.013 | 0.415 | 0.001 |
| R.S | <0.001 | <0.001 | <0.001 | <0.001 | 0.106 | <0.001 |
| M.R.S | 0.183 | 0.076 | 0.003 | <0.001 | 0.246 | 0.001 |
| Source of Variation | Variance Ratio | |||||
| Mannoheptulose | Perseitol | Glucose | Fructose | Sucrose | Starch | |
| Maturity (M) | 7.64 | 35.41 | 41.88 | 31.32 | 8.68 | 5.26 |
| Ripeness (R) | 353.98 | 350.40 | 145.81 | 97.98 | 1.00 | 38.57 |
| Storage (S) | 12.52 | 0.13 | 15.45 | 1.59 | 0.13 | 34.37 |
| M.R | 0.30 | 8.80 | 0.09 | 4.00 | 7.48 | 8.68 |
| M.S | 0.51 | 2.65 | 1.64 | 4.48 | 0.89 | 6.98 |
| R.S | 28.01 | 39.05 | 46.38 | 52.87 | 2.64 | 32.91 |
| M.R.S | 1.72 | 2.63 | 6.27 | 8.51 | 1.42 | 7.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burdon, J.; Billing, D.; Bowen, J.; Boldingh, H. Non-Structural Carbohydrate Composition of ‘Hass’ Avocado Fruit Is Affected by Maturity, Storage, and Ripening. Horticulturae 2024, 10, 866. https://doi.org/10.3390/horticulturae10080866
Burdon J, Billing D, Bowen J, Boldingh H. Non-Structural Carbohydrate Composition of ‘Hass’ Avocado Fruit Is Affected by Maturity, Storage, and Ripening. Horticulturae. 2024; 10(8):866. https://doi.org/10.3390/horticulturae10080866
Chicago/Turabian StyleBurdon, Jeremy, David Billing, Judith Bowen, and Helen Boldingh. 2024. "Non-Structural Carbohydrate Composition of ‘Hass’ Avocado Fruit Is Affected by Maturity, Storage, and Ripening" Horticulturae 10, no. 8: 866. https://doi.org/10.3390/horticulturae10080866
APA StyleBurdon, J., Billing, D., Bowen, J., & Boldingh, H. (2024). Non-Structural Carbohydrate Composition of ‘Hass’ Avocado Fruit Is Affected by Maturity, Storage, and Ripening. Horticulturae, 10(8), 866. https://doi.org/10.3390/horticulturae10080866






