Dormancy Release and Seed Germination in Tulipa saxatilis (Liliaceae) Coupled with Effects of Fertilization Schemes for Bulblet Development from Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ecological Profiling of Tulipa saxatilis
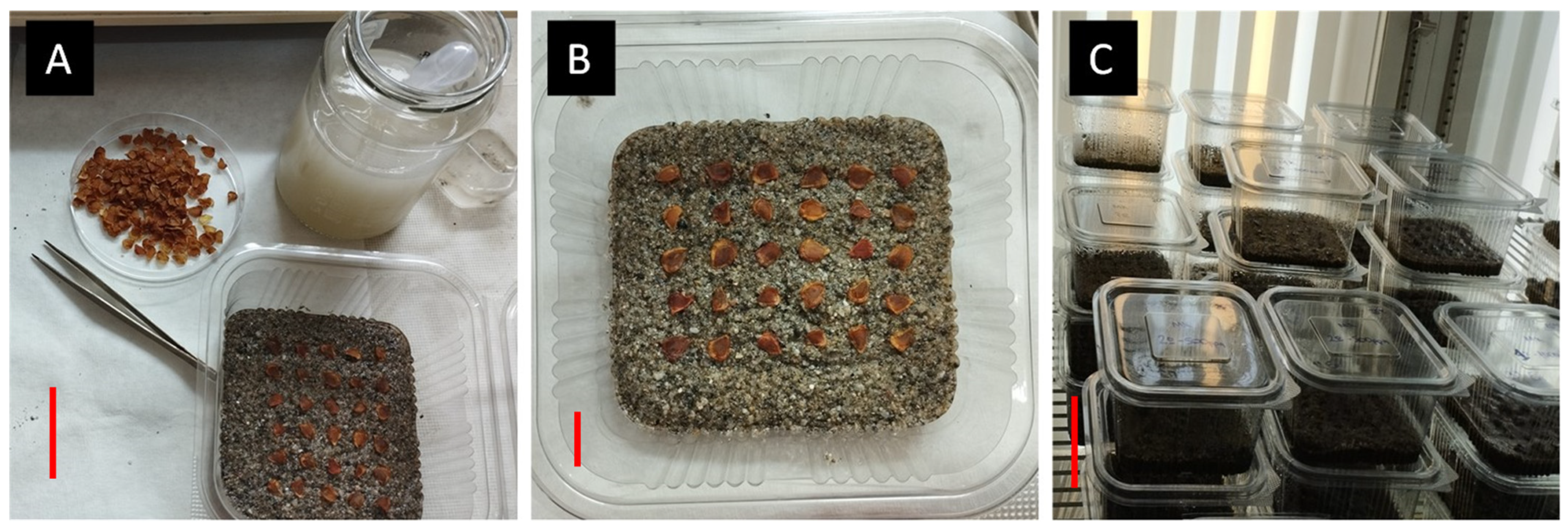
2.3. Effect of Temperature on Seed Germination
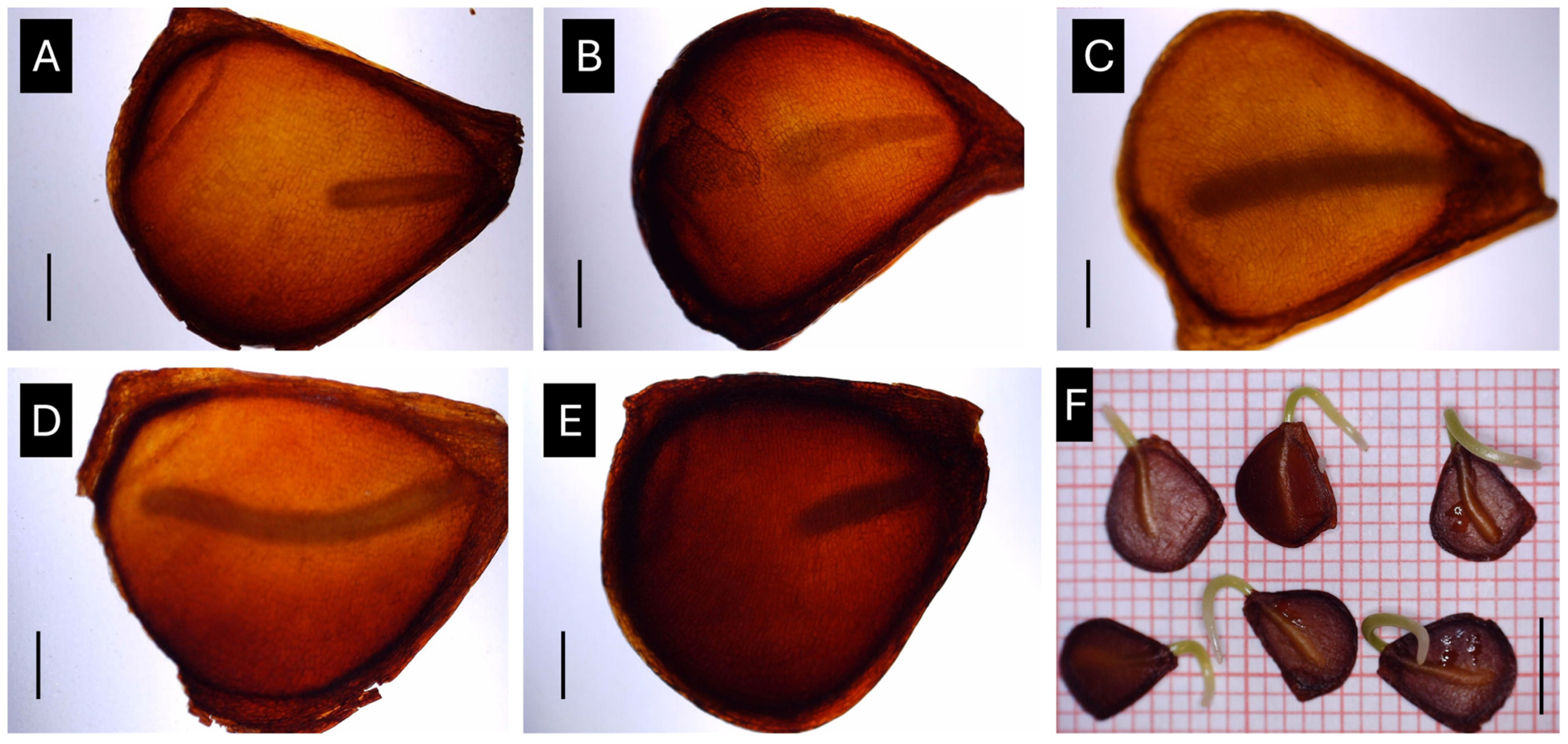
2.4. Seed Embryo Testing and Effect of Temperature on Embryonic Growth
2.5. Seedling Production
2.6. Statistical Analysis
3. Results
3.1. Bioclimatic Profiling
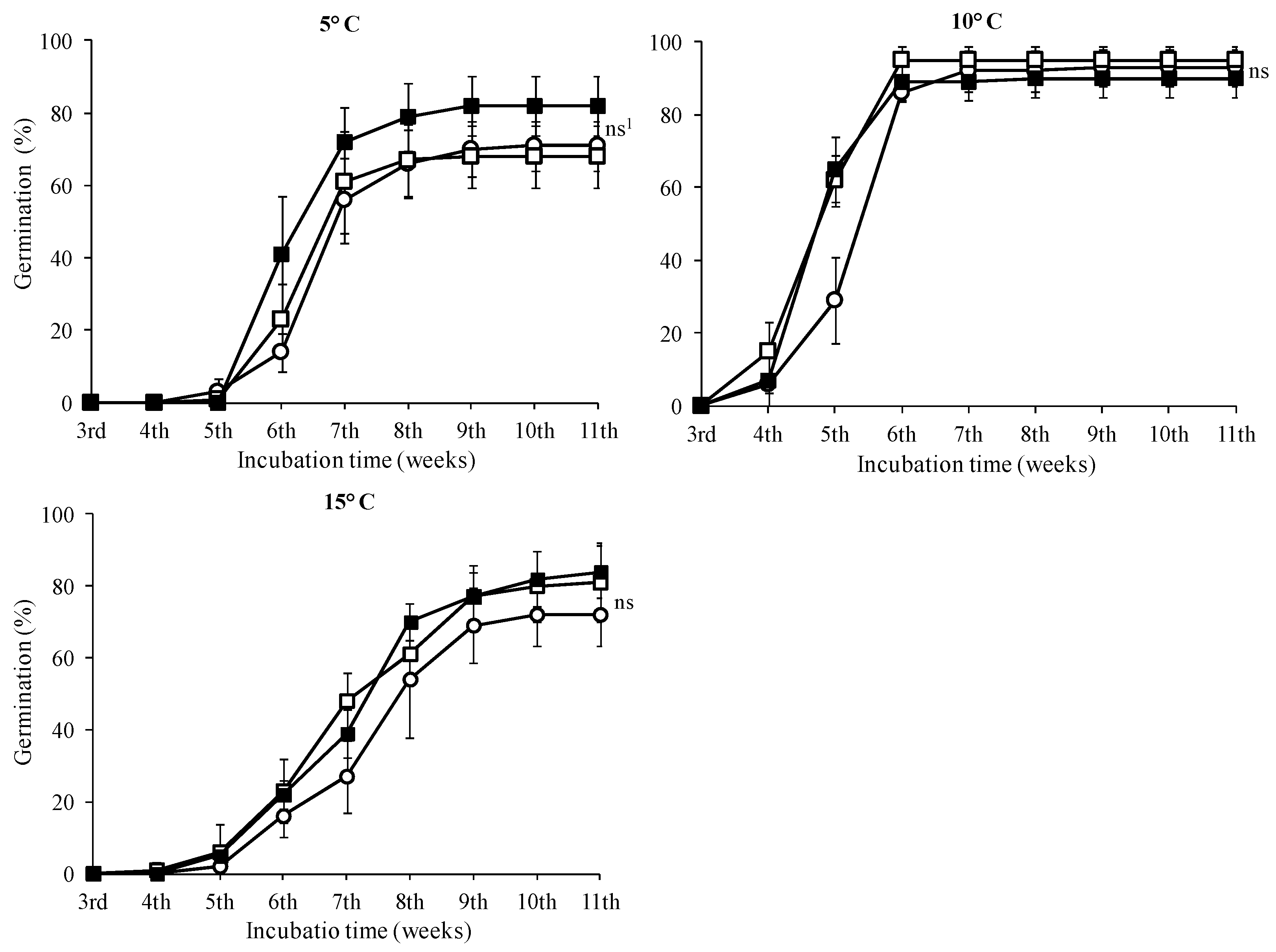
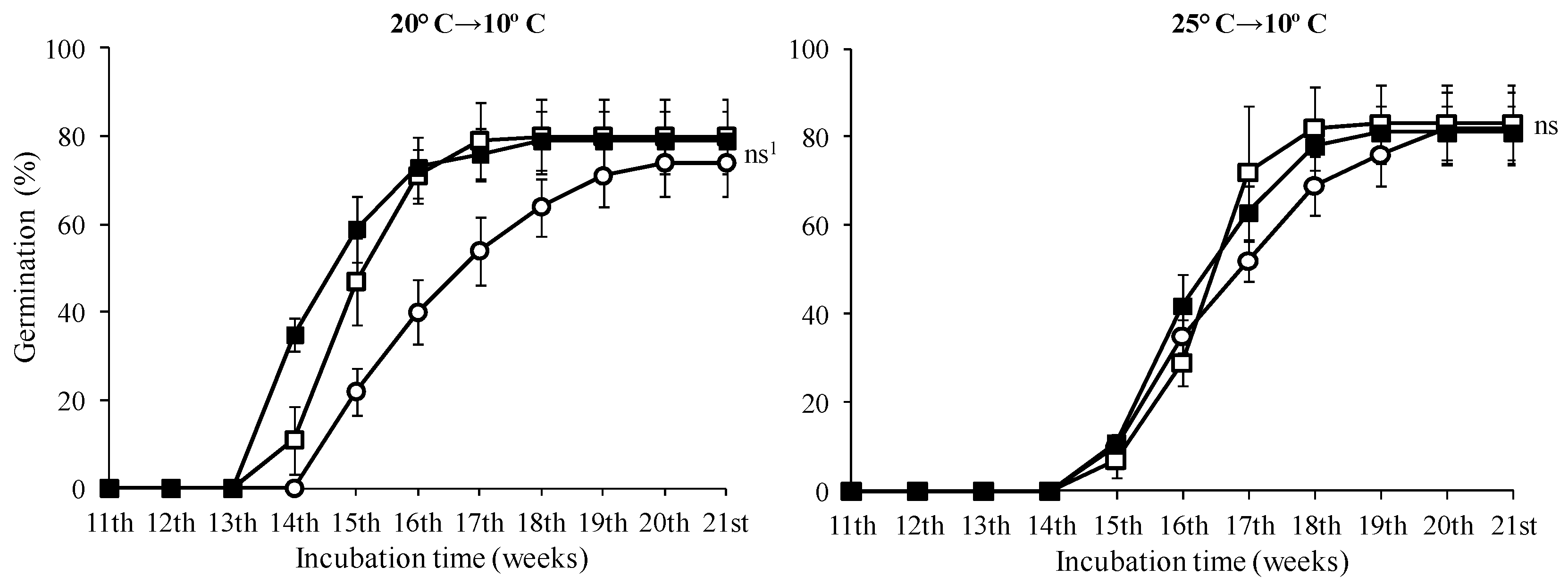
3.2. Seed Germination Tests
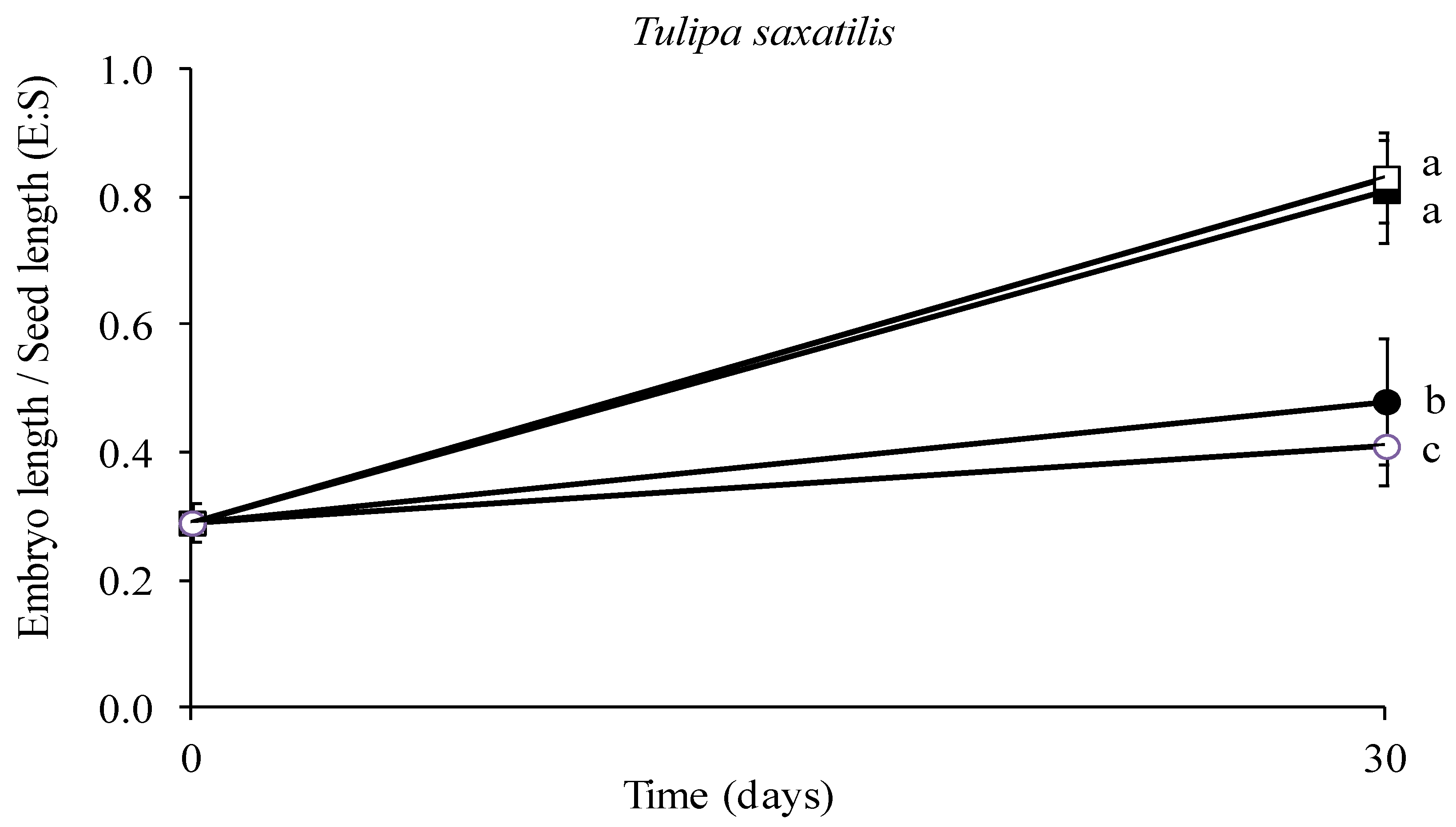
3.3. Effect of Temperature on Embryo Growth
3.4. Bulblet Production from Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marasek-Ciolakowska, A.; Nishikawa, T.; Shea, D.J.; Okazaki, K. Breeding of lilies and tulips-Interspecific hybridization and genetic background. Breed. Sci. 2018, 68, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Bilias, F.; Karagianni, A.-G.; Ipsilantis, I.; Samartza, I.; Krigas, N.; Tsoktouridis, G.; Matsi, T. Adaptability of wild-growing tulips of Greece: Uncovering relationships between soil properties, rhizosphere fungal morphotypes and nutrient content profiles. Biology 2023, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, J.; Xue, L.; Dai, H.; Lei, J. Seed morphology and germination of native Tulipa species. Agriculture 2023, 13, 466. [Google Scholar] [CrossRef]
- Mackay, A.C.; Mcgill, C.R.; Fountain, D.W.; Southward, R.C. Seed dormancy and germination of a panel of New Zealand plants suitable for re-vegetation. N. Z. J. Bot. 2002, 40, 373–382. [Google Scholar] [CrossRef]
- Pipinis, E.; Hatzilazarou, S.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Samartza, I.; Plastiras, I.; Kriemadi, E.; Bareka, P.; et al. Effect of temperature on breaking of morphophysiological dormancy and seed germination leading to bulblet production in two endemic tulip species from Greece. Plants 2023, 12, 1859. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Classification, biogeography, and phylogenetic relationships of seed dormancy. In Seed Conservation: Turning Science into Practice; Smith, R.D., Dickie, J.B., Simon, H., Pritchard, H.W., Probert, R.J., Eds.; The Royal Botanic Gardens, Kew: London, UK, 2003; pp. 518–544. Available online: https://www.researchgate.net/publication/265158564_Chapter_28_Classification_Biogeography_and_Phylogenetic_Relationships_of_Seed_Dormancy#fullTextFileContent (accessed on 30 May 2024).
- Finch-Savage, W.E.; Footitt, S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J. Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; ISBN 9780124166776. [Google Scholar]
- Samartza, I.; Tsaballa, A.; Sakellariou, M.; Mahnev, P.; Tsiripidis, I.; Krigas, N.; Tsoktouridis, G. Taxonomic and molecular characterization of 15 wild-growing tulip species of Greece using the internal transcribed spacer (ITS) nuclear marker in combination with the psbA-trnH and trnL/trnF plastid markers. Biotechnol. Biotechnol. Equip. 2024, 38, 2337694. [Google Scholar] [CrossRef]
- Krigas, N.; Lykas, C.; Ipsilantis, I.; Matsi, T.; Weststrand, S.; Havström, M.; Tsoktouridis, G. Greek tulips: Worldwide electronic trade over the internet, global ex situ conservation and current sustainable exploitation challenges. Plants 2021, 10, 580. [Google Scholar] [CrossRef]
- Strid, A. Atlas of the Aegean Flora. Part 1: Text & Plates. Part 2: Maps, 1st ed.; Englera 33 (1 & 2); Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, Germany, 2016; ISBN 9783921800973. [Google Scholar] [CrossRef]
- Bareka, P.; Kriemadi, E.; Samartza, I.; Koutsovoulou, K.; Krigas, N. Tulipa saxatilis Sieber ex Spreng—Near Threatened (NT). The IUCN Red List of Threatened Species 2024, submitted. Available online: https://www.iucnredlist.org/ (accessed on 30 July 2024).
- Rouhi, H.R.; Shakarami, R.; Tavakkol Afshari, R. Seed Treatments to Overcome Dormancy of Waterlily Tulip (Tulipa kaufmanniana Regel.). Austr. J. Crop Sci. 2010, 4, 718–721. Available online: https://www.cropj.com/tavakol_4_9_2010_718_721.pdf (accessed on 30 July 2024).
- Hatzilazarou, S.; Pipinis, E.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Avramakis, M.; Samartza, I.; Plastiras, I.; Kriemadi, E.; et al. Influence of temperature on seed germination of five wild-growing Tulipa species of Greece associated with their ecological profiles: Implications for conservation and cultivation. Plants 2023, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing 2021. Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 20 June 2024).
- Hijmans, R.J.; van Etten, J. Raster: Geographic Analysis and Modeling with Raster Data. 2012. Available online: http://raster.r-forge.r-project.org/ (accessed on 10 June 2024).
- International Seed Testing Association. International Rules for Seed Testing. Seed Sci. Technol. 1999, 27, 25–30. [Google Scholar]
- Thanos, C.A.; Kadis, C.C.; Skarou, F. Ecophysiology of germination in the aromatic plants thyme, savory and oregano (Labiatae). Seed Sci. Res. 1995, 5, 161–170. [Google Scholar] [CrossRef]
- Snedecor, G.; Cochran, W. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Klockars, A.J.; Sax, G. Multiple Comparisons; Quantitative applications in the social sciences, No. 61.; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1986; ISBN 0-8039-2051-2. [Google Scholar]
- Carasso, V.; Hay, F.R.; Probert, R.J.; Mucciarelli, M. Temperature control of seed germination in Fritillaria tubiformis subsp. moggridgei (Liliaceae) a rare endemic of the South-west Alps. Seed Sci. Res. 2011, 21, 33–38. [Google Scholar] [CrossRef][Green Version]
- Luo, M.; Gao, J.; Liu, R.; Wang, S.; Wang, G. Morphological and anatomical changes during dormancy break of the seeds of Fritillaria taipaiensis. Plant Signal. Behav. 2023, 18, 2194748. [Google Scholar] [CrossRef]
- Gashi, B.; Osmani, M.; Aliu, S. Breaking seed dormancy of Tulipa scardica Bornm. and Tulipa kosovarica Kit Tan, Shuka & Krasniqi by pre-chilling, plant growth regulators and some chemical treatments. Acta Agric. Slov. 2019, 113, 203–210. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, L.-W.; Zhao, J.; Xue, L.; Dai, H.-P.; Xing, G.-M.; Lei, J.-J. Practical methods for breaking seed dormancy in a wild ornamental tulip species Tulipa thianschanica Regel. Agronomy 2020, 10, 1765. [Google Scholar] [CrossRef]
- van Tuyl, J.; Creij, M.G.M. Tulip: Tulipa gesneriana and Tulipa hybrids. In Flower Breeding & Genetics: Issues, Challenges and Opportunities for the 21st Century; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 623–641. [Google Scholar] [CrossRef]
- Lazare, S.; Zaccai, M. Flowering pathway is regulated by bulb size in Lilium longiflorum (Easter lily). Plant Biol. 2016, 18, 577–584. [Google Scholar] [CrossRef]
- Alsaleh, T.; Awadh, A. The Effect of the Size of the Bulbs and the Type of Fertilizer in the Number and Length of Leaves and Flower Production in Lilium longiflorum. Marsh Bull. 2020, 15, 92–102. Available online: https://www.iasj.net/iasj/download/bd0dc098b3f5c795 (accessed on 3 July 2024).
- Kumar, N.; Prasad, V.; Kerketta, A.; Das, K. Effect of organic and inorganic fertilizer on vegetative growth parameter of Lilium (Lilium longiflorum) cv. pavia under shade net condition. Int. J. Environ. Clim. Change 2023, 13, 323–328. [Google Scholar] [CrossRef]
- Niedziela, C.E.; Kim, S.H.; Nelson, P.V.; De Hertogh, A.A. Effects of N–P–K deficiency and temperature regime on the growth and development of Lilium longiflorum ‘Nellie White’ during bulb production under phytotron conditions. Sci. Hortic. 2008, 116, 430–436. [Google Scholar] [CrossRef]
- Wang, Y.; Bu, H.; Wang, H.; Zhang, P.; Jin, L. Effects of applying nitrogen and potassium on Lilium lancifolium growth and accumulation of secondary metabolites in bulbs. Horticulturae 2023, 9, 396. [Google Scholar] [CrossRef]




| Seed Treatment | Incubation Temperatures | ||
|---|---|---|---|
| 5 °C | 10 °C | 15 °C | |
| 0 mg·L−1 GA3 | 71.00 b 1 ± 6.83 | 93.00 a 1 ± 5.03 | 72.00 b ± 8.64 |
| 500 mg·L−1 GA3 | 68.00 b ± 8.64 | 95.00 a ± 3.83 | 81.00 b ± 11.02 |
| 1000 mg·L−1 GA3 | 82.00 a ± 8.33 | 90.00 a ± 5.16 | 84.00 a ± 7.30 |
| Length (mm) | Width (mm) | Weight (g) | |
|---|---|---|---|
| Fertilization (F) | * | ns | * |
| Control | 6.28 b 1 ± 0.12 | 3.96 a ± 0.07 | 0.042 ab ± 0.001 |
| Chemical fertilization (ChF) | 6.65 a ± 0.13 | 3.86 a ± 0.07 | 0.040 b ± 0.001 |
| Integrated nutrient management (INM) | 6.86 a ± 0.14 | 4.02 a ± 0.08 | 0.046 a ± 0.002 |
| Biostimulant | 6.67 a ± 0.12 | 3.86 a ± 0.07 | 0.041 b ± 0.001 |
| Seed pre-treatment with GA3 | * | * | * |
| 0 mg·L−1 GA3 | 6.83 a ± 0.11 | 4.07 a ± 0.06 | 0.045 a ± 0.001 |
| 500 mg·L−1 GA3 | 6.43 b ± 0.12 | 3.78 b ± 0.07 | 0.040 b ± 0.001 |
| 1000 mg·L−1 GA3 | 6.58 ab ± 0.11 | 3.93 ab ± 0.06 | 0.041 b ± 0.001 |
| F × GA3 | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozoni, M.; Samartza, I.; Pipinis, E.; Kostas, S.; Anestis, I.; Karapatzak, E.; Bareka, P.; Hatzilazarou, S.; Tsoktouridis, G.; Krigas, N. Dormancy Release and Seed Germination in Tulipa saxatilis (Liliaceae) Coupled with Effects of Fertilization Schemes for Bulblet Development from Seedlings. Horticulturae 2024, 10, 820. https://doi.org/10.3390/horticulturae10080820
Kozoni M, Samartza I, Pipinis E, Kostas S, Anestis I, Karapatzak E, Bareka P, Hatzilazarou S, Tsoktouridis G, Krigas N. Dormancy Release and Seed Germination in Tulipa saxatilis (Liliaceae) Coupled with Effects of Fertilization Schemes for Bulblet Development from Seedlings. Horticulturae. 2024; 10(8):820. https://doi.org/10.3390/horticulturae10080820
Chicago/Turabian StyleKozoni, Marianthi, Ioulietta Samartza, Elias Pipinis, Stefanos Kostas, Ioannis Anestis, Eleftherios Karapatzak, Pepy Bareka, Stefanos Hatzilazarou, Georgios Tsoktouridis, and Nikos Krigas. 2024. "Dormancy Release and Seed Germination in Tulipa saxatilis (Liliaceae) Coupled with Effects of Fertilization Schemes for Bulblet Development from Seedlings" Horticulturae 10, no. 8: 820. https://doi.org/10.3390/horticulturae10080820
APA StyleKozoni, M., Samartza, I., Pipinis, E., Kostas, S., Anestis, I., Karapatzak, E., Bareka, P., Hatzilazarou, S., Tsoktouridis, G., & Krigas, N. (2024). Dormancy Release and Seed Germination in Tulipa saxatilis (Liliaceae) Coupled with Effects of Fertilization Schemes for Bulblet Development from Seedlings. Horticulturae, 10(8), 820. https://doi.org/10.3390/horticulturae10080820








