Discrimination of Fungicide-Contaminated Lettuces Based on Maximum Residue Limits Using Spectroscopy and Chemometrics
Abstract
1. Introduction
2. Materials and Methods
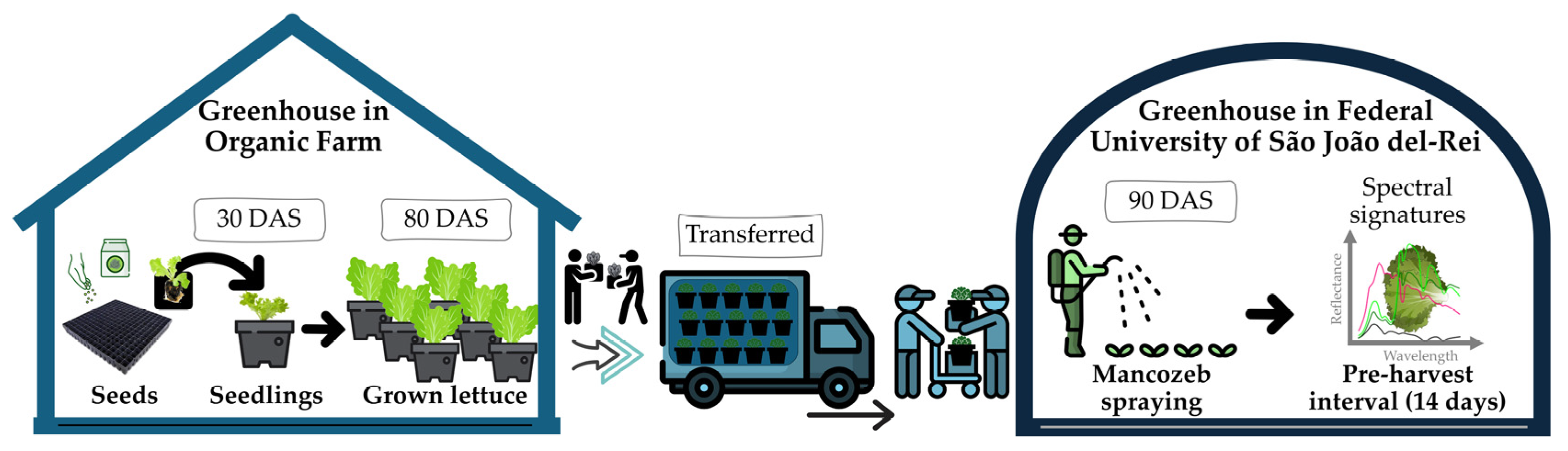
2.1. Plant Material, Experimental Design, and Location
2.2. Fungicide Spraying
2.3. Reflectance Measurements
2.4. Dithiocarbamate Analytical Determination
2.5. Chemometric Analysis
3. Results
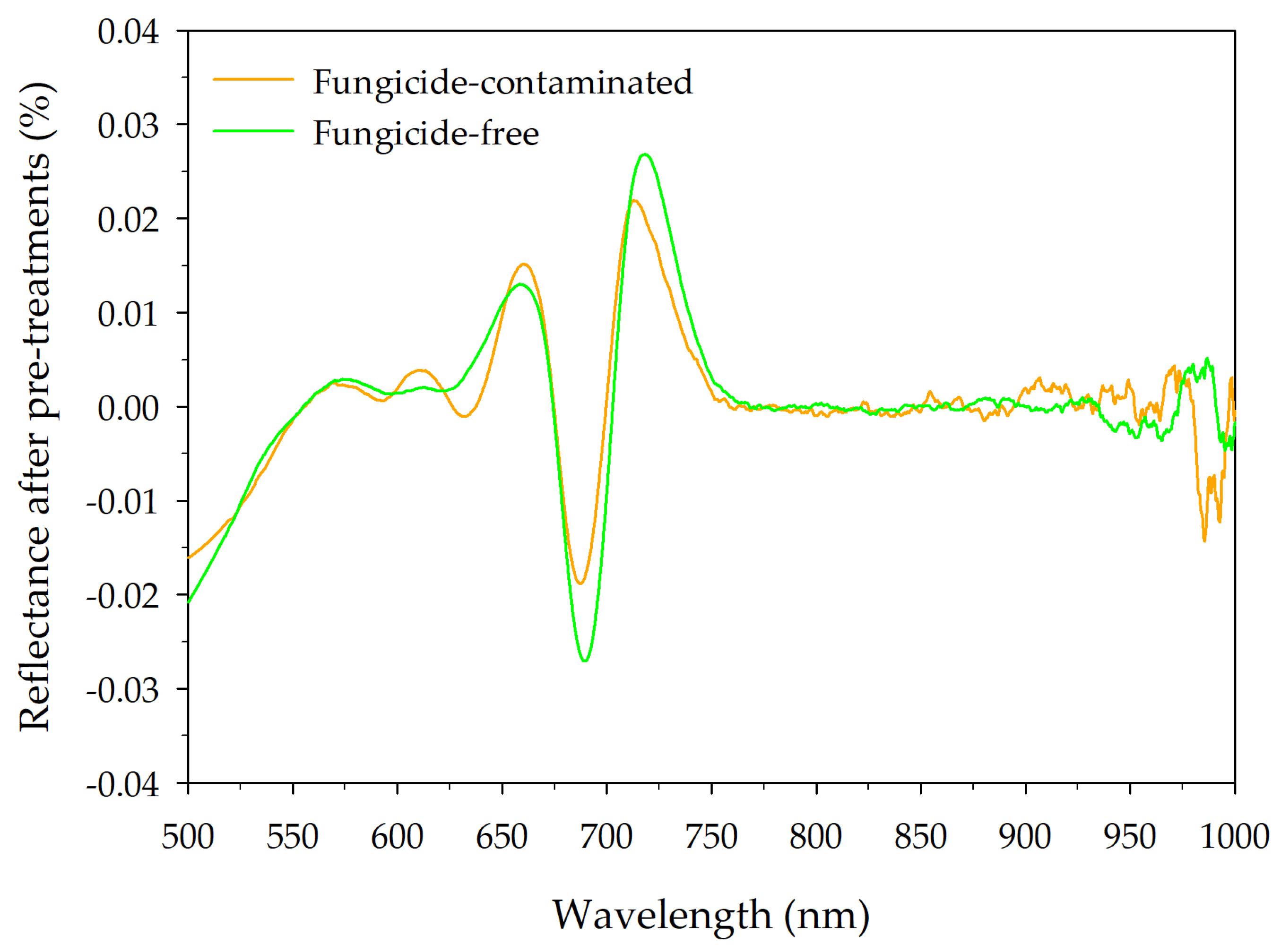
3.1. CS2 Behaviour in Lettuce and NIR Spectral Signatures
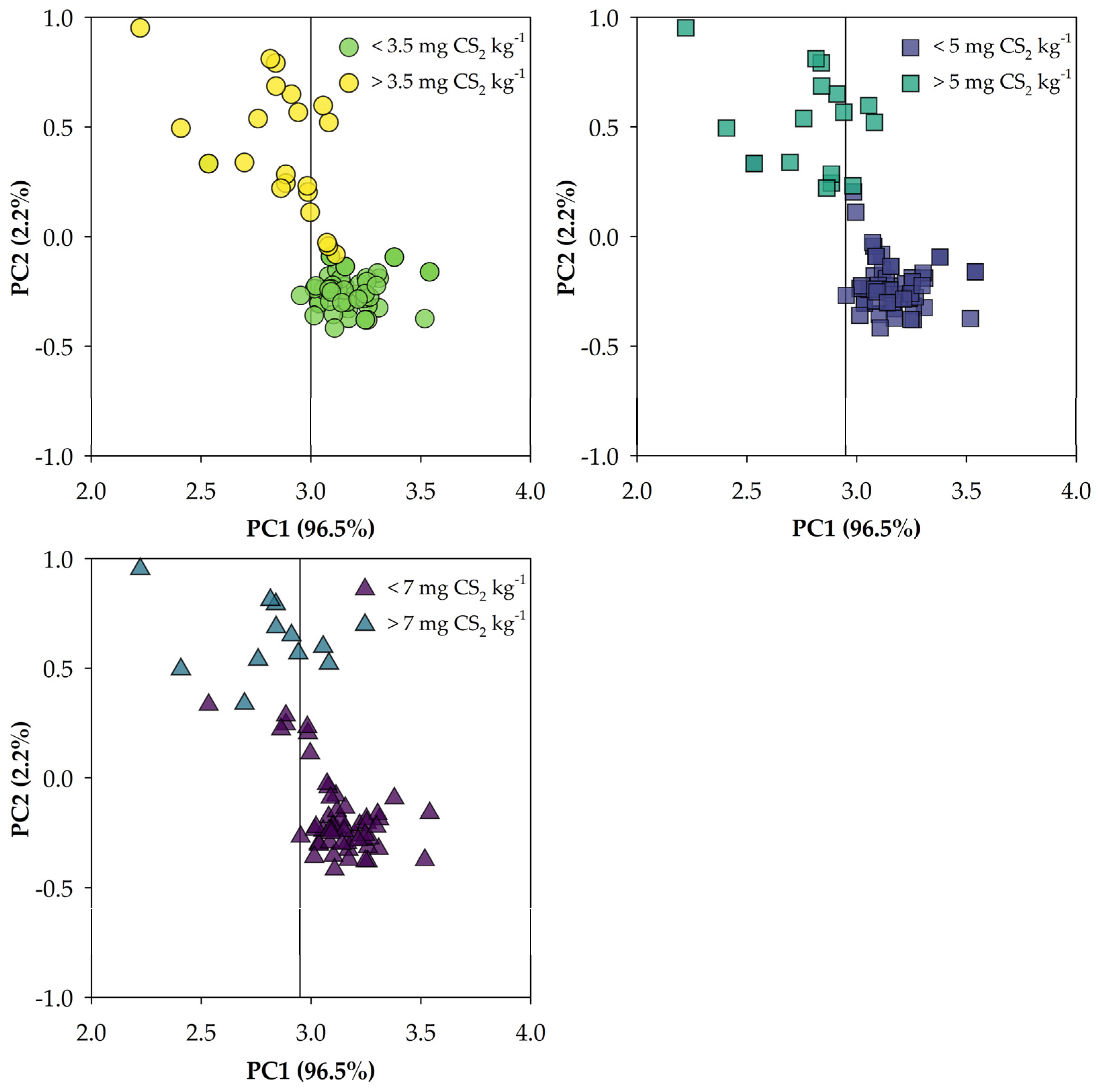
3.2. Principal Component Analysis
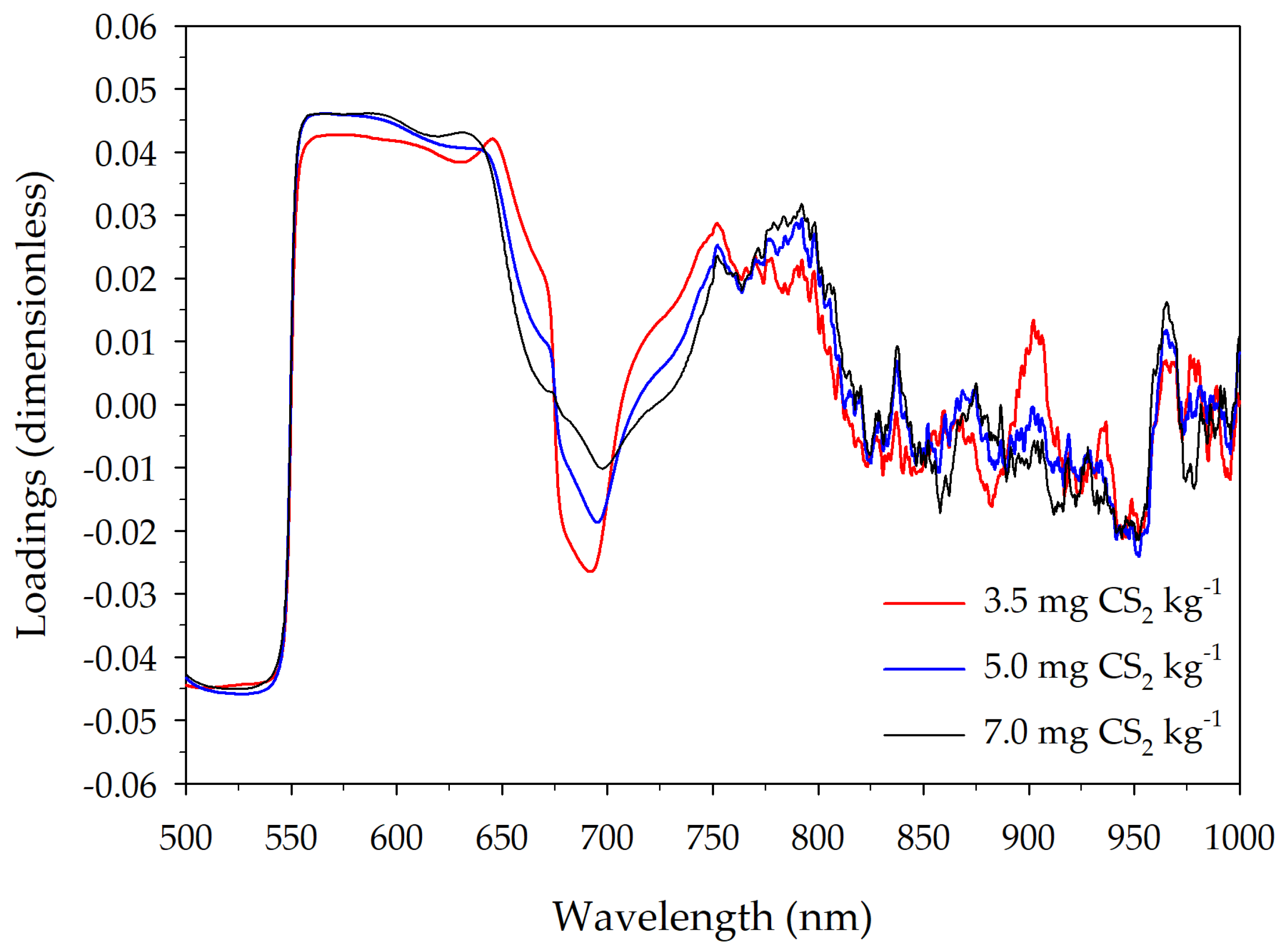
3.3. Classification by PLS-DA
4. Discussion
5. Directions for Practical Applications and Further Research
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yazdi, M.; Kolahi, M.; Mohajel Kazemi, E.; Goldson Barnaby, A. Study of the Contamination Rate and Change in Growth Features of Lettuce (Lactuca sativa Linn.) in Response to Cadmium and a Survey of Its Phytochelatin Synthase Gene. Ecotoxicol. Environ. Saf. 2019, 180, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, A.; Magdas, D.A.; Feher, I.; Puscas, R.H.; Hategan, A.R.; Mirel, V.; Mirel, S.; Crivii, C. Characterization of the Maximal Nutritional Value of Lactuca sativa (Lettuce) Based upon Elemental Analysis. Anal. Lett. 2023, 56, 331–343. [Google Scholar] [CrossRef]
- López-Fernández, O.; Rial-Otero, R.; Simal-Gándara, J. Factors Governing the Removal of Mancozeb Residues from Lettuces with Washing Solutions. Food Control 2013, 34, 530–538. [Google Scholar] [CrossRef]
- Choubbane, H.; Ouakhssase, A.; Chahid, A.; Taourirte, M.; Aamouche, A. Pesticides in Fruits and Vegetables from the Souss Massa Region, Morocco. Food Addit. Contam. Part B 2022, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.; Deng, K.; Liu, S.; Li, B.; Li, Y. Monitoring and Dietary Exposure Assessment of Pesticide Residues in Strawberry in Beijing, China. Food Addit. Contam. Part B 2022, 15, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gaouar, Z.L.; Chefirat, B.; Saadi, R.; Djelad, S.; Rezk-Kallah, H. Pesticide Residues in Tomato Crops in Western Algeria. Food Addit. Contam. Part B 2021, 14, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, S.; Fuentes, M.; Valenzuela, M.; Zhao, G.; Liu, S.; Lu, H.; Correa, A. Pesticide Residues in Ready-to-Eat Leafy Vegetables from Markets of Santiago, Chile, and Consumer’s Risk. Food Addit. Contam. Part B 2019, 12, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Shoeibi, S.; Amini, M.; Amirahmadi, M.; Rastegar, H.; Ghaffarian, A.; Ghazi-Khansari, M. Monitoring Dithiocarbamate Fungicide Residues in Greenhouse and Non-Greenhouse Tomatoes in Iran by HPLC-UV. Food Addit. Contam. Part B 2012, 5, 87–92. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, J.C.; Galvan, D.; Kato, L.S.; Conte-Junior, C.A. Consumption of Fruits and Vegetables Contaminated with Pesticide Residues in Brazil: A Systematic Review with Health Risk Assessment. Chemosphere 2023, 322, 138244. [Google Scholar] [CrossRef]
- Soylak, M.; Ozalp, O.; Uzcan, F. Determination of Trace Ziram in Food by Magnesium Hydroxide Coprecipitation with Indirect Detection by Flame Atomic Absorption Spectrometry (FAAS). Anal. Lett. 2023, 56, 1525–1534. [Google Scholar] [CrossRef]
- Crnogorac, G.; Schwack, W. Residue Analysis of Dithiocarbamate Fungicides. Trends Anal. Chem. 2009, 28, 40–50. [Google Scholar] [CrossRef]
- Pereira, S.I.; Figueiredo, P.I.; Barros, A.S.; Dias, M.C.; Santos, C.; Duarte, I.F.; Gil, A.M. Changes in the Metabolome of Lettuce Leaves Due to Exposure to Mancozeb Pesticide. Food Chem. 2014, 154, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Belpoggi, F.; Soffritti, M.; Guarino, M.; Lambertini, L.; Cevolani, D.; Maltoni, C. Results of Long-Term Experimental Studies on the Carcinogenicity of Ethylene-Bis-Dithiocarbamate (Mancozeb) in Rats. Ann. N. Y. Acad. Sci. 2002, 982, 123–136. [Google Scholar] [CrossRef] [PubMed]
- JMPR Joint Meeting on Pesticide Residues—FAO/WHO—Mancozeb. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation93/mancoz.pdf (accessed on 1 June 2024).
- PMRA Pest Management Regulatory Agency—Proposed Maximum Residue Limit PMRL2018-27, Ethylene Bis-Dithiocarbamate (EBDC) Fungicides: Mancozeb, Metiram, Maneb and Zineb. Available online: https://www.canada.ca/content/dam/hc-sc/documents/services/consumer-product-safety/pesticides-pest-management/public/consultations/proposed-maximum-residue-limit/2018/ethylene_bis_dithiocarbamate/PMRL2018-27-eng.pdf (accessed on 1 June 2024).
- JMPR Joint Meeting on Pesticide Residues—FAO/WHO—Pesticides Database: Dithiocarbamates. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=105 (accessed on 1 June 2024).
- HSE Health & Safety Executive—The Great Britain MRL Statutory Register. Available online: https://secure.pesticides.gov.uk/MRLs/search (accessed on 1 June 2024).
- MARD Ministry of Agriculture and Rural Development—Pesticide Residues Databases. Available online: https://data.gov.il/dataset/mrl/resource/3ee1cd66-6176-4c1f-8d38-8f36a1461426 (accessed on 1 June 2024).
- APVMA Federal Register of Legislation—Australia New Zealand Food Standards Code—Schedule 20—Maximum Residue Limits. Available online: https://www.legislation.gov.au/F2015L00468/2023-02-21/text (accessed on 31 May 2024).
- MPI Ministry for Primary Industries—Maximum Residue Levels for Agricultural Compounds. Available online: https://www.mpi.govt.nz/agriculture/agricultural-compounds-vet-medicines/maximum-residue-levels-agricultural-compounds/ (accessed on 1 June 2024).
- JFCRF Japan Food Chemical Research Foundation—Table of MRLs for Agricultural Chemicals: Dithiocarbamates. Available online: http://db.ffcr.or.jp/front/ (accessed on 1 June 2024).
- CFS Centre for Food Safety—Hong Kong Pesticide MRL Database. Pesticide Residues in Food Regulation (Cap. 132CM). Available online: https://www.cfs.gov.hk/english/mrl/index.php (accessed on 1 June 2024).
- EPA Environmental Protection Agency—Mancozeb, Maneb, Metiram, and Thiram; Tolerance Actions. Available online: https://www.federalregister.gov/documents/2014/05/14/2014-10955/mancozeb-maneb-metiram-and-thiram-tolerance-actions (accessed on 1 June 2024).
- MFDS Ministry of Food and Drug Safety—Pesticides and Veterinary Drugs Information. Available online: http://www.foodsafetykorea.go.kr/residue/prd/mrls/list.do?menuKey=1&subMenuKey=161 (accessed on 1 June 2024).
- Veiga-del-Baño, J.M.; Martínez-López, S.; Pérez-Lucas, G.; Cuenca-Martínez, J.J.; Andreo-Martínez, P. Trends in Dithiocarbamates Food Research: A Bibliometric Vision. Chemosphere 2023, 313, 137342. [Google Scholar] [CrossRef] [PubMed]
- EFSA. The 2010 European Union Report on Pesticide Residues in Food. EFSA J. 2013, 11, 3130. [Google Scholar] [CrossRef]
- OJEU Official Journal of the European Union—Commission Implementing Regulation (EU) 2020/2087. Document 32020R2087. Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/2087/oj (accessed on 1 June 2024).
- Al-Nidawi, M.; Özalp, Ö.; Alshana, U.; Soylak, M. Cloud Point Microextraction Prior to Flame-Atomic Absorption Spectrometry for the Determination of Zinc Ethylene-1,2-Bisdithiocarbamate (Zineb) in Food and Environmental Samples. Anal. Lett. 2024, 57, 1313–1324. [Google Scholar] [CrossRef]
- Pu, H.; Xie, A.; Sun, D.W.; Kamruzzaman, M.; Ma, J. Application of Wavelet Analysis to Spectral Data for Categorization of Lamb Muscles. Food Bioprocess Technol. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Moura, L.d.O.; Lopes, D.C.; Steidle Neto, A.J.; de Castro Louback Ferraz, L.; de Almeida Carlos, L.; Martins, L.M. Evaluation of Techniques for Automatic Classification of Lettuce Based on Spectral Reflectance. Food Anal. Methods 2016, 9, 1799–1806. [Google Scholar] [CrossRef]
- Gonzálvez, A.; Garrigues, S.; Armenta, S.; de la Guardia, M. Determination at Low Ppm Levels of Dithiocarbamate Residues in Foodstuff by Vapour Phase-Liquid Phase Microextraction-Infrared Spectroscopy. Anal. Chim. Acta 2011, 688, 191–196. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Pu, H.; Sun, D.W.; Gao, W.; Xiong, Z. Use of Hyperspectral Imaging to Discriminate the Variety and Quality of Rice. Food Anal. Methods 2015, 8, 515–523. [Google Scholar] [CrossRef]
- Aouidi, F.; Dupuy, N.; Artaud, J.; Roussos, S.; Msallem, M.; Perraud-Gaime, I.; Hamdi, M. Discrimination of Five Tunisian Cultivars by Mid InfraRed Spectroscopy Combined with Chemometric Analyses of Olive Olea Europaea Leaves. Food Chem. 2012, 131, 360–366. [Google Scholar] [CrossRef]
- Diago, M.P.; Fernandes, A.M.; Millan, B.; Tardaguila, J.; Melo-Pinto, P. Identification of Grapevine Varieties Using Leaf Spectroscopy and Partial Least Squares. Comput. Electron. Agric. 2013, 99, 7–13. [Google Scholar] [CrossRef]
- Garrett, R.; Schmidt, E.M.; Pereira, L.F.P.; Kitzberger, C.S.G.; Scholz, M.B.S.; Eberlin, M.N.; Rezende, C.M. Discrimination of Arabica Coffee Cultivars by Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Chemometrics. LWT—Food Sci. Technol. 2013, 50, 496–502. [Google Scholar] [CrossRef]
- Steidle Neto, A.J.; Lopes, D.C.; Toledo, J.V.; Zolnier, S.; Silva, T.G.F. Classification of Sugarcane Varieties Using Visible/near Infrared Spectral Reflectance of Stalks and Multivariate Methods. J. Agric. Sci. 2018, 156, 537–546. [Google Scholar] [CrossRef]
- Simko, I.; Jimenez-Berni, J.A.; Furbank, R.T. Detection of Decay in Fresh-Cut Lettuce Using Hyperspectral Imaging and Chlorophyll Fluorescence Imaging. Postharvest Biol. Technol. 2015, 106, 44–52. [Google Scholar] [CrossRef]
- Diezma, B.; Lleó, L.; Roger, J.M.; Herrero-Langreo, A.; Lunadei, L.; Ruiz-Altisent, M. Examination of the Quality of Spinach Leaves Using Hyperspectral Imaging. Postharvest Biol. Technol. 2013, 85, 8–17. [Google Scholar] [CrossRef]
- Huang, W.; Li, J.; Wang, Q.; Chen, L. Development of a Multispectral Imaging System for Online Detection of Bruises on Apples. J. Food Eng. 2015, 146, 62–71. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.d.M.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Zeitschrift 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.E. Spectrophotometric Determination of Dithiocarbamate Residues on Food Crops. Anal. Chem. 1964, 36, 221–224. [Google Scholar] [CrossRef]
- Keppel, G.E. Collaborative Study of the Determination of the Dithiocarbamate Residues by a Modified Carbon Disulfide Evolution Method. J. Assoc. Off. Anal. Chem. 1971, 54, 528–532. [Google Scholar] [CrossRef]
- Caldas, E.D.; Miranda, M.C.C.; Conceição, M.H.; De Souza, L.C.K.R. Dithiocarbamates Residues in Brazilian Food and the Potential Risk for Consumers. Food Chem. Toxicol. 2004, 42, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.C.; Steidle Neto, A.J. Classification and Authentication of Plants by Chemometric Analysis of Spectral Data. Compr. Anal. Chem. 2018, 80, 105–125. [Google Scholar]
- Moscetti, R.; Haff, R.P.; Stella, E.; Contini, M.; Monarca, D.; Cecchini, M.; Massantini, R. Feasibility of NIR Spectroscopy to Detect Olive Fruit Infested by Bactrocera Oleae. Postharvest Biol. Technol. 2015, 99, 58–62. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, Y.; Loraamm, R.W.; Nie, C.; Wang, J.; Zhang, J. Spectral Analysis of Winter Wheat Leaves for Detection and Differentiation of Diseases and Insects. F. Crop. Res. 2014, 156, 199–207. [Google Scholar] [CrossRef]
- Martens, H.; Næs, T. Multivariate Calibration; John Wiley & Sons: Hoboken, NJ, USA, 1992; Volume 1, ISBN 0471930474. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Wang, L.; Mizaikoff, B. Application of Multivariate Data-Analysis Techniques to Biomedical Diagnostics Based on Mid-Infrared Spectroscopy. Anal. Bioanal. Chem. 2008, 391, 1641–1654. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised Pattern Recognition in Food Analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef] [PubMed]
- Saporta, G. Probabilités, Analyse Des Données et Statistique, 3rd ed.; TECHNIP: Paris, France, 2011; ISBN 978-2710809807. [Google Scholar]
- Westad, F.; Marini, F. Validation of Chemometric Models—A Tutorial. Anal. Chim. Acta 2015, 893, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, H.; Xu, H.; Ying, Y. Near Infrared Spectroscopy for on/in-Line Monitoring of Quality in Foods and Beverages: A Review. J. Food Eng. 2008, 87, 303–313. [Google Scholar] [CrossRef]
- Agelet, L.E.; Hurburgh, C.R. Limitations and Current Applications of Near Infrared Spectroscopy for Single Seed Analysis. Talanta 2014, 121, 288–299. [Google Scholar] [CrossRef]
- Devaux, M.F.; Bertrand, D.; Robert, P.; Qannari, M. Application of Multidimensional Analyses to the Extraction of Discriminant Spectral Patterns from NIR Spectra. Appl. Spectrosc. 1988, 42, 1015–1019. [Google Scholar] [CrossRef]
- Congalton, R.G. A Review of Assessing the Accuracy of Classifications of Remotely Sensed Data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Salkind, N.J. Encyclopedia of Measurement and Statistics, 1st ed.; Salkind, N.J., Ed.; SAGE Publications: Newcastle upon Tyne, UK, 2007; ISBN 1412916119. [Google Scholar]
- Gold, K.M.; Townsend, P.A.; Herrmann, I.; Gevens, A.J. Investigating Potato Late Blight Physiological Differences across Potato Cultivars with Spectroscopy and Machine Learning. Plant Sci. 2020, 295, 110316. [Google Scholar] [CrossRef] [PubMed]
- Fantke, P.; Juraske, R. Variability of Pesticide Dissipation Half-Lives in Plants. Environ. Sci. Technol. 2013, 47, 3548–3562. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, B.; Mohajerani, E.; Jamshidi, J.; Minaei, S.; Sharifi, A. Non-Destructive Detection of Pesticide Residues in Cucumber Using Visible/near-Infrared Spectroscopy. Food Addit. Contam. Part A 2015, 32, 857–863. [Google Scholar] [CrossRef]
- Rojas, F.S.; Ojeda, C.B. Recent Development in Derivative Ultraviolet/Visible Absorption Spectrophotometry: 2004–2008: A Review. Anal. Chim. Acta 2009, 635, 22–44. [Google Scholar] [CrossRef]
- Yu, G.; Li, H.; Li, Y.; Hu, Y.; Wang, G.; Ma, B.; Wang, H. Multiscale Deepspectra Network: Detection of Pyrethroid Pesticide Residues on the Hami Melon. Foods 2023, 12, 1742. [Google Scholar] [CrossRef]
- Steidle Neto, A.J.; Moura, L.d.O.; Lopes, D.C.; Carlos, L.A.; Martins, L.M.; De Castro Louback Ferraz, L. Non-Destructive Prediction of Pigment Content in Lettuce Based on Visible–NIR Spectroscopy. J. Sci. Food Agric. 2017, 97, 2015–2022. [Google Scholar] [CrossRef]
- Dias, M.C.; Figueiredo, P.; Duarte, I.F.; Gil, A.M.; Santos, C. Different Responses of Young and Expanded Lettuce Leaves to Fungicide Mancozeb: Chlorophyll Fluorescence, Lipid Peroxidation, Pigments and Proline Content. Photosynthetica 2014, 52, 148–151. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Gaiad, J.E.; Hidalgo, M.J.; Avanza, M.V.; Pellerano, R.G. Classification of Cowpea Beans Using Multielemental Fingerprinting Combined with Supervised Learning. Food Control 2019, 95, 232–241. [Google Scholar] [CrossRef]
- Borràs, E.; Amigo, J.M.; Van Den Berg, F.; Boqué, R.; Busto, O. Fast and Robust Discrimination of Almonds (Prunus Amygdalus) with Respect to Their Bitterness by Using near Infrared and Partial Least Squares-Discriminant Analysis. Food Chem. 2014, 153, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Robotti, E.; Quasso, F.; Mazzucco, E.; Calabrese, G.; Marengo, E. Fast Classification of Hazelnut Cultivars through Portable Infrared Spectroscopy and Chemometrics. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2018, 189, 427–435. [Google Scholar] [CrossRef] [PubMed]

| Country/Organisation | Crop | † MRLs (mg CS2 kg−1) | Reference |
|---|---|---|---|
| Canada | Lettuce | 0.1 | [15] |
| Codex Alimentarius (FAO/WHO) | Lettuce (head) | 0.5 | [16] |
| Great Britain | Lettuce | 5.0 | [17] |
| Israel | Lettuce | 5.0 | [18] |
| Australia | Leafy vegetables | 5.0 | [19] |
| New Zealand | Vegetables | 7.0 | [20] |
| Japan | Lettuce (leaf) | 10.0 | [21] |
| Hong Kong | Lettuce (head/leaf) | 0.5/18.0 | [22] |
| United States of America | Lettuce (head/leaf) | 3.5/18.0 | [23] |
| South Korea | Lettuce (head/leaf) | 20.0/10.0 | [24] |
| Detector: 2048-element linear silicon CCD array sensor | Signal-to-noise ratio: 250:1 (full signal) |
| Wavelength range (grating): 400–1000 nm | Display: organic light-emitting diode (128 × 64 pixels) |
| Optical resolution: 1.3 nm (full width at half maximum) | Light source (range): 360–1100 nm (tungsten-halogen) |
| Integration time: 870 μs to 65 s | Light source (lifetime): 500–10,000 h |
| Entrance aperture: 25 μm width slit | Battery: rechargeable lithium-ion |
| Fiber optic connector: type SMA 905 | Data storage: SD card (2 GB capacity) |
| Time (days) | Dithiocarbamate (mg CS2 kg−1) | Standard Error (mg CS2 kg−1) |
|---|---|---|
| 1 | 10.3 | ±1.70 |
| 3 | 5.6 | ±1.11 |
| 5 | 2.4 | ±0.87 |
| 7 | 1.0 | ±0.50 |
| 9 | 0.7 | ±0.23 |
| 11 | 0.4 | ±0.17 |
| 13 | 0.1 | ±0.08 |
| Calibration with Cross-Validation | External Validation | ||||
|---|---|---|---|---|---|
| 3.5 mg CS2 kg−1 | |||||
| >3.5 | ≤3.5 | >3.5 | ≤3.5 | ||
| >3.5 | 97.73% | 3.85% | >3.5 | 97.47% | 5.47% |
| ≤3.5 | 2.27% | 96.15% | ≤3.5 | 2.53% | 94.53% |
| 5.0 mg CS2 kg−1 | |||||
| >5.0 | ≤5.0 | >5.0 | ≤5.0 | ||
| >5.0 | 97.96% | 0.00% | >5.0 | 97.51% | 0.00% |
| ≤5.0 | 2.04% | 100.00% | ≤5.0 | 2.49% | 100.00% |
| 7.0 mg CS2 kg−1 | |||||
| >7.0 | ≤7.0 | >7.0 | ≤7.0 | ||
| >7.0 | 98.15% | 0.00% | >7.0 | 96.99% | 5.03% |
| ≤7.0 | 1.85% | 100.00% | ≤7.0 | 3.01% | 94.97% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steidle Neto, A.J.; de Lima, J.L.M.P.; Jardim, A.M.d.R.F.; Lopes, D.d.C.; Silva, T.G.F.d. Discrimination of Fungicide-Contaminated Lettuces Based on Maximum Residue Limits Using Spectroscopy and Chemometrics. Horticulturae 2024, 10, 828. https://doi.org/10.3390/horticulturae10080828
Steidle Neto AJ, de Lima JLMP, Jardim AMdRF, Lopes DdC, Silva TGFd. Discrimination of Fungicide-Contaminated Lettuces Based on Maximum Residue Limits Using Spectroscopy and Chemometrics. Horticulturae. 2024; 10(8):828. https://doi.org/10.3390/horticulturae10080828
Chicago/Turabian StyleSteidle Neto, Antonio José, João L. M. P. de Lima, Alexandre Maniçoba da Rosa Ferraz Jardim, Daniela de Carvalho Lopes, and Thieres George Freire da Silva. 2024. "Discrimination of Fungicide-Contaminated Lettuces Based on Maximum Residue Limits Using Spectroscopy and Chemometrics" Horticulturae 10, no. 8: 828. https://doi.org/10.3390/horticulturae10080828
APA StyleSteidle Neto, A. J., de Lima, J. L. M. P., Jardim, A. M. d. R. F., Lopes, D. d. C., & Silva, T. G. F. d. (2024). Discrimination of Fungicide-Contaminated Lettuces Based on Maximum Residue Limits Using Spectroscopy and Chemometrics. Horticulturae, 10(8), 828. https://doi.org/10.3390/horticulturae10080828







