Abstract
Climacteric fruits undergo a characteristic ripening process regulated by ethylene, a key plant hormone. Extending the shelf life of these fruits while preserving their postharvest quality poses a significant challenge for the food industry. This review provides a comprehensive overview of physiological and molecular strategies to delay ethylene-mediated ripening in climacteric fruits and their impact on shelf life, postharvest quality, sensory attributes, and volatile compounds. Additionally, it examines the role of ethylene in fruit ripening, analysing various ethylene managing strategies including ethylene inhibitors, ethylene adsorbents, and ethylene scavengers by catalytic oxidation. This review concludes with future research directions including molecular and genetic approaches for reducing ethylene production or responsiveness in fruits, integrated strategies, environmental considerations, and commercial applications for improving postharvest handling and fruit quality.
1. Introduction
Ripening is a complex, genetically programmed, and irreversible phenomenon involving a series of physiological, biochemical, and organoleptic changes. Since this process is unstoppable, there is a finite time for commercialization and consumption where the product exhibits desirable quality attributes. Once this time has elapsed, ripening becomes an adversary as it leads to processes such as weight, size, and firmness reduction, organoleptic degradation, or loss in bioactive compounds, resulting in an inedible and unmarketable product, leading to economic and environmental losses.
According to the Ministry of Agriculture, Fisheries and Food of the Government of Spain, in its report entitled “More food, less waste” [1], global food losses and waste in 2020 amounted to one-third of the world’s food production intended for human consumption. This translates into losses of approximately 1.3 billion tons per year, a quantity sufficient for feeding 2 billion people. In Europe, this figure stands at 89 million tons and that for Spain is 7.7 million tons. In economic terms, global food losses and waste would amount to throwing away more than €782.397 billion (excluding environmental and social costs).
According to De Laurentiis [2], households in the European Union waste about 17 billion kilograms of fresh fruit and vegetables each year, which is 35.3 kg per person per year. On average, 29% of fresh fruits and vegetables are wasted in households. Furthermore, according to the programme “Informe Semanal: Comer bien, tirar menos” by Radio Televisión Española (RTVE) [3], aired on 11 March 2023, more than two-fifths of all fruit, vegetable, and green crops are wasted because they are “ugly”. This subjective criterion prevails over other objectives such as firmness, flavour, or internal composition of these foods.
Based on the aforementioned, proper management of ripening-related processes can provide more marketing and consumption time for the customer, thereby avoiding waste and subsequent economic losses and environmental problems for the entire production chain.
1.1. Ethylene: A Ripening Plant Hormone
Ethylene is a plant hormone produced by plants and fruits that remains in gaseous state under normal conditions (25 °C temperature and 1 atm pressure). It is biologically active in minimal amounts and its effects are commercially crucial in fruit and vegetable ripening [4]. The role of ethylene as a potent regulator of plant growth has been established over the last hundred years, but its effects have been known for several centuries. The use of ethylene to accelerate fruit ripening has been known since ancient times. Examples of this phenomenon include the ripening of apples in southern Italy using quinces for joint preservation or the ripening of mangoes in India using straw combustion. It is even known that in ancient Egyptian civilization, superficial cuts were made in the skin of figs to stimulate their ripening. Subsequently, it was discovered that these cuts or scarifications promote fruit stress, leading to increased respiration and ethylene production [5].
Phytohormones play a role in many aspects of plant development. Ethylene was one of the first plant hormones discovered. Its discovery arose from a remarkably curious fact. Although by the mid-19th century it was clear that the presence of gaseous materials in the air could modify plant growth, it was not until the late 19th century that the Russian researcher Dimitry Neljubow identified ethylene, an active component of street lamp gas, as the generator of a strange growth habit in pea seedlings suffering from etiolation [6]. The first evidence that plant material produces a gas that affects the growth of nearby plants was discovered by Cousins in 1910 [7], who hypothesized that gases emitted by oranges caused banana ripening in mixed commercial shipments. However, since healthy oranges produce very little ethylene because they are non-climacteric fruits, the origin of this ethylene was from oranges infected with fungi. In 1917, Sarah Doubt successfully correlated the presence of ethylene with the stimulation of leaf and fruit abscission [8].
In 1924, Frank E. Denny [9] observed that farmers cultivating Florida lemons stored their fruits in sheds with kerosene lamps, thinking that heat caused them to lose their green colour. Upon investigation, Denny discovered that it was the ethylene produced by those lamps that induced the colour change in lemons from green to yellow, a process later known as degreening. Gane [10] demonstrated in 1934 that fruits during ripening synthesize ethylene. He provided chemical evidence that ethylene was indeed mostly produced by ripest bananas, demonstrating that plants produce ethylene themselves, confirming Cousins’ hypothesis. He later found that ethylene was also produced by other fruits and could promote seed germination [11]. Crocker reported in 1935 [12] that ethylene acts similarly to auxins, being involved in plant growth and the senescence of vegetative tissues in Arabidopsis thaliana L. Therefore, it was established that ethylene is a plant hormone.
Subsequently, between the 1940s and the early 1970s, methods for eliminating this phytohormone to extend the shelf life of plant products began to be proposed [13]. Southwick & Smock showed in 1943 [14] that by using activated charcoal with bromine as an adsorbent for ethylene, the shelf life of ‘McIntosh’ apples could be extended by a month. In 1971, Scott and colleagues [15] proposed the use of ultraviolet light to eliminate ethylene and thus extend the shelf life of fruits, although these methods were still far from industrial application.
In recent years, multiple procedures have been developed to eliminate or inactivate ethylene and its effects. Among them, oxidative ethylene removers (such as potassium permanganate or titanium dioxide) and ethylene inhibitors (such as salicylic acid or 1-MCP) stand out. Currently, this field faces the following challenges:
- Observing the effect of different ethylene removal methods on as many foods as possible;
- Discovering to what extent food quality is maintained through these methods;
- Establishing which of them are truly applicable on an industrial scale and refining these methods to make them more effective.
Ethylene can be classified according to its origin as endogenous and exogenous. Endogenous ethylene is produced by a fruit as a result of its internal synthesis. In contrast, exogenous ethylene is produced by other agents, which can be biological, such as other adjacent fruits, or of another nature such as the combustion of vegetables like straw or stubble or combustion in vehicle engines [16].
Regarding the capacity of this gas to act, effects of ethylene have been recorded at very low concentrations, even below 0.001 μL per litre of air [17]. While it is true that in the initial stages of fruit development, a high presence of this gas can be beneficial as it promotes and accelerates their development, in later stages, especially during postharvest, it can be detrimental by accelerating senescence and reducing their commercial life [18,19]. This rapid ripening favoured by the presence of ethylene affects most of the qualitative parameters of fruits, from physical parameters such as weight or firmness to biochemical parameters such as antioxidant capacity, soluble solids, pH, or acidity.
1.2. Scope and Structure of This Review
This review aims to provide a comprehensive examination of the ripening dynamics of climacteric fruits, with a specific focus on ethylene control and removal. By synthesizing current research findings, theoretical frameworks, and practical applications, this review seeks to deepen our understanding of the interplay between ethylene and fruit ripening processes, elucidating the molecular mechanisms, physiological effects, and practical implications of ethylene biology in the horticultural industry.
To achieve these objectives, this review will be structured into several interrelated sections, each addressing specific aspects of climacteric fruit ripening and ethylene biology:
- Climacteric fruits and ethylene: This section will provide a comprehensive overview of climacteric fruits and their characteristic ripening process. It will define climacteric fruits, delineate the stages of their ripening process, and discuss the physiological changes associated with ripening. Additionally, it will highlight the pivotal role of ethylene as a master regulator of climacteric fruit ripening, emphasizing its multifaceted functions and importance in fruit development and maturation.
- Regulation of ethylene biosynthesis and signalling: This section will delve into the molecular mechanisms underlying ethylene biosynthesis, perception, and signal transduction in climacteric fruits. It will explore the regulatory pathways governing ethylene production, receptor-mediated signalling cascades, and downstream responses in fruit tissues. Additionally, it will discuss the environmental and hormonal factors that modulate ethylene biosynthesis and signalling pathways, providing insights into the complexity of ethylene regulation during fruit ripening.
- Physiological and molecular effects of ethylene on fruit ripening: This section will examine the specific molecular and physiological effects of ethylene on climacteric fruit ripening processes. It will elucidate how ethylene influences key ripening-related events, such as fruit softening, flavour development, colour changes, and aroma production. By integrating molecular biology, biochemistry, and physiology, this section will offer a comprehensive understanding of ethylene-mediated ripening processes at the cellular and tissue levels.
- Ethylene managing strategies: This section will be destined to go deep in different strategies to remove, inhibit, or reduce the effect that ethylene could have according to its interaction with climacteric and non-climacteric fruits.
- Practical implications and future directions: This final section will discuss the practical implications of ethylene biology for agricultural practices, postharvest management, and fruit quality enhancement. It will highlight potential strategies for manipulating ethylene levels, optimizing postharvest handling techniques, and improving fruit quality and shelf life. Additionally, it will identify emerging research trends and future directions in ethylene research, pointing towards new opportunities for innovation and advancement in this field.
Overall, this review aims to provide a comprehensive synthesis of current knowledge on the interrelationship between climacteric fruit ripening and ethylene biology, offering valuable insights into the mechanisms, regulation, and practical applications of ethylene in fruit production and postharvest handling.
2. Climacteric Fruits and Ethylene
Fruit ripening has always been the subject of intense study due to its relevance to the nutritional characteristics that define its quality [20,21]. As mentioned earlier, depending on the respiration pattern displayed, fruits can be divided into climacteric fruits, which exhibit an increase in respiration rate mediated by a sudden rise in ethylene, and non-climacteric fruits, in which there is no increase in respiration rate or accumulation of ethylene [22].
Both climacteric and non-climacteric fruits show common pathways regarding the signal transduction pathway in response to ethylene [23] and accumulate abscisic acid (ABA), especially at the beginning of ripening [24,25]. The accumulation of ABA precedes and, therefore, modulates ethylene production in climacteric fruits and triggers ripening in non-climacteric ones while they are still in the plant [25,26]. Recently, the fundamental role of abscisic acid (ABA) in non-climacteric fruits, especially in Fragaria × ananassa, affecting cell wall modification was demonstrated. This is because ABA suppresses the production of pectin esterase and polygalacturonase enzymes, which degrade the cell wall and promote softening of the affected fruits [27,28].
The focus on the ripening process of climacteric fruits can be divided into several sequential stages, each characterized by specific physiological and biochemical changes (Figure 1):
- Pre-climacteric phase: At the beginning of fruit development, climacteric fruits are in the pre-climacteric phase. During this stage, ethylene production and respiration rates are relatively low. The fruits are typically firm, green, and physiologically immature. Although metabolic processes are occurring, they are not yet at levels indicative of ripening.
- Climacteric peak: As fruits reach maturity, they undergo a dramatic increase in ethylene biosynthesis and respiration, marking the climacteric peak. This peak is a pivotal event in the ripening process and triggers a cascade of biochemical and physiological changes. One of the most notable transformations is the conversion of starches into sugars, leading to increased sweetness. Additionally, the fruit softens as cell wall components break down, resulting in changes in texture and juiciness. Other changes include alterations in pigmentation, aroma development, and flavour enhancement.
- Climacteric phase: Following the climacteric peak, fruits enter the climacteric phase, characterized by sustained ethylene production and ongoing metabolic activity. Ripening processes initiated during the peak continue, albeit at a slower pace. This phase is crucial for the completion of ripening, as fruits continue to develop desirable sensory attributes and undergo structural modifications indicative of ripeness.
- Post-climacteric phase: Eventually, climacteric fruits enter the post-climacteric phase, marked by a decline in ethylene production and respiration rates. While fruits remain physiologically ripe during this phase, they may exhibit signs of senescence, such as loss in firmness, increased susceptibility to decay, and decline in sensory quality.
The ripening process of climacteric fruits is governed by a complex network of hormonal, genetic, and environmental factors. Ethylene, in particular, plays a central role in coordinating ripening-related processes by regulating gene expression, enzyme activities, and physiological responses. Additionally, interactions between ethylene and other hormones, such as auxins, abscisic acid, and gibberellins, further modulate ripening dynamics.
There are two ethylene production systems according to the developmental stages of plant tissues and fruits: system I and system II.
- System I of ethylene production is associated with vegetative tissues and fruits in the early stages of development and is characterized by low rates of ethylene production, auto-inhibitory character (a process by which ethylene induces and controls its own production), and absence of relevant peaks in the production of this phytohormone [29,30].
- On the other hand, system II is present in more advanced development processes, especially in climacteric fruits, and is characterized by high rates of ethylene emission, feedback (higher ethylene concentration in the environment implies higher production of the same), and a high peak of ethylene production at the onset of physiological maturity [29].
The systems I and II of climacteric fruits are identical in the ethylene synthesis pathway. They exhibit different response patterns to exogenous ethylene, which may be related to the distinct properties of the ACS and ACO isoenzymes.
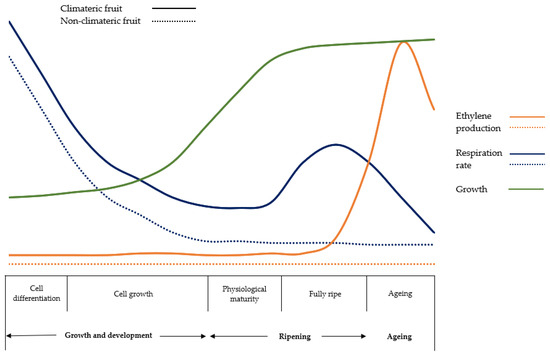
Figure 1.
Respiration rate and ethylene production during postharvest (own source: http://hdl.handle.net/10952/6740 (accessed on 20 May 2023)).
As shown in Figure 1, climacteric fruits exhibit a peak in respiration and ethylene production during the ripening process and are capable of ripening even after harvest, whereas non-climacteric fruits do not show any peak in respiration and cannot ripen after harvest [31]. The differences between the two types of fruits are detailed below:
Firstly, climacteric fruits contain both the system I and system II of ethylene production, whereas non-climacteric fruits only emit ethylene through the so-called system I. This means that, at the onset of ripening, climacteric fruits experience a peak in respiration, followed by massive ethylene production [32]. As mentioned earlier, system II is characterized by feedback in ethylene production, where more ethylene in the environment leads to higher production. This maintains the ethylene peak until reaching an overripe state [30]. In contrast, for non-climacteric fruits, once the point of physiological maturity is reached, ethylene production remains, without significant changes, at a basal level [33,34]
Secondly, both climacteric and non-climacteric fruits show little difference during the developmental phase, as they only produce minimal amounts of ethylene. However, during the full ripening period, the amount of ethylene produced by climacteric fruits is much higher than that of non-climacteric fruits.
Thirdly, the application of exogenous ethylene is only effective in the early stages of development of climacteric fruits on the tree, which can lead to increased respiration and autocatalysis of endogenous ethylene. This reaction is irreversible because artificially applying ethylene accelerates the fruit ripening process, and as mentioned earlier, ripening cannot be stopped.
Finally, increasing the concentration of exogenous ethylene advances the appearance of the respiratory peak in climacteric fruits, but the intensity of the respiratory peak remains unchanged. However, for non-climacteric fruits, increasing the concentration of exogenous ethylene may increase respiratory intensity but not the duration of the respiratory peak [35].
The biochemical changes induced by ethylene, along with the microbiological damage caused by bacteria or fungi, are the main causes of deterioration in climacteric fruits, even under low-temperature storage conditions [36,37]. Consequently, an appropriate strategy must be adopted to prevent ethylene accumulation in order to prolong the postharvest life of such plant products and thereby reduce losses [38]. The effect of ethylene must be blocked or eliminated to improve its quality and extend its shelf life. Furthermore, its application should not only be limited to storage but should also be applied to handling and all stages after harvest [39]. To effectively eliminate or block the effect of ethylene, a thorough understanding of available methods, their industrial application, effectiveness, and cost is necessary.
The physiological maturity or ripening of a fruit is defined, according to the dictionary of the Royal Spanish Academy as “the state of development in which a fruit meets the requirements to be consumed or used by the consumer for a particular purpose”. In these terms, two categories can be distinguished depending on whether, once harvested, they continue to ripen, known as climacteric fruits, or if their ripening is interrupted, known as non-climacteric fruits. For climacteric fruits, the ripening process is initiated by changes in their hormonal composition. It is not a gradual process; there is a peak of ripeness followed by ageing or overripening [5].
The onset of climacteric ripening is a well-defined process characterized by a rapid increase in the rate of respiration and ethylene production by the fruit. Climacteric fruits have the ability to continue ripening even when separated from the mother plant, provided they have reached a physiological state that ensures ethylene production. Examples of such fruits include apple, avocado, peach, or tomato [38,40,41,42].
Conversely, regarding non-climacteric fruits, the ripening process is gradual and continuous. They lack the ability to continue ripening once separated from the mother plant, so it must be ensured that they have reached an appropriate state of maturity for consumption at the time of harvest. Citrus fruits, grapes, pomegranates, or raspberries fall into this category of non-climacteric fruits [33,43].
About the effect of ethylene on non-climacteric fruits, although they do not show a clear increase in ethylene production rates during ripening, in certain cases, their exposure to exogenously applied ethylene can stimulate certain ripening-related processes, such as the inhibition of senescence, the inhibition of the development of physiological disorders, and the inhibition of colour changes [44]. Exogenous ethylene can react with ethylene receptors both in the early and late stages, leading to accelerated ripening. However, in this case, once exogenous ethylene application is stopped, the fruits will return to their pretreatment levels of respiration and ethylene production [35,45]. This process is widely applied in citrus fruits, for example, in lemons. These fruits are harvested without reaching their physiological ripeness (colour change from green to yellow). Subsequently, when they are ready for consumption, they undergo a process called “degreening” in which controlled ethylene is introduced into lemon storage chambers until they reach their optimal ripeness for consumption and then return to their pretreatment levels of respiration and ethylene production [46].
As an example, exposure to 1-MCP had an effect on delaying rachis browning in table grape varieties (Thompson Seedless’), thus suggesting the possible involvement of the ethylene signalling pathway in the regulation of the rachis browning process of non-climacteric fruit [47]. Another example can be found on pomegranates, where the main problems associated with their postharvest storage are related to weight loss and shrivelling, as well as peel discolouration. Previous studies indicated that exposure to ethylene blockers such as 1-MCP reduced skin shrivelling [48] and the incidence of peel browning [49].
Depending on the type of fruit and the ripening stage it is in, ethylene can have both positive and negative effects. The beneficial effects of ethylene include stimulating ripening in climacteric fruits until they reach the optimal consumption stage, developing colour through pigment synthesis (anthocyanin and lycopene), and chlorophyll degradation (a process known as degreening, commonly applied in the case of lemons). The negative effects of ethylene, especially during the postharvest of climacteric fruits, are numerous. Excessive softening, weight loss, loss in bioactive compounds, and emission of unpleasant volatile compounds in fruit [50]; leaf and flower abscission in higher plants; accelerated hardening of vegetables; increased susceptibility to bacterial or fungal pathogens; stimulation of sprouting; changes in shape; and appearance of reddish spots are some of them [43,51]. These undesirable changes often occur due to accelerated ripening caused by exposure to ethylene emitted by adjacent fruits and/or to ethylene generated as a contaminant in locations where the product is placed, whether in greenhouses, trucks, ships, or airplanes during storage and transport. Therefore, it is crucial to reduce surrounding ethylene as well as inhibit ethylene biosynthesis to minimize its impact on the product.
3. Regulation of Ethylene Biosynthesis and Signalling
3.1. Biosynthesis of Ethylene in Climacteric Fruits and the Enzymes Involved
As mentioned earlier, ethylene is involved in many aspects of plant development, including seed germination, root hair development in roots, seedling growth, leaf and petal abscission, fruit ripening, and plant organ senescence [4,52]. Ethylene production is regulated by internal signals during development and in response to biotic (pathogen attack) and abiotic environmental stimuli, such as wounds, hypoxia, ozone, cold, or freezing. Regulation can also occur at the level of perception or signal transduction [53,54].
Many plant tissues produce ethylene at concentrations that are mostly low. It was not until the discovery of gas chromatography that deeper knowledge of ethylene synthesis and metabolism began [55,56]. Bradford [57] clarified and explained the ethylene synthesis transduction pathway through a series of experiments in apples, summarizing it as a chain of reactions starting from the amino acid methionine and ending with ethylene synthesis, resulting in a series of intermediate products: methionine → S-adenosyl-L-methionine (SAM) → 1-aminocyclopropane-1-carboxylic acid (ACC) → ethylene.
Ethylene originates from the third and fourth carbon of methionine. During the cycle, methionine binds to adenosine to form SAM; subsequently, SAM, apart from giving rise to ACC, can produce 5′-methylthioadenosine (MTA), which, after the cycle repeat reaction and hydrolysis, regenerates methionine, leading to high rates of ethylene production without the need for high levels of intracellular methionine [55]. This ensures that the amino acid content in plants is ready for a new cycle of ethylene production [56,57,58]. This important discovery was made thanks to the work of Professor Shan Fa Yang [4]; therefore, this ethylene production cycle was named the “Yang cycle” (Figure 2).
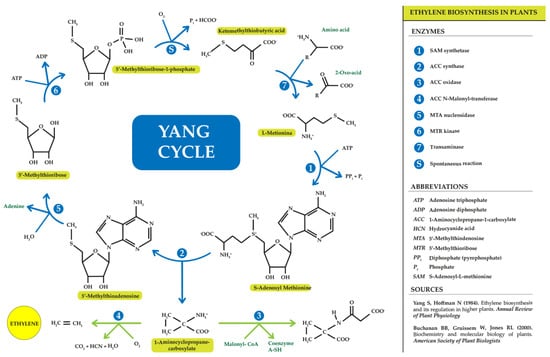
Figure 2.
Yang cycle (own adaptation: http://hdl.handle.net/10952/6740 (accessed on 20 May 2023)) [4,59].
During ethylene synthesis, the activity of three enzymes is regulated: SAM synthetase (SAMS), ACC synthase (ACS), and ACC oxidase (ACO). SAM synthesized by the enzyme SAMS is also involved in other biochemical pathways such as that related to polyamine synthesis [60,61,62]. On the other hand, the ACS enzyme catalyses the breakdown of SAM into ACC and MTA, thus being the key point that limits the speed of the entire pathway. ACS is present in the cytoplasm forming monomers, dimers, or even trimers, with monomers exhibiting the highest catalytic activity. In biochemical assays carried out by Yamagami and colleagues in 2003 [63], the analysis of the amino acid sequence of the enzymes showed that all ACS isoforms depend on pyridoxal phosphate (PLP) coenzymes. Therefore, inhibitors of enzymes competing for PLP such as aminoethoxyvinylglycine (AVG) and aminooxyacetic acid (AOA) can be used to inhibit ethylene synthesis [64]. In addition, cobalt ion and low oxygen levels can also affect ethylene production by inhibiting the final step of the pathway, catalysed by ACC oxidase, to finally give ethylene. Finally, ACO forms ethylene, CO2, and cyanide from ACC. This latter component (cyanide) is detoxified by beta-cyanoalanine synthase to beta-cyanoalanine to prevent toxicity of accumulated cyanide during high rates of ethylene biosynthesis [64]. In 2002, Chung and colleagues [65] discovered that the ACO enzyme is widely distributed in various plant tissues, and its function depends on the effects of ferrous ion (Fe2+) and oxygen (O2), and therefore various chelating agents can inhibit its activity. The activity of this enzyme also depends on ascorbic acid or vitamin C as a co-substrate, establishing a direct relationship between the concentration of this acid and ethylene production [66]. There is still no clear evidence about the cellular localization of the ACO enzyme, although several authors indicate that it could be found in the cytosol [67,68], but this is still unknown [69].
Recent advances have shown that ethylene synthesis and metabolism in plants are closely correlated with environmental factors such as light and temperature. There are also other processes in which the three key enzymes mentioned are involved that can regulate ethylene production. For example, Argueso and colleagues reported [70] that the expression of multiple ACS genes (ACS2, ACS4 and ACS5) is induced by wounds, increasing ethylene synthesis.
3.2. Ethylene Perception and Signalling
Researchers’ understanding of the ethylene signal transduction pathways primarily stems from genetic and molecular biology studies of Arabidopsis thaliana L mutants [71]. The initial step in ethylene perception involves its binding to receptors for this molecule in plants and fruits. Cells possess a family of five receptors located in the endoplasmic reticulum (ER) membrane that are homologous to bacterial histidine kinases, namely, ETR1, ETR2, EIN4, ERS1, and ERS2, corresponding to ethylene receptors 1, 2, ethylene insensitive 4, and ethylene response sensor 1, 2, respectively, which bind to ethylene with the assistance of a copper cofactor in the transmembrane domain of the protein receptor [71,72,73].
While some receptor specialization has been recognized, it is believed that they primarily function by modulating the activity of the kinase called CTR1. Inactivation of this kinase in the presence of ethylene results in decreased levels of phosphorylation of the transmembrane protein located in the endoplasmic reticulum, EIN2 [74,75,76]. Two different responses have been characterized by studying EIN2 activity. On one hand, there is a rapid growth inhibition once the fruit is harvested, which occurs within minutes of hormone exposure [76,77]. On the other hand, many other changes, possibly slower, are induced by this hormone, including alterations at the transcriptional level in hundreds of genes [77,78]. Target genes include those involved in ripening, seed germination, wound response, senescence, and other phenomena whose functions are still unknown. This entire process is schematically illustrated in Figure 3.
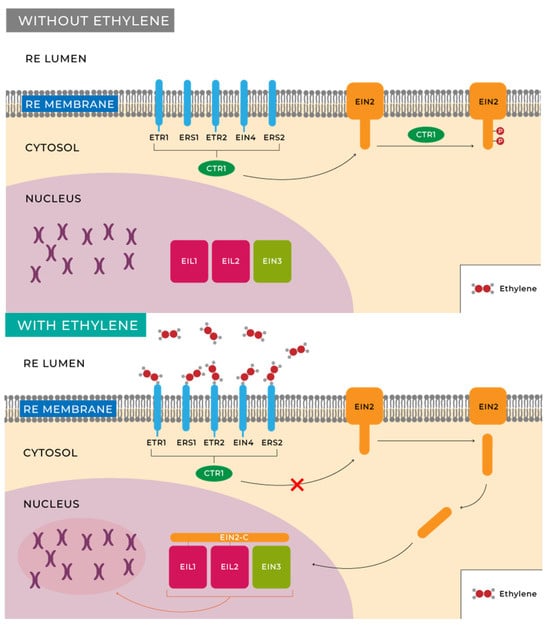
Figure 3.
Ethylene perception and signalling (own source: http://hdl.handle.net/10952/6740 (accessed on 20 May 2023)).
Despite the growing evidence that CTR1 is directly regulated by ethylene receptors, the biochemical events controlling such regulation are not well understood. The involvement of a MAPK cascade and its role in ethylene signal transmission are still unclear, and more conclusive genetic evidence is needed. Another question to be addressed is the function of EIN2, a protein that plays an essential role in mediating all known ethylene responses. Regarding events occurring in the nucleus, determining how ethylene controls the stability/activity of primary transcription factors, such as EIN3, EIL1, or EIL2, represents another challenge, as well as determining the subsequent steps in gene regulation. Experiments using global gene expression profiling show that hundreds of genes are induced or repressed by ethylene. In the future, it will be important to discern how many of these genes are immediate targets of EIN3 and, in the long term, to categorize the transcriptional networks involved in ethylene responses.
Ethylene receptors, along with other two-component receptors—those having a part that receives the extracellular stimulus and another part that modifies a transcription factor regulating gene expression to rapidly adapt to environmental changes (such as phytochromes and cytokinin receptors)—are believed to have been acquired by plants from cyanobacteria, which allowed for the contribution of chloroplasts through endosymbiosis, as they are homologous to some bacterial receptors [79,80,81,82,83,84].
Data from recent phylogenetic analysis suggest a common origin for the ethylene-binding domain in cyanobacteria and plants [85]. Therefore, it is interesting to note that ethylene binding has been observed in at least one cyanobacterium, Synechocystis sp., which has a functional ethylene receptor regulating cell surface properties affecting biofilm formation and phototaxis, i.e., the ability of cells to orient in response to light intensity [86,87,88]. Furthermore, ethylene-binding affinities to some of these cyanobacteria and to the ethylene receptor of Synechocystis sp. are similar to those observed in plants [89], demonstrating conservation of this domain among these organisms. However, the primitive prokaryotic organism from which ethylene receptors first emerged remains unknown [71].
Plants contain multiple isoforms of ethylene receptors. Early studies identified ethylene-binding sites in plant endoplasmic reticulum (ER) membranes [90,91], a finding corroborated by subsequent research [92,93,94,95,96,97]. In Arabidopsis thaliana L., the five aforementioned isoforms (ETR1, ETR2, ERS1, ERS2, and EIN 4) have been identified [55,98,99,100]. Mutations in any of these receptors prevent ethylene binding to them and lead to a plant showing ethylene insensitivity [55,86,98,101]. Additionally, some mutations in these receptors do not affect ethylene binding but prevent signalling through the receptor, also resulting in ethylene insensitivity [86].
4. Physiological and Molecular Effects of Ethylene on Fruit Ripening
Ethylene-mediated fruit ripening involves a complex interplay of physiological and biochemical changes that occur in response to ethylene signalling. These changes are crucial for transforming immature, unripe fruit into fully mature, ripe fruit with desirable sensory attributes. Understanding the intricate mechanisms underlying ethylene-mediated ripening provides insights into the regulation of key ripening processes and offers opportunities for improving postharvest fruit quality and shelf life.
4.1. Effect of Ethylene Over Shelf Life, Quality and Disease Resistance
Phytohormone is also a key player in how long fruits and vegetables stay fresh. For example, ethylene speeds up the ripening process, which is great for achieving peak flavour and texture. However, beyond this point, ethylene can cause them to overripen and spoil much faster [51]. Ethylene also initiates the ageing process in plants, known as senescence, and induces abscission. Consequently, this results in a decline in the overall quality of the produce, affecting both its appearance and texture [53]. Another crucial role of the ethylene is stimulating the production of enzymes that degrade cell wall components, resulting in the softening of fruit tissue. This softening increases the susceptibility of the fruit to mechanical damage and microbial decay [102]. Lastly, ethylene increases the respiration rate in fruits, leading to a faster depletion of their energy reserves. This elevated metabolic activity accelerates the loss in firmness, flavour, and nutritional value, thereby contributing to a reduced shelf life [103].
Ethylene plays a crucial role in impacting the quality of fruits and vegetables, but its effects can be a double-edged sword if not carefully managed. Firstly, ethylene is known to induce the production of enzymes that break down cell walls, which leads to the softening of fruits. While this is essential for ripening, it can also result in a loss in texture and firmness, qualities highly valued by consumers [104]. Another effect of ethylene is the degradation of chlorophylls. This process affects the vibrant green colour of many fruits and vegetables, making them appear less fresh and less appealing. In addition, ethylene causes overripening, provoking a significant drop in overall quality and visual appeal. This deterioration can make fresh produce look unappetizing and less desirable [105]. Furthermore, the presence of ethylene during storage and transport is a significant issue. It can speed up the ripening process before the produce reaches consumers, resulting in a noticeable decline in quality by the time it reaches the shelves [106].
Ethylene also plays a multifaceted role in fruit disease resistance, acting as both a promoter and a suppressor depending on the context. Its influence is nuanced and varies with different factors. On one hand, ethylene can boost the fruit’s defence mechanisms against pathogens. For example, research on ‘Kyoho’ grapes shows that treating them with ethephon, a compound that releases ethylene, before ripening can enhance the expression of genes related to both fruit colouration and disease resistance. This treatment led to improved resistance against Botrytis cinerea, a common fungal pathogen [107].
Firstly, the ethylene response involves a variety of genes that are differentially expressed when plants are challenged by pathogens. For instance, in a study on potato, a total of 1226 ethylene-specific differentially expressed genes (DEGs) were identified, including those encoding for transcription factors, kinases, defence enzymes, and disease resistance-related genes [108]. These genes are part of the plant’s immune system, helping to activate defence responses. Secondly, transcription factors such as those from the APETALA2/ethylene response factor (AP2/ERF) family play a pivotal role in plant disease resistance. They act downstream of mitogen-activated protein kinase (MAPK) cascades and regulate the expression of genes associated with hormonal signalling pathways, biosynthesis of secondary metabolites, and formation of physical barriers [109,110]. For example, the ERF gene from the tomato is known to confer resistance to Pseudomonas syringae pv. tomato by activating genes encoding antifungal proteins and proteins involved in oxidative burst. Ethylene’s role in disease resistance is closely linked to its biosynthesis and signalling pathways. In terms of disease resistance, the glutathione metabolism pathway, which includes key enzymes like glutathione S-transferase (GST), plays an important role in response to ethylene stimulus [111].
4.2. Effect of Ethylene on the Physical Characteristics of Fruits during Ripening
Ethylene is responsible for a series of physiological changes that transform the physical characteristics of fruits, directly affecting important physical parameters that impact their commercial value, such as weight, size, and texture. Controlling the presence of ethylene has a positive effect on maintaining the physical characteristics of fruits during postharvest storage.
Fruit weight is an important parameter for fruit producers, especially from an economic perspective, making the control of fruit weight loss crucial. This loss is associated with excessive water loss due to transpiration, which is related to low relative humidity (RH). Water loss after harvesting is an unavoidable phenomenon, resulting in weight loss, size reduction, wilting, abnormal textures, and quality degradation. The dehydration of peaches can be prevented by maintaining a high environment RH (90–95%), while also controlling air speed and using physical or chemical barriers [112,113].
Different authors have shown evidence that by controlling and eliminating ethylene, weight loss during postharvest storage can be reduced. Emadpour and colleagues [42] observed a weight loss reduction of only 3% during the storage of peaches using an ethylene eliminator like potassium permanganate. Mansourbahmani and colleagues [41] recorded a weight loss reduction of only 2% in ‘Valouro’ tomatoes when ethylene concentration in the storage chamber was controlled with potassium permanganate mixed with zeolite after 35 days of storage. Alonso-Salinas and colleagues [114] recorded weight reductions of between 3% and 7% in pears stored in an atmosphere with ethylene reduction systems, compared to weight losses of 10% and 17% in pears stored without ethylene control systems over a 28-day period.
Regarding fruit size during storage, it tends to show a similar trend as fruit weight [115]. These authors also observed that the decrease in the diameter of peaches, compared with weight loss, was less sensitive to the presence of ethylene in the storage atmosphere. The presence of ethylene promotes accelerated respiration, which causes a greater decrease in weight and size due to increased water loss [36]. In a study conducted with pears stored for 28 days, it was observed that the reduction in size was around 20% when ethylene was not removed from the storage chamber and between 5% and 10% when different ethylene removal techniques, such as potassium permanganate filters combined with ultraviolet light, were used [114].
Firmness is one of the main quality attributes that determine the acceptance of the product by the consumer. On one hand, weight loss influences firmness in a directly proportional relationship. On the other hand, ethylene significantly affects fruit firmness by triggering cell wall hydrolysis, which leads to fruit softening [116,117]. In addition, the expression of polygalacturonase-related genes is associated with ethylene production [118]. The action of this enzyme is considered key in the softening of fruits, increasing its activity in those treatments where the exposure to ethylene was higher [119]. Wu and colleagues [113] demonstrated that the effect of ethylene scavengers on apricots resulted in significantly higher firmness compared to control fruit.
Álvarez-Hernández and colleagues [120] showed that apricots with an ethylene removal system based on potassium permanganate and sepiolite sachets recorded 20% more firmness than the control treatment after 36 days of storage. Mansourbahmani and colleagues [41] recorded approximately 15% preservation of firmness on the final day when applying their ethylene removal method in tomatoes. In a study on peaches stored with and without an ethylene removal system, Alonso-Salinas and colleagues [115] observed that the firmness of the fruits from which ethylene was removed from the storage atmosphere maintained the same firmness during the first 7 days, tending then to equalize with the texture of the peaches stored without ethylene removers. These same authors observed, in a trial conducted with pears stored at 1 °C and 8 °C, that removing ethylene from the storage atmosphere reduced firmness by 10% and 28%, respectively, while not removing ethylene reduced firmness by 29% (1 °C) and 46% (8 °C) [114].
4.3. Effect of Ethylene on the Biochemical Characteristics of Fruits during Ripening
Ethylene is a crucial plant hormone that plays a significant role in the regulation of fruit ripening [121]. During ripening, ethylene significantly impacts the biochemical characteristics of fruits, enhancing attributes such as sweetness, maturity index, and antioxidant capacity, while modulating acidity, pH, and the concentration of bioactive compounds [122]. Understanding these changes during ripening is essential for optimizing postharvest handling and improving consumer satisfaction [123].
Ethylene significantly influences the accumulation of solid soluble content (SSC) in fruits. During postharvest ripening of climacteric fruits, such as peaches, sugars displace acids by certain metabolic processes, increasing SSC and giving the fruit a sweet taste. Therefore, SSC is a key indicator of the ripening stage of the fruit [124,125]. The increase in SSC primarily comprises an increase in sugars such as glucose, fructose, and sucrose [126]. This process is facilitated by the ethylene-induced activation of enzymes like invertase and amylase, which catalyse the breakdown of starches into simpler sugars [127]. Total acidity (TA) of fruits generally decreases during ethylene-induced ripening. This decline is attributed to the metabolic conversion of organic acids, such as citric and malic acids, into sugars and other metabolites [128]. The reduction in acidity not only affects the taste, making the fruit less sour, but also impacts the overall flavour profile and consumer preference [129]. The pH of fruits typically rises during ethylene-mediated ripening. This increase in pH correlates with the decrease in TA, reflecting the metabolic changes occurring within the fruit tissues. Monitoring pH changes is crucial for understanding the ripening dynamics and the biochemical environment of the fruit [130].
Maturity index (MI) defined as the ratio of SSC to TA, serves as an indicator of fruit ripeness. Ethylene accelerates the ripening process, thereby increasing the MI. A higher MI indicates a more advanced stage of ripeness, characterized by optimal sugar levels and reduced acidity, which are desirable traits for market quality and consumer acceptance, [131] but if the maturity index (MI) becomes too high due to the effect of ethylene, several negative outcomes can occur: (1) Overripening: Fruits may become too soft and mushy, leading to a loss in structural integrity and an undesirable texture for consumers [132]. (2) Flavour deterioration: Overripened fruits often experience changes in flavour, becoming overly sweet and losing the balance of acidity and sweetness that defines optimal ripeness [133]; (3) nutrient degradation: ascorbic acid (vitamin C), phenolic compounds, and antioxidant capacity (ORAC) initially can increase with ripening due to ethylene being able to stimulate the expression of genes involved in the ascorbate biosynthetic pathway and favouring the activity of phenylalanine ammonia-lyase (PAL), leading to increased synthesis of phenolic compounds (such as flavonoids and anthocyanins) and contributing to the accumulation of antioxidant capabilities. On the contrary, excessive ethylene exposure and overripening can lead to the degradation of these compounds due to oxidative degradation and metabolic consumption, reducing the fruit’s nutritional value and antioxidant capacity [123,134,135]; (4) Increased susceptibility to diseases and pests: Overripened fruits are more prone to infections and infestations, leading to higher postharvest losses and a decrease in marketable yield [136]. (5) Economic losses: Fruits that are too ripe may have a shorter shelf life and become unmarketable, resulting in economic losses for producers and retailers [137,138].
Different studies performed by Alonso et al. in peaches, tomatoes, and pears [114,115,139] showed favourable responses to the preservation of the quality and shelf life of these fruits thanks to the different methodologies applied for the elimination of ethylene through oxidation reactions mediated by KMnO4 and UV-C. Other studies have reported a less successful effect of ethylene scavengers on peach fruit conserved for 36 days at 0 °C [42,140] or in apricots stored at 15 °C [120].
4.4. Effect of Ethylene on the Organoleptic Characteristics of Fruits during Ripening
Fruit ripening brings out the best taste and scent, which influences the fruit’s flavour. Fruits go through biochemical changes when they ripen, such as colour breakdown, starch hydrolysis, sugar and acid metabolism, volatile production, and cell wall disintegration, all of which affect flavour. These modifications add to the typical aroma of ripe fruits as well as their sweet and sour taste. Furthermore, when fruit ripens, its texture improves, becoming crisper, juicier, or melty, intensifying the taste release in the mouth. Ethylene gas regulates the ripening process and contributes to the softening and flavouring of fruit. Fruit ripening is an intricate process that involves a range of physiological and biochemical changes that have a big impact on the fruit’s flavour [141,142,143].
The flavour changes associated with ethylene involve a network of genes, transcription factors, and metabolic pathways. Firstly, specific genes responsible for the synthesis of flavour compounds as genes encoding enzymes like lipoxygenases, hydroperoxide lyases, and alcohol dehydrogenases are involved in the formation of volatile compounds that contribute to fruit aroma [144]. Additionally, genes related to the biosynthesis of sugars, acids, and secondary metabolites like carotenoids and flavonoids are also ethylene-responsive and contribute to flavour [145]. Secondly, transcription factors such as the RIN (RIPENING INHIBITOR), NOR (NONRIPENING), and CNR (COLOURLESS NONRIPENING) play crucial roles in the regulation of ripening-related genes. These factors can directly or indirectly influence the expression of genes involved in flavour compound biosynthesis [144]. For instance, the transcription factor HY5 has been shown to bind to the promoter of SWEET12c, a gene involved in sugar transport, thereby modulating sugar content and influencing sweetness in tomato fruit [145]. Finally, ethylene influences several metabolic pathways that are directly related to flavour development. The Lox pathway is responsible for the production of volatile compounds that contribute to aroma [146]. The glycolytic pathway and tricarboxylic acid (TCA) cycle are involved in sugar and acid metabolism, affecting sweetness and sourness [147]. Ethylene also affects the isoprenoid pathway, which includes the biosynthesis of carotenoids, contributing to both colour and flavour [145].
Ethylene is a highly volatile substance produced by many fruits during their ripening process, and it is thus considered a maturation hormone. These fruits ripen due to the action of ethylene, as increased concentrations accelerate the maturation process, altering the colour, firmness, flavour, and characteristic aromas of each fruit. During maturation, the starch in fruits is converted into sugars (fructose and glucose), tannins are reduced—these are compounds characteristic of unripe fruit that impart a bitter taste—and the pH increases, decreasing acidity. All these changes lead to modifications in the organoleptic characteristics of the fruit [148].
Volatile compounds, which undergo modifications during ripening, play a significant role in the flavour of foods. These compounds belong to various chemical families, with the most notable being hydrocarbons, aldehydes, ketones, alcohols, carboxylic acids, esters, and lactones [149,150].
Several authors have investigated the control of ethylene synthesis on the production of volatile compounds during fruit ripening. These changes in the aromatic profile have led to greater sensory acceptance by consumers of the final product [151,152].
Moreover, if ethylene control, through its inhibitors, is combined with other evaluative parameters such as different storage conditions—controlled or dynamic atmospheres, partial oxygen pressure, temperature, or fruit CO2 production—it could offer commercial benefits. These conditions can slow down the respiration rate of the fruit, partially inhibit ethylene synthesis, and result in improvements in sensory parameters such as acidity and firmness, while also enhancing aroma formation and the synthesis of aromatic compounds [114,139,151,153,154,155].
Another crucial parameter in the sensory evaluation of fruits is colour, which influences flavour perception and is affected by ethylene synthesis and the presence of its inhibitors. This has led to positive evaluations from consumer panels analysing these products [29,105,153,156].
5. Ethylene Managing Strategies
Given that fruits and vegetables ripen faster regardless of whether ethylene exposure is exogenous or endogenous, it is essential to eliminate both types [51,157,158]. Therefore, strategies that allow for controlling ethylene levels in storage environments of climacteric fruits are crucial for reducing postharvest losses of products and maintaining the organoleptic quality of fruits [159].
Over the past two decades, various methods for ethylene control have been developed, becoming increasingly effective in achieving minimal ethylene concentrations in fruit storage and transportation environments. These methods can be classified into three well-defined categories:
- Ethylene inhibitors:
- ○
- 1-MCP: 1-methylcyclopropene;
- ○
- SA: salicylic acid;
- ○
- AVG: aminoethoxyvinylglycine;
- ○
- AOA: amino-oxyacetic acid.
- Ethylene adsorbents:
- ○
- Zeolite;
- ○
- Activated carbon;
- ○
- Metal–organic frameworks;
- ○
- Silica gel.
- Ethylene scavengers by catalytic oxidation:
- ○
- KMnO4: potassium permanganate;
- ○
- UV radiation;
- ○
- TiO2: titanium dioxide;
- ○
- O3: ozone;
- ○
- Palladium;
- ○
- Cold plasma and other technologies.
5.1. Ethylene Inhibitors
Ethylene inhibitors act at some point in the biosynthesis, signalling, or perception pathways of ethylene as previously developed. By delaying or slowing down these metabolic processes, they hinder the natural progression of product ripening.
5.1.1. 1-Methylcyclopropene (1-MCP)
1-MCP is a chemically synthesized molecule belonging to the family of small-ring hydrocarbons (Figure 4). Under normal environmental conditions (25 °C and 1 atm), 1-MCP is gaseous; therefore, in its commercial form, it is encapsulated in γ-cyclodextrins that dissolve with the relative humidity of the environment, releasing this gas [160,161].
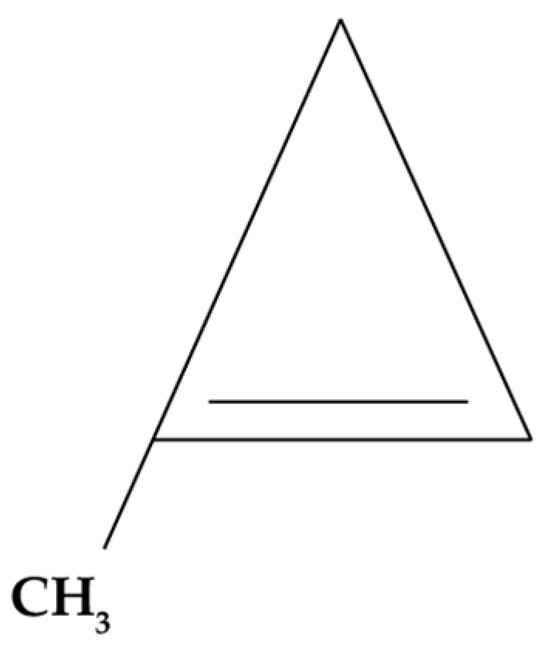
Figure 4.
1-MCP molecular structure.
This molecule is characterized by having a molecular structure similar to ethylene. This causes it to compete with this phytohormone for the ethylene membrane receptors (ETR1, ERS1, and EIN4) located on the plasma membranes of plant cells, inhibiting the ethylene response [29]. This mechanism can be observed in Figure 5.
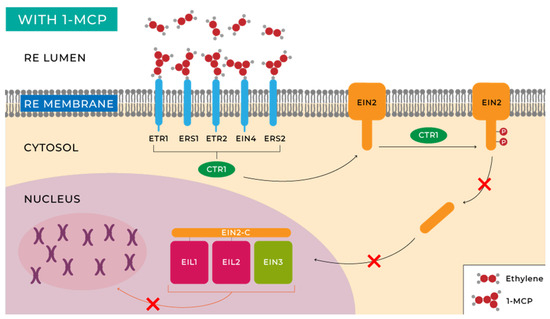
Figure 5.
Ethylene perception and signalling with 1-MCP (own source: http://hdl.handle.net/10952/6740 (accessed on 20 May 2023)).
Additionally, 1-MCP interacts with ethylene transmembrane receptors in a more intense and rapid manner than ethylene itself, facilitating the binding of 1-MCP to these receptors [162]. Other studies have shown that 1-MCP has other functions besides competing for ethylene reception; however, these mechanisms have not yet been discovered [163]. The effects of applying this molecule have been widely studied in the last 15 years (Table 1).
However, 1-MCP shows lower effectiveness than other ethylene scavengers or deactivators such as palladium compounds or the use of potassium permanganate as ethylene oxidants [29,41]. The reason for this is that 1-MCP is limited to competing with ethylene for cellular receptors, thereby partially preventing its reactivation but not eliminating it. Additionally, 1-MCP does not block all ethylene receptors in plant cells, thus its effectiveness is lower than expected [164].

Table 1.
Studies on the application of 1-MCP in different formats and concentrations on the preservation of various fruits.
Table 1.
Studies on the application of 1-MCP in different formats and concentrations on the preservation of various fruits.
| Format | Concentration | Fruit | Conditions | Significative Results | Reference |
|---|---|---|---|---|---|
| Gas | 0.5 µL L−1 | ‘Raf’ Tomato | 10 °C, 90% RH, 7 days 20 °C, 90% RH, 4 days | It reduces both ethylene production and respiration rate and, in turn, delays weight, soluble solids content, and total acidity changes. | [165] |
| Gas | 1 µL L−1 | Pear cv: ‘Gorham’ ‘Gran Champion’ ‘La France’ ‘Gold La France’ | 20 °C, 25 days | Reduction in ethylene production, significant delay in the expression of genes related to the change in skin colour of pears, and preservation of chlorophyll and fruit firmness. | [166] |
| Gas Gas | 0.1 µL L−1 0.035 µL L−1 | ‘Unicorn’ Tomato | 10 °C, 85% RH, 15 days | The treatment using a higher concentration of 1-MCP showed a higher conservation of lycopene and weight. | [167] |
| Gas | 0.9 µL L−1 | ‘Hayward’ Kiwi | 20 °C, 95% RH, 20 days | Inhibition of ethylene production and respiration rate, delay of rot incidence, weight loss, increase in soluble solids content and total bacterial count. Improved preservation of firmness, chlorophyll, total acidity, ascorbic acid, and antioxidant capacity. | [168] |
| Micro-bubbles | 100–400 ppb | ‘Khai’ Banana | 25 °C, 85% RH, 12 days | Reduced respiration rate and ethylene production. Higher preservation of total chlorophyll content, colour, firmness, total soluble solids, antioxidant capacity, and total phenolic compounds. | [169] |
| Gas | 100 nL L−1 | ‘Gold’ and ‘Rainbow’ Papaya | 22 °C, 85% RH, 25 days 10 °C, 85% RH, 25 days | Fruits treated with high concentrations of 1-MCP showed increased firmness and delayed colour variation, meaning delayed ripening. The authors claim that commercial application could lead to a 30% reduction in papaya ripening. | [170] |
5.1.2. Salicylic Acid (SA)
Salicylic acid (SA) (Figure 6) is a phenolic compound found in plants that is currently considered an endogenous plant hormone [171]. This molecule regulates many processes such as stress response, plant development, thermogenesis, photosynthesis, stomatal behaviour, transpiration, ion absorption and transport, seed germination, and glycolysis, among others [172,173].
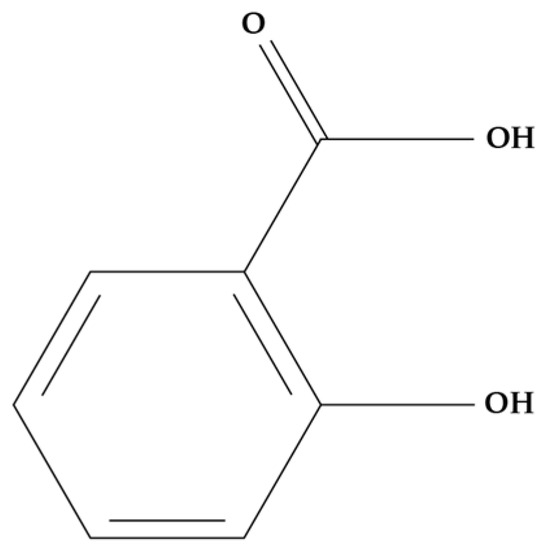
Figure 6.
SA molecular structure.
It acts as an antagonist of ACC oxidase (ACO), inhibiting its action; this enzyme is crucial in the process of ethylene production. ACO oxidizes ACC to ethylene in its final stage [174]. It has been demonstrated that SA inhibits ethylene production in various plants. Leslie and Romani in 1988 [175] observed that applying a concentration of 80 μM of SA during washing of pears (Pyrus communis L.) reduced the ethylene production rate by 80% after 24 h. Srivastava & Dwivedi in 2000 [176] applied 100 μM of SA to bananas, achieving, approximately, a 50% reduction in respiration rate. These authors also observed a reduction in the activity of cellulase and polygalacturonase enzymes after SA application. Babalar and colleagues [173] applied SA at a concentration of 2 mM, achieving effective reduction in both fungal rot and respiration rate in ‘Selva’ variety strawberries. In ‘Canino’ apricots, Elmenofy et al. in 2021 [177] compared the application of two ethylene inhibitors such as SA (4 mM) and AVG (150 mg L−1), recommending the application of SA (4 mM) which yielded optimal yield, quality, and preservation of this apricot variety when grown under Egyptian environmental conditions, where the research was conducted. Lastly, Mansourbahmani and colleagues [41] compared different ethylene removal methods, including SA. These authors concluded that applying a 1% SA solution to ‘Valouro’ variety tomatoes reduced ethylene production by 25%, which was lower than other investigated methods such as 1-MCP application or ethylene oxidation via the use of palladium, potassium permanganate, or UV-C radiation.
Recently, applications of SA as an elicitor have been discovered. It refers to a substance or signal that triggers a specific response in a cell, organism, or system. In plants, elicitors can be molecules like phytohormones, which activate specific receptors on the surface of cells, leading to a cascade of intracellular signalling events ultimately resulting in a cellular response. Gong and colleagues [178] reported in their review that SA application during fruit formation (lemon, pomegranate, mango, and strawberry among others) improved phenylpropanoid metabolism and carotenoid biosynthesis, increasing accumulation of flavonoids, ascorbic acid, and carotenoids, thereby enhancing antioxidant activity in the subsequently harvested fruit.
5.1.3. Aminoethoxyvinylglycine (AVG)
Similar to SA, aminoethoxyvinylglycine (AVG) (Figure 7) is a compound that inhibits ethylene production by intervening at some point in the metabolic pathways that leads to its production. Specifically, AVG is an inhibitor of ACC synthase (ACS) as it binds to its coenzyme, pyridoxal phosphate (PLP), preventing the conversion of SAM to ACC and thereby affecting the subsequent production of ethylene [179,180,181].
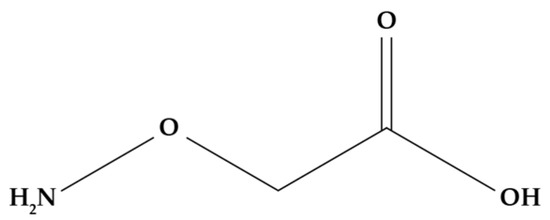
Figure 7.
AVG molecular structure.
The postharvest application of AVG has been studied in various fruits: Salveit [182] applied a 10 mM solution of AVG in tomatoes (Lycopersicon esculentum Mill.) of the ‘Castlemart’ variety, resulting in a 30% reduction in respiration rate and a 70% reduction in ethylene production rate of the fruits. Ozturk and colleagues [183] applied 200 mg L−1 of AVG in plums two weeks before harvest in the ‘Black Beauty’, ‘Black Amber’, and ‘Fibre’ plum varieties. Results showed a 60% reduction in ethylene production and respiration rate. They also observed a delay in the colour change from green to red and prevented firmness loss (70% firmer fruits in treated fruits) and weight loss (15% more weight in treated fruits). The same author in 2019 applied 225 mg L−1 of AVG in kiwifruits (Actinia deliciosa L.) of the ‘Hayward’ variety, observing similar results, but no differences were observed with the control in the analysis of ascorbic acid and total flavonoids with the application of AVG [184]. Similar effects were observed by Yuan & Carbaugh [185] in apples of the ‘Golden Supreme’ and ‘Golden Delicious’ varieties, Muñoz-Robredo and colleagues [186] in apricots of the ‘Patterson’ variety, Xie and colleagues [187] in pears of the ‘Starkrimson’ variety, Kim and colleagues [188] in plums of the ‘Formosa’ variety, and Win and colleagues [189] in persimmons of the ‘Sangjudungsi’ variety.
However, possible negative effects on fruit aroma development have been reported, resulting in loss in intensity [190,191]. Additionally, its cost is too high for industrial application [180].
5.1.4. Aminooxyacetic Acid (AOA)
Unlike the compounds mentioned earlier (1-MCP, SA, and AVG), AOA (Figure 8) is a nonspecific inhibitor of all enzymes that use PLP as a coenzyme, including ACC synthase, so its application could decrease ethylene synthesis [180,192,193]. Due to its nonspecificity, it may interfere with other enzymatic reactions, negatively affecting other physiological processes [194].
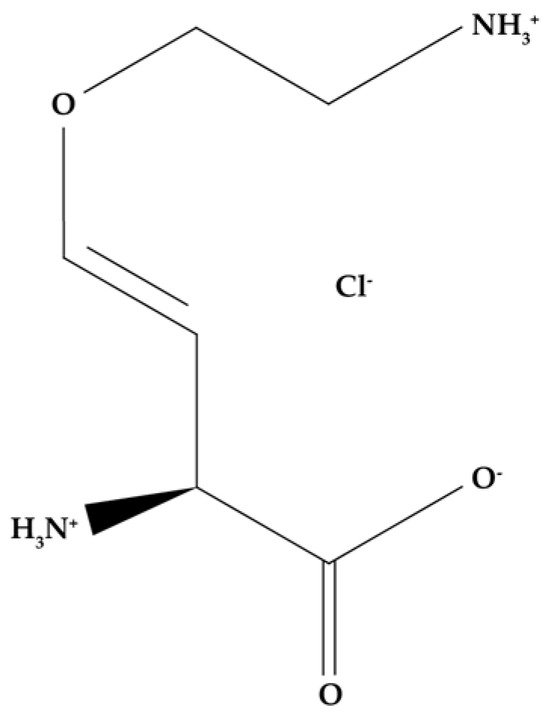
Figure 8.
AOA molecular structure.
Bulantseva and colleagues [195] applied a solution of AOA at 10 mg L−1 to bananas harvested at three different ripening stages (green, green-yellow, and bright yellow). Those treated and harvested in the green and green-yellow stages had ethylene production rates about 30% lower than controls at the same ripening stages. Lima and colleagues [196] immersed the roots of sweet potatoes of the ‘BRS Rubissol’ variety in 1 mg L−1 of AOA, resulting in better preservation of weight, total sugars, and pH and a reduction in sprouting compared to untreated potatoes, thereby extending the shelf life of treated potatoes.
To date, the application of this compound has been mainly limited to the preservation of harvested flowers [197], while its application in fruits and vegetables has not been sufficiently tested.
5.2. Ethylene Adsorbents
Methods for trapping ethylene and minimizing its action are based on two physical phenomena: adsorption and absorption. Adsorption occurs when a particle adheres to the surface of a solid material. On the other hand, in absorption, the particle remains within the solid material. Many materials are capable of carrying out adsorption/absorption processes of ethylene, and they have been used to develop ethylene scavengers. Among them, zeolites, activated carbon, and metal–organic frameworks stand out.
5.2.1. Zeolite
Zeolites (Figure 9) [198] are three-dimensional microporous structures of crystalline aluminosilicates. Zeolites have negative charges in their structure that are balanced with alkali or alkaline earth ions [199]. The pore size of zeolites ranges from 3 to 12 Å, which gives them the capacity to adsorb many chemical substances with a certain degree of specificity, including ethylene [18].
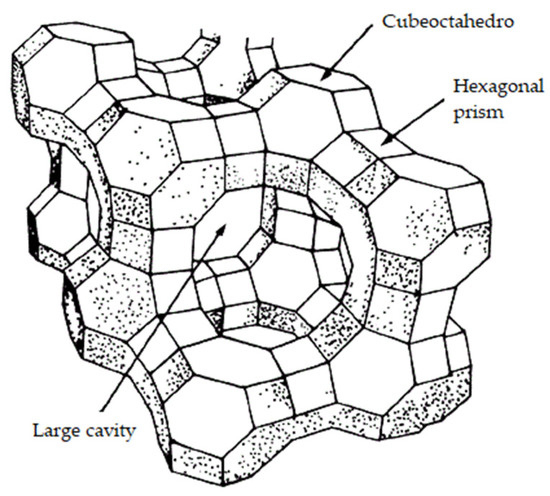
Figure 9.
Zeolite typical structure (own adaptation from [199]).
Ethylene can be absorbed within the structure of zeolite and adsorbed on the surface of the zeolite structure [200]. In the case of natural zeolites, a large pore diameter (12 Å) favours ethylene (diameter 3.9 Å) to pass through the pore openings and be absorbed into the interior of the zeolite [201]. There are two theories for explaining this phenomenon. The first refers to a cation–π interaction (non-covalent molecular interaction between a cation and an electron-rich system) between the double-bond electrons of ethylene and metallic cations of the zeolite. The second theory involves a resulting CH-O interaction between the hydrogen atoms of ethylene and oxygen atoms on the surface of the zeolite [18,199,202].
De Bruijin and colleagues [203] applied zeolites (composed of 53% clinoptilolite, 40% mordenite, and 7% quartz) as adsorbents in the conservation of tomatoes of the ‘Medano’ variety and observed a 50% reduction in ethylene concentration measurements up to day 10 of storage at 10 °C. From that point on, ethylene levels between the control treatment and the treatment with zeolite adsorbents equalized. These authors concluded that it may be an effective method for ethylene capture but that it alone is not highly effective. Mariah and colleagues [204] reached a similar conclusion in their review work.
However, despite the fact that zeolites do not cause a significant environmental impact and are non-toxic, their ethylene retention capacity is low and nonspecific for this molecule [112].
5.2.2. Activated Carbon
The use of activated carbon dates back to 1940. Southwick and Smock as early as in 1943 [14] used brominated charcoal to remove ethylene from the storage atmosphere of ‘McIntosh’ apples, which allowed for a considerable extension of the fruit’s life in that controlled atmosphere for a period of 2 to 3 months.
Activated carbon traps ethylene by adsorption (Figure 10) [205]. Its effectiveness depends on factors such as the contact surface, pore size, temperature, and relative humidity of the environment [193]. Granular powder activated carbon is the most common form used for ethylene capture with this methodology. Bailén and colleagues [206] confirmed that at an ethylene concentration ranging from 1 to 7.5 μL L−1 in the air, granular activated carbon of 20 to 60 meshes (a unit of measure for the pore diameter of a sieve in particle size analysis, in this case between 0.841 and 0.177 mm) could adsorb 70% of the available ethylene, while powdered activated carbon of 100 to 400 meshes (between 0.149 and 0.037 mm) could only adsorb 40%.
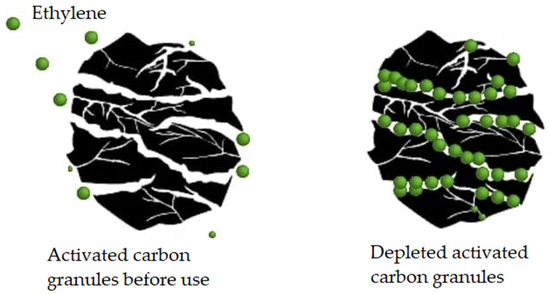
Figure 10.
Active carbon acts as an ethylene adsorbent. When this phytohormone penetrates through the structural cracks in the active carbon, it remains adhered to the walls, thus preventing its effect (own adaptation from [205]).
Jaimun and Sabgsywan [207] applied activated carbon to chitosan-coated papers (aminopolysaccharide biopolymer) and vanillin to compare its effect with other ethylene scavengers such as zeolite in the transport boxes of ‘Nam Dok Mai’ mangoes preserved for 30 days at 13 °C. In their results, they observed that the application of zeolite in this context was more effective than activated carbon for ethylene adsorption, although the differences between these treatments were low (1.91 mL L−1 of ethylene for zeolite, 2.31 mL L−1 of ethylene for activated carbon, and 3.21 mL L−1 of ethylene for the control treatment at 28 days of trial). No differences were observed between applying activated carbon or zeolite in the rest of the analysed parameters (weight, firmness, titratable acidity, and soluble solids), but differences were observed with the control treatment, thus maintaining the quality of mangoes treated with both adsorbent substances during the storage time.
The main advantages of activated carbon as an ethylene remover are its environmental friendliness due to its carbon composition, low toxicity, availability, and low cost. However, its nonspecific adsorption nature is a significant limitation for its widespread use as an ethylene adsorbent alone, so it is usually used as an adjunct along with other ethylene scavengers and removers such as potassium permanganate (KMnO4) or palladium [18,29].
5.2.3. Metal–Organic Frameworks
Metal–organic frameworks (MOFs) are a type of synthetic porous material formed by metal ions or groups of ions bonded to organic molecules. The combination of different organic and inorganic molecules provides flexibility in terms of pore size, shape, and structure [208]. MOFs have an exceptionally high adsorption surface area (from 1000 to 3000 m2 g−1), greater than zeolites (320 m2 g−1) and activated carbon (827 to 1120 m2 g−1) [209]. MOFs can selectively adsorb volatile compounds such as ethylene [208,210].
Awalgaonkar [164] found that MOFs had better ethylene inactivation capacity than potassium permanganate under low and controlled relative humidity conditions. Chopra and colleagues [210] reported that MOFs do not adsorb ethylene as efficiently when there is water in the storage environment. This is indeed a problem for its implementation at an industrial level since the storage environments of plant products typically have relative humidity values between 90 and 95%. Although this method is effective for ethylene capture, it has not yet been approved by the U.S. Food and Drug Administration for use in the food industry [18]. Further research and development of this product are necessary to enable its application.
As seen thus far, ethylene traps, while effective at “capturing” ethylene, are not efficient at preventing its action. Some of the main drawbacks of these technologies are as follows:
- Ethylene is only adsorbed on the surface or absorbed into the interior of these materials but cannot be decomposed.
- Desorption phenomena (the opposite process of adsorption/absorption) may occur, whereby a substance is released from or through a surface.
- Over time, the effectiveness of adsorption/absorption tends to decrease as these materials easily become saturated and require replacement.
5.3. Ethylene Removal by Catalytic Oxidation
This method of ethylene elimination relies on chemical oxidation–reduction reactions in which ethylene irreversibly dissociates into CO2 and water. The effectiveness of this method depends on the oxidizing capacity of the various compounds that can be used for this purpose.
5.3.1. Potassium Permanganate (KMnO4)
Potassium permanganate acts on the double bond of the ethylene molecule to oxidize it in the presence of water. Ethylene, when oxidized by potassium permanganate, initially forms acetaldehyde, which is subsequently oxidized to acetic acid and then to carbon dioxide and water.
The redox reaction caused by potassium permanganate results in a colour change in the permanganate itself, shifting from purple (MnO4 ions) to brown (MnO2). The studies conducted using potassium permanganate as an ethylene remover are quite diverse. Its effects have been tested in various applications involving apples [211], apricots [120], blueberries [212], tomatoes [41,139,213], pears [114,214], and peaches [42,115], among others. Many studies conducted with this molecule aim to enhance ethylene removal by increasing the contact surface of potassium permanganate with this phytohormone. This is achieved by mixing it with inert materials such as zeolites or activated carbon mentioned earlier, compounds that also aid in the absorption and adsorption of ethylene (see Table 2).

Table 2.
Some studies on the application of potassium permanganate, in different formats, in the preservation of fruits.
This molecule has significant industrial applications due to its low production cost, ease of incorporation into storage and transportation systems, and high effectiveness in ethylene removal. The main drawback lies in its rapid consumption and potential toxicity upon contact with food. However, it is worth mentioning that this issue has been addressed by presenting it (in most of its commercial forms) in easily replaceable sachets when they become saturated.
5.3.2. UV Radiation
Short-wave UV radiation between 100 and 280 nm (UV-C) is also capable of acting on the double bond of ethylene, generating oxidizing radicals that ultimately produce CO2 and water in the reaction process, a phenomenon known as photocatalysis [219,220]. Additionally, this radiation can produce small amounts of ozone at these wavelengths, a molecule that also acts on ethylene, as will be discussed in the following points.
Bu and colleagues [221] applied UV-C radiation at 254 nm to tomatoes of the ‘Zhenzhu’ variety. Despite the direct impact of UV radiation on the fruit potentially causing adverse effects, these researchers observed a significant 20% reduction in the ethylene production rate. Mabusela and colleagues [222] in their review article studied ultraviolet radiation at 254 nm and 185 nm in a vacuum environment. At both wavelengths, ethylene elimination was observed through two pathways: firstly, the direct action of UV-C radiation on the double bond of ethylene and, secondly, by producing ROS by reacting with water and oxygen from the environment (considering that the relative humidity of fruit storage environments shows values around 90% and 21% for oxygen). These ROS then react with ethylene, producing CO2 and water (Figure 11).
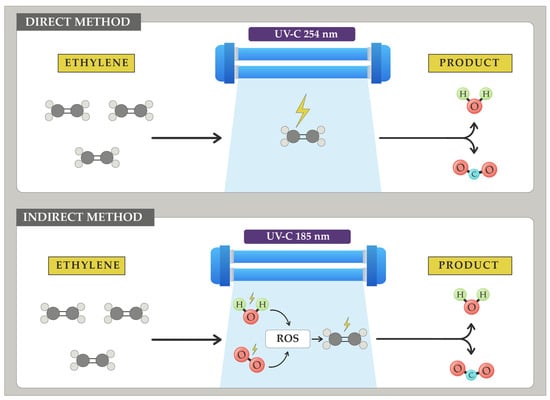
Figure 11.
How two different wavelengths of UV-C radiation affect ethylene removal. The first one, at 254 nm, produces an indirect ethylene elimination by producing free radicals that act on ethylene. The second wavelength works directly on the ethylene molecules (own adaptation from [222]).
The effectiveness of the treatments studied by Mabusela and colleagues [222] depends on numerous factors that greatly influence the ethylene removal rate. One example is relative humidity. Water is the main source of ROS when attacked by radiation; therefore, low relative humidity significantly affects ethylene removal using this method. Currently, there is no consensus on the performance of this ethylene oxidation technique.
Additionally, UV-C radiation has been extensively studied for its ability to eliminate spores of microorganisms present in fruit storage environments [223]. This is an effect that must be considered, as fungal and bacterial diseases, among many other effects, increase the ethylene production rate in infected fruits [222].
This method has numerous advantages, such as ease of implementation, low energy consumption, effective ethylene removal under optimal conditions, and the elimination of spores, minimizing fungal and bacterial infections. However, it has several disadvantages that, if not considered, would reduce its effectiveness:
- Relative humidity: As mentioned earlier, environmental water is the main source of certain ROS, crucial for the efficacy of this method. The lower the relative humidity, the lower the ethylene removal achievable with UV-C radiation [224].
- Initial ethylene concentration: Increasing the initial concentration of ethylene, while keeping the photon energy constant, reduces the energy received by a greater number of ethylene molecules, thus reducing ethylene removal [157,225].
- Oxygen concentration: Oxygen is the precursor of certain ROS and ozone that facilitate this process. A low concentration of this gas will hinder ethylene removal [225].
- Direct incidence on fruits: When UV-C radiation is directly targeted at fruits, it causes structural changes that negatively affect their quality [222].
5.3.3. Titanium Dioxide (TiO2)
Photocatalysis is another approach for eliminating ethylene [16,219,226]. Titanium dioxide (TiO2) is a semiconductor material and one of the most frequently employed photocatalysts for ethylene degradation, a capacity primarily attributed to its unique photochemical reactivity and physical properties, which include high brightness (due to high refractive index) and resistance to discolouration [29].
The complete mechanism of organic compound oxidation by UV light radiation on titanium dioxide is described by Pathak and colleagues [225]. In summary, the reaction is based on the irradiation with UV wavelengths (hν) (around 240–380 nm) which causes titanium dioxide to generate electrons (e-) and other effects (reaction 1). These, when acting on the water in the environment, produce highly reactive hydroxyl radicals (·OH) (reaction 2). These radicals then react with organic compounds, resulting in CO2 and water (reaction 3) [219,220].
- -
- Reaction 1: TiO2 + hν (UV energy/radiation) → TiO2 + e− + h+
- -
- Reaction 2: H2O + h+ → ·OH + H+
- -
- Reaction 3: ·OH + Organic compound (ethylene) → CO2 + H2O
Although the mechanism of photocatalytic oxidation has been described [225], the exact mechanism of reaction with ethylene remains a topic of debate due to the presence of various reaction intermediates that have not been fully elucidated [157].
Numerous studies have been conducted on the application of this ethylene elimination technology. Alves and colleagues [227] applied it to cherry tomatoes stored at 18 °C with a relative humidity of 85%. The treatment involved passing a constant airflow through the titanium dioxide and UV light system. During the storage period, cherry tomatoes treated with titanium dioxide irradiated with UV light showed a lower concentration of ethylene, lower respiration rate, lower total soluble solids content, and higher concentrations of lycopene and titratable acidity compared to the control, demonstrating that fruits treated with photocatalysis did not reach full ripeness. Li and colleagues [228] obtained similar results using the same tomato variety for their study.
In recent years, many studies on the effect of titanium dioxide–UV on ethylene have focused on its application as a component of films or fruit packaging. Among them, Fonseca and colleagues [229] evaluated the efficacy of polyethylene foam nets coated with a photocatalytic nanocomposite composed of gelatine and titanium dioxide for degrading the ethylene produced by papayas (Carica papaya L.) of the ‘Golden’ variety. The fruits treated with gelatine–titanium dioxide and irradiated with UV light showed a 60% lower ethylene accumulation than the fruit in the control group after four days of storage, as well as higher firmness and lower ripening index (soluble solids/titratable acidity ratio). Ghosh and colleagues [230] applied photoactivated titanium dioxide and chitosan to form a film to cover peppers of the ‘Tejaswani’ variety stored at room temperature (25 °C). The treated peppers maintained CSS and had higher firmness, cell integrity, hydration, and colour. De Chiara and colleagues [231] applied a powder composed of titanium dioxide and silicon dioxide in different proportions for the preservation of ‘Camone’ tomatoes. In tomatoes treated with an 80–20 ratio (TiO2–SiO2), ethylene completely degraded after 14 days of study. However, the fruits in this treatment did not reach the red colouration stage.
Titanium dioxide is categorized as a food additive by the U.S. Food and Drug Administration under code 21CFR73.575 and is inexpensive. This makes it cost-effective for application in fruit storage and transportation. However, there are some drawbacks to consider for its implementation:
- It requires very controlled storage conditions as the effectiveness of titanium dioxide depends on numerous variables such as humidity, temperature, or UV light intensity [157,193,206].
- It is not a selective method, as seen in the summary of the reaction triggered by titanium dioxide and UV radiation. In addition to ethylene, it acts on other organic compounds such as aromatic compounds, which can affect the organoleptic quality of the fruits [232].
- It can increase the storage temperature, which may lead to fruit damage [233].
- If UV radiation, essential for this method to be effective, directly impacts the fruits, it can lead to negative consequences such as loss in aroma, rupture of cell membranes, and degradation of structures [234].
5.3.4. Ozone (O3)
Ozone, typically produced with UV lamps, is a strongly oxidizing molecule that can also be used as an ethylene eliminator [219]. Ozone, being a highly reactive gas, acts on the double bond of ethylene, breaking it and dissociating this phytohormone into CO2 and water according to the following reaction: O3 + C2H4 → CO2 + H2O. The application of this method is straightforward. It involves passing a flow of air from fruit storage facilities (rich in ethylene) through an ozone generator and returning it to the warehouse free of ethylene [235]. Liang and colleagues [236] analysed ethylene production, firmness, vitamin C content, malondialdehyde content, levels of ascorbate peroxidase, peroxidase, and aromatic compound contents of ‘Hard Pink’ tomatoes stored for 25 days at 10 °C and treated with 17.14 mg (m3)−1 of ozone for 1 h at the start of the study. The results showed that ozone treatment delayed the loss in firmness, bioactive compounds, and aromatic compounds and reduced the ethylene production rate by 50% during the first 15 days. Toti and colleagues [237] treated ‘Cantaloupe’ melons with 0.3 ppm of ozone at 6 °C for 13 days. Their results indicated that ozone treatment reduced the activity of cell wall enzymes (α-arabinopyranosidase, β-galactopyranosidase, and polygalacturonase) responsible for their degradation and hence the softening of melons. Ethylene concentration was also reduced. With all this, the quality of the treated melons was maintained for a longer period. More recently, Gao and colleagues [238] treated ‘Muscat Hamburg’ grapes, despite being non-climacteric fruits, with 14.98 mg (m3)−1 of ozone. The results revealed a reduction in ethylene production close to 30% in the fruits subjected to the treatment. Additionally, there was maintenance of antioxidant capacity with higher concentrations of enzymes such as catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), and polyphenol oxidase (PPO), capable of removing reactive oxygen species (ROS) from plant tissues. Furthermore, a lower incidence of fungal diseases was observed in the treated fruits. Although considered safe by the FDA (U.S. Food and Drug Administration) and not complicated for industrial implementation, ozone presents several drawbacks:
- Handling is very challenging due to its easy decomposition into oxygen [159].
- If certain levels are exceeded, ozone shifts from offering significant improvements to being harmful as it can destroy tissues, leading to wounds that can promote ethylene production due to stress [219].
- Even though it is safe at low concentrations, ozone begins to have harmful effects on human health from 5 ppm onwards, including vision problems, sensation of asphyxia, headaches, pulmonary oedema, and coma when concentrations reach 50 ppm [239].
5.3.5. Palladium
Palladium is a scarce metal with numerous applications, including its use as a catalyst in chemical reactions and in medicine for surgical material production. It also has the ability to absorb hydrogen into its metallic structure and purify hydrocarbons in the automotive industry. This latter application prompted its use as a potential ethylene eliminator through catalysis. Similar to other ethylene oxidants, palladium irreversibly decomposes ethylene into CO2 and water.
Bailén and colleagues [206] employed a mixture of granulated (20–60 mesh) activated carbon with 1% palladium on three tomato varieties, ‘Beef’, ‘Mendez’, and ‘Raf’. Within the first 50 h, nearly 60% ethylene elimination was achieved in all three varieties, along with better preservation of firmness and colour, both internally and externally. Mok and colleagues [240] applied a mixture of ZSM-5 zeolite and 0.36% palladium in the conservation of ‘Fuji’ apples, introduced into quartz tubes through which air from the storage chamber passed. The results showed nearly 100% ethylene elimination after 40 days of storage. Tzeng and colleagues [241] observed a 99% ethylene removal in banana preservation using a similar mixture and application method.
Mansourbahmani and colleagues [41] employed palladium with nano zeolites in various concentrations (0%, 1%, 2.5%, and 5%) and compared this ethylene removal method with other ethylene eliminators or inhibitors such as potassium permanganate, 1-MCP, and UV-C, concluding that the palladium method was the most effective.
Although this method does not have many drawbacks, its industrial application proves to be very complicated due to palladium being a very scarce and difficult-to-extract metal and consequently extremely expensive. Additionally, the country with the highest production of this element worldwide is the Russian Federation, which is currently subject to numerous economic and commercial sanctions (due to the invasion of Ukraine), further increasing its price.
5.3.6. Cold Plasma and Other Technologies
Cold plasma technology has emerged as a promising method for ethylene scavenging, offering a non-thermal and eco-friendly approach to extend the shelf life and maintain the quality of fresh produce. This promising and novel method stands out (1) for being a non-thermal technology, which means it does not rely on heat to achieve its effects, and is particularly beneficial for ethylene scavenging as it avoids thermal damage to the produce, maintaining its nutritional and sensory properties; (2) as favouring microbial decontamination where cold plasma has shown effectiveness in microbial decontamination, enzyme denaturation, and pesticide degradation, which are all crucial for preserving the quality and safety of food products; and (3) for its versatility and applications where cold plasma technology allows for its application in various forms, such as direct treatment, plasma-treated water, and functional coatings. This flexibility makes it suitable for a wide range of uses in the food industry [242]. In addition, cold plasma is considered an eco-friendly technology due to its low energy requirements and minimal environmental impact. It represents a sustainable option for ethylene scavenging and food preservation [243]. The potential of cold plasma technology in food processing is vast, with ongoing research exploring its scalability and commercial viability. Future advancements are expected to further enhance its effectiveness and applicability in the food industry [242].
Other novel advances and developments in ethylene scavenging beyond cold plasma technology are as follows: (1) The first is nanotechnology, where the incorporation of nanoparticles into polymer matrices has been shown to play a major role in reducing the permeability of gases and the absorption of ethylene. This approach enhances the effectiveness of packaging materials in controlling ethylene levels around fresh produce [105]. For this reason, nanotechnology is at the forefront of advancing ethylene scavenging techniques to maintain the quality and extend the shelf life of fruits and vegetables. For example, nanosilica has been isolated from rice straw and used in a polyvinyl alcohol (PVA) formulation to coat paperboard, which showed improved barrier, mechanical, and surface properties, along with higher ethylene scavenging activity compared to uncoated paperboard [244]. Also, palladium encapsulated nanofibres has been developed for ethylene scavenging. Encapsulation of 1–2% PdCl2 in nanomats has increased the ethylene scavenging capacity significantly, proving to be effective in the presence of fruits like sapota [245]. (2) The second is mechanochemical synthesis, where recent studies have investigated the use of mechanochemically synthesized absorbents for ethylene removal. These materials have shown promising ethylene scavenging activity and offer a safer, innovative, and eco-friendlier approach to active packaging [246]. This process involves the use of mechanical force to induce chemical reactions, which can be used to synthesize metal–organic frameworks (MOFs) that are effective in ethylene scavenging [247].
6. Practical Implications and Future Directions
6.1. Energy Consumption
Controlled atmosphere storage or refrigeration require significant energy inputs to maintain optimal storage conditions. High energy consumption associated with refrigeration systems contributes to greenhouse gas emissions and environmental footprint, highlighting the need for energy-efficient technologies and alternative cooling methods [248,249,250].
6.2. Food, Microbiological, and Chemical Safety
Ensuring the safety of ethylene inhibition strategies involves addressing potential risks to human health, worker safety, and food quality: On one hand, workers involved in handling ethylene inhibition chemicals or applying postharvest treatments may be exposed to potential health hazards, such as respiratory irritation or skin sensitization. Proper training, personal protective equipment (PPE), and adherence to safety protocols are essential for minimizing chemical exposure risks and ensuring worker safety. On the other hand, ethylene inhibition treatments and packaging materials must comply with regulatory standards and food safety guidelines to prevent contamination and ensure consumer protection. Residual chemicals or contaminants from postharvest treatments or packaging materials should be carefully monitored and controlled to maintain food safety and quality throughout the supply chain. In addition, improper handling or storage of fruits treated with ethylene inhibition strategies may increase the risk of microbial contamination and foodborne illness. Effective sanitation practices, temperature control, and hygienic handling procedures are essential for minimizing microbial risks and ensuring food safety from farm to fork [160,251,252,253,254].
6.3. Exploring Sustainable and Eco-Friendly Alternatives
Packaging plays a critical role in maintaining fruit quality and extending shelf life by creating protective barriers against external factors, including ethylene exposure, physical damage, and microbial contamination. Increased adoption of single-use plastics in packaging can exacerbate plastic pollution and waste management issues, necessitating the adoption of recyclable or biodegradable packaging alternatives and promoting circular economy initiatives. Utilizing biodegradable or compostable packaging materials derived from renewable sources, such as bioplastics or cellulose-based films, can help to reduce plastic waste and promote circular economy principles, in addition to exploring innovative packaging designs and technologies that enhance gas barrier properties, extend shelf life, and minimize resource consumption throughout the packaging lifecycle. Innovation of smart packaging solutions equipped with sensors, indicators, and active components for real-time monitoring and control of ethylene levels, fruit quality, and storage conditions enhances supply chain transparency, traceability, and product integrity, ensuring end-to-end quality assurance and consumer confidence [255,256,257,258,259,260,261,262,263].
6.4. Biocontrol Agents
Biocontrol agents offer sustainable alternatives to chemical treatments, providing effective control of postharvest pathogens and decay while promoting environmental sustainability and food safety. Implementing integrated pest management (IPM) strategies that incorporate biocontrol agents can reduce reliance on synthetic pesticides and minimize environmental contamination risks [264,265].
6.5. Commercial and Industrial Applications
The implementation of ethylene inhibition strategies in the fruit industry encompasses various postharvest handling and storage practices aimed at extending shelf life, preserving quality, and ensuring market competitiveness.
Pretreatments and harvest treatments may be employed to mitigate ethylene production and enhance fruit quality. Techniques such as preharvest stress conditioning, including deficit irrigation or exposure to plant growth regulators, can modulate ethylene biosynthesis and improve fruit firmness, colour development, and flavour. Additionally, preharvest application of natural or bio-based ethylene inhibitors may offer sustainable alternatives to synthetic chemicals, reducing environmental impacts and chemical residues. Harvesting fruits at the appropriate stage of maturity, using sharp tools to prevent mechanical damage, and handling fruits gently to avoid bruising or injury are essential considerations. Prompt removal of harvested fruits from the field and rapid cooling to reduce respiration rates and ethylene production are key steps in preserving postharvest quality and extending shelf life [266,267,268,269].
Efficient transportation and distribution networks are essential for minimizing postharvest losses and ensuring timely delivery of fresh fruits to markets. Temperature-controlled transport vehicles, such as refrigerated trucks or containers, maintain optimal storage conditions throughout transit, preventing temperature fluctuations and ethylene build-up. Monitoring devices and data loggers enable real-time tracking of environmental parameters, ensuring compliance with quality standards and regulatory requirements [270,271].
Proper handling and display practices at retail outlets are crucial for preserving fruit quality and enhancing consumer appeal. Temperature-controlled display cases, ethylene filters, and airflow management systems help minimize ethylene exposure and maintain freshness. Training retail staff on proper handling techniques, including gentle handling, regular rotation of stock, and removal of damaged or overripe fruits, ensures optimal product presentation and reduces waste [272].
Finally, as for consumer education and behaviour change, it is possible to promote eco-friendly alternatives, sustainable consumption behaviours, and waste reduction strategies through education campaigns, labelling initiatives, and consumer engagement programmes [273,274].
7. Conclusions
In conclusion, this review provides a comprehensive examination of ethylene-mediated ripening in climacteric fruits and the strategies employed to delay this process, thereby enhancing postharvest quality. Beginning with an introduction to climacteric fruits and the pivotal role of ethylene in their ripening, this review delineated the scope and structure of the discussion. Ethylene, as a plant hormone, plays a fundamental role in initiating and coordinating fruit ripening, orchestrating a cascade of physiological and biochemical changes. Various ethylene inhibition strategies were explored, encompassing chemical inhibitors, temperature control, modified atmospheres, and controlled atmosphere storage. These strategies act by reducing ethylene levels or its effects, thereby extending shelf life and preserving fruit quality. Understanding ethylene production and receptor mechanisms is crucial for effective inhibition strategies. Insights into ethylene biosynthesis, enzyme regulation, and receptor targeting were discussed to elucidate the underlying processes.
This review highlighted the significant impact of ethylene inhibition on shelf life extension and postharvest quality. Examples and case studies underscored the efficacy of these strategies in maintaining firmness, colour, sugar content, and nutritional value while reducing decay and pathogen development. Sensory quality, including flavour, aroma, and texture, emerged as a key consideration in ethylene inhibition. Examination of sensory attributes and consumer preferences emphasized the importance of treated fruits in meeting market demands. Furthermore, alterations in volatile compounds due to ethylene inhibition were investigated, shedding light on aroma profiles and the potential for off-flavours. Molecular and genetic approaches offered promising avenues for reducing ethylene responsiveness and developing genetically modified varieties with improved shelf life. Integrated approaches, combining multiple inhibition strategies, demonstrated enhanced efficacy and synergy between postharvest treatments and packaging techniques. Commercial and industrial applications showcased success stories and economic benefits, illustrating the real-world impact of ethylene inhibition strategies. Looking ahead, future directions in ethylene inhibition encompass emerging research areas and technological advancements, including nanomaterials and precision agriculture. Leveraging these innovations holds the promise of further enhancing efficacy, sustainability, and market competitiveness in the fruit industry. Finally, environmental and safety considerations underscored the importance of exploring sustainable and eco-friendly alternatives, ensuring responsible stewardship of resources and minimizing adverse impacts on the environment and human health. In essence, this review underscores the critical role of ethylene inhibition in preserving postharvest quality, driving innovation, and shaping the future of the fruit industry. By embracing emerging technologies, advancing scientific knowledge, and fostering collaborative efforts, stakeholders can continue to unlock new opportunities and address challenges in ethylene-mediated ripening, ultimately benefiting producers, consumers, and the environment.
Author Contributions
Conceptualization, R.A.-S., J.R.A.-M., S.L.-M. and A.J.P.-L.; methodology, R.A.-S., J.R.A.-M., S.L.-M. and A.J.P.-L.; software, R.A.-S. and J.R.A.-M.; validation, J.R.A.-M., S.L.-M. and A.J.P.-L.; formal analysis, R.A.-S., J.R.A.-M., S.L.-M. and A.J.P.-L.; investigation, R.A.-S., J.R.A.-M., S.L.-M. and A.J.P.-L.; resources, J.R.A.-M. and S.L.-M.; data curation, R.A.-S., J.R.A.-M. and A.J.P.-L.; writing---original draft preparation, R.A.-S., J.R.A.-M., S.L.-M. and A.J.P.-L.; writing---review and editing, R.A.-S., J.R.A.-M., S.L.-M. and A.J.P.-L.; visualization, A.J.P.-L., J.R.A.-M. and S.L.-M.; supervision, J.R.A.-M. and S.L.-M.; project administration, J.R.A.-M. and S.L.-M.; funding acquisition, J.R.A.-M. and S.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
Nuevas Tecnologías Agroalimentarias (KEEPCOOL): CFE/KE/76-19; “Cátedra Emprendimiento en el Ámbito Agroalimentario” from Universidad Católica San Antonio de Murcia (UCAM) and Banco Santander: CFE/KE/76-19.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors gratefully acknowledge Antonio Cerdá (Cátedra UCAM-Banco Santander de Emprendimiento en el Ámbito Agroalimentario director), FECOAM (“Federación de Cooperativas Agrarias de Murcia”), especially Antonio Sanz, Lola Mondéjar, and Miriam Monje for their help with figures.
Conflicts of Interest
The doctoral thesis (Ph.D.) of Ramiro Alonso Salinas, co-author of this work, is being co-financed by the company Nuevas Tecnologías Agroalimentarias (KEEPCOOL) within the UCAM Universidad Católica de Murcia Industrial Doctorate Program. The rest of the authors of this article declare that their contribution to this research was carried out in the absence of commercial or financial relationships that could be constructed as possible conflicts of interest.
References
- Ministerio de Agricultura, Alimentación y Medio Ambiente. Estrategia Más Alimento, Menos Desperdicio. 2013, 60. Aprovecha los alimentos (alimentosdespana.es). Available online: https://www.alimentosdespana.es/es/estrategia-alimentos-espana/aprovecha-los-alimentos/#:~:text=Esta%20iniciativa%20del%20Ministerio%20de%20Agricultura%2C%20Pesca%20y,contribuir%20a%20la%20reducci%C3%B3n%20del%20desperdicio%20de%20alimentos (accessed on 25 June 2024).
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying Household Waste of Fresh Fruit and Vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Informe Semanal RTVE. Comer Bien, Tirar Menos. 2023. Available online: https://www.rtve.es/play/videos/informe-semanal/comer-bien-tirar-menos/6832236/ (accessed on 25 June 2024).
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and Its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Kader, A. Tecnología Postcosecha de Cultivos Hortofrutícolas; UCANR Publications: Irvine, CA, USA, 2011; Volume 311, ISBN 1601077440. [Google Scholar]
- Neljubow, D. Uber Die Horizontale Nutation Der Stengel von Pisum Sativum Und Einiger Anderen Planzen. Bot. Centralbl. Beih. 1901, 10, 128–139. [Google Scholar]
- Cousins, H.H., III. Agricultural Experiments: Citrus. Annu. Rep. Dep. Agric. Jam. 1910, 1–7. [Google Scholar]
- Doubt, S.L. The Response of Plants to Illuminating Gas. Bot. Gaz. 1917, 63, 209–224. [Google Scholar] [CrossRef]
- Denny, F.E. Effect of Ethylene Upon Respiration of Lemons. Bot. Gaz. 1924, 77, 322–329. [Google Scholar] [CrossRef]
- Gane, R. Production of Ethylene by Some Ripening Fruits. Nature 1934, 134, 1008. [Google Scholar] [CrossRef]
- Gane, R. The Formation of Ethylene by Plant Tissues, and Its Significance in the Ripening of Fruits. J. Pomol. Hortic. Sci. 1935, 13, 351–358. [Google Scholar] [CrossRef]
- Crocker, W.; Hitchcock, A.E.; Zimmerman, P.W. Similarities in the Effects of Ethylene and the Plant Auxins. Contrib. Boyce Thompson Inst. 1935, 7, 231–248. [Google Scholar]
- Forsyth, F.R.; Eaves, C.A.; Lightfoot, H.J. Storage Quality of McIntosh Appels as Affected Nby Removal of Ethylene from the Storage Atmosphere. Can. J. Plant Sci. 1969, 49, 567–572. [Google Scholar] [CrossRef][Green Version]
- Southwick, F.W.; Smock, R.M. Lengthening the Storage Life of Apples By Removal of Volatile Materials From the Storage Atmosphere. Plant Physiol. 1943, 18, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.J.; Wills, R.B.H.; Patterson, B.D. Removal by Ultra-Violet Lamp of Ethylene and Other Hydrocarbons Produced by Bananas. J. Sci. Food Agric. 1971, 22, 496–497. [Google Scholar] [CrossRef]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Artés-Hernández, F. Potassium Permanganate-Based Ethylene Scavengers for Fresh Horticultural Produce as an Active Packaging. Food Eng. Rev. 2019, 11, 159–183. [Google Scholar] [CrossRef]
- Awalgaonkar, G.; Beaudry, R.; Almenar, E. Ethylene-Removing Packaging: Basis for Development and Latest Advances. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3980–4007. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Nazar, R.; Khan, M.I.R.; Khan, N.A. Variation in Photosynthesis and Growth of Mustard Cultivars: Role of Ethylene Sensitivity. Sci. Hortic. 2012, 135, 1–6. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic Regulation of Fruit Development and Ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Carrari, F.; Fernie, A.R. Metabolic Regulation Underlying Tomato Fruit Development. J. Exp. Bot. 2006, 57, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Figueroa, C.R.; Nair, H. “Movers and Shakers” in the Regulation of Fruit Ripening: A Cross-Dissection of Climacteric versus Non-Climacteric Fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.; Yuan, B.; Guo, Y. The Role of Abscisic Acid in Fruit Ripening and Responses to Abiotic Stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Martínez-Madrid, M.C.; Romojaro, F. Ethylene Biosynthesis and Polyamine and ABA Levels in Cut Carnations Treated with Aminotriazole. J. Am. Soc. Hortic. Sci. JASHS 1999, 124, 81–85. [Google Scholar] [CrossRef]
- Symons, G.M.; Chua, Y.-J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal Changes during Non-Climacteric Ripening in Strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked Regulatory Loops of ABA Catabolism and Biosynthesis Coordinate Fruit Growth and Ripening in Woodland Strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, E11542–E11550. [Google Scholar] [CrossRef] [PubMed]
- Forlani, S.; Masiero, S.; Mizzotti, C. Fruit Ripening: The Role of Hormones, Cell Wall Modifications, and Their Relationship with Pathogens. J. Exp. Bot. 2019, 70, 2993–3006. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef] [PubMed]
- Lara, I.; Vendrell, M. Cold-Induced Ethylene Biosynthesis Is Differentially Regulated in Peel and Pulp Tissues of ‘Granny Smith’ Apple Fruit. Postharvest Biol. Technol. 2003, 29, 109–119. [Google Scholar] [CrossRef]
- Sdiri, S.; Navarro, P.; Monterde, A.; Benabda, J.; Salvador, A. New Degreening Treatments to Improve the Quality of Citrus Fruit Combining Different Periods with and without Ethylene Exposure. Postharvest Biol. Technol. 2012, 63, 25–32. [Google Scholar] [CrossRef]
- Mathooko, F.M.; Kubo, Y.; Inaba, A.; Nakamura, R. Characterization of the Regulation of Ethylene Biosynthesis in Tomato Fruit by Carbon Dioxide and Diazocyclopentadiene. Postharvest Biol. Technol. 1995, 5, 221–233. [Google Scholar] [CrossRef]
- Alberto, O.; Alzate, T. Hallazgos De La Biosíntesis Del Etileno En Frutas Climatéricas Y De Los Factores Que Afectan La Ruta Metabólica. Rev. Aliment. Hoy 2014, 22, 46–63. [Google Scholar]
- Asif, M.H.; Pathak, N.; Solomos, T.; Trivedi, P.K. Effect of Low Oxygen, Temperature and 1-Methylcyclopropene on the Expression of Genes Regulating Ethylene Biosynthesis and Perception during Ripening in Apple. S. Afr. J. Bot. 2009, 75, 137–144. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Liu, C.; Chen, L.; Ma, J.; Sheng, J.; Shen, L. Inhibition of Nitric Oxide Synthesis Delayed Mature-Green Tomato Fruits Ripening Induced by Inhibition of Ethylene. Sci. Hortic. 2016, 211, 95–101. [Google Scholar] [CrossRef]
- Li, Y.; Golding, J.B.; Arcot, J.; Wills, R.B.H. Continuous Exposure to Ethylene in the Storage Environment Adversely Affects “Afourer” Mandarin Fruit Quality. Food Chem. 2018, 242, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Xie, X.-L.; Liu, S.-C.; Chen, K.-S.; Yin, X.-R. Auto- and Mutual-Regulation between Two CitERFs Contribute to Ethylene-Induced Citrus Fruit Degreening. Food Chem. 2019, 299, 125163. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; Golding, J.B. Reduction of Energy Usage in Postharvest Horticulture through Management of Ethylene. J. Sci. Food Agric. 2015, 95, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Cara, B.; Giovannoni, J.J. Molecular Biology of Ethylene during Tomato Fruit Development and Maturation. Plant Sci. 2008, 175, 106–113. [Google Scholar] [CrossRef]
- Mansourbahmani, S.; Ghareyazie, B.; Zarinnia, V.; Kalatejari, S.; Mohammadi, R.S. Study on the Efficiency of Ethylene Scavengers on the Maintenance of Postharvest Quality of Tomato Fruit. J. Food Meas. Charact. 2018, 12, 691–701. [Google Scholar] [CrossRef]
- Emadpour, M.; Ghareyazie, B.; Kalaj, Y.R.; Entesari, M.; Bouzari, N. Effect of the Potassium Permanganate Coated Zeolite Nanoparticles on the Quality Characteristic and Shelf Life of Peach and Nectarine. Int. J. Agric. Technol. 2015, 11, 1411–1421. [Google Scholar]
- Gavin, C.; Barzallo, D.; Vera, H.; Lazo, R. Revisión Bibliográfica: Etileno En Poscosecha, Tecnologías Para Su Manejo y Control. Ecuadorian Sci. J. 2021, 5, 163–178. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Chalupowicz, D.; Gamrasni, D.; Goldberg, T.; Nerya, O.; Ben-Arie, R.; Porat, R. Effects of the Ethylene-Action Inhibitor 1-Methylcyclopropene on Postharvest Quality of Non-Climacteric Fruit Crops. Postharvest Biol. Technol. 2016, 111, 322–329. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Hu, Y.X.; Liu, J.; Wang, H.; Wei, J.H.; Sun, P.D.; Wu, L.F.; Zheng, H.J. Progress of Ethylene Action Mechanism and Its Application on Plant Type Formation in Crops. Saudi J. Biol. Sci. 2020, 27, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Serna-Escolano, V.; Gimenez, M.J.; Garcia-Pastor, M.E.; Dobon-Suarez, A.; Pardo-Pina, S.; Zapata, P.J. Effects of Degreening Treatment on Quality and Shelf-Life of Organic Lemons. Agronomy 2022, 12, 270. [Google Scholar] [CrossRef]
- Li, L.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Porat, R.; Lichter, A. The Effects of 1-Methylcyclopropane and Ethylene on Postharvest Rachis Browning in Table Grapes. Postharvest Biol. Technol. 2015, 107, 16–22. [Google Scholar] [CrossRef]
- Özdemir, A.; Çandır, E. Impact of Hot Water and Modified Atmosphere Packaging Treatments on the Postharvest Quality of Pomegranate Fruit Cv. Hicaznar. Tarım Bilim. Derg. 2021, 27, 304–311. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Zhang, Y.-H.; Li, L.-L.; Li, Y.-X. Effects of 1-MCP on peel browning of pomegranates. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium, 31 October 2008; pp. 275–282. [Google Scholar]
- Flores, F.; El Yahyaoui, F.; de Billerbeck, G.; Romojaro, F.; Latché, A.; Bouzayen, M.; Pech, J.; Ambid, C. Role of Ethylene in the Biosynthetic Pathway of Aliphatic Ester Aroma Volatiles in Charentais Cantaloupe Melons. J. Exp. Bot. 2002, 53, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Saltveit, M.E. Effect of Ethylene on Quality of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Chapter 3—The Biosynthesis of Ethylene, 2nd ed.; Abeles, F.B., Morgan, P.W., Saltveit, M.E.B.T.-E., Eds.; Academic Press: New York, NY, USA, 1992; pp. 26–55. ISBN 978-0-08-091628-6. [Google Scholar]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Lee, Y.; Seo, J. Ethylene Scavenging Systems in Packaging of Fresh Produce: A Review. Food Rev. Int. 2021, 37, 155–176. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Estelle, M.A.; Somerville, C.; Kende, H. Insensitivity to Ethylene Conferred by a Dominant Mutation in Arabidopsis Thaliana. Science 1988, 241, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.; Kunishi, A. Stimulation of Ethylene Production in Apple Tissue Slices by Methionine. Plant Physiol. 1966, 41, 376–382. [Google Scholar] [CrossRef]
- Bradford, K.J. Shang Fa Yang: Pioneer in Plant Ethylene Biochemistry. Plant Sci. 2008, 175, 2–7. [Google Scholar] [CrossRef]
- Zhang, T.C. Ethylene Biosynthesis and Signal Pathway Model. Chin. Bull. Bot. 2006, 23, 519–530. [Google Scholar]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; American Society of Plant Biologists: Chichester, UK, 2000. [Google Scholar]
- Li, J.-F.; Qu, L.-H.; Li, N. Tyr152 Plays a Central Role in the Catalysis of 1-Aminocyclopropane-1-Carboxylate Synthase. J. Exp. Bot. 2005, 56, 2203–2210. [Google Scholar] [CrossRef]
- Ravanel, S.; Gakière, B.; Job, D.; Douce, R. The Specific Features of Methionine Biosynthesis and Metabolism in Plants. Proc. Natl. Acad. Sci. USA 1998, 95, 7805–7812. [Google Scholar] [CrossRef] [PubMed]
- Pretel, M.; Serrano, M.; Amoros, A.; Romojaro, F. Ripening and Ethylene Biosynthesis in Controlled Atmosphere Stored Apricots. Eur. Food Res. Technol. 1999, 209, 130–134. [Google Scholar] [CrossRef]
- Yamagami, T.; Tsuchisaka, A.; Yamada, K.; Haddon, W.F.; Harden, L.A.; Theologis, A. Biochemical Diversity among the 1-Amino-Cyclopropane-1-Carboxylate Synthase Isozymes Encoded by the Arabidopsis Gene Family. J. Biol. Chem. 2003, 278, 49102–49112. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, A.; Van Der Straeten, D. Ethylene Biosynthesis and Signaling: An Overview. In Plant Hormones; Litwack, G., Ed.; Vitamins & Hormones; Academic Press: Cambridge, MA, USA, 2005; Volume 72, pp. 399–430. [Google Scholar]
- Chung, M.-C.; Chou, S.-J.; Kuang, L.-Y.; Charng, Y.-Y.; Yang, S.F. Subcellular Localization of 1-Aminocyclopropane-1-Carboxylic Acid Oxidase in Apple Fruit. Plant Cell Physiol. 2002, 43, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Ono, E.; Mizutani, M. Evolution and Diversity of the 2-Oxoglutarate-Dependent Dioxygenase Superfamily in Plants. Plant J. Mol. Biol. 2014, 78, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Van Der Straeten, D. 1-Aminocyclopropane-1-Carboxylic Acid (ACC) in Plants: More than Just the Precursor of Ethylene! Front. Plant Sci. 2014, 5, 640. [Google Scholar] [CrossRef]
- Hudgins, J.W.; Ralph, S.G.; Franceschi, V.R.; Bohlmann, J. Ethylene in Induced Conifer Defense: CDNA Cloning, Protein Expression, and Cellular and Subcellular Localization of 1-Aminocyclopropane-1-Carboxylate Oxidase in Resin Duct and Phenolic Parenchyma Cells. Planta 2006, 224, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Vaughan-Hirsch, J.; van de Poel, B. The Regulation of Ethylene Biosynthesis: A Complex Multilevel Control Circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.T.; Hansen, M.; Kieber, J.J. Regulation of Ethylene Biosynthesis. J. Plant Growth Regul. 2007, 26, 92–105. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene Signaling in Plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Javid, E.; Corpas Iguarán, E.; Alberto, O.; Alzate, T. Hallazgos de la biosíntesis del etileno en frutas climatéricas y de los factores que afectan la ruta metabólica. Aliment. Hoy 2014, 22, 46–63. [Google Scholar]
- Stearns, J.C.; Glick, B.R. Transgenic Plants with Altered Ethylene Biosynthesis or Perception. Biotechnol. Adv. 2003, 21, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 Phosphorylates the Central Regulator EIN2 to Control Ethylene Hormone Signaling from the ER Membrane to the Nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef]
- Qiao, H.; Shen, Z.; Huang, S.C.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and Subcellular Trafficking of ER-Tethered EIN2 Control Response to Ethylene Gas. Science 2012, 338, 390–393. [Google Scholar] [CrossRef]
- Merchante, C.; Brumos, J.; Yun, J.; Hu, Q.; Spencer, K.R.; Enríquez, P.; Binder, B.M.; Heber, S.; Stepanova, A.N.; Alonso, J.M. Gene-Specific Translation Regulation Mediated by the Hormone-Signaling Molecule EIN2. Cell 2015, 163, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M.; Mortimore, L.A.; Stepanova, A.N.; Ecker, J.R.; Bleecker, A.B. Short-Term Growth Responses to Ethylene in Arabidopsis Seedlings Are EIN3/EIL1 Independent. Plant Physiol. 2004, 136, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.N.; Zhong, S.; Weirauch, M.T.; Hon, G.; Pelizzola, M.; Li, H.; Huang, S.-S.C.; Schmitz, R.J.; Urich, M.A.; Kuo, D.; et al. Temporal Transcriptional Response to Ethylene Gas Drives Growth Hormone Cross-Regulation in Arabidopsis. eLife 2013, 2, e00675. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Stock, A.M. Biological Insights from Structures of Two-Component Proteins. Annu. Rev. Microbiol. 2009, 63, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, D.M.; Grossman, A.R. Similarity of a Chromatic Adaptation Sensor to Phytochrome and Ethylene Receptors. Science 1996, 273, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Rujan, T.; Richly, E.; Hansen, A.; Cornelsen, S.; Lins, T.; Leister, D.; Stoebe, B.; Hasegawa, M.; Penny, D. Evolutionary Analysis of Arabidopsis, Cyanobacterial, and Chloroplast Genomes Reveals Plastid Phylogeny and Thousands of Cyanobacterial Genes in the Nucleus. Proc. Natl. Acad. Sci. USA 2002, 99, 12246–12251. [Google Scholar] [CrossRef] [PubMed]
- Mount, S.M.; Chang, C. Evidence for a Plastid Origin of Plant Ethylene Receptor Genes. Plant Physiol. 2002, 130, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic Gene Transfer: Organelle Genomes Forge Eukaryotic Chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Shiu, S.-H.; Armitage, J.P. Two-Component Systems and Their Co-Option for Eukaryotic Signal Transduction. Curr. Biol. 2011, 21, R320–R330. [Google Scholar] [CrossRef] [PubMed]
- Hérivaux, A.; Dugé de Bernonville, T.; Roux, C.; Clastre, M.; Courdavault, V.; Gastebois, A.; Bouchara, J.-P.; James, T.Y.; Latgé, J.-P.; Martin, F.; et al. The Identification of Phytohormone Receptor Homologs in Early Diverging Fungi Suggests a Role for Plant Sensing in Land Colonization by Fungi. mBio 2017, 8, e01739-16. [Google Scholar] [CrossRef] [PubMed]
- Rivarola, M.; McClellan, C.A.; Resnick, J.S.; Chang, C. ETR1-Specific Mutations Distinguish ETR1 from Other Arabidopsis Ethylene Receptors as Revealed by Genetic Interaction with RTE1. Plant Physiol. 2009, 150, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.I.; Esch, J.J.; Hall, A.E.; Binder, B.M.; Schaller, G.E.; Bleecker, A.B. A Copper Cofactor for the Ethylene Receptor ETR1 from Arabidopsis. Science 1999, 283, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Lacey, R.F.; Binder, B.M. Ethylene Regulates the Physiology of the Cyanobacterium Synechocystis Sp. PCC 6803 via an Ethylene Receptor. Plant Physiol. 2016, 171, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.J.; Lacey, R.F.; Binder Bickford, A.B.; Beshears, C.P.; Gilmartin, C.J.; Binder, B.M. Cyanobacteria Respond to Low Levels of Ethylene. Front. Plant Sci. 2019, 10, 950. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.E.; Bengochea, T.; Cairns, A.J.; Dodds, J.H.; Hall, M.A. Studies on Ethylene Binding by Cell-Free Preparations from Cotyledons of Phaseolus Vulgaris L.: Subcellular Localization. Plant Cell Environ. 1982, 5, 101–107. [Google Scholar] [CrossRef]
- Evans, D.E.; Dodds, J.H.; Lloyd, P.C.; Apgwynn, I.; Hall, M.A. A Study of the Subcellular Localisation of an Ethylene Binding Site in Developing Cotyledons of Phaseolus vulgaris L. by High Resolution Autoradiography. Planta 1982, 154, 48–52. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Randlett, M.D.; Findell, J.L.; Schaller, G.E. Localization of the Ethylene Receptor ETR1 to the Endoplasmic Reticulum of Arabidopsis. J. Biol. Chem. 2002, 277, 19861–19866. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Shakeel, S.N.; Bowers, J.; Zhao, X.-C.; Etheridge, N.; Schaller, G.E. Ligand-Induced Degradation of the Ethylene Receptor ETR2 through a Proteasome-Dependent Pathway in Arabidopsis. J. Biol. Chem. 2007, 282, 24752–24758. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Cui, M.-L.; Sun, H.-J.; Takada, K.; Mori, H.; Kamada, H.; Ezura, H. Subcellular Localization and Membrane Topology of the Melon Ethylene Receptor CmERS1. Plant Physiol. 2006, 141, 587–597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhong, S.; Lin, Z.; Grierson, D. Tomato Ethylene Receptor-CTR Interactions: Visualization of NEVER-RIPE Interactions with Multiple CTRs at the Endoplasmic Reticulum. J. Exp. Bot. 2008, 59, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-H.; Rivarola, M.; Resnick, J.S.; Maggin, B.D.; Chang, C. Subcellular Co-Localization of Arabidopsis RTE1 and ETR1 Supports a Regulatory Role for RTE1 in ETR1 Ethylene Signaling. Plant J. Cell Mol. Biol. 2008, 53, 275–286. [Google Scholar] [CrossRef]
- Grefen, C.; Städele, K.; Růzicka, K.; Obrdlik, P.; Harter, K.; Horák, J. Subcellular Localization and in Vivo Interactions of the Arabidopsis Thaliana Ethylene Receptor Family Members. Mol. Plant 2008, 1, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Kwok, S.F.; Bleecker, A.B.; Meyerowitz, E.M. Arabidopsis Ethylene-Response Gene ETR1: Similarity of Product to Two-Component Regulators. Science 1993, 262, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Chang, C.; Sun, Q.; Meyerowitz, E.M. Ethylene Insensitivity Conferred by Arabidopsis ERS Gene. Science 1995, 269, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Sakai, H.; Nourizadeh, S.; Chen, Q.G.; Bleecker, A.B.; Ecker, J.R.; Meyerowitz, E.M. EIN4 and ERS2 Are Members of the Putative Ethylene Receptor Gene Family in Arabidopsis. Plant Cell 1998, 10, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bleecker, A.B. Ethylene-Binding Sites Generated in Yeast Expressing the Arabidopsis ETR1 Gene. Science 1995, 270, 1809–1811. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.; Grierson, D. Ethylene Biosynthesis and Action in Tomato: A Model for Climacteric Fruit Ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Kays, S.J.; Paull, R.E. Postharvest Biology; Exon Press: Athens, Greece, 2004; ISBN 1888186542/9781888186543. [Google Scholar]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Xie, X.; Chen, K.; Grierson, D. Ethylene† and Fruit Softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Khajavi, M.Z.; Ahmadi, S.; Mortazavian, A.M.; Abdolshahi, A.; Rafiee, S.; Farhoodi, M. Novel Strategies to Control Ethylene in Fruit and Vegetables for Extending Their Shelf Life: A Review. Int. J. Environ. Sci. Technol. 2021, 19, 4599–4610. [Google Scholar] [CrossRef]
- Mope, C.; Adegoroye, A.; Oluwalade, T.A.; Adeyelu, A.A. Ethylene Management in Fresh Produce Transport. Asian J. Food Res. Nutr. 2024, 3, 60–71. [Google Scholar]
- Dong, T.; Zheng, T.; Fu, W.; Guan, L.; Jia, H.; Fang, J. The Effect of Ethylene on the Color Change and Resistance to Botrytis Cinerea Infection in ‘Kyoho’ Grape Fruits. Foods 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, L.; Yang, Y.; Guo, X.; Chen, G.; Xiong, X.; Dong, D.; Li, G. Transcriptome Analysis Reveals That Exogenous Ethylene Activates Immune and Defense Responses in a High Late Blight Resistant Potato Genotype. Sci. Rep. 2020, 10, 21294. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Sun, P.; Li, Z.-Y.; Zhang, F.-J.; Wang, X.-F.; You, C.-X.; Zhang, C.-L.; Zhang, Z. Plant Disease Resistance Outputs Regulated by AP2/ERF Transcription Factor Family. Stress Biol. 2024, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambantham, K.; Durairaj, S.; Saravanan, S.; Karikalan, K.; Muralidaran, S.; Islam, V.I.H. Role of Ethylene Response Transcription Factor (ERF) and Its Regulation in Response to Stress Encountered by Plants. Plant Mol. Biol. Report. 2015, 33, 347–357. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Etheridge, N.; Schaller, G.E. Ethylene Signal Transduction. Ann. Bot. 2005, 95, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, M.H.; Artés-Hernández, F.; Ávalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Ventura-Sobrevilla, J.M.; Martínez-Hernández, G.B. Current Scenario of Adsorbent Materials Used in Ethylene Scavenging Systems to Extend Fruit and Vegetable Postharvest Life. Food Bioprocess Technol. 2018, 11, 511–525. [Google Scholar] [CrossRef]
- Wu, B.; Guo, Q.; Wang, G.; Peng, X.; Wang, J.; Che, F. Effects of Different Postharvest Treatments on the Physiology and Quality of ‘Xiaobai’ Apricots at Room Temperature. J. Food Sci. Technol. 2015, 52, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Salinas, R.; Acosta-Motos, J.R.; Pérez-López, A.J.; Noguera-Artiaga, L.; Núñez-Delicado, E.; Burló, F.; López-Miranda, S. Effect of Combination of KMnO4 Oxidation and UV-C Radiation on Postharvest Quality of Refrigerated Pears Cv. “Ercolini”. Horticulturae 2022, 8, 1078. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; Acosta-Motos, J.R.; Núñez-Delicado, E.; Gabaldón, J.A.; López-Miranda, S. Combined Effect of Potassium Permanganate and Ultraviolet Light as Ethylene Scavengers on Post-Harvest Quality of Peach at Optimal and Stressful Temperatures. Agronomy 2022, 12, 616. [Google Scholar] [CrossRef]
- Zhou, H.-W.; Dong, L.i.; Ben-Arie, R.; Lurie, S. The Role of Ethylene in the Prevention of Chilling Injury in Nectarines. J. Plant Physiol. 2001, 158, 55–61. [Google Scholar] [CrossRef]
- Fan, X.; Xi, Y.; Zhao, H.; Liu, B.; Cao, J.; Jiang, W. Improving Fresh Apricot (Prunus armeniaca L.) Quality and Antioxidant Capacity by Storage at near Freezing Temperature. Sci. Hortic. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Hayama, H.; Shimada, T.; Fujii, H.; Ito, A.; Kashimura, Y. Ethylene-Regulation of Fruit Softening and Softening-Related Genes in Peach. J. Exp. Bot. 2006, 57, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Crisosto, C.H.; Garner, D.; Basinal, L.M. Effect of Continuous Exposure to Exogenous Ethylene during Cold Storage on Postharvest Decay Development and Quality Attributes of Stone Fruits and Table Grapes. Postharvest Biol. Technol. 2003, 27, 243–254. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Miranda-Molina, F.D.; Artés-Hernández, F. Postharvest Quality Retention of Apricots by Using a Novel Sepiolite–Loaded Potassium Permanganate Ethylene Scavenger. Postharvest Biol. Technol. 2020, 160, 111061. [Google Scholar] [CrossRef]
- Pech, J.-C.; Purgatto, E.; Bouzayen, M.; Latché, A. Ethylene and Fruit Ripening. In Annual Plant Reviews Volume 44: The Plant Hormone Ethylene; Wiley: Hoboken, NJ, USA, 2018; pp. 275–304. ISBN 9781119312994. [Google Scholar]
- Pott, D.; Vallarino, J.; Osorio, S. Metabolite Changes during Postharvest Storage: Effects on Fruit Quality Traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.; Serrano, M.; Valero, D.; Aguilar, G. Influence of Postharvest Technologies and Handling Practices on Phytochemicals in Fruits and Vegetables: Chemistry and Human Health. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 609–628. ISBN 9781119157946. [Google Scholar]
- Zhang, B.; Peng, B.; Zhang, C.; Song, Z.; Ma, R. Determination of Fruit Maturity and Its Prediction Model Based on the Pericarp Index of Absorbance Difference (IAD) for Peaches. PLoS ONE 2017, 12, e0177511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shao, X.; Wei, Y.; Xu, F.; Wang, H. At-Harvest Fruit Maturity Affects Sucrose Metabolism during Cold Storage and Is Related to Chilling Injury in Peach. J. Food Sci. Technol. 2020, 57, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Beckles, D.M. Factors Affecting the Postharvest Soluble Solids and Sugar Content of Tomato (Solanum lycopersicum L.) Fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Brecht, J.; Yahia, E. Postharvest Physiology. In The Mango, 2nd Edition: Botany, Production and Uses; CABI: Wallingford, UK, 2009; pp. 484–528. ISBN 978-1-84593-489-7. [Google Scholar]
- Brizzolara, S.; Manganaris, G.; Fotopoulos, V.; Watkins, C.; Tonutti, P. Primary Metabolism in Fresh Fruits During Storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- McBey, D.; Nadathur, S. Flavors, Taste Preferences, and the Consumer: Taste Modulation and Influencing Change in Dietary Patterns for a Sustainable Earth. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2024; pp. 629–647. ISBN 9780323916523. [Google Scholar]
- Hossain, M.A.; Karim, M.; Juthee, S. Postharvest Physiological and Biochemical Alterations in Fruits: A Review. Fundam. Appl. Agric. 2020, 5, 453–469. [Google Scholar] [CrossRef]
- Prasad, K.; Jacob, S.; Siddiqui, M. Fruit Maturity, Harvesting, and Quality Standards. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Academic Press: Cambridge, MA, USA, 2017; pp. 41–69. [Google Scholar]
- Singh, D.; Sharma, R.; Kesharwani, A. Postharvest Losses of Horticultural Produce. In Postharvest Handling and Diseases of Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–24. ISBN 9781003045502. [Google Scholar]
- Mohammed, M.; Khalid Alqahtani, N.; Munir, M. Artificial Ripening Technologies for Dates. In New Discoveries in the Ripening Processes; BoD–Books on Demand: Norderstedt, Germany, 2023; ISBN 978-0-85014-126-9. [Google Scholar]
- Madani, B.; Mirshekari, A.; Yahia, E.; Golding, J. Sapota (Manilkara achras Forb.): Factors Influencing Fresh and Processed Fruit Quality. Hortic. Rev. 2018, 45, 105–142. [Google Scholar]
- Arabia, A.; Munné-Bosch, S.; Muñoz, P. Ascorbic Acid as a Master Redox Regulator of Fruit Ripening. Postharvest Biol. Technol. 2024, 207, 112614. [Google Scholar] [CrossRef]
- Ludwig-Ohm, S.; Dirksmeyer, W.; Klockgether, K. Approaches to Reduce Food Losses in German Fruit and Vegetable Production. Sustainability 2019, 11, 6576. [Google Scholar] [CrossRef]
- Gallagher, M.S.; Mahajan, P.V. The stability and shelf life of fruit and vegetables. In Food and Beverage Stability and Shelf-Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; Chapter 22; pp. 641–656. [Google Scholar]
- Debebe, S. Post-Harvest Losses of Crops and Its Determinants in Ethiopia: Tobit Model Analysis. Agric. Food Secur. 2022, 11, 13. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Núñez-Delicado, E.; Acosta-Motos, J.R. Novel Combination of Ethylene Oxidisers to Delay Losses on Postharvest Quality, Volatile Compounds and Sensorial Analysis of Tomato Fruit. LWT 2022, 170, 114054. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene Scavengers for Active Packaging of Fresh Food Produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Zerbini, P. Role of Maturity for Improved Flavour. Fruit Veg. Flavour Recent Adv. Future Prospect. 2008, 180–199. [Google Scholar] [CrossRef]
- Cruz-Hernández, A.; Paredes-López, O. Fruit Quality: New Insights for Biotechnology. Crit. Rev. Food Sci. Nutr. 2012, 52, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Li, J.; Gong, H.; Fan, X.; Yang, Y.; Liu, X.; Yin, X. Transcriptome Co-Expression Network Analysis Identifies Key Genes and Regulators of Ripening Kiwifruit Ester Biosynthesis. BMC Plant Biol. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xu, Y.; Deng, Y.; Xie, Y.; Gao, Z.; Lang, Z.; Niu, Q. Key Transcription Factors Regulate Fruit Ripening and Metabolite Accumulation in Tomato. Plant Physiol. 2024, 195, 2256–2273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, F.; Chen, X.; Wu, W.; Wang, L.; Shi, J.; Huang, Y.; Shen, Y.; Wu, G.; Guo, J. Characterization of Key Aroma Compounds and Regulation Mechanism of Aroma Formation in Local Binzi (Malus pumila × Malus asiatica) Fruit. BMC Plant Biol. 2022, 22, 532. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Delrot, S.; Liang, Z. From Acidity to Sweetness: A Comprehensive Review of Carbon Accumulation in Grape Berries. Mol. Hortic. 2024, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Z.; Peydayesh, M.; Zhang, B.; Jia, X.; Huang, Q. Ethylene Control in Fruit Quality Assurance: A Material Science Perspective. Aggregate 2024, e565. [Google Scholar] [CrossRef]
- Datta, H.S. Physiological Approaches for Regulation of Fruit Ripening: A Review. IJCS 2019, 7, 4587–4597. [Google Scholar]
- Abbas, F.; Zhou, Y.; O’Neill Rothenberg, D.; Alam, I.; Ke, Y.; Wang, H.-C. Aroma Components in Horticultural Crops: Chemical Diversity and Usage of Metabolic Engineering for Industrial Applications. Plants 2023, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Liao, B.; Li, R.; Liu, Z. Changes in Taste and Volatile Compounds and Ethylene Production Determined the Eating Window of ‘Xuxiang’ and ‘Cuixiang’ Kiwifruit Cultivars. Postharvest Biol. Technol. 2022, 194, 112093. [Google Scholar] [CrossRef]
- Wendt, L.; Soldateli, F.; Both, V.; Thewes, F.; Ludwig, V.; Berghetti, M.; Ferrão, T.; Wagner, R.; Brackmann, A. Effects of Ethylene Control and Dynamic Controlled Atmosphere Storage on ‘Galaxy’ Apple Quality. J. Plant Growth Regul. 2022, 42, 701–718. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; González-Báidez, A.; Pérez-López, A.J.; Noguera-Artiaga, L.; Núñez-Delicado, E.; Carbonell-Barrachina, Á.; Acosta-Motos, J.R. Effect of Potassium Permanganate, Ultraviolet Radiation and Titanium Oxide as Ethylene Scavengers on Preservation of Postharvest Quality and Sensory Attributes of Broccoli Stored with Tomatoes. Foods 2023, 12, 2418. [Google Scholar] [CrossRef] [PubMed]
- Büchele, F.; Khera, K.; Thewes, F.; Kittemann, D.; Neuwald, D. Dynamic Control of Atmosphere and Temperature Based on Fruit CO2 Production: Practical Application in Apple Storage and Effects on Metabolism, Quality, and Volatile Profiles. Food Bioprocess Technol. 2023, 16, 2497–2510. [Google Scholar] [CrossRef]
- Zaitoon, A.; Luo, X.; Lim, L.-T. Triggered and Controlled Release of Active Gaseous/Volatile Compounds for Active Packaging Applications of Agri-Food Products: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 541–579. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Ayhan, Z. Combined Effects of Ethylene Scavenging-Active Packaging System and Modified Atmosphere to Reduce Postharvest Losses of Ethylene Sensitive Produce: Banana and Kiwifruit. Packag. Technol. Sci. 2023, 36, 951–967. [Google Scholar] [CrossRef]
- Pathak, N.; Caleb, O.J.; Geyer, M.; Herppich, W.B.; Rauh, C.; Mahajan, P. V Photocatalytic and Photochemical Oxidation of Ethylene: Potential for Storage of Fresh Produce—A Review. Food Bioprocess Technol. 2017, 10, 982–1001. [Google Scholar] [CrossRef]
- Pathak, N. Photocatalysis and Vacuum Ultraviolet Light Photolysis as Ethylene Removal Techniques for Potential Application in Fruit Storage. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2019. [Google Scholar]
- Janjarasskul, T.; Suppakul, P. Active and Intelligent Packaging: The Indication of Quality and Safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef] [PubMed]
- Baswal, A.K.; Ramezanian, A. 1-Methylcyclopropene Potentials in Maintaining the Postharvest Quality of Fruits, Vegetables, and Ornamentals: A Review. J. Food Process. Preserv. 2021, 45, 15129. [Google Scholar] [CrossRef]
- Watkins, C. 1-Methylcyclopropene (1-MCP) Based Technologies for Storage and Shelf Life Extension. Int. J. Postharvest Technol. Innov. 2006, 1, 62–68. [Google Scholar] [CrossRef]
- Tsantili, E.; Gapper, N.E.; Arquiza, J.M.R.A.; Whitaker, B.D.; Watkins, C.B. Ethylene and α-Farnesene Metabolism in Green and Red Skin of Three Apple Cultivars in Response to 1-Methylcyclopropene (1-MCP) Treatment. J. Agric. Food Chem. 2007, 55, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Yarılgaç, T.; Kadim, H.; Ozturk, B. Role of Maturity Stages and Modified-Atmosphere Packaging on the Quality Attributes of Cornelian Cherry Fruits (Cornus mas L.) throughout Shelf Life. J. Sci. Food Agric. 2019, 99, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Awalgaonkar, G.S. Development of active packaging trays with ethylene removing capacity. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2018. [Google Scholar]
- Guillén, F.; Castillo, S.; Zapata, P.J.; Martínez-Romero, D.; Serrano, M.; Valero, D. Efficacy of 1-MCP Treatment in Tomato Fruit. 1. Duration and Concentration of 1-MCP Treatment to Gain an Effective Delay of Postharvest Ripening. Postharvest Biol. Technol. 2007, 43, 23–27. [Google Scholar] [CrossRef]
- Charoenchongsuk, N.; Matsumoto, D.; Itai, A.; Murayama, H. Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae 2018, 4, 22. [Google Scholar] [CrossRef]
- Taye, A.M.; Tilahun, S.; Seo, M.H.; Park, D.S.; Jeong, C.S. Effects of 1-MCP on Quality and Storability of Cherry Tomato (Solanum lycopersicum L.). Horticulturae 2019, 5, 29. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Xu, J.; Liu, T.; Dong, S. Effectiveness of Lysozyme Coatings and 1-MCP Treatments on Storage and Preservation of Kiwifruit. Food Chem. 2019, 288, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pongprasert, N.; Srilaong, V.; Kaewsukseang, S. 1-MCP Micro-Bubbles Delaying Postharvest Ripening of “Khai” Banana. In Proceedings of the III Asia Pacific Symposium on Postharvest Research, Education and Extension: APS2014, Hochiminh City, Vietnam, 5 October 2018; Volume 1213, pp. 245–250. [Google Scholar]
- Manenoi, A.; Bayogan, E.R.V.; Thumdee, S.; Paull, R.E. Utility of 1-Methylcyclopropene as a Papaya Postharvest Treatment. Postharvest Biol. Technol. 2007, 44, 55–62. [Google Scholar] [CrossRef]
- Wang, J.; Allan, A.C.; Wang, W.Q.; Yin, X.R. The Effects of Salicylic Acid on Quality Control of Horticultural Commodities. New Zealand J. Crop Hortic. Sci. 2022, 50, 99–117. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Serrano, M.; Valero, D. Effect of Salicylic Acid Treatment on Reducing Chilling Injury in Stored Pomegranates. Postharvest Biol. Technol. 2009, 53, 152–154. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of Pre- and Postharvest Salicylic Acid Treatment on Ethylene Production, Fungal Decay and Overall Quality of Selva Strawberry Fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of Salicylic Acid on Post-Harvest Physiology of Horticultural Crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Leslie, C.A.; Romani, R.J. Inhibition of Ethylene Biosynthesis by Salicylic Acid. Plant Physiol. 1988, 88, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.K.; Dwivedi, U.N. Delayed Ripening of Banana Fruit by Salicylic Acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Elmenofy, H.M.; Okba, S.K.; Salama, A.M.; Alam-Eldein, S.M. Yield, Fruit Quality, and Storability of “Canino” Apricot in Response to Aminoethoxyvinylglycine, Salicylic Acid, and Chitosan. Plants 2021, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Bi, Y.; Li, Y.; Wang, Y.; Prusky, D.; Alkan, N. Preharvest Elicitors Spray Improves Antioxidant Activity, Alleviates Chilling Injury, and Maintains Quality in Harvested Fruit. Horticulturae 2022, 8, 1208. [Google Scholar] [CrossRef]
- Johnson, D.S.; Colgan, R. Low Ethylene Controlled Atmosphere Induces Adverse Effects on the Quality of ‘Cox’s Orange Pippin’ Apples Treated with Aminoethoxyvinylglycine during Fruit Development. Postharvest Biol. Technol. 2003, 27, 59–68. [Google Scholar] [CrossRef]
- Balaguera-López, H.; Salamanca-Gutiérrez, F.; García, J.; Herrera, A. Ethylene and Maturation Retardants in the Postharvest of Perishable Horticultural Products. A Review. Rev. Colomb. Cienc. Hortícolas 2014, 8, 302–313. [Google Scholar] [CrossRef]
- Capitani, G.; Tschopp, M.; Eliot, A.C.; Kirsch, J.F.; Grütter, M.G. Structure of ACC Synthase Inactivated by the Mechanism-Based Inhibitor l-Vinylglycine. FEBS Lett. 2005, 579, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Saltveit, M.E. Aminoethoxyvinylglycine (AVG) Reduces Ethylene and Protein Biosynthesis in Excised Discs of Mature-Green Tomato Pericarp Tissue. Postharvest Biol. Technol. 2005, 35, 183–190. [Google Scholar] [CrossRef]
- Ozturk, B.; Kucuker, E.; Yıldız, K.; Celik, S.M. Changes of Bioactive Compounds and Ethylene Production of Japanese Plums Treated with Pre-Harvest Aminoethoxyvinylglycine. Int. J. Food Prop. 2015, 18, 2165–2186. [Google Scholar] [CrossRef]
- Ozturk, B.; Uzun, S.; Karakaya, O. Combined Effects of Aminoethoxyvinylglycine and MAP on the Fruit Quality of Kiwifruit during Cold Storage and Shelf Life. Sci. Hortic. 2019, 251, 209–214. [Google Scholar] [CrossRef]
- Yuan, R.; Carbaugh, D.H. Effects of NAA, AVG, and 1-MCP on Ethylene Biosynthesis, Preharvest Fruit Drop, Fruit Maturity, and Quality of ‘Golden Supreme’ and ‘Golden Delicious’ Apples. HortScience Horts 2007, 42, 101–105. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Rubio, P.; Infante, R.; Campos-Vargas, R.; Manríquez, D.; González-Agüero, M.; Defilippi, B.G. Ethylene Biosynthesis in Apricot: Identification of a Ripening-Related 1-Aminocyclopropane-1-Carboxylic Acid Synthase (ACS) Gene. Postharvest Biol. Technol. 2012, 63, 85–90. [Google Scholar] [CrossRef]
- Xie, X.; Einhorn, T.; Wang, Y. Inhibition of Ethylene Biosynthesis and Associated Gene Expression by Aminoethoxyvinylglycine and 1-Methylcyclopropene and Their Consequences on Eating Quality and Internal Browning of ‘Starkrimson’ Pears. J. Am. Soc. Hortic. Sci. J. Amer. Soc. Hort. Sci. 2015, 140, 587–596. [Google Scholar] [CrossRef]
- Kim, Y.T.; Ha, S.T.T.; Chun, I.; In, B.C. Inhibition of Ethylene Binding and Biosynthesis Maintains Fruit Quality of “Formosa” Plums during Postharvest Storage. Hortic. Sci. Technol. 2021, 39, 368–378. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Lwin, H.P.; Lee, E.J.; Kang, I.K.; Lee, J. Effects of 1-Methylcyclopropene and Aminoethoxyvinylglycine Treatments on Fruit Quality and Antioxidant Metabolites in Cold-Stored “Sangjudungsi” Persimmons. Hortic. Environ. Biotechnol. 2021, 62, 891–905. [Google Scholar] [CrossRef]
- Salazar, N.A.; Molina-Corral, F.J.; Aguilar, G.; Otero, A.; Sepulveda, D.R.; Olivas, G. Volatile Production by ‘Golden Delicious’ Apples Is Affected by Preharvest Application of Aminoethoxyvinylglycine. Sci. Hortic. 2011, 130, 436–444. [Google Scholar] [CrossRef]
- Romani, R.; Labavitch, J.; Yamashita, T.; Hess, B.; Rae, H. Preharvest AVG Treatment of ‘Bartlett’ Pear Fruits: Effects on Ripening, Color Change, and Volatiles. J. Am. Soc. Hortic. Sci. 1983, 108, 1046–1049. [Google Scholar] [CrossRef]
- López, P.; Neisa, D.P.; Bacca, C.; Flórez, V.J. Evaluación de Preservantes Florales En La Poscosecha de Tres Variedades de Clavel Estándar. Agron. Colomb. 2008, 26, 116–126. [Google Scholar]
- Martínez-Romero, D.; Bailén, G.; Serrano, M.; Guillén, F.; Valverde, J.M.; Zapata, P.; Castillo, S.; Valero, D. Tools to Maintain Postharvest Fruit and Vegetable Quality through the Inhibition of Ethylene Action: A Review. Crit. Rev. Food Sci. Nutr. 2007, 47, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Stabyl, G.; Basel, R.; Reid, M.; Dodge, L. Efficacies of Commercial Anti Ethylene Products for Fresh Cut Flowers. HortTechnology 1993, 3, 199–202. [Google Scholar] [CrossRef]
- Bulantseva, E.A.; Nguyen, T.T.; Ruzhitsky, A.O.; Protsenko, M.A.; Korableva, N.P. The Effect of Ethylene Biosynthesis Regulators on Metabolic Processes in the Banana Fruits in Various Physiological States. Appl. Biochem. Microbiol. 2009, 45, 93–96. [Google Scholar] [CrossRef]
- Lima, P.C.C.; Santos, M.N.D.; Guimaraes, M.E.D.; de Araujo, N.O.; Krause, M.R.; Finger, F.L. Ethylene and Its Inhibitors Affect the Quality of Processed Sweet Potatoes. Food Sci. Technol. 2021, 41, 825–832. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Timofeeva, G.V.; Andreev, I.M.; Golivanov, Y.Y.; Bogoutdinova, L.R.; Baranova, E.N.; Khaliluev, M.R. Aminooxyacetic Acid (AOA), Inhibitor of 1-Aminocyclopropane-1-Carboxilic Acid (ACC) Synthesis, Suppresses Self-Incompatibility-Induced Programmed Cell Death in Self-Incompatible Petunia hybrida L. Pollen Tubes. Protoplasma 2020, 257, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Instituto Latinoamericano de la Comunicación Educativa ¿Que Es Una Zeolita? Available online: http://bibliotecadigital.ilce.edu.mx/sites/ciencia/volumen1/ciencia2/55/htm/sec_3.html (accessed on 24 May 2024).
- Patdhanagul, N.; Rangsriwatananon, K.; Siriwong, K.; Hengrasmee, S. Combined Modification of Zeolite NaY by Phenyl Trimethyl Ammonium Bromide and Potassium for Ethylene Gas Adsorption. Microporous Mesoporous Mater. 2012, 153, 30–34. [Google Scholar] [CrossRef]
- Coloma, A.; Rodríguez, F.; Bruna, J.; Guarda, A.; Galotto, M. Development of an Active Film with Natural Zeolite as Ethylene Scavenger. J. Chil. Chem. Soc. 2014, 59, 2409–2414. [Google Scholar] [CrossRef]
- Erdoğan, B.; Sakızcı, M.; Yörükoğulları, E. Characterization and Ethylene Adsorption of Natural and Modified Clinoptilolites. Appl. Surf. Sci. 2008, 254, 2450–2457. [Google Scholar] [CrossRef]
- Patdhanagul, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Ethylene Adsorption on Cationic Surfactant Modified Zeolite NaY. Microporous Mesoporous Mater. 2010, 131, 97–102. [Google Scholar] [CrossRef]
- de Bruijn, J.; Gomez, A.; Loyola, C.; Melin, P.; Solar, V.; Abreu, N.; Azzolina-Jury, F.; Valdes, H. Use of a Copper- and Zinc-Modified Natural Zeolite to Improve Ethylene Removal and Postharvest Quality of Tomato Fruit. Crystals 2020, 10, 471. [Google Scholar] [CrossRef]
- Mariah, M.A.; Vonnie, J.M.; Erna, K.H.; Nur’Aqilah, N.M.; Huda, N.; Abdul Wahab, R.; Rovina, K. The Emergence and Impact of Ethylene Scavengers Techniques in Delaying the Ripening of Fruits and Vegetables. Membranes 2022, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Carbotecnia ¿Qué Es El Carbón Activado y Para Qué Sirve? Available online: https://www.carbotecnia.info/aprendizaje/carbon-activado/que-es-carbon-activado/ (accessed on 15 February 2024).
- Bailén, G.; Guillén, F.; Castillo, S.; Zapata, P.J.; Serrano, M.; Valero, D.; Martínez-Romero, D. Use of a Palladium Catalyst to Improve the Capacity of Activated Carbon to Absorb Ethylene, and Its Effect on Tomato Ripening. Span. J. Agric. Res. 2007, 5, 579–586. [Google Scholar] [CrossRef]
- Jaimun, R.; Sangsuwan, J. Efficacy of Chitosan-Coated Paper Incorporated with Vanillin and Ethylene Adsorbents on the Control of Anthracnose and the Quality of Nam Dok Mai Mango Fruit. Packag. Technol. Sci. 2019, 32, 383–394. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential Applications of Metal-Organic Frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Dhumal, S.; Abeli, P.; Beaudry, R.; Almenar, E. Metal-Organic Frameworks Have Utility in Adsorption and Release of Ethylene and 1-Methylcyclopropene in Fresh Produce Packaging. Postharvest Biol. Technol. 2017, 130, 48–55. [Google Scholar] [CrossRef]
- Shorter, A.J.; Scott, K.J.; Ward, G.; Best, D.J. Effect of Ethylene Absorption on the Storage of Granny Smith Apples Held in Polyethylene Bags. Postharvest Biol. Technol. 1992, 1, 189–194. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Rodríguez-Hernández, A.M.; Castillo-Campohermoso, M.A.; Artés-Hernández, F. An Innovative Ethylene Scrubber Made of Potassium Permanganate Loaded on a Protonated Montmorillonite: A Case Study on Blueberries. Food Bioprocess Technol. 2019, 12, 524–538. [Google Scholar] [CrossRef]
- Mujtaba, A.; Masud, T.; Butt, S.J.; Qazalbash, M.; Fareed, W.; Shahid, A. Potential Role of Calcium Chloride, Potassium Permanganate and Boric Acid on Quality Maintenance of Tomato Cv. Rio Grandi at Ambient Temperature. Int. J. Biosci. 2014, 5, 9–20. [Google Scholar]
- Scott, K.J.; Wills, R.B.H. Reduction of Brown Heart in Pears by Absorption of Ethylene from the Storage Atmosphere. Aust. J. Exp. Agric. 1974, 14, 266–268. [Google Scholar] [CrossRef]
- Illeperuma, C.K.; Nikapitiya, C. Extension of the Postharvest Life of `Pollock’ Avocado Using Modified Atmosphere Packaging. Fruits 2002, 57, 287–295. [Google Scholar] [CrossRef]
- García, J.; Herrera, A.; García, J. Conservación Del Fruto de Banano Bocadillo (Musa AA Simmonds) Con La Aplicación de Permanganato de Potasio (KMnO4). Rev. Colomb. De Cienc. Hortícolas 2012, 6, 161–171. [Google Scholar] [CrossRef]
- Çelik, S.; Bal, E. The Effects of Postharvest Treatments of Salicylic Acid and Potassium Permanganate on the Storage of Kiwifruit. Bulg. J. Agric. Sci. 2010, 16, 576–584. [Google Scholar]
- Giraldo, E.; Szczerbanik, M.J.; Scottpez, K.J.; Paton, J.E.; Best, D.J. Effects of Polyethylene Bags, Ethylene Absorbent and 1-Methylcyclopropene on the Storage of Japanese Pears. J. Hortic. Sci. Biotechnol. 2005, 80, 162–166. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Jeong, G.H.; Kim, S.-W. Ethylene Gas Decomposition Using ZSM-5/WO3-Pt-Nanorod Composites for Fruit Freshness. ACS Sustain. Chem. Eng. 2019, 7, 11250–11257. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active Packaging from Chitosan-Titanium Dioxide Nanocomposite Film for Prolonging Storage Life of Tomato Fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C Irradiation Inhibits the Production of Ethylene and the Activity of Cell Wall-Degrading Enzymes during Softening of Tomato (Lycopersicon esculentum L.) Fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Mabusela, B.P.; Belay, Z.A.; Godongwana, B.; Pathak, N.; Mahajan, P.V.; Caleb, O.J. Advances in Vacuum Ultraviolet Photolysis in the Postharvest Management of Fruit and Vegetables Along the Value Chains: A Review. Food Bioprocess Technol. 2022, 15, 28–46. [Google Scholar] [CrossRef]
- Collazo Cordero, C.; Charles, F.; Aguiló-Aguayo, I.; Marín-Sáez, J.; Lafarga, T.; Abadias, M.; Vinas, I. Decontamination of Listeria Innocua from Fresh-Cut Broccoli Using UV-C Applied in Water or Peroxyacetic Acid, and Dry-Pulsed Light. Innov. Food Sci. Emerg. Technol. 2019, 52, 438–449. [Google Scholar] [CrossRef]
- Orhewere, A.; Ajayi, O.; Ajayi, A. Advances in the Development of a Tomato Postharvest Storage System: Towards Eradicating Postharvest Losses. J. Phys. Conf. Ser. 2019, 1378, 22064. [Google Scholar] [CrossRef]
- Pathak, N.; Caleb, O.J.; Rauh, C.; Mahajan, P. V Efficacy of Photocatalysis and Photolysis Systems for the Removal of Ethylene under Different Storage Conditions. Postharvest Biol. Technol. 2019, 147, 68–77. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Alves, M.J.D.; Nobias, M.C.; Soares, L.S.; Coelho, D.S.; Maraschin, M.; Basso, A.; Moreira, R.; Jose, H.J.; Monteiro, A.R. Physiological Changes in Green and Red Cherry Tomatoes after Photocatalytic Ethylene Degradation Using Continuous Air Flux. Food Sci. Technol. Int. 2023, 29, 3–12. [Google Scholar] [CrossRef]
- Li, W.H.; Liu, Z.Y.; Li, X.J.; Li, X.H. Quality Maintenance of 1-Methylcyclopropene Combined with Titanium Dioxide Photocatalytic Reaction on Postharvest Cherry Tomatoes. J. Food Process. Preserv. 2022, 46, e16500. [Google Scholar] [CrossRef]
- Fonseca, J.D.; Pabon, N.Y.L.; Nandi, L.G.; Valencia, G.A.; Moreira, R.; Monteiro, A.R. Gelatin-TiO2-Coated Expanded Polyethylene Foam Nets as Ethylene Scavengers for Fruit Postharvest Application. Postharvest Biol. Technol. 2021, 180, 111602. [Google Scholar] [CrossRef]
- Ghosh, A.; Saha, I.; Fujita, M.; Debnath, S.C.; Hazra, A.K.; Adak, M.K.; Hasanuzzaman, M. Photoactivated TiO2 Nanocomposite Delays the Postharvest Ripening Phenomenon through Ethylene Metabolism and Related Physiological Changes in Capsicum Fruit. Plants 2022, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- de Chiara, M.L.V.; Pal, S.; Licciulli, A.; Amodio, M.L.; Colelli, G. Photocatalytic Degradation of Ethylene on Mesoporous TiO2/SiO2 Nanocomposites: Effects on the Ripening of Mature Green Tomatoes. Biosyst. Eng. 2015, 132, 61–70. [Google Scholar] [CrossRef]
- Chawengkijwanich, C.; Hayata, Y. Efficiency of TiO2 Photocatalytic Reaction on Delay of Fruit Ripening and Removal of Off-Flavors from the Fruit Storage Atmosphere. Trans. ASABE 2006, 49, 833–837. [Google Scholar] [CrossRef]
- Fonseca, J.D.; Alves, M.J.D.; Soares, L.S.; Moreira, R.; Valencia, G.A.; Monteiro, A.R. A Review on TiO2-Based Photocatalytic Systems Applied in Fruit Postharvest: Set-Ups and Perspectives. Food Res. Int. 2021, 144, 110378. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Wang, B.; Jin, W.F. Experimental Investigation on Decomposition of Ethylene by Ozone: Harmful Product, Food Safety, and Control Strategy. J. Food Process. Preserv. 2021, 45, e15861. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Ji, L.L.; Chen, C.K.; Dong, C.H.; Wang, C.R. Effects of Ozone Treatment on the Storage Quality of Post-Harvest Tomato. Int. J. Food Eng. 2018, 14, 20180012. [Google Scholar] [CrossRef]
- Toti, M.; Carboni, C.; Botondi, R. Postharvest Gaseous Ozone Treatment Enhances Quality Parameters and Delays Softening in Cantaloupe Melon during Storage at 6 °C. J. Sci. Food Agric. 2018, 98, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.C.; Lin, Q.; Dong, C.H.; Ji, H.P.; Yu, J.Z.; Chen, C.K.; Zhu, Z.Q.; Ban, Z.J.; Zhang, N.; Bao, Y.Y. Effects of Ozone Concentration on the Postharvest Quality and Microbial Diversity of Muscat Hamburg Grapes. RSC Adv. 2020, 10, 9037–9045. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.S.; Huang, Z.C.; Qu, K.; Shi, W.B.; Peng, Z.M.; Zeng, L.M.; Xie, S.D.; Zhang, Y.H. Impacts of Synoptic Circulation on Surface Ozone Pollution in a Coastal Eco-City in Southeastern China during 2014–2019. J. Environ. Sci. 2023, 127, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Mok, Y.S.; Kim, S.G.; Han, J.; Nguyen, D.B.; Lee, H.W.; Jeon, H.; Kim, S.B. Removal of Dilute Ethylene Using Repetitive Cycles of Adsorption and Plasma-Catalytic Oxidation over Pd/ZSM-5 Catalyst. J. Phys. D Appl. Phys. 2020, 53, 334002. [Google Scholar] [CrossRef]
- Tzeng, J.H.; Weng, C.H.; Huang, J.W.; Shiesh, C.C.; Lin, Y.H.; Lin, Y.T. Application of Palladium-Modified Zeolite for Prolonging Post-Harvest Shelf Life of Banana. J. Sci. Food Agric. 2019, 99, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Yadav, S.K. Recent Advances in Cold Plasma Technology for Food Processing. Food Eng. Rev. 2022, 14, 555–578. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold Plasma Technology: Advanced and Sustainable Approach for Wastewater Treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kardam, S.K.; Kadam, A.A.; Singh, S.P.; Kumar, V.; Gaikwad, K.K. Ethylene Gas Scavenging Properties of Nano-Silica Isolated from Rice Straw and of Its PVA-Based Formulation Coated on Duplex Paperboard Made from Softwood and Rice Straw. Biomass Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Gundewadi, G.; Rudra, S.G.; Prasanna, R.; Banerjee, T.; Singh, S.K.; Dhakate, S.R.; Gupta, A.; Anand, A. Palladium Encapsulated Nanofibres for Scavenging Ethylene from Sapota Fruits. Front. Nutr. 2022, 9, 994813. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, D.; Gracia-Vallés, N.; Clavero, E.; Silva, F.; Nerín, C. Mechanochemically Scaled-Up Alpha Cyclodextrin Nanosponges: Their Safety and Effectiveness as Ethylene Scavenger. Nanomaterials 2022, 12, 2900. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Kathuria, A.; Gaikwad, K.K. Metal–Organic Frameworks for Active Food Packaging. A Review. Environ. Chem. Lett. 2022, 20, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Büchele, F.; Hivare, K.; Khera, K.; Thewes, F.R.; Argenta, L.C.; Hoffmann, T.G.; Mahajan, P.V.; Prange, R.K.; Pareek, S.; Neuwald, D.A. Novel Energy-Saving Strategies in Apple Storage: A Review. Sustainability 2024, 16, 1052. [Google Scholar] [CrossRef]
- Abedrabboh, O.; Koç, M.; Biçer, Y. Sustainability Performance of Space-Cooling Technologies and Approaches. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 9017–9042. [Google Scholar] [CrossRef]
- Yenare, R.; Sonawane, C.; Sur, A.; Singh, B.; Panchal, H.; Kumar, A.; Sadasivuni, K.K.; Siddiqui, M.; Bhalerao, Y. A Comprehensive Review of Portable Cold Storage: Technologies, Applications, and Future Trends. Alex. Eng. J. 2024, 94, 23–33. [Google Scholar] [CrossRef]
- Marimuthu, S.; Saikumar, A.; Badwaik, L. Food Losses and Wastage within Food Supply Chain: A Critical Review of Its Generation, Impact, and Conversion Techniques. Waste Dispos. Sustain. Energy 2024, 1–16. [Google Scholar] [CrossRef]
- Onyeaka, H.; Ghosh, S.; Obileke, K.; Miri, T.; Odeyemi, O.; Nwaiwu, O.; Tamasiga, P. Preventing Chemical Contaminants in Food: Challenges and Prospects for Safe and Sustainable Food Production. Food Control 2023, 155, 110040. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, G.; Song, J.; Dai, J.; Shi, W.; Wang, L. Food Safety Practices of Food Handlers in China and Their Correlation with Self-Reported Foodborne Illness. J. Food Prot. 2024, 87, 100202. [Google Scholar] [CrossRef] [PubMed]
- Usman, A. Influence of Agricultural Practices on Food Safety in Nigeria. Int. J. Food Sci. 2024, 7, 43–54. [Google Scholar] [CrossRef]
- Kumar, N.; Devgan, K.; Kumar, A.; Kaur, P.; Mahajan, P. Active and Passive Modified Atmosphere Packaging. In Nonthermal Food Engineering Operations; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 225–259. ISBN 9781119776468. [Google Scholar]
- Nian, L.; Wang, M.; Sun, X.; Zeng, Y.; Xie, Y.; Cheng, S.; Cao, C. Biodegradable Active Packaging: Components, Preparation, and Applications in the Preservation of Postharvest Perishable Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2024, 64, 2304–2339. [Google Scholar] [CrossRef] [PubMed]
- Ozal, G.; Ilyasova, C.; Ilgiz, V. Post-Harvest Storage and Processing Technology in Russia: Reducing Yield Loss. Techno Agric. Stud. Res. 2024, 1, 28–40. [Google Scholar]
- Mavai, S.; Bains, A.; Sridhar, K.; Rashid, S.; Elossaily, G.M.; Ali, N.; Chawla, P.; Sharma, M. Formulation and Application of Poly Lactic Acid, Gum, and Cellulose-Based Ternary Bioplastic for Smart Food Packaging: A Review. Int. J. Biol. Macromol. 2024, 268, 131687. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Prasad, A.; Sonika; Katiyar, V. State of Art Review on Sustainable Biodegradable Polymers with a Market Overview for Sustainability Packaging. Mater. Today Sustain. 2024, 26, 100776. [Google Scholar] [CrossRef]
- Singh, A.; Itkor, P.; Lee, M.; Shin, J.; Lee, Y.S. Promoting Sustainable Packaging Applications in the Circular Economy by Exploring and Advancing Molded Pulp Materials for Food Products: A Review. Crit. Rev. Food Sci. Nutr. 2022, 63, 11010–11025. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Abdelkhalek, A. Packaging of the Future: Smart Technologies and Food Quality and Safety. In Intelligent Packaging; Academic Press: Cambridge, MA, USA, 2024; pp. 1–30. ISBN 9780443153884. [Google Scholar]
- Arijeniwa, F.; Akinsemolu, A.; Chukwugozie, D.; Onawo, G.; Ochulor, C.; Uju, N.; Kawino, D.; Onyeaka, H. Closing the Loop: A Framework for Tackling Single-Use Plastic Waste in the Food and Beverage Industry through Circular Economy—A Review. J. Environ. Manag. 2024, 359, 120816. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Ting, H.; Atanda, A. Enhancing Supply Chain Traceability through Blockchain and IoT Integration: A Comprehensive Review. Green Intell. Syst. Appl. 2024, 4, 11–28. [Google Scholar] [CrossRef]
- Ninama, N.; Gangal, L.; Khayum, A.; Harshitha, S.B.; Divya, C.; Sowmya, H.M.; Singh, A. Anushi Post-Harvest Biotechnology or Genetic Engineering Solutions: Extending Shelf Life and Reducing Food Waste. J. Adv. Biol. Biotechnol. 2024, 27, 1–26. [Google Scholar] [CrossRef]
- Shi, C.; Xiang, L.; Jiahu, G. Exploring the Frontier of Fruit Diseases Management: Advances in Nano-Based and Biocontrol Strategies and Underlying Action Mechanism. S. Afr. J. Bot. 2024, 166, 612–623. [Google Scholar] [CrossRef]
- Doerflinger, F.; Al Shoffe, Y.; Sutano, G.; Nock, J.F.; Watkins, C. Preharvest 1-Methylcyclopropene (1-MCP) Treatment Effects on Quality of Spot and Strip Picked ‘Gala’ Apples at Harvest and after Storage as Affected by Postharvest 1-MCP and Temperature Conditioning Treatments. Sci. Hortic. 2023, 325, 112682. [Google Scholar] [CrossRef]
- Zhu, M.; Li, J.; Liu, Y.; Wang, Q.; Fan, Z.; Zeng, J.; Yu, J. Preharvest Nano-Calcium Reduces the Table Grape Berry Abscission by Regulating Ethylene Production During Storage. J. Plant Growth Regul. 2023, 43, 1400–1409. [Google Scholar] [CrossRef]
- Opara, U.; Pathare, P. Mechanical Damage of Fresh Produce: An Overview. In Mechanical Damage in Fresh Horticultural Produce: Measurement, Analysis and Control; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–19. ISBN 978-981-99-7095-7. [Google Scholar]
- Baloyi, R.G.; Mafeo, T.P.; Mathaba, N. Effect of Pre- and Post-Harvest Factors on ‘Benny’ Valencia Fruit Rind Phenolics on Mitigation of Chilling and Non-Chilling Disorders during Cold Storage. J. Hortic. Postharvest Res. 2023, 6, 299–316. [Google Scholar] [CrossRef]
- Rattanakaran, J.; Saengrayap, R.; Prahsarn, C.; Kitazawa, H.; Chaiwong, S. Application of Room Cooling and Thermal Insulation Materials to Maintain Quality of Okra during Storage and Transportation. Horticulturae 2021, 7, 188. [Google Scholar] [CrossRef]
- Waldhans, C.; Albrecht, A.; Ibald, R.; Wollenweber, D.; Sy, S.-J.; Kreyenschmidt, J. Temperature Control and Data Exchange in Food Supply Chains: Current Situation and the Applicability of a Digitalized System of Time–Temperature-Indicators to Optimize Temperature Monitoring in Different Cold Chains. J. Packag. Technol. Res. 2024, 8, 79–93. [Google Scholar] [CrossRef]
- Bisht, A.; Singh, S. Postharvest Losses and Management of Horticultural Produce: A Review. J. Sci. Res. Rep. 2024, 30, 305–320. [Google Scholar] [CrossRef]
- Gupta, N.; Daruwalla, F. Examining the Effectiveness of Green Marketing Communication on Consumer Behavior towards Sustainable Purchases. Educ. Adm. Theory Pract. 2024, 30, 6861–6868. [Google Scholar] [CrossRef]
- Uda, S.; Basrowi, B. Environmental Education Using SARITHA-Apps to Enhance Environmentally Friendly Supply Chain Efficiency and Foster Environmental Knowledge towards Sustainability. Uncertain Supply Chain Manag. 2024, 12, 359–372. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).