1. Introduction
Fresh fruits of horticultural crops are rich in soluble sugars, mainly glucose, fructose and sucrose, which determine their flavor and quality. The soluble sugars that accumulate in fruits are mainly produced through photosynthesis of source tissues (mainly adult leaf or stem), and sucrose acts as the dominant carbohydrate form for long-distance transportation in the vascular phloem [
1]. After it reaches sink tissues (root, young leaf, flower, seed, fruit and so on), sucrose can be degraded into hexoses (glucose and fructose), re-synthesized or transported into the vacuole for storage [
2]. Sucrose synthase and invertase are two important types of enzymes responsible for soluble sugar metabolism for plant growth and development [
3]. Invertase, also known as sucrase or β-fructofuranosidase (EC3.2.1.26), can cleave sucrose irreversibly into glucose and fructose through primary carbon metabolism [
4]. Sucrose catabolism, which is mediated by invertases, provides the carbon skeleton, energy sources or signaling molecules for physiological activities and plays multiple roles in plant growth, development and stress response [
4,
5].
According to the pH optima, various invertase isoenzymes can be divided into two types: the acid invertases (AINVs) and the alkaline/neutral invertases (A/NINVs) [
5]. AINVs with an optimum pH of 3.5–5.5 can hydrolyze sucrose, raffinose, stachyose and 1-kestose and belong to the glycoside hydrolase 32 (GH32) family [
5,
6]. Based on the subcellular localization mode, AINVs are classified into cell wall invertases (CWINs) and vacuolar invertases (VINs), which are located in the extracellular space and bind tightly to the cell wall and vacuole, respectively [
4,
5]. A/NINVs, also called cytoplasmic invertases, specifically hydrolyze sucrose, have an optimum pH of 7.0–7.8 [
4,
5,
7]. A/NINVs belong to the GH100 family and show no sequence homology and structure similarity to AINVs [
5].
In plant cells, vacuoles act as important organelles for maintaining turgor pressure, controlling metabolites storage and so on, playing vital roles in growth and development [
8]. Over the past years, multiple functions of
VINs in plant growth, development and stress response have been reported. For example, Arabidopsis (
Arabidopsis thaliana)
VINs regulate root and hypocotyl elongation [
9,
10]; rice (
Oryza sativa L.)
OsINV2 and
OsINV3 affect important traits concerning grain size and weight [
11,
12,
13]; cotton (
Gossypium hirsutum)
GhVIN1 controls flower development, male and female fertilities and fiber cell elongation [
14,
15]; and sorghum (
Sorghum bicolor)
SbSAI-2 affects the sugar compositions and contents in adult stems [
16,
17]. Moreover, under abiotic or biotic stresses, tea (
Camellia sinensis L.)
CsINV5 and cucumber (
Cucumis sativus L.)
CsVI1 and
CsVI2 were induced by cold acclimation or drought [
18,
19,
20], sweet potato [
Ipomoea batatas (L.) Lam.]
IbVIN was induced in response to
Ceratocystis fimbriata Ellis and Halsted infection [
21], and therefore, the VIN activity positively regulated stress resistance by altering sugar metabolism.
The development and ripening of fresh fruit, as an important sink organ of horticultural crops, was also regulated by
VINs. For instance, loquat [
Eriobotrya japonica (Thunb.) Lindl.]
EjVIN was induced by hexose treatments, and its overexpression in tobacco plants affected growth and sugar metabolism [
6]; antisense inhibition of the muskmelon (
Cucumis melo L.)
VIN gene
MAI1 altered plant growth, fruit development and soluble sugar accumulation [
22]. In the date palm (
Phoenix dactylifera) genome, copy numbers of
VIN gene showed a negative correlation with the sucrose content in ripe fruits [
23]. Pear (
Pyrus bretschneideri Rehd)
PbrvacInv1 was expressed mainly in early stages of fruit development, and its transient overexpression in ripe fruits affected the soluble sugar composition [
24]. During bilberry (
Vaccinium myrtillus L.) fruit development and towards ripening, the gene expression and enzymatic activity of
VINs significantly increased, which was accompanied by an up-regulation profile of hexose contents [
25].
Red pitaya (
Hylocereus polyrhizus), also known as pitahaya or dragon fruit, belongs to the
Hylocereus genus of the Cactaceae family and originates from South and Central America [
26]. Red pitaya, acting as a new tropical and perennial fruit crop, has been widely cultivated in the south and southwest of China in recent years. Red pitaya fruit is rich in multiple natural compounds such as soluble sugars, organic acids, vitamin C, oligosaccharides, polyphenols, flavonoids and betalains [
26,
27]. Among them, soluble sugars are the key determinants that affect the fruit flavor and quality. Over the past years, several studies had reported the accumulation mechanism, candidate genes and possible regulatory models of soluble sugar metabolism in red pitaya fruits. During red pitaya fruit development and towards ripening, starch degrades rapidly, promoting the rapid accumulation of soluble sugars [
28]. Correlation analyses of soluble sugar contents, enzymatic activities and the expression of candidate genes related to soluble sugar metabolism suggested that members of
VINs,
A/NINVs and so on may be involved in soluble sugar metabolism during red pitaya fruit development and towards ripening [
29]. Moreover, a WRKY transcription factor, HpWRKY3, transcriptionally activated the expressions of sucrose metabolic genes
HpINV2 and
HpSuSy1, which regulated sugar accumulation in red pitaya fruit [
30]. Meanwhile, the publishing of pitaya genome sequence data provided a good basis for studying the formation and regulation of fruit flavor and quality [
31]. Collectively, several candidate genes that are associated with soluble sugar accumulation in red pitaya fruit have been reported, and further studies of their physiological functions are still lacking.
In our earlier unpublished work, a VIN isoform isolated from the transcriptome sequencing of red pitaya fruit was mainly expressed in the adult stem (main source tissue for photosynthesis) and fruit pulp (approximately 23 days after flowering) and weakly expressed in fruit pulp at the ripening stage (approximately 30 days after flowering). Hence, we speculated that other VIN isoforms may participate in soluble sugar accumulation during the ripening of red pitaya fruit. In the present study, the VIN gene family was isolated based on the pitaya genome sequence, and sequence alignment and expression pattern detection were conducted. Furthermore, the VIN enzymatic activities during fruit development and towards ripening were measured, and candidate VIN genes that contributed to the VIN enzymatic activity during fruit development were screened. Moreover, to better characterize the physiological functions of the candidate VIN genes, subcellular localization and enzymatic property assays were further conducted. Overall, this study not only facilitates our understanding of the molecular mechanism of soluble sugar metabolism in red pitaya fruit but also provides the target gene for regulating and improving fruit flavor and quality.
2. Materials and Methods
2.1. Materials and Sampling
Red pitaya cultivar ‘Zihonglong’ (
Hylocereus polyrhizus) was used as the study material and planted in an orchard that is located in Zhenning county, Anshun city, Guizhou province, China. Plants with a similar growth vigor and no visible pests and diseases, checked by observation in the orchard, were selected to harvest the samples. The day when the flowers were fully flowering for artificial pollination was recorded as 0 days after flowering (DAF). According to the developmental stage division of red pitaya fruit [
32], fruits were harvested during six developmental stages (10, 20, 23, 25, 27 and 30 DAF). At least 15 fruits were picked at each developmental stage and randomly mixed into three biological repetitions. The fruit pulp was frozen in liquid nitrogen, then ground into powder and stored at −80 °C for using.
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 plants were cultivated for mesophyll protoplast isolation. The invertase-deficient Saccharomyces cerevisiae strain SEY2102 (MATα; ura3-52; leu2-3112; his4-519; suc2-Δ9; gal2), which lacked the cell wall-bound invertase, was used for the heterologous expression of candidate invertase genes.
2.2. Enzymatic Activity Determination of Red Pitaya Tissues
The stem and fruit pulp samples (50–100 mg) stored at −80 °C were weighed. The total protein extraction and enzyme activity determination of vacuolar invertase were conducted according to the instructions of the Acid Invertase Activity Assay Kit (BC0560, Solarbio Co. Ltd., Beijing, China). The protein concentrations were measured using a Bradford Assay Kit (PC0010, Solarbio Co. Ltd., Beijing, China) and using Bovine Serum Albumin as the standard.
2.3. Total RNA Extraction and cDNA Synthesis
The total RNA from the tissues was isolated using the EASYspin Plus Complex Plant RNA Kit (RN53, Aidlab Biotech Co. Ltd., Beijing, China) according to the manufacturer’s description. The quality and concentration of total RNA were detected using agarose gel electrophoresis and a spectrophotometer (NanoPhotometer, IMPLEN Co. Ltd., Munich, Germany). The first-strand cDNA was synthesized from the total RNA (1–1.5 µg) using the PrimeScriptTM 1st Strand cDNA Synthesis Kit (6110A, TaKaRa Co. Ltd., Dalian, China). Finally, the cDNA was diluted using sterile water and stored at −20 °C.
2.4. Genome-Wide Isolation and Sequence Analysis of Red Pitaya VINs
The AtVIN1 and AtVIN2 (accession numbers: At1g12240 and At1g62660) sequences were downloaded from the Arabidopsis Information Resource database (
https://www.arabidopsis.org/, accessed on 17 March 2023). The amino acid sequences of AtVIN1 and AtVIN2 were then used as queries to conduct a Blastp search in the pitaya genome database (
http://pitayagenomic.com/, accessed on 17 March 2023) with default parameters. The obtained candidate genes were confirmed by a Blastp search against the UniProtKB/SwissProt database (
https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 17 March 2023).
The theoretical isoelectric point (pI) and relative molecular weight (Mw) of each amino acid sequence were calculated using the online Compute pI/Mw program (
https://web.expasy.org/compute_pi/, accessed on 30 March 2023), and the grand average of hydropathicity (GRAVY) was computed using the online ProtParam program (
https://web.expasy.org/protparam/, accessed on 20 March 2023). Genomic sequences and coding sequences of
HpVINs were downloaded from the pitaya genome database (
http://pitayagenomic.com/, accessed on 26 March 2023). Introns and exons of
HpVINs were predicted using the online Magic-BLAST program (
https://ncbi.github.io/magicblast/, accessed on 26 March 2023) and displayed using the Gene Structure Display Server program (
https://gsds.gao-lab.org/, version 2.0, accessed on 26 March 2023). The transmembrane structure was predicted using the online DeepTMHMM program (
https://dtu.biolib.com/DeepTMHMM, accessed on 3 April 2023). Conserved domains of amino acid sequences were analyzed using the online HMMER tool (
https://www.ebi.ac.uk/Tools/hmmer/, accessed on 7 April 2023).
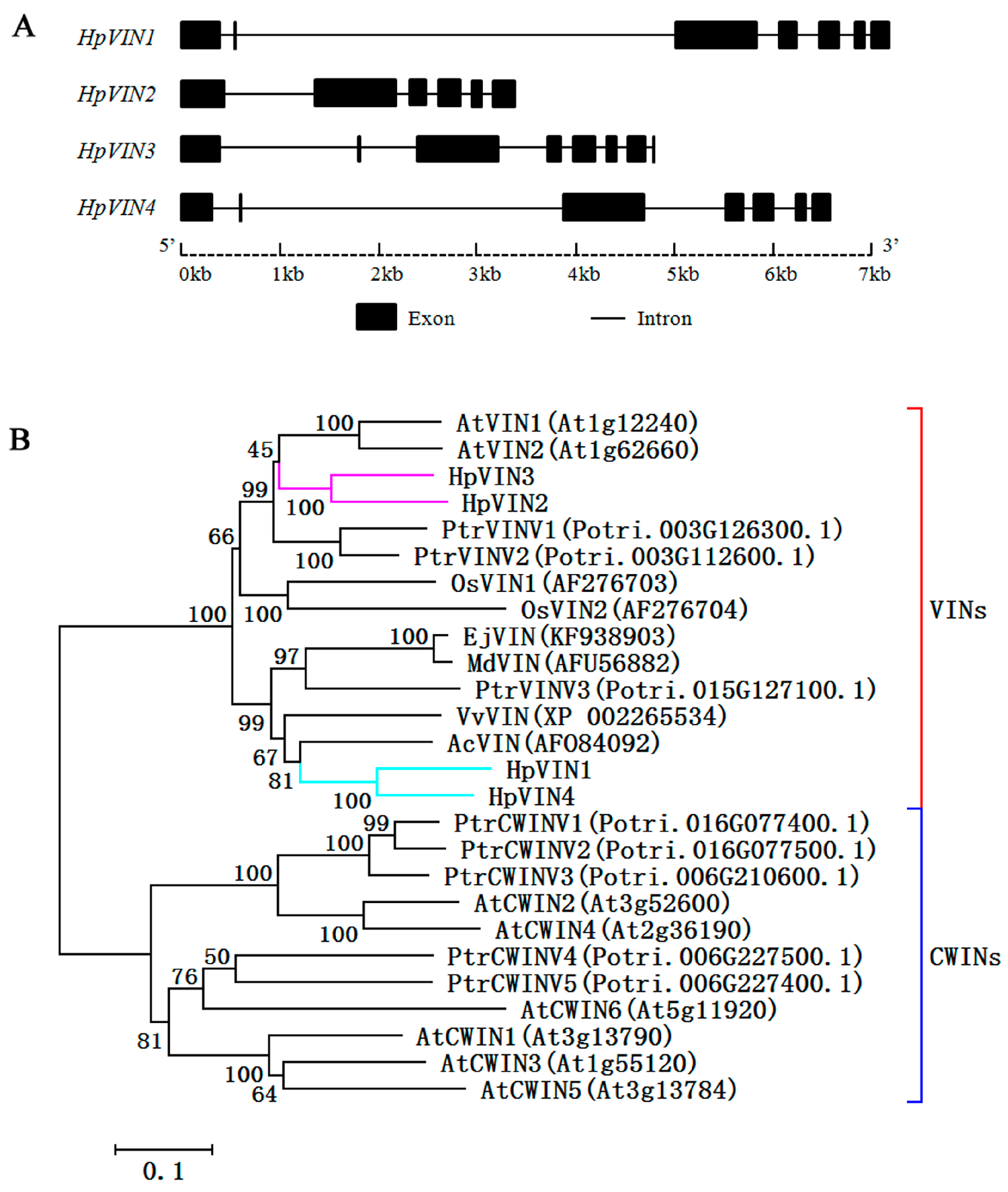
Multiple alignment of the amino acid sequences was conducted using Clustal W software (version 2.1), and the results were displayed using GENDOC software (version 2.7). A phylogenetic tree was constructed using MEGA7.0 software via the Neighbor-Joining method with the Jones–Taylor–Thornton model, and the bootstrap test was calculated 1000 times.
2.5. Gene Expression Analysis
The Fragments Per Kilobase of exon model per Million mapped fragments (FPKM) values of candidate genes at four stages during flower bud differentiation and five stages during flowering were searched in the pitaya genome database (
http://pitayagenomic.com/, accessed on 3 May 2023) according to the gene ID. The FPKM values of transcripts at five stages during red pitaya fruit development, as well as in adult stem and ripe fruit pulp tissues, were obtained from transcriptome sequencing data [
32,
33].
A quantitative real-time PCR (qRT-PCR) assay was conducted to detect the gene expression patterns at five stages during fruit development. The primer pair
HpVIN4-qRT-F/R was designed for PCR amplification using Primer Premier 5.0 software (
Supplementary Table S1). To ensure the amplification specificity, the sequence alignment of the expected PCR product was confirmed by a Blastn search in the pitaya genome database (
http://pitayagenomic.com/, accessed on 11 May 2023). The PCR reaction was carried out on the real-time PCR detection system (CFX96, Bio-Rad Co. Ltd., Hercules, CA, USA). The total volume for PCR amplification was 10.0 µL, including the cDNA template at 0.5 µL, primer pairs at 0.2 µL + 0.2 µL, SYBR Green Fast qPCR Mix (RK02001, Biomarker Co. Ltd., Beijing, China) at 5 µL and sterile water at 4.1 µL. The reaction procedure was as follows: 95 °C/3 min for denaturation, 95 °C/5 s for denaturation and 60 °C/30 s for annealing and amplification for a total 40 cycles. The gene expression level was normalized using the housekeeping gene
β-ACT as the internal control (
Supplementary Table S1). Each reaction of both the target gene and housekeeping gene contained three technical repeats. The relative gene expression level was calculated by using the 2
−ΔΔCt method, and the gene expression level of the fruit pulp sample at 20 DAF was set to “1”.
2.6. Subcellular Localization Determination
Using the cDNA of fruit pulp (at 30 DAF) as the RT-PCR template, the coding sequence of
HpVIN4 without the stop codon was amplified by the high-fidelity DNA polymerase (P515, Vazyme Co. Ltd., Nanjing, China) under the primer pair
HpVIN4-GFP-F/R (
Supplementary Table S1). The amplified products were isolated and inserted into the 16318-hGFP vector using the ClonExpress
® II One Step Cloning Kit (C112, Vazyme Co. Ltd., Nanjing, China). After verification using sequencing, the construct, named HpVIN4::hGFP, was isolated. The 16318-hGFP and HpVIN4::hGFP fusion constructs were transformed into the
Arabidopsis thaliana mesophyll protoplast for transient expression using a PEG-mediated method [
34]. Meanwhile, the
Arabidopsis thaliana VAMP711 gene that was used as the tonoplast marker gene and fused using red fluorescence protein (RFP) [
35] was transiently expressed together with 16318-hGFP or HpVIN4::hGFP. After 24 h, the fluorescence signal was observed using confocal laser microscope (LSM510, ZEISS Co. Ltd., Oberkochen, Germany).
2.7. Complementation Assay in Baker’s Yeast
The entire coding sequence of
HpVIN4 was amplified using the primer pair
HpVIN4-Yeast-F/R (
Supplementary Table S1) and then inserted into the yeast shuttle vector pDR196. After verification via sequencing, the pDR196 and pDR196::HpVIN4 constructs were transformed into the invertase-deficient baker’s yeast strain SEY2102 using a PEG/LiAc method with the Frozen Yeast Transformation Kit (SK2400, Coolaber Co. Ltd., Beijing, China). After being screened on the synthetic complete (SC) medium without uracil at 30 °C, transformants were obtained after PCR verification and cultured in the lipid SC/-ura medium.
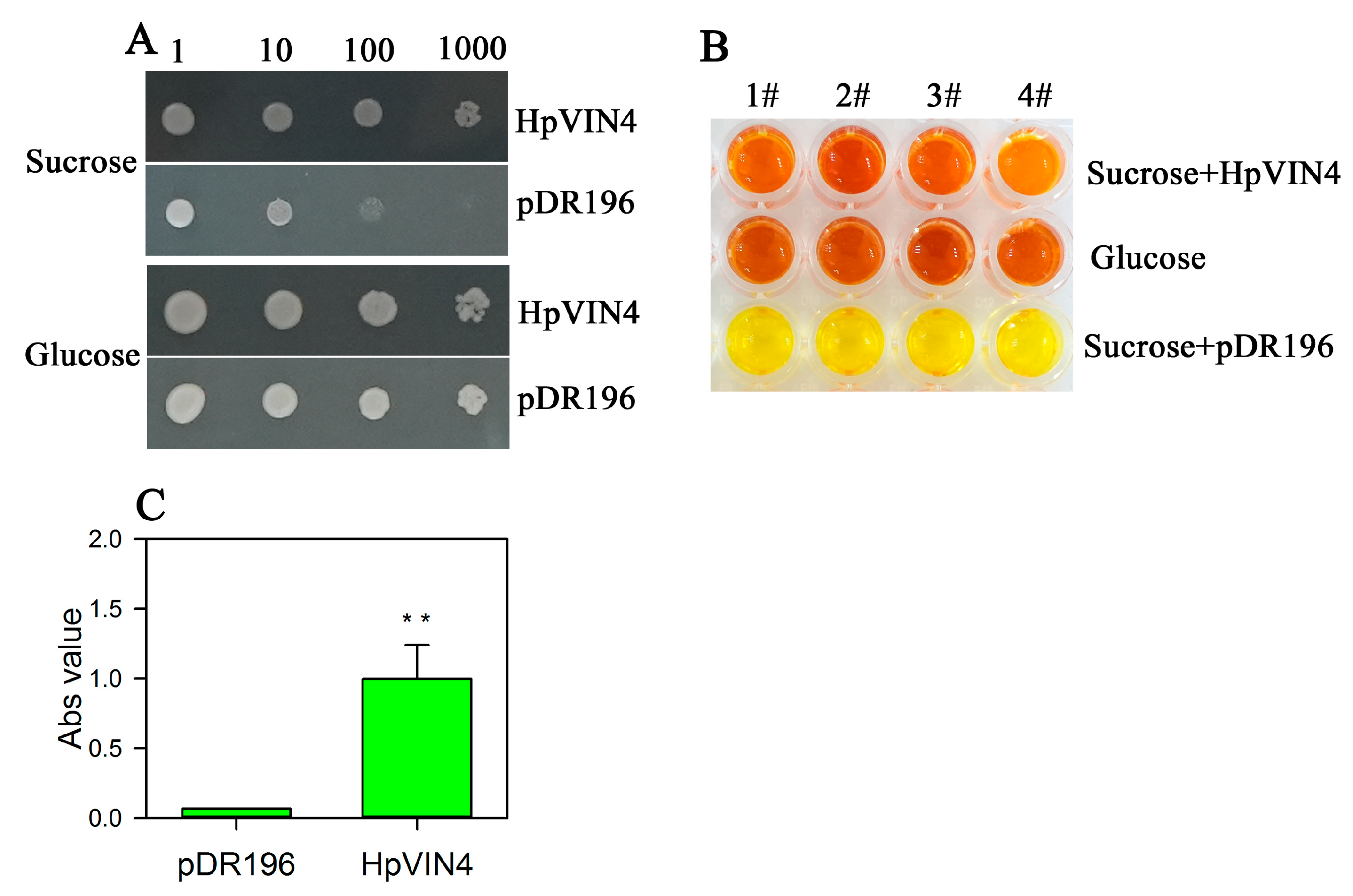
Yeast clones were inoculated and cultured in liquid SC/-ura medium (2% glucose as carbon source) at 30 °C to the logarithmic phase (OD600 = 0.6–0.8). Yeast cells were collected by centrifugation and washed twice with sterile water. After removing the supernatant, the OD600 value of the yeast cells was adjusted to 1.0 with sterile water. The OD600 value was diluted to 10−1, 10−2 and 10−3 using sterile water. A yeast cell solution of 1.0 µL was transferred into the solid SC/-ura medium (glucose or sucrose as the sole carbon source) and cultured at 30 °C. Four independent clones of yeast cells carrying pDR196 or pDR196::HpVIN4 were tested. After 48 h, the growth status of the yeast cells was observed and captured with pictures.
Yeast cells at the logarithmic phase (OD600 = 0.6–0.8) were washed twice with sterile water and collected after centrifugation. Then, the yeast cells were incubated in phosphate buffer (pH = 5.0) containing sucrose at 30 °C for 12 h, and the supernatant was isolated. The sugar content in the supernatant was detected using the DNS (3, 5-Dinitro-2-hydroxybenzoic acid) method, using glucose as the positive control. The absorbance value at 540 nm was measured with a Microplate Spectrophotometer (Multiskan GO, Thermo Fisher Co. Ltd., Agawam, MA, USA).
2.8. Enzymatic Activity Detection of Recombinant Protein
Yeast cells at the logarithmic phase (OD600 = 0.6–0.8) were collected and washed twice with phosphate buffer (pH = 4.0). Then, the yeast cells were broken by vortexing with steel balls, and the total protein was extracted by dissolving them with phosphate buffer. The protein concentrations were detected using the Bradford Assay Kit (PC0010, Solarbio Co. Ltd., Beijing, China).
To identify sucrose hydrolysis activity, the reaction mixture contained yeast total protein (about 1.0 µg), sucrose (60 mmol·L−1) and phosphate buffer (pH = 4.0). The reaction was incubated at 37 °C for 12 h and terminated at 90 °C for 5 min. The supernatant was collected by centrifugation for HPLC (high-performance liquid chromatography) detection. Reaction products were detected using the HPLC system (Agilent-1200, Agilent Co. Ltd., Santa Clara, CA, USA) using a ZORBAX Carbohydrate Analysis column (Zorbax Carbohydrate, Agilent Co. Ltd., Santa Clara, CA, USA). Glucose, fructose and sucrose were used as standards to determine the retention time and identify product types.
The optimum pH of enzymatic activity was detected under a series of phosphate buffers with pH values of 3.0–8.0. The reaction mixture contained yeast total protein (about 1.0 µg), sucrose (100 mmol·L−1) and phosphate buffers. The reaction was incubated at 37 °C for 30 min and terminated at 90 °C for 5 min. To measure the enzymatic activity under different substrate concentrations, a series of sucrose solutions with varying final sucrose concentrations (10–600 mmol·L−1) were used. The reaction mixture contained yeast total protein (about 1.0 µg), sucrose and phosphate buffer (pH = 4.0). After being incubated at 37 °C for 40 min and terminated, the products were determined using the DNS method.
2.9. Statistical Analysis
The significant difference testing among samples was conducted using the t-test analysis. Microsoft Excel 2007 was used for data processing, and Sigmaplot 12.0 software was employed for mapping.
4. Discussion
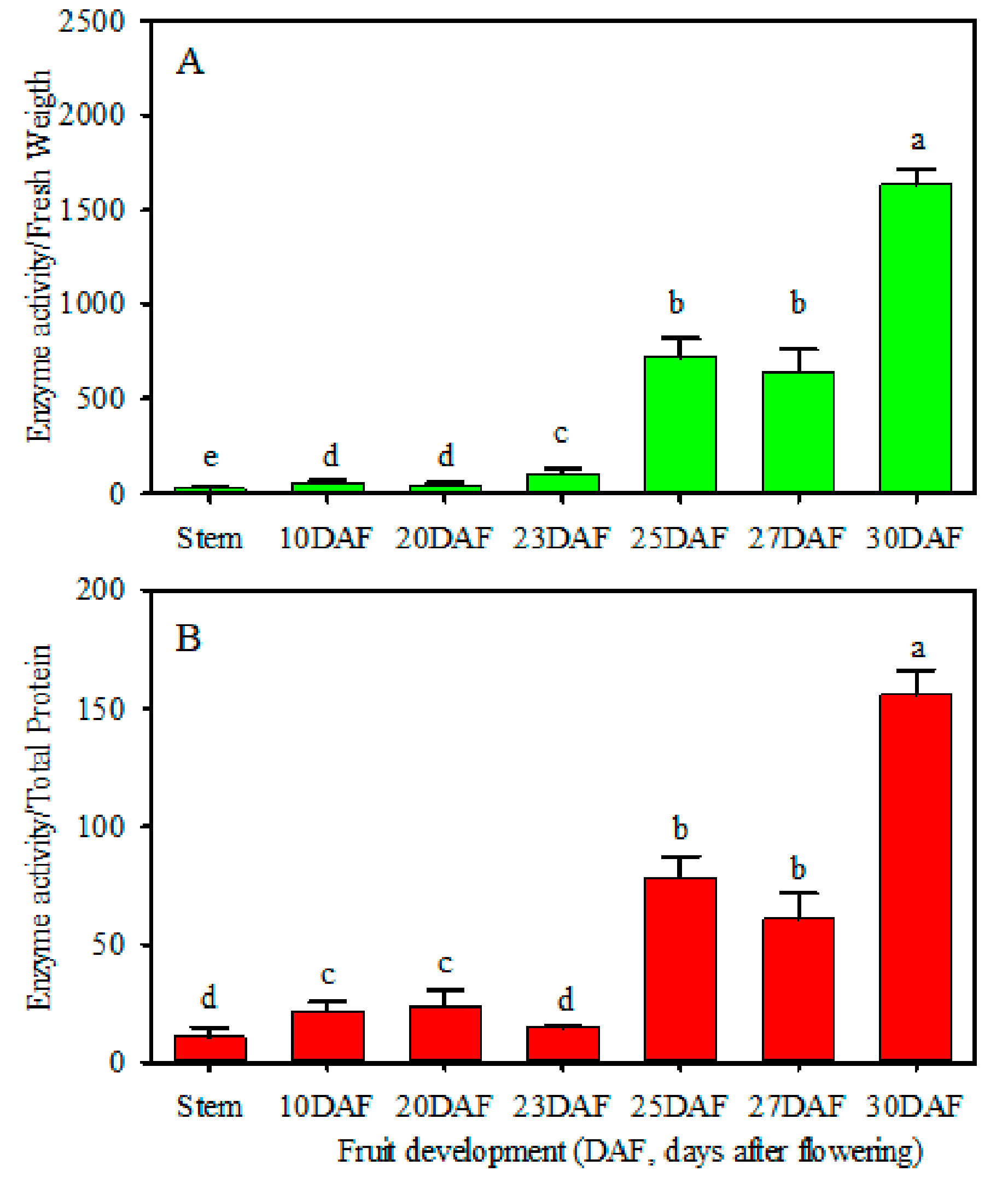
The vacuoles of plant cells are vital organelles that function as reservoirs for metabolites such as sugars, acids, pigments and so on [
8]. Therefore, transportation processes across the tonoplast or metabolism inside the vacuole are critical for regulating metabolite accumulation. Recent studies have demonstrated that most soluble sugars are stored in plant vacuoles, which occupy up to 90% of the total cell volume [
37]. In this situation, functioning as a class of enzymes involved in sucrose catabolism in plant vacuoles, VIN can regulate the soluble sugar contents and compositions in plant tissues. During the early stages of red pitaya (
Figure 1), loquat [
6] and bilberry [
25] fruit development, it was shown that the VIN activities are low and accompanied by low contents of soluble sugars, which suggested that VIN may play no role or have very weak effects. Therefore, it speculated that other types of invertases, such as A/NINVs or CWINs, may be responsible for maintaining sugar metabolism to fulfill fruit development during the early stages [
5]. During red pitaya fruit development and towards ripening, abundant hexoses, mainly glucose and fructose, were accumulated, with low sucrose content, indicating that sucrose catabolism played crucial roles in soluble sugar metabolism [
28,
29,
30]. The present study as well as a previous study [
29] revealed that the VIN enzymatic activity increased during red pitaya fruit development and towards ripening (
Figure 1). Therefore, it speculated that, similar to loquat and bilberry fruit [
6,
25], VINs may play vital roles in soluble sugar accumulation in red pitaya fruits during development and towards ripening. Herein, we conducted a genome-wide isolation and sequence analysis of the red pitaya VIN gene family. Focusing on the candidate
VIN gene that may contribute to VIN’s enzymatic activity during fruit development and towards ripening, we further conducted a subcellular localization assay and enzymatic activity identification to elucidate its physiological role in soluble sugar accumulation.
4.1. HpVIN4 Was the Major VIN Gene Expressed in Red Pitaya Fruit during Development and towards Ripening
Based on genome-wide analyses, it was reported that plant
VINs were usually encoded by small-scale gene families, which contained several members varying from two to eight in
Arabidopsis thaliana [
9,
10], rice [
11,
12,
13], sorghum [
16], cucumber [
19,
20] and pear [
24]. In this study, we conducted a genome-wide isolation for the red pitaya VIN gene family and also identified four
VIN isoforms, which was comparable to the
VIN gene number of rice [
11,
12,
13] and cucumber [
19,
20].
However, it has been reported that nonfunctional events of
VINs were very common in higher plants, indicating that some members lost their sucrose hydrolysis activity [
36]. It has been proved that the β-fructosidase motif (NDPD/NG) of AINVs is crucial for sucrose hydrolysis [
4,
5]. A genomic structure comparison showed that the β-fructosidase motif was partly encoded by the second exon [
5]. In some plant species, such as pear [
24], sugarcane (
Saccharum bicolor) [
38] and moso bamboo (
Phyllostachys edulis) [
39], the second exon missing in some
VINs resulted in an incomplete β-fructosidase motif, which suggested the loss of sucrose hydrolysis activity. Of four
HpVINs, the β-fructosidase motif of the HpVIN2 protein was incomplete due to the second exon missing (
Figure 2A and
Figure 3), indicating that it may be a non-functional
VIN. Additionally,
HpVIN2-related transcripts could not be detected in the main tissues (
Figure 4A,B and
Supplementary Table S3), which suggested that it may have no functions in the growth and development of red pitaya. On the contrary, the HpVIN1, HpVIN3 and HpVIN4 proteins all contained the complete β-fructosidase motif and other essential motifs for sucrose hydrolysis (
Figure 3), indicating that they may be functional
VINs.
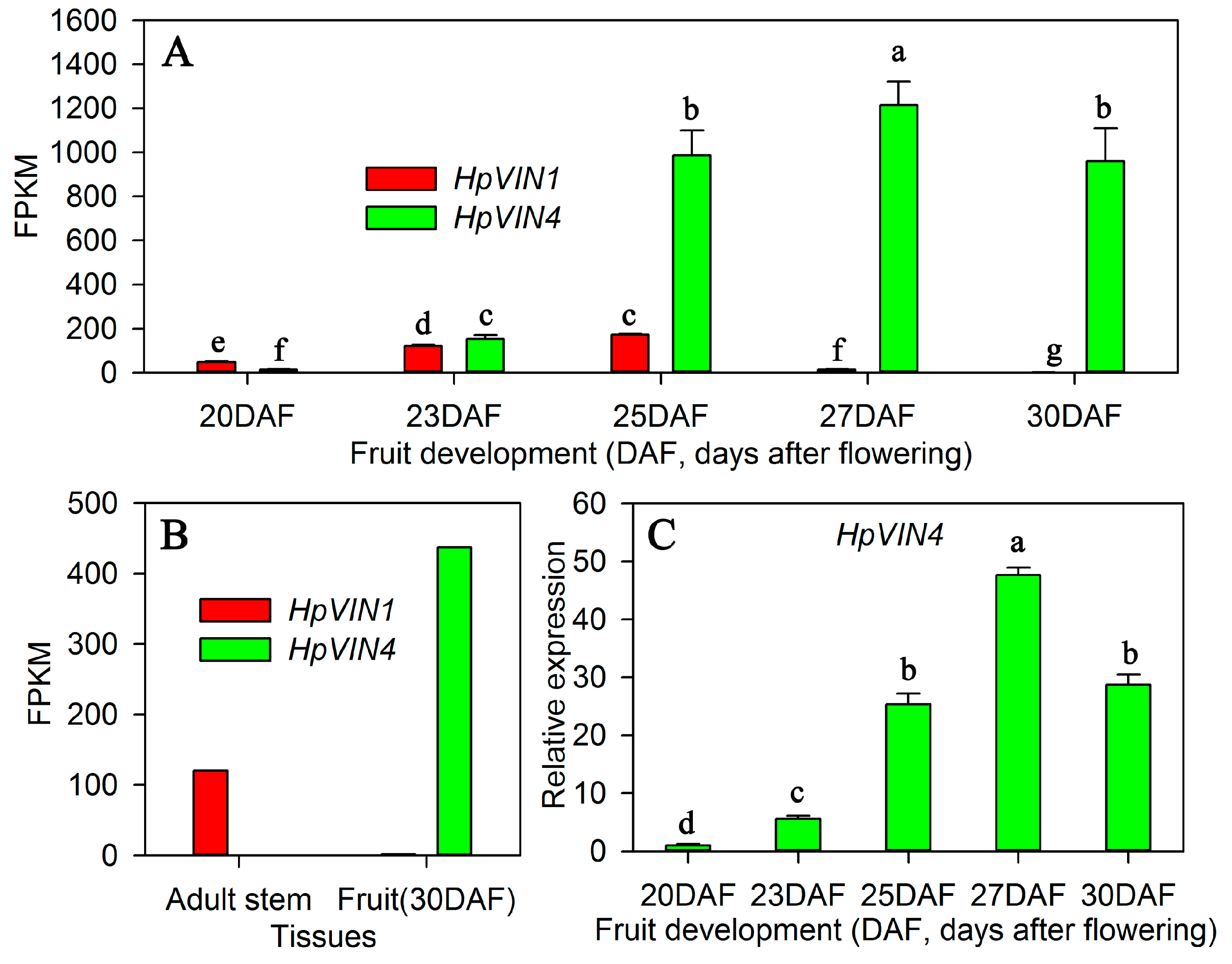
Towards fruit ripening, the expression level of
HpVIN4 was much higher than that of
HpVIN1 and
HpVIN3 (
Figure 4A). Moreover, the expression pattern of the
HpVIN4 gene showed a similar trend with the VIN enzymatic activity (
Figure 1 and
Figure 4A,C). Taken together, it suggested that
HpVIN4 was the main contributor of the VIN activity towards ripening. However, the expression pattern of the
HpVIN4 gene was not completely consistent with the trend of VIN enzymatic activities. In particular, the gene expression level at 27 DAF was the highest (
Figure 4A,C), but the VIN enzymatic activity at 30 DAF was the highest (
Figure 1). The inconsistency between gene expression and enzymatic activities may be attributed to the post-translational regulation by invertase inhibitors, which are bound to the sucrose binding site and repress the invertase activity [
40]. Next, it will be intriguing and worthy to explore the regulation mode of the VIN enzymatic activity by invertase inhibitors in red pitaya fruit.
4.2. HpVIN4 Protein Was Localized to the Vacuole
The multiple subcellular localization patterns of
VINs reflected the differences in their physiological functions. Although the VIN gene family has been isolated from many species, subcellular location assays were conducted only through sequence prediction using bioinformatics tools [
6,
15,
16,
17,
18,
19,
20,
21,
22]. To date, subcellular localization modes of VIN members including OsVIN2 [
12], OsVIN3 [
13], PbrvacInv1 [
24] and AtVIN1 [
36] only had been confirmed by experiments. Furthermore, the conserved motif analyses and site mutation verification proved the subcellular localization mechanism of the
Arabidopsis thaliana AtVIN1 protein [
36]. The AtVIN1 protein was successfully located to vacuoles through the endoplasmic reticulum–Golgi matrix–vacuole pathway. In particular, the AtVIN1 protein was carried by vesicles derived from the endoplasmic reticulum and then inserted into the tonoplast; after N-terminal processing mediated by vacuolar proteases, the mature AtVIN1 protein was released into the vacuole lumen. The N-terminal of the AtVIN1 protein contained complex NTPP regions including the dileucine, BR and TMD domains, which determined the correct location to the vacuole [
36].
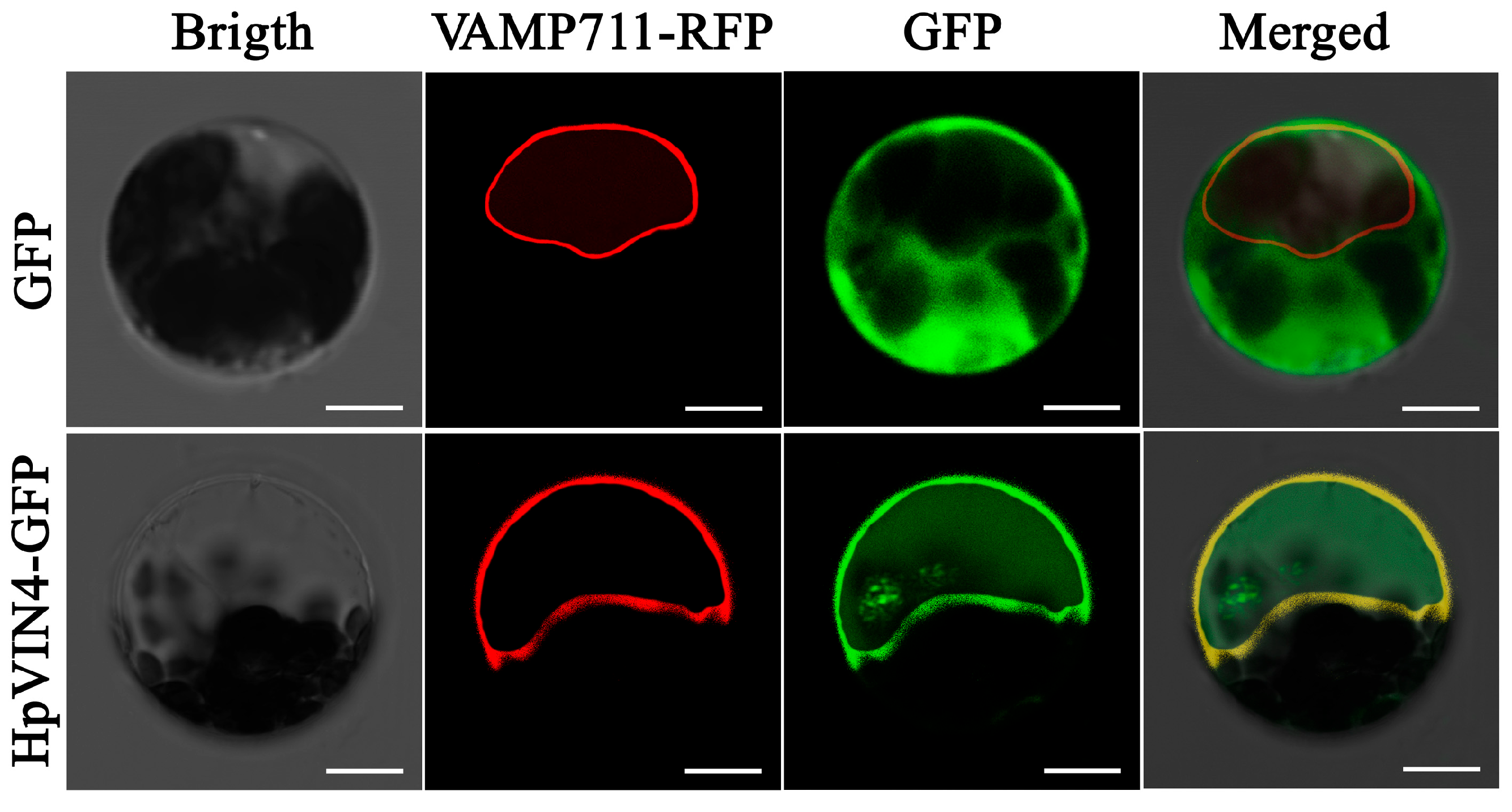
Sequence analysis showed that the HpVIN4 protein contained the dileucine, BR and TMD domains in the N-terminal (
Figure 3), which was similar to
Arabidopsis thaliana AtVIN1. Using a transient expression assay in
Arabidopsis thaliana mesophyll protoplasts, it was revealed that most HpVIN4 proteins were located in the tonoplast with a few in the vacuolar lumen (
Figure 5). Given the vacuole location pathway of the AtVIN1 protein, it speculated that an incomplete processing of the N-terminal of the HpVIN4 protein by the
Arabidopsis thaliana vacuolar protease may lead to HpVIN4 protein accumulation in the tonoplast. Collectively, according to the conserved motif analyses and transient expression assay, it was suggested that the HpVIN4 protein was located in the vacuole.
4.3. HpVIN4 Was a Functional VIN with Typical Characteristics Similar to Other VINs
The HpVIN4 protein contains several essential motifs responsible for sucrose hydrolysis, suggesting that HpVIN4 may be a functional VIN. However, a further verification of its enzymatic activity was necessary to clarify the physiological functions. The invertase-deficient yeast strains SEY2102 and SEY6210 are commonly used expression hosts that were employed to verify the enzymatic activity of foreign invertase genes using a growth complementation assay in vivo. For example, using sucrose as the sole carbon source, wheat (
Triticum aestivum L) Ta-A/NINV1, chili pepper (
Capsicum annuum L.) CaVINV1, cassava (
Manihot esculenta Crantz) MeNINV1 and sweet potato [
Ipomoea batatas (L.) Lam.] Ibβfruct2 could restore the growth of the SEY2102 or SEY6210 yeast strains in vivo, which proved that they were functional invertase genes [
41,
42,
43,
44]. As expected, HpVIN4 could also complement yeast SEY2102 growth in vivo (
Figure 6A). Meanwhile, we detected a potential product from the sucrose solution after incubated it with yeast cells expressing HpVIN4 (
Figure 6B). The result showed that glucose, functioning as a carbon source for yeast growth, may be generated from sucrose hydrolysis by the HpVIN4 protein.
Furthermore, the enzymatic properties of the HpVIN4 recombinant protein were analyzed in vitro. From the HPLC identification, it clearly demonstrated that the HpVIN4 protein could cleave sucrose into glucose and fructose (
Figure 7). Furthermore, the HpVIN4 protein had an optimum pH of 4.0 for sucrose hydrolysis (
Figure 8A), which was consistent with the pH-dependent feature of VIN enzymatic activities [
5,
6,
42]. Meanwhile, the HpVIN4 protein had an estimated Km value of 5.15 ± 1.03 mmol·L
−1 for sucrose hydrolysis (
Figure 8B), which was also comparable to the Km value of AtVIN1 (5.65 ± 0.7 mmol·L
−1) [
10]. Taken together, the in vivo and in vitro assays both demonstrated that the HpVIN4 protein was a typical VIN with similar enzymatic characteristics to other plant VINs.
5. Conclusions
In the current study, we investigated the VIN activities during red pitaya fruit development and towards ripening, conducted genome-wide isolation, sequence alignment, phylogenetic relationship analysis of the red pitaya VIN gene family, subcellular localization and an enzymatic properties assay for the candidate HpVIN4 gene. The enzymatic activities of VIN in adult stem tissues and fruit pulps at 10 DAF, 20 DAF and 23 DAF were low, up-regulated towards fruit ripening and reached their highest level at 30 DAF. The changes in the VIN enzymatic activities in red pitaya fruit pulp were consistent with the phenomenon that a large number of hexoses accumulated towards fruit ripening. Subsequently, we identified four red pitaya VIN members from the genome database. Sequence alignment suggested that the HpVIN1, HpVIN3 and HpVIN4 proteins contained essential motifs for correct targeting to the vacuole and conserved motifs responsible for sucrose binding and hydrolysis. Through gene expression analyses, it was revealed that HpVIN4 is the major VIN gene expressed in red pitaya fruit during development and towards ripening. Finally, a transient expression assay in Arabidopsis thaliana mesophyll protoplasts suggested that the HpVIN4 protein was located in the vacuole. The enzymatic activities of the HpVIN4 protein were identified in vivo and in vitro through heterologous expression in the invertase-deficient yeast strain. HpVIN4 could complement the yeast growth using sucrose as the sole carbon source, suggesting that it had sucrose hydrolysis activity. Using the recombinant protein from yeast cells, it was demonstrated that the HpVIN4 protein could cleave sucrose into glucose and fructose at an optimum pH of 4.0 and an estimated Km value of 5.15 ± 1.03 mmol·L−1. The HpVIN4 protein shared typical characteristics of VIN enzymatic activities, which were similar to other plant VINs. Taken together, these findings are beneficial to understanding the molecular mechanisms of VIN genes involved in soluble sugar accumulation in red pitaya fruit and provide functional target genes for regulating the fruit flavor and quality. In the future, on one hand, the physiological functions of VINs in red pitaya fruits need to be elucidated through overexpression and gene interference, and on the other hand, the gene expression of HpVIN4 at the transcriptional level and the regulation mechanism of VIN enzyme activities at the post-translational level need to be further researched.