Are Cytological and Morphological Analyses Sufficient in Ploidy Determination of Watermelon Haploid Plants?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Ploidy Level
Flow Cytometry Method
Stomatal Examinations
2.2.2. Determining Morphological Differences between Haploid and Double Haploid Plants
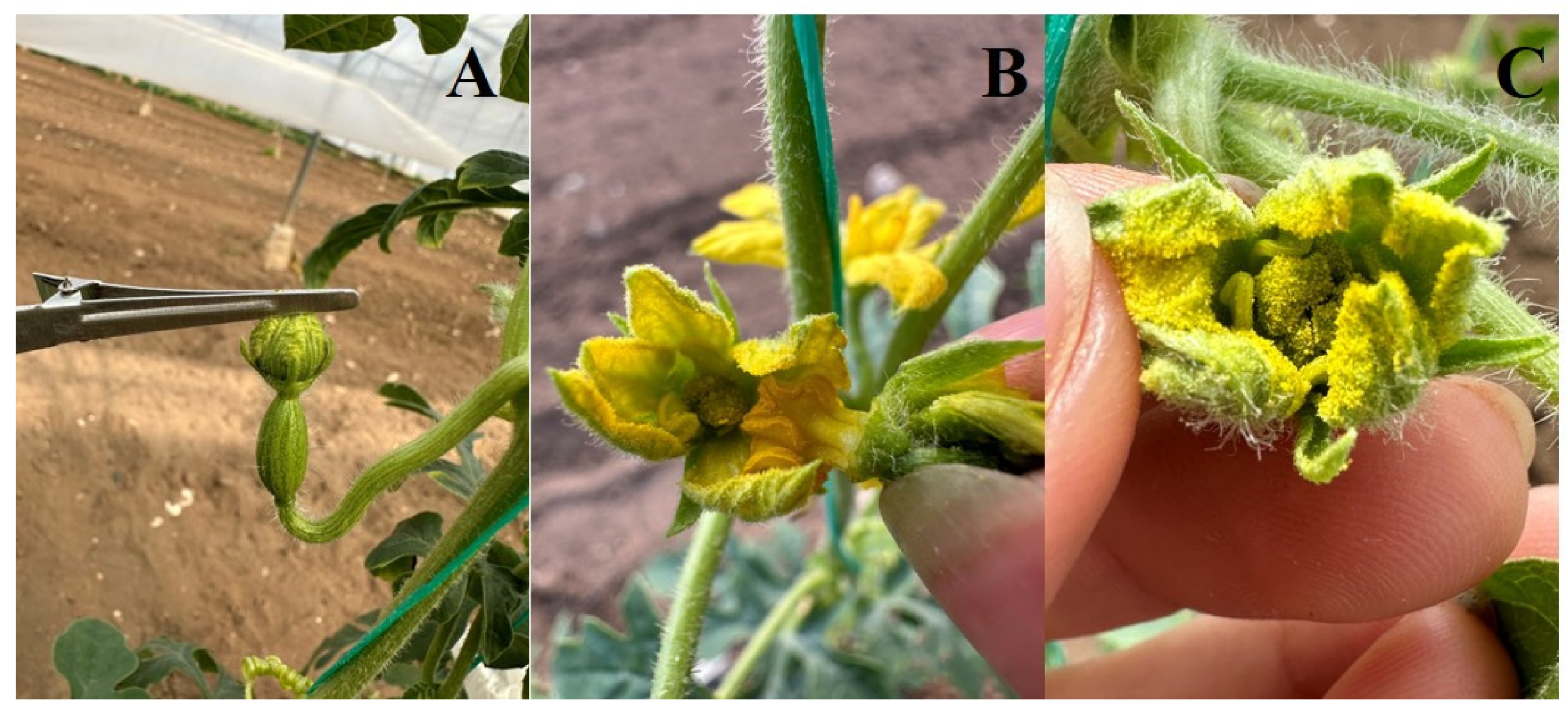
Pollen Analysis
Determination of In Vitro Pollen Viability Rate (%)
Determination of In Vitro Pollen Germination Rate (%)
2.2.3. Inbreeding Success (%)
2.2.4. Statistical Analysis
3. Results
3.1. Determination of Ploidy Levels of Plants
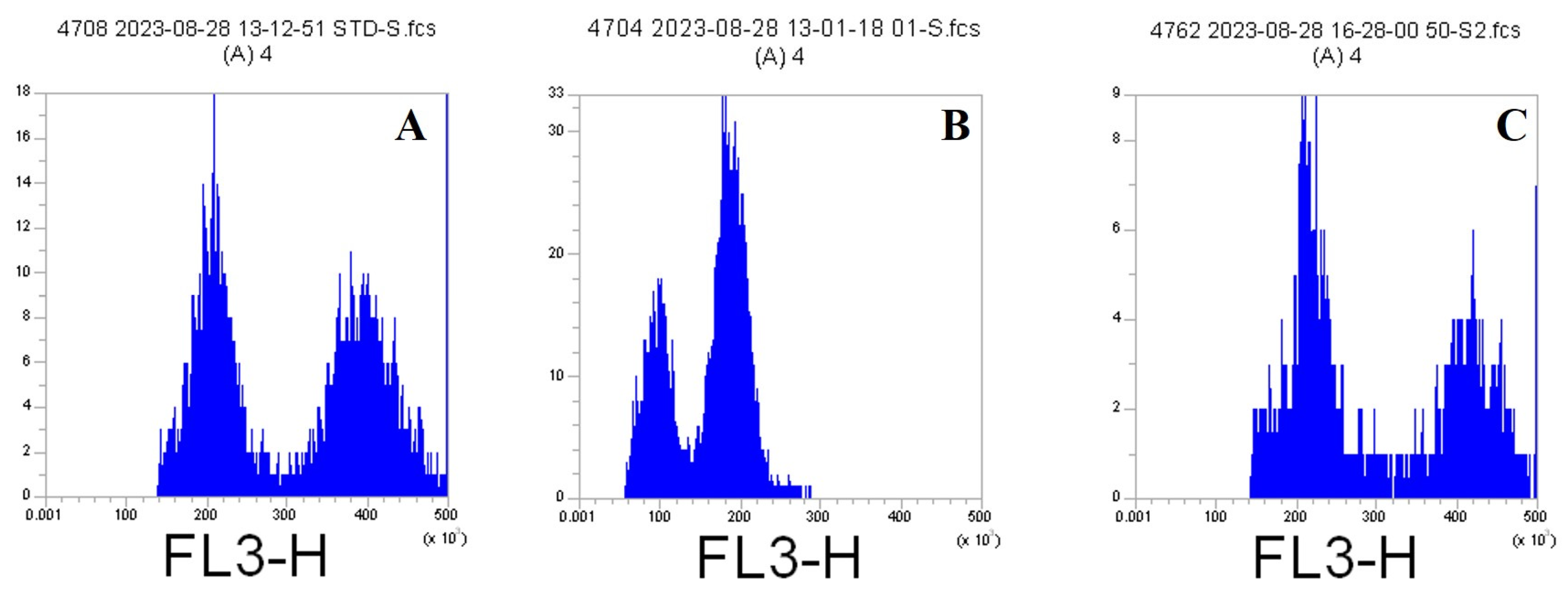
3.1.1. Flow Cytometry
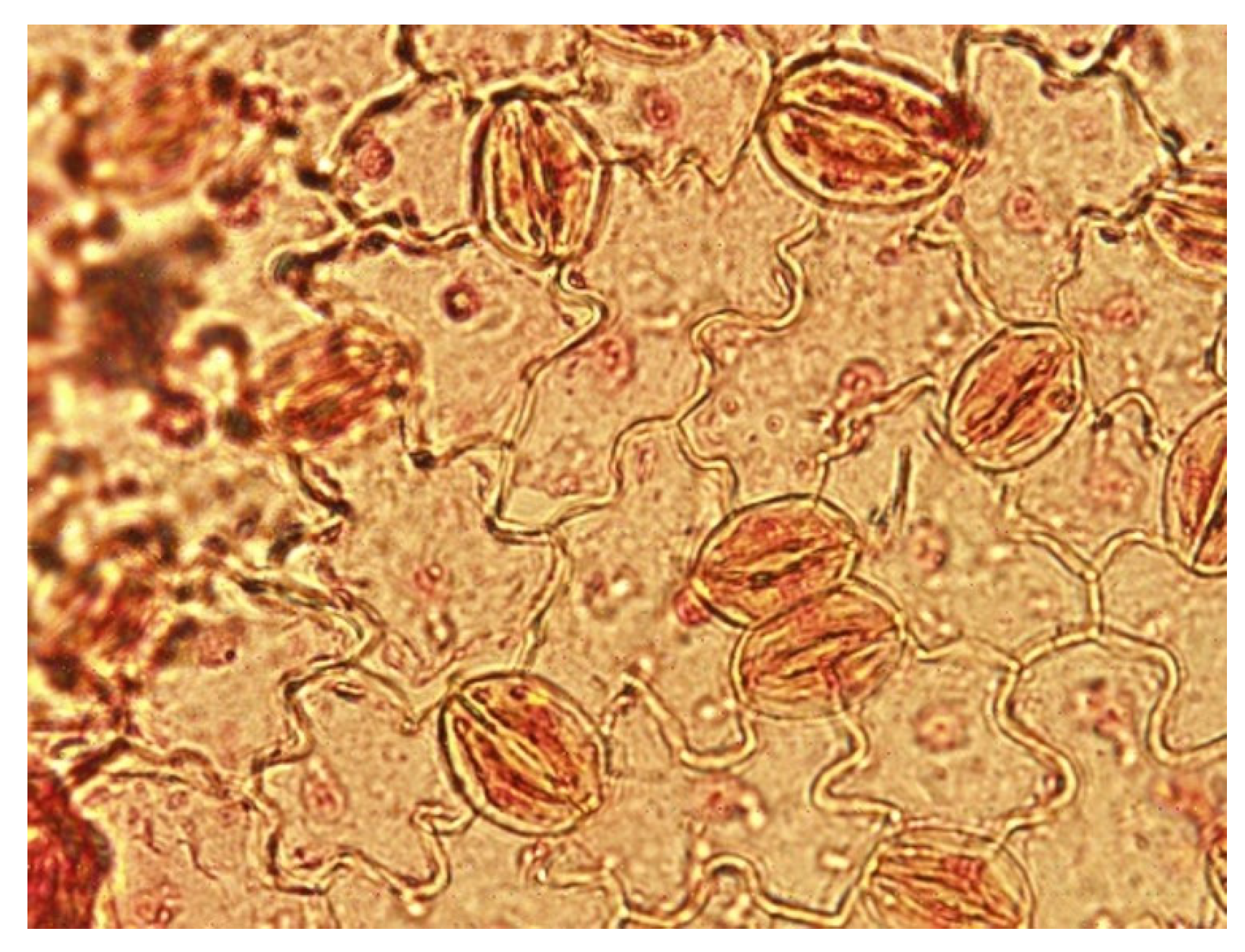
3.1.2. Stomatal Examinations
3.2. Determining Morphological Differences between Haploid and Double Haploid Plants
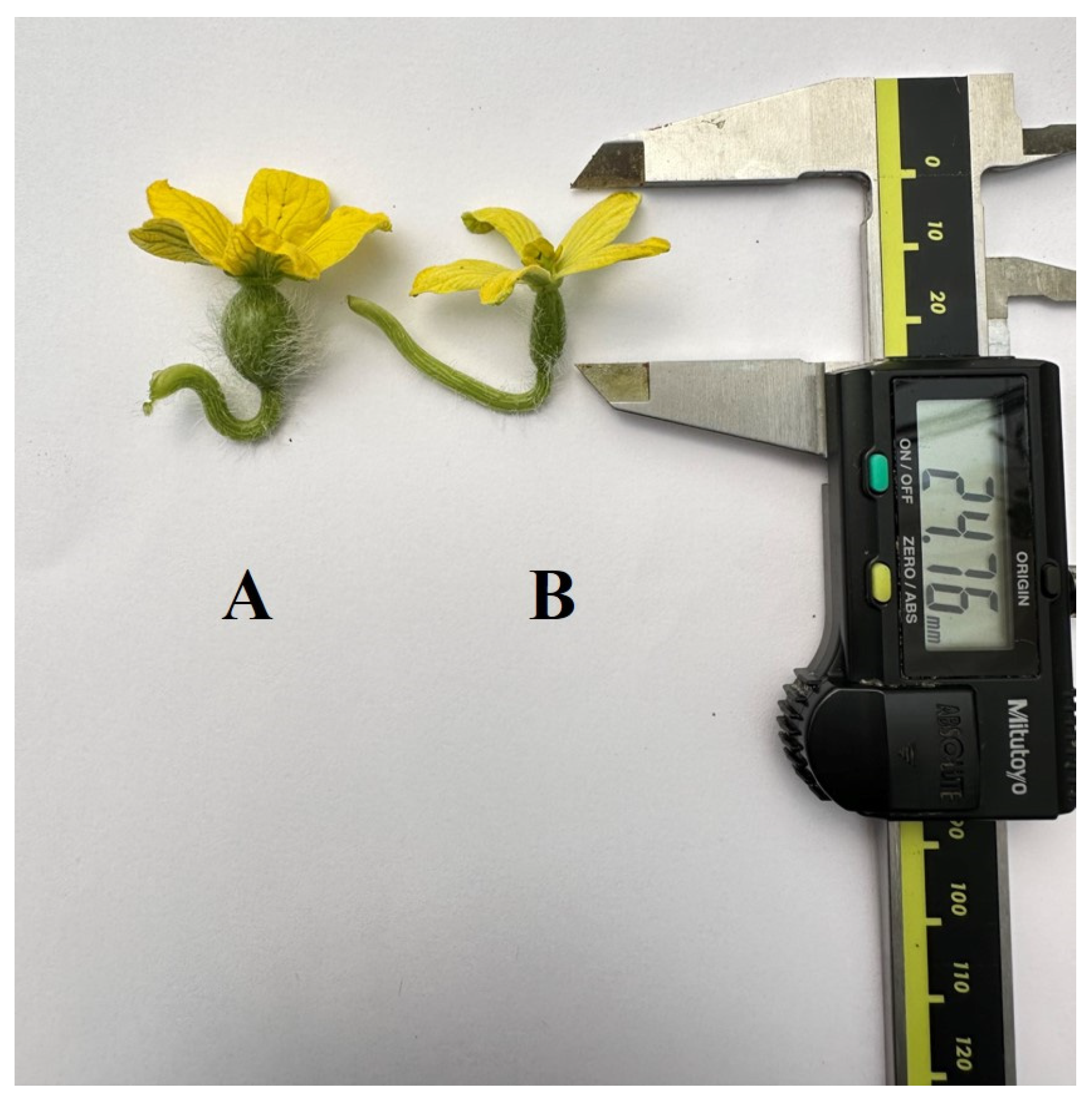
3.2.1. Flower Characteristics of Haploid and Double Haploid Plants
3.2.2. Pollen Analysis
3.3. Inbreeding Success
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dou, J.; Kang, Q.; Li, T.; Umer, M.J.; Alharthi, B.; Liu, D.; Yang, S.; Niu, H.; Ma, C.; Zhu, H.; et al. Construction and application of a new watermelon germplasm with the phenotype of dwarf and branchless. Funct. Integr. Genom. 2023, 23, 310. [Google Scholar] [CrossRef] [PubMed]
- Jordana, C.N.; Stapleton, S.C.; Colee, J.C.; Lee, S.; Gao, Z.; Ray, Z.T.; Anrecio, L.R.; Freed, D.J.; Zhao, X. How Does Watermelon Grafting Impact Fruit Yield and Quality? A Systematic Review. HortScience 2023, 58, 836–845. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, J.; Zhao, H.; Zong, M.; Li, M.; Gong, G.; Wang, J.; Zhang, J.; Ren, Y.; Zhang, H.; et al. Production of double haploid watermelon via maternal haploid induction. Plant Biotechnol. J. 2023, 21, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nation. Available online: http://www.fao.org (accessed on 31 March 2024).
- TÜİK. Türkiye İstatistik Kurumu (TÜİK). Available online: https://www.tuik.gov.tr/ (accessed on 31 March 2024).
- Sarı, N.; Solmaz, İ. Doubled Haploid Production in Watermelon. Methods Mol. Biol. 2021, 2289, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.C.; Coleman, J.D.; Kearns, A. Plant Cell Culture; Bios Scientific Publications: London, UK, 2003; p. 192. [Google Scholar]
- Baktemur, G.; Keleş, D.; Kara, E.; Taşkın, H. Effects of genotype and nutrient medium on obtaining haploid plants through ovary culture in cucumber. Mol. Biol. Rep. 2022, 49, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Yiqin, G.; Bo, D.; Tonghui, R. Induction of watermelon anther callus. Hubei Agric. Sci. 2005, 5, 93–95. [Google Scholar]
- Zhu, Y.; Sun, Z.; Sun, D.; Deng, Y.; Wang, Z.; Liu, J. Advances of watermelon anther culture technology. China Cucurbits Veg. 2010, 23, 28–31. [Google Scholar]
- Taskin, H.; Büyükalaca, S.; Keleş, D.; Ekbiç, E. Induction of microspore-derived embryos by anther culture in selected pepper genotypes. Afr. J. Biotechnol. 2011, 10, 17116–17121. [Google Scholar]
- Zhu, Y.; Sun, D.; Deng, Y.; An, G.; Li, W.; Liu, J. Effects of preliminary treatment on watermelon anther culture callus induction. China Cucurbits Veg. 2012, 25, 17–19. [Google Scholar]
- Keleş, D.; Pınar, H.; Ata, A.; Taşkın, H.; Yıldız, S.; Büyükalaca, S. Effect of pepper types on obtaining spontaneous doubled haploid plants via anther culture. HortScience 2015, 50, 1671–1676. [Google Scholar] [CrossRef]
- Ata, A.; Keleş, D.; Taşkın, H.; Büyükalaca, S. Effects of season, genotype, and nutrient medium on pepper anther culture and microspore development. Turk. J. Agric. For. 2019, 43, 123–137. [Google Scholar] [CrossRef]
- Zou, T.; Su, H.N.; Wu, Q.; Sun, X.W. Haploid induction via unfertilized ovary culture in watermelon. Plant Cell Tissue Organ Cult. 2018, 135, 179–187. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Sun, D.X.; Deng, Y.; An, G.L.; Li, W.H.; Si, W.J.; Liu, J.P.; Sun, X.W. Comparative transcriptome analysis of the effect of different heat shock periods on the unfertilized ovule in watermelon (Citrullus lanatus L.). J. Integr. Agr. 2020, 19, 528–540. [Google Scholar] [CrossRef]
- Baktemur, G.; Taşkın, H.; Büyükalaca, S. Comparison of different methods for separation of haploid embryo induced through irradiated pollen and their economic analysis in melon (Cucumis melo var. inodorus). Sci. World J. 2013, 2013, 529502. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, H.; Yücel, K.N.; Baktemur, G.; Çömlekçioğlu, S.; Büyükalaca, S. Effects of different genotypes and gamma ray doses on haploidization with irradiated pollen technique in watermelon (Citrullus lanatus L.). Can. J. Plant Sci. 2013, 93, 1165–1168. [Google Scholar] [CrossRef]
- Baktemur, G.; Yücel, N.K.; Taşkın, H.; Çömlekçioğlu, S.; Büyükalaca, S. Effects of different genotypes and gamma ray doses on haploidization using irradiated pollen technique in squash. Turk. J. Biol. 2014, 38, 318–327. [Google Scholar] [CrossRef]
- Yıldız, Ç.; Koruk, M.; Doğan, A.; Ellialtıoğlu, Ş.Ş. Parthenogenetic Embryogenesis Frequencies of Different Cucumber Genotypes by Irradiated Pollen Pollination and Haploid Embryo Development. Turk. J. Nat. Sci. 2020, 9, 69–73. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Ai, G.; Chen, J.; Guo, D.; Zhu, Z.; Zhu, X.; Tian, S.; Wang, J.; Liu, M.; et al. Creation of a watermelon haploid inducer line via ClDMP3-mediated single fertilization of the central cell. Hortic. Res. 2023, 10, 6. [Google Scholar] [CrossRef]
- Heiser, C.B.J.R. Peppers. In Evoluation of Crop Plants; Simmands, N.W., Ed.; Longman Science & Technology Report: London, UK, 1976; pp. 265–268. [Google Scholar]
- Andrews, J. Peppers. The Domasticated Capsicum; University of Texas Press: Austin, TX, USA, 1985. [Google Scholar]
- Dong, Y.Q.; Zhao, W.X.; Li, X.H.; Liu, X.C.; Gao, N.N.; Huang, J.H.; Wang, W.Y.; Xu, X.L.; Tang, Z.H. Androgenesis, Gynogenesis, and Parthenogenesis Haploids in Cucurbit Species. Plant Cell Rep. 2016, 35, 1991–2019. [Google Scholar] [CrossRef]
- Keller, J. Culture of Unpollinated Ovules, Ovaries and Flower Buds in Some Species of The Genus Allium and Haploid Induction Via Gynogenesis in Onion (Allium cepa L.). Euphytica 1990, 47, 241–247. [Google Scholar] [CrossRef]
- Yaralı, F.; Yanmaz, R. Utilization of Haploidy Techniques in Breeding of Allium Species. Türk Bilimsel Derlemeler Derg. 2013, 6, 44–50. Available online: https://dergipark.org.tr/en/download/article-file/417890 (accessed on 31 March 2024). (In Turkish).
- Sari, N.; Abak, K.; Pitrat, M. Comparison of ploidy level screening methods in watermelon: Citrullus lanatus (Thunb.) Matsum. and Nakai. Sci. Hortic. 1999, 82, 265–277. [Google Scholar] [CrossRef]
- Demirel, E.; Onus, A.N. Obtaining Haploid Embryo and Plant by Gynogenesis in Some Cucumber (Cucumis sativus L.) Cultivars and Types. Uluslararası Tarım Yaban Hayatı Bilim. Derg. 2021, 7, 360–367. (In Turkish) [Google Scholar] [CrossRef]
- Kurtar, E.S.; Uzun, S.; Esendal, E. Haploid Plant Propagation by Anther Culture of Squash (Cucurbita pepo L.). Ondokuz Mayıs Üniversitesi Ziraat Fakültesi Derg. 1998, 14, 33–45. (In Turkish) [Google Scholar]
- Kurtar, E.S.; Balkaya, A.; Ozbakir, M.; Ofluoglu, T. Induction of haploid embryo and plant regeneration via irradiated pollen technique in pumpkin (Cucurbita moschata Duchesne ex. Poir). Afr. J. Biotechnol. 2009, 8, 5944–5951. [Google Scholar]
- Kurtar, E.S.; Balkaya, A.; Göçmen, M.; Karaağaç, O. Dihaploidization in Squash Genotypes (Cucurbita spp) as a Rootstock Candidates for Cucumber (Cucumis sativus L.) via Irradiated Pollen Technique. Selcuk J. Agric. Food Sci. 2017, 31, 34–41. (In Turkish) [Google Scholar] [CrossRef]
- Supena, E.D.J.; Muswita, W.; Suharsono, S.; Custers, J.B.M. Evaluation of crucial factors for implementing shed-microspore culture of Indonesian hot pepper (Capsicum annuum L.). Sci. Hortic. 2006, 107, 226–232. [Google Scholar] [CrossRef]
- İlhan, M.; Kurtar, E.S. Doublehaploidization Efficiency of Selected Pepper Genotypes Via In Vitro Anther Culture. Selcuk. J. Agric. Food Sci. 2022, 36, 253–259. [Google Scholar] [CrossRef]
- Zahroh, Z.A. Dihaploidization in Some Indonesian Pepper Genotypes via Anther Culture. Master’s Thesis, Sakarya University, Sakarya, Türkiye, 2022. (In Turkish). [Google Scholar]
- Güner, N.; Wehner, T.C. The genes of watermelon. HortScience 2004, 39, 1175–1182. [Google Scholar] [CrossRef]
- Wehner, T.C. Watermelon. In Vegetables I; Handbook of Plant Breeding; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; Volume 1. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Wijesinghe, S.A.E.C.; Evans, L.J.; Kirkland, L.; Rader, R. A global review of watermelon pollination biology and ecology: The increasing importance of seedless cultivars. Sci. Hortic. 2020, 271, 109493. [Google Scholar] [CrossRef]
- Abacı, Z.T.; Asma, B.M. Pollen Vitality, Germination Conditions and Pollen Tube Lenght Investigation of Hybrid Apricot Genotypes. Anadolu J. Agric. Sci. 2014, 29, 12–19. (In Turkish) [Google Scholar] [CrossRef]
- Kılıç, T.; Doğan, E.; Dursun, H.B.; Çamurcu, S.; Ünsal, H.T.; Kazaz, S. Effects of Pollen Holding Duration in Some Rose Species and Varieties on Pollen Viability and Germination. J. Agric. Fac. Bursa Uludag Univ. 2020, 34, 173–184. (In Turkish) [Google Scholar]
- Çetin, M.; Soylu, A. Standart Ayva Çeşitlerinin Döllenme Biyolojisi Üzerinde Araştırmalar. Bahçe 2006, 35, 83–96. (In Turkish) [Google Scholar]
- Albertos, P.; Wagner, K.; Poppenberger, B. Cold stress signalling in female reproductive tissues. Plant Cell Environ. 2019, 42, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. The effect of temperature on pollen germination, pollen tube growth, and stigmatic receptivity in peach. Plant Biol. 2005, 7, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Karabıyık, Ş. Effects of temperature on pollen viability and in vivo pollen tube growth in Citrus sinensis. J. Appl. Bot. Food Qual. 2022, 95, 100–104. [Google Scholar] [CrossRef]
- Bykova, O.; Chuine, I.; Morin, X.; Higgins, S.I. Temperature dependence of the reproduction niche and its relevance for plant species distributions. J. Biogeogr. 2012, 39, 2191–2200. [Google Scholar] [CrossRef]
- Snider, J.L.; Oosterhuis, D.M.; Kawakami, E.M. Diurnal pollen tube growth is slowed by high temperature in field-grown Gossypium hirsutum pistils. J. Plant Physiol. 2011, 168, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Guo, S.; Wang, D.; Zhong, Y.; Chen, M.; Chen, C.; Cheng, D.; Liu, Z.; An, T.; Li, J.; et al. ZmCOI2a and ZmCOI2b redundantly regulate anther dehiscence and gametophytic male fertility in maize. Plant J. 2022, 110, 849–862. [Google Scholar] [CrossRef]
- Cuevas, J.; Rallo, L.; Rapoport, H.F. Procedure to study ovule sensescence inolive. Acta Hortic. 1994, 356, 252–255. [Google Scholar] [CrossRef]
- Carpenedo, S.; Raseira, M.C.B.; Byrne, D.H.; Franzon, R.C. The effect of heat stress on the reproductive structures of peach. J. Am. Pomol. Soc. 2017, 71, 114–120. [Google Scholar]
- Karabıyık, Ş.; Eti, S. Determination of Flowering Dates and Pollen Properties of Some Pecan Nut Cultivars in Adana Ecological Conditions. Turk. J. Agric. Food Sci. Technol. 2018, 6, 1795–1801. (In Turkish) [Google Scholar] [CrossRef][Green Version]
- Cimen, B. Induction of Polyploidy in C35 Citrange through In Vitro Colchicine Treatments of Seed-Derived Explants. Int. J. Fruit Sci. 2020, 20, 1929–1941. [Google Scholar] [CrossRef]
- Sarı, N. Karpuzlarda Işınlanmış Polen Uyartımıyla Haploid Bitki Eldesi Üzerine Genotipin ve Mevsimin Etkisi Ile Işınlama Yerine Geçebilecek Uygulamalar Üzerinde Araştırmalar. Ph.D. Thesis, Cukurova University, Adana, Türkiye, 1994. (In Turkish). [Google Scholar]
- Şimşek, İ.; Göçmen, M.; Sarı, N. Diploid ve tetraploid karpuz bitkilerinde morfolojik ve sitolojik farklılıkların belirlenmesi. Derim 2013, 30, 1–14. (In Turkish) [Google Scholar]
- Norton, J.D. Testing of Plum Pollen Viability with Tetrazolium Salts. Proc. J. Am. Soc. Hortic. 1996, 89, 132–134. [Google Scholar]
- Karabıyık, Ş.; Sarıdaş, M.A.; Eti, S.; Paydaş Kargı, S. The Effects of Boron and Calcium Applications on Pollen Characteristics and Distorted Fruit Formation in Sweet ann Strawberry Varieties. Bahçe 2017, 46, 271–279. [Google Scholar]
- Adıgüzel, P.; Solmaz, İ.; Karabıyık, Ş.; Sarı, N. Comparison on Flower, Fruit and Seed Characteristics of Tetraploid and Diploid Watermelons (Citrullus lanatus Thunb. Matsum. and Nakai). Int. J. Agric. Environ. Food Sci. 2022, 6, 704–710. [Google Scholar] [CrossRef]
- Yıldırım, K.C.; Demir, İ. Changing in Pollen Viability with Storage Time, Temperature and Plant Situation. In Proceedings of the Turkey 5th Seed Congress with International Participation and Sectoral Business Forum, Diyarbakir, Türkiye, 19–23 October 2014. [Google Scholar]
- Gyulai, G.; Gémesné, J.A.; Sági, Z.; Venczel, G.; Pintér, P.; Kristóf, Z.; Törjék, O.; Heszky, L.; Bottka, S.; Kiss, J.; et al. Doubled haploid development and PCRanalysis of F1 hybrid derived DH-R2 paprika (Capsicum annuum L.) lines. J. Plant Physiol. 2000, 156, 168–174. [Google Scholar] [CrossRef]
- Ari, E.; Bedir, H.; Yildirim, S.; Yıldırım, T. Androgenic Responses of 64 Ornamental Pepper (Capsicum annuum L.) Genotypes to Shed-Microspore Culture in Autumn Season. Turk. J. Biol. 2016, 40, 706–717. [Google Scholar] [CrossRef]
- Bat, H.; Altındağ, F.N.; Yiğit, M.A.; Ellialtıoğlu, Ş.Ş.; Çölekçioğlu, N. Ploidy estimation in pepper and eggplant via stomata characteristics. Int. J. Agric. For. Life Sci. 2021, 5, 139–146. [Google Scholar]
- Eş, E. Stimulation of Autopolyploid in Phaseolus vulgaris L. Master’s Thesis, Namık Kemal University, Tekirdağ, Türkiye, 2011. (In Turkish). [Google Scholar]
- Usman, M.; Fatima, B.; Gillani, K.A.; Khan, M.S.; Khan, M.M. Exploitation of otential target tissues to develop polyploids in citrus. Pak. J. Bot. 2008, 40, 1755–1766. [Google Scholar]
- Sukamto, L.A.; Ahmad, F.; Hawo, A.H. Pengaruh Oryzalin Terhadap Tingkat Ploidi Tanaman Garut (Maratana arundinacea L.). Bul. Littro 2010, 21, 93–102. [Google Scholar]
- Moghbel, N.; Borujeni, M.K.; Bernard, F. Colchicine effect on the DNA content and stomata size of Glycyrrhiza glabra var. glandulifera and Carthamus tinctorius L. cultured in vitro. J. Genet. Eng. Biotechnol. 2015, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.M.; Fazil, R.; Alam, M.A.; Saifuddin, M.; Zakaria, A.J. Effects of temperature treatment on seed germination, root development and seedling growth of Citrullus lanatus (watermelon). Bulg. J. Agric. Sci. 2020, 26, 558–566. [Google Scholar]
- Jibril, S.M.; Bello, H.J. Leaf epidermal structures and stomata ontogeny in some members of the family Cucurbitaceae. Int. J. Plant Soil. Sci. 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Çelikli, F.B. Effect of Different Nutrient Media and Irradiation Doses on Haploid Embriyo and Plant Formation in Galia and Kırkagac Melon (Cucumis melo L.) Genotypes. Master’s Thesis, Akdeniz University, Antalya, Türkiye, 2016. (In Turkish). [Google Scholar]
- Şensoy, A.S.; Ercan, N.; Ayar, F.; Temirkaynak, M. The Evaluation of Pollen Viability and the Determine Some Pollen Characteristics in Some Species of Cucurbitaceae Family. Akdeniz Univ. J. Fac. Agric. 2003, 16, 1–6. (In Turkish) [Google Scholar]
- Koyuncu, F. Response of In Vitro Pollen Germination and Pollen Tube Growth of Strawberry Cultivars to Temperature. Eur. J. Hortic. Sci. 2006, 71, 125–128. [Google Scholar]
- Voyiatzsis, D.G.; Paraskevopoulou-Paroussi, G. Factors Affecting the Quality and in Vitro Germination Capacity of Strawberry Pollen. Int. J. Fruit Sci. 2005, 5, 25–35. [Google Scholar] [CrossRef]
- Sánchez, M.; Velásquez, Y.; González, M.; Cuevas, J. Pollination Effectiveness of the Hoverfly Eristalinus aeneus (Scopoli, 1763) in Diploid and Triploid Associated Watermelon Crop. Insects 2022, 13, 1021. [Google Scholar] [CrossRef]
- Sawe, T.; Eldegard, K.; Totland, Ø.; Macrice, S.; Nielsen, A. Enhancing pollination is more effective than increased conventional agriculture inputs for improving watermelon yields. Ecol. Evol. 2020, 10, 5343–5353. [Google Scholar] [CrossRef]
- Sravani, V.; Ashok, P.; Sasikala, K.; Babu, B.R. Effect of Intergeneric Pollen on Induction of Parthenocarpy in Watermelon (Citrullus lanatus Thunb.). Int. J. Curr. Microbiol. App. Sci. 2018, 7, 890–895. [Google Scholar] [CrossRef]
- Şimşek, İ. Obtaining Tetraploid Lines That Is Aimed for Developing Seedless Watermelon (Citrullus lanatus (Thunb.) Matsum and Nakai) Varieties. Master’s Thesis, Cukurova University, Adana, Türkiye, 2011. (In Turkish). [Google Scholar]




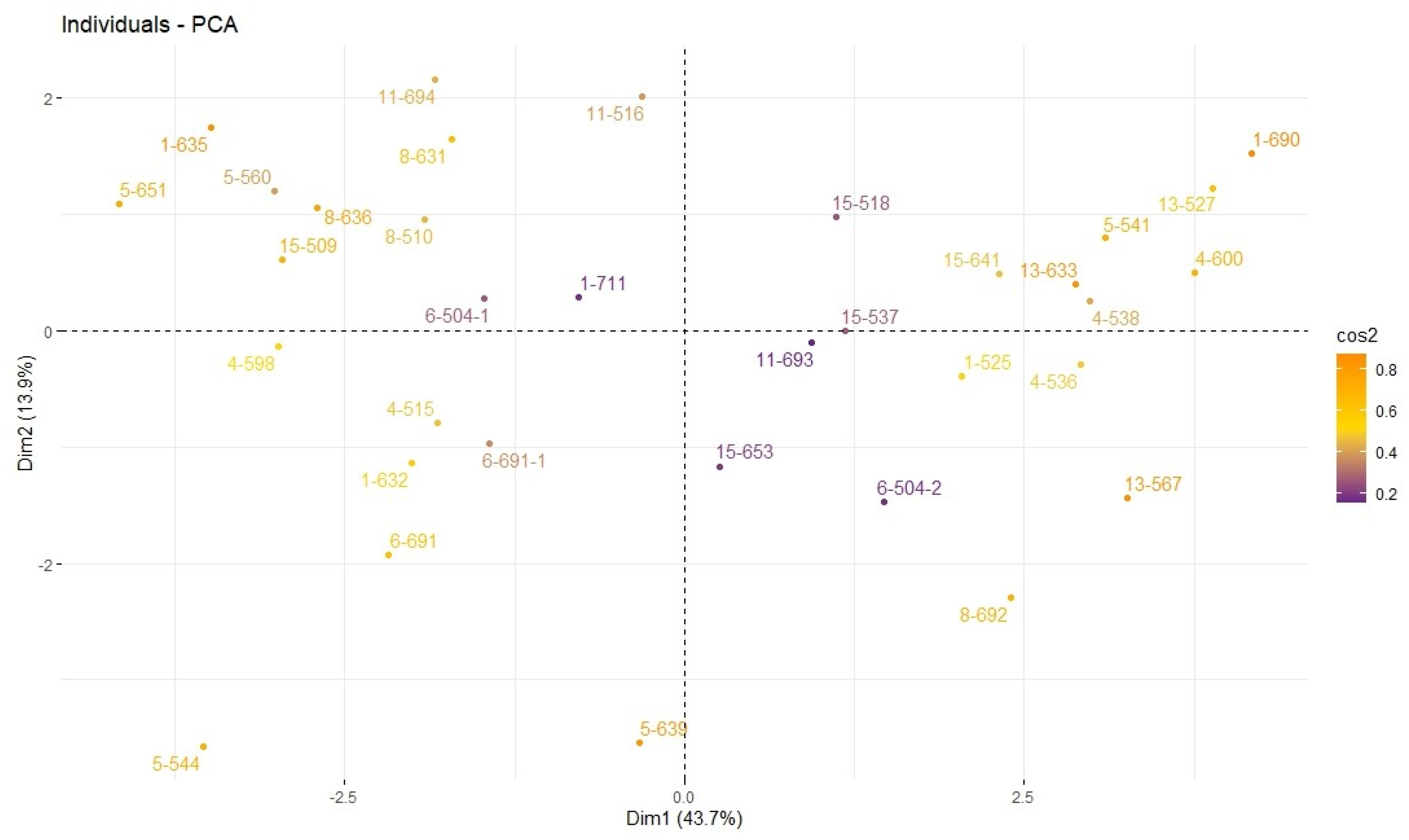


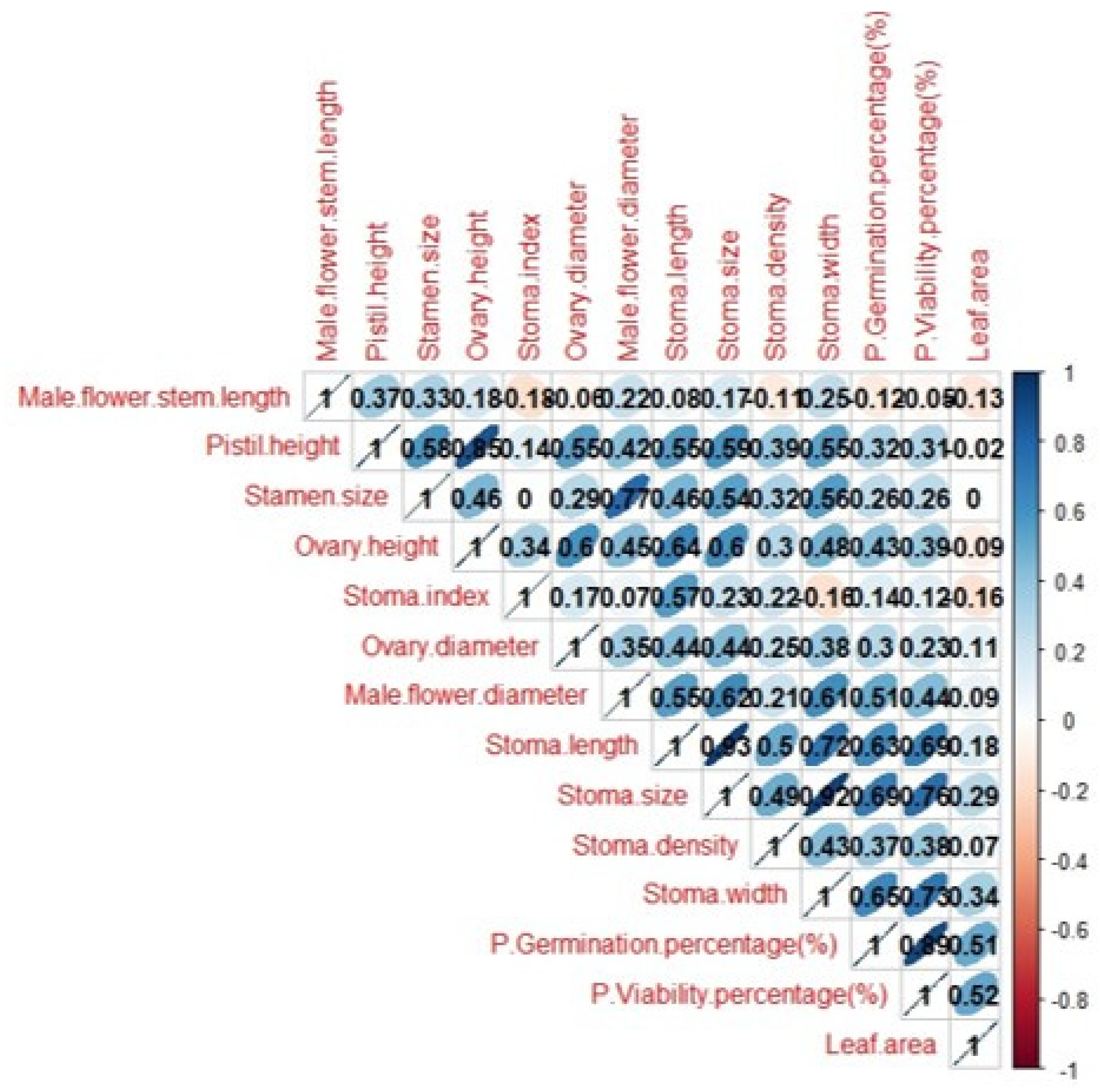
| Genotypes/Plants | Ploidy Level | Stomatal Length (μm) | Stomatal Width (μm) | Stomatal Size (μm) | Stomatal Index (μm) | Stomatal Density (mm2) | Leaf Area (cm2) |
|---|---|---|---|---|---|---|---|
| 1-632 | H | 11.40m-p | 7.45lm | 77.57n-s | 1.40b-h | 7.33r | 60.23l |
| 1-690 | DH | 12.91c-f | 9.49a-d | 122.65a-e | 1.36d-ı | 31.33cd | 68.42k |
| 1-525 | DH | 12.45e-h | 8.77e-h | 109.23e-h | 1.42b-g | 29.33efg | 106.75cd |
| 1-711 | DH | 11.44ı-l | 7.83klm | 89.60j-o | 1.46bcd | 16.67n | 70.59jk |
| 1-635 | H | 9.32qr | 7.29m | 67.87s | 1.28hıj | 7.33r | 76.03ıj |
| 4-515 | H | 11.28ı-m | 7.58lm | 85.53k-q | 1.49bc | 28.33gh | 83.28gh |
| 4-600 | DH | 12.82def | 9.80abc | 125.85abc | 1.31g-j | 21.33lm | 105.43cde |
| 4-536 | DH | 13.26b-e | 9.38a-e | 124.33a-d | 1.42b-g | 5.32st | 94.77f |
| 4-538 | H | 14.57a | 8.64f-j | 125.94abc | 1.69a | 24.00jk | 57.81lm |
| 4-598 | H | 10.50l-p | 7.63lm | 80.30m-s | 1.37c-ı | 6.67rs | 121.22b |
| 5-639 | DH | 11.60h-k | 8.69f-j | 100.89g-j | 1.33e-j | 32.67c | 132.74a |
| 5-541 | DH | 12.60d-g | 9.37a-e | 118.03b-f | 1.35d-j | 14.67op | 78.18hı |
| 5-544 | H | 9.80pqr | 7.27m | 71.27rs | 1.35d-j | 5.00o | 134.70a |
| 5-651 | H | 10.16jn-q | 7.65lm | 77.72n-s | 1.33f-j | 4.67t | 41.71p |
| 5-560 | DH | 9.07r | 7.95klm | 72.19qrs | 1.14k | 11.00n | 52.64mn |
| 6-504-2 | H | 13.82abc | 7.71lm | 106.62f-ı | 1.79a | 28.67q | 46.73m-p |
| 6-691-1 | H | 11.73g-j | 8.47h-k | 99.63g-k | 1.38c-h | 22.67kl | 33.22qr |
| 6-504-1 | DH | 11.04j-n | 7.35m | 81.02m-s | 1.51b | 26.67hı | 48.00nop |
| 6-691 | H | 11.06j-n | 8.04jkl | 89.01j-p | 1.38c-ı | 28.33gh | 43.93op |
| 8-510 | H | 10.75k-p | 8.50g-k | 91.40j-n | 1.27ıj | 13.33p | 67.71k |
| 8-692 | DH | 13.45bcd | 9.89ab | 133.32a | 1.36d-j | 40.00a | 101.06def |
| 8-636 | H | 10.79j-o | 7.42lm | 80.07m-s | 1.45bcd | 14.67op | 49.01no |
| 8-631 | H | 10.99j-n | 7.60lm | 83.53l-r | 1.45b-e | 13.33p | 48.30nop |
| 11-516 | H | 11.60h-k | 8.08ı-l | 93.79ı-m | 1.43b-f | 31.67cd | 26.51q |
| 11-693 | DH | 12.97c-f | 8.71e-ı | 113.12c-g | 1.49bc | 30.33def | 42.97op |
| 11-694 | DH | 9.84pqr | 7.63lm | 75.16p-s | 1.29hıj | 15.67no | 34.84q |
| 13-633 | DH | 12.47e-h | 9.27b-f | 116.05b-f | 1.35d-j | 25.67ıj | 72.85ıjk |
| 13-567 | DH | 13.98ab | 9.28a-f | 129.77ab | 1.51b | 29.00fg | 123.84b |
| 13-527 | DH | 12.64d-g | 9.77abc | 123.55a-d | 1.29hıj | 31.67cd | 98.70ef |
| 15-641 | DH | 13.02c-f | 9.94a | 129.91ab | 1.31g-j | 20.00m | 86.13g |
| 15-653 | DH | 11.03j-n | 8.08ı-l | 89.17j-p | 1.37d-ı | 36.33b | 111.12c |
| 15-537 | DH | 12.12f-ı | 9.16c-g | 111.01d-g | 1.32f-j | 28.00gh | 76.16ıj |
| 15-518 | DH | 10.97j-n | 8.82d-h | 96.68h-l | 1.25jk | 31.00cde | 67.50k |
| 15-509 | H | 9.86o-r | 7.69lm | 75.84o-s | 1.28hıj | 24.00jk | 60.72l |
| LSD | 0.95 *** | 0.67 *** | 14.17 *** | 1.19 *** | 1.81 *** | 6.78 *** |
| Genotypes/Plants | Ploidy Level | Male Flower Diameter (mm) | Stamen Size (mm) | Male Flower Pedicel Length (mm) | Ovary Height (mm) | Ovary Diameter (mm) | Pistil Height (mm) | Female Flower Pedicel Length (mm) |
|---|---|---|---|---|---|---|---|---|
| 1-632 | H | 22.68gh | 6.27j-m | 12.10kl | 9.43hıj | 6.15h-k | 4.80j-m | 16.74kl |
| 1-690 | DH | 36.04 | 10.65ab | 22.59cde | 15.53bc | 11.77ab | 7.37a-d | 34.48b |
| 1-525 | DH | 27.20f | 7.94e-h | 16.90f-ı | 14.79cd | 10.56b | 6.23d-ı | 27.22c-f |
| 1-711 | DH | 22.78gh | 7.00g-k | 24.13bcd | 10.93f-ı | 8.12c-f | 5.81f-j | 15.05l-o |
| 1-635 | H | 19.28ıjk | 6.54jkl | 26.40abc | 7.10k-m | 5.39j-m | 5.53g-l | 22.99e-j |
| 4-515 | H | 18.35jkl | 7.74e-ı | 12.87ı-l | 6.48l-o | 5.72ı-l | 5.45g-l | 18.20jkl |
| 4-600 | DH | 32.20c | 8.07efg | 20.57d-g | 15.26cd | 12.18a | 7.83ab | 31.68bc |
| 4-536 | DH | 31.32cd | 6.83h-l | 16.66g-j | 17.97a | 10.64b | 6.83a-f | 27.14d-f |
| 4-538 | H | 28.77def | 8.33def | 25.71abc | 17.35ab | 4.62l-o | 7.58abc | 23.05e-j |
| 4-598 | H | 19.34ıjk | 6.73ı-l | 22.70b-e | 7.86j-m | 6.44g-j | 4.87jkl | 7.24p |
| 5-639 | DH | 17.90j-m | 5.88klm | 9.90l | 7.74j-m | 5.26j-n | 4.31lm | 15.60lmn |
| 5-541 | DH | 36.65 | 9.36cd | 22.24cde | 17.51a | 7.18e-h | 7.95a | 20.90g-k |
| 5-544 | H | 16.58k-n | 3.80o | 11.04l | 4.75o | 3.22p | 2.21o | 20.62g-k |
| 5-651 | H | 13.75nop | 5.19mn | 23.12b-e | 5.55no | 4.09m-p | 2.98no | 30.57bcd |
| 5-560 | DH | 39.71a | 9.52bc | 13.14ı-l | 5.96mno | 4.81lmn | 3.53mn | 17.41kl |
| 6-504-2 | H | 35.75 | 9.45cd | 11.21l | 14.77cd | 6.41g-j | 5.35h-l | 10.22op |
| 6-691-1 | H | 18.11jkl | 5.75lm | 13.69ı-l | 9.65hıj | 6.90f-ı | 4.96ı-l | 9.12p |
| 6-504-1 | DH | 21.84ghı | 5.93klm | 16.60g-j | 12.66ef | 9.07c | 5.58f-l | 19.55h-l |
| 6-691 | H | 13.28op | 4.08no | 12.33jkl | 9.02ıjk | 6.48g-j | 4.36lm | 11.42nop |
| 8-510 | H | 20.52hıj | 7.76e-ı | 23.02b-e | 7.23k-n | 3.35op | 4.43klm | 27.42c-f |
| 8-692 | DH | 29.10def | 7.89e-ı | 11.70kl | 9.29hıj | 6.26 | 5.71f-k | 17.37kl |
| 8-636 | H | 15.14mno | 6.45jkl | 19.45e-h | 8.08jkl | 6.28hıj | 5.58f-l | 25.53efg |
| 8-631 | H | 30.54cde | 8.46cde | 21.12def | 9.45hıj | 7.08e-h | 4.84jkl | 22.71f-j |
| 11-516 | H | 23.99g | 6.30j-m | 25.78abc | 15.50bc | 6.32g-j | 7.15a-e | 23.74e-h |
| 11-693 | DH | 29.07def | 8.30def | 15.98h-k | 11.21fgh | 8.76cd | 5.34h-l | 18.64ı-l |
| 11-694 | DH | 11.33p | 8.56cde | 13.22ı-l | 16.37abc | 7.35e-h | 7.45a-d | 25.52efg |
| 13-633 | DH | 37.78ab | 9.44cd | 18.76e-h | 13.49de | 7.61d-g | 7.48a-d | 24.95efg |
| 13-567 | DH | 27.66ef | 8.69cde | 14.17ı-l | 13.46de | 8.06c-f | 6.63b-h | 27.83cde |
| 13-527 | DH | 37.28ab | 10.89a | 29.36a | 10.35ghı | 7.11e-h | 6.66b-g | 39.57a |
| 15-641 | DH | 31.20cd | 8.72cde | 27.04ab | 12.12efg | 4.89k-n | 6.25d-ı | 23.43e-ı |
| 15-653 | DH | 21.76ghı | 7.26f-j | 13.30ı-l | 12.52ef | 8.32cde | 6.49c-h | 19.62h-l |
| 15-537 | DH | 21.71ghı | 8.75cde | 24.32bcd | 12.50ef | 6.50g-j | 6.03e-j | 16.45klm |
| 15-518 | DH | 30.49cde | 8.76cde | 26.42abc | 12.69ef | 4.66lmn | 6.48c-h | 26.20def |
| 15-509 | H | 16.30lmn | 6.87h-l | 19.64 | 9.37hıj | 3.99nop | 5.81f-j | 11.75m-p |
| LSD | 2.96 *** | 1.17 *** | 4.37 *** | 1.95 *** | 1.31 *** | 1.30 *** | 4.2 *** |
| Viability Rate (%) | Germination Rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| Genotype/Plant | Periods | Genotype Mean | Genotype/Plant | Periods | Genotype Mean | ||
| 1st Period | 2nd Period | 1st Period | 2nd Period | ||||
| 1-632 H | 20.27pqr | 74.50gh | 47.39GH | 1-632 H | 13.19rs | 56.43h | 34.81H |
| 1-690 DH | 61.38jk | 80.23fg | 70.80B | 1-690 DH | 19.02opq | 69.35cde | 44.18CDE |
| 1-525 DH | 0 | 86.85c-f | 43.42HI | 1-525 DH | 7.28t | 39.15j | 23.21I |
| 1-711 DH | 18.30qrs | 58.94k | 38.62IJ | 1-711 DH | 4.10tuv | 22.59nop | 13.35J |
| 4-515 H | 4.32vwx | 43.40m | 23.86K | 4-515 H | 0 | 0 | 0 |
| 4-600 DH | 7.34uvw | 86.20def | 46.77GH | 4-600 DH | 8.71st | 69.07cde | 38.89FG |
| 4-536 DH | 12.52stu | 89.55a-e | 51.03FG | 4-536 DH | 13.27rs | 73.85bc | 43.56DE |
| 4-538 H | 25.77op | 88.89b-e | 57.33DE | 4-538 H | 7.24t | 63.40fg | 35.32GH |
| 5-639 DH | 0 | 95.11ab | 47.56GH | 5-639 DH | 30.42kl | 75.31b | 52.86A |
| 5-541 DH | 32.88n | 93.19a-d | 63.04C | 5-541 DH | 24.53mno | 71.44bcd | 47.98BC |
| 5-544 H | 0 | 93.66abc | 46.83GH | 5-544 H | 14.47qr | 73.99bc | 44.23CDE |
| 6-504-2 H | 16.10q-t | 66.22ıj | 41.16I | 6-504-2 H | 12.98rs | 65.05efg | 39.01FG |
| 6-691-1 H | 5.54u-x | 50.95l | 28.24K | 6-691-1 H | 5.66tu | 24.74mn | 15.20J |
| 6-504-1 DH | 0 | 22.64pq | 11.32L | 6-504-1 DH | 0 | 13.04rs | 6.52K |
| 6-691 H | 9.38tuv | 59.77jk | 34.57J | 6-691 H | 6.10t | 43.93ıj | 25.02I |
| 8-510 H | 4.45vwx | 47.27lm | 25.86K | 8-510 H | 0 | 0 | 0 |
| 8-692 D | 1.94wx | 15.40rst | 8.67LM | 8-692 DH | 26.23lmn | 67.50def | 46.87CD |
| 8-636 H | 4.23vwx | 0 | 25.77K | 8-636 H | 0 | 0 | 0 |
| 11-516 H | 0 | 0 | 0 | 11-516 H | 0 | 7.68st | 3.84KL |
| 11-693 DH | 73.06hı | 89.53a-e | 81.28A | 11-693 DH | 15.70qr | 32.42k | 24.06 |
| 11-694 DH | 0 | 94.69ab | 68.98B | 11-694 DH | 0 | 0 | 0 |
| 12-P61 DH | 14.69rst | 90.48a-d | 52.58EF | 12-P61 DH | 0.33uv | 43.34ıj | 21.84I |
| 12-P88 DH | 1.94wx | 15.40rst | 8.67LM | 12-P88 DH | 29.18klm | 59.58gh | 44.38CDE |
| 13-633 DH | 30.49no | 91.03a-d | 60.76CD | 13-633 DH | 32.65k | 69.11422cde | 50.88AB |
| 13-567 DH | 62.19jk | 96.49a | 79.34A | 13-567 DH | 16.64qr | 64.68efg | 40.66EF |
| 13-527 DH | 14.98rst | 95.78ab | 55.38EF | 13-527 DH | 7.04t | 87.72a | 47.38BCD |
| 15-641 DH | 72.03hı | 89.92a-e | 80.97A | 15-641 DH | 0.33uv | 46.75ı | 23.54I |
| 15-653 DH | 0 | 48.21lm | 24.11K | 15-653 D H | 39.97j | 65.10efg | 52.54A |
| 15-537 DH | 6.17u-x | 96.02a | 51.10FG | 15-537 DH | 7.98st | 39.68j | 23.83I |
| 15-518 DH | 80.49fg | 83.22ef | 81.86A | 15-518 DH | 18.05pqr | 65.10efg | 41.58EF |
| Period mean | 17.27 B | 57.93 A | Period mean | 10.03B | 39.17A | ||
| LSD | period: 1.18 *** | genotype: 5.02 *** | genotypes x period: 7.10 *** | LSD | period: 0.94 *** | genotype: 3.99 *** | genotypes x period: 5.65 *** |
| Genotype/Plant | Inbreeding Success in the First Period (%) | Inbreeding Success in the Second Period (%) |
|---|---|---|
| 1-690 | 0 | 25.00 |
| 1-525 | 0 | 42.86 |
| 1-711 | 0 | 33.33 |
| 4-515 | 0 | 25.00 |
| 4-600 | 0 | 60.00 |
| 4-536 | 0 | 50.00 |
| 5-639 | 0 | 50.00 |
| 5-541 | 0 | 66.67 |
| 6-504-2 | 0 | 25.00 |
| 6-691 | 0 | 20.00 |
| 11-693 | 0 | 75.00 |
| 13-633 | 0 | 50.00 |
| 13-567 | 0 | 66.67 |
| 13-527 | 0 | 33.33 |
| 15-641 | 0 | 66.67 |
| 15-653 | 0 | 20.00 |
| 15-537 | 0 | 60.00 |
| 15-518 | 0 | 66.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, E.; Taşkın, H.; Karabıyık, Ş.; Solmaz, İ.; Sarı, N.; Karaköy, T.; Baktemur, G. Are Cytological and Morphological Analyses Sufficient in Ploidy Determination of Watermelon Haploid Plants? Horticulturae 2024, 10, 818. https://doi.org/10.3390/horticulturae10080818
Kara E, Taşkın H, Karabıyık Ş, Solmaz İ, Sarı N, Karaköy T, Baktemur G. Are Cytological and Morphological Analyses Sufficient in Ploidy Determination of Watermelon Haploid Plants? Horticulturae. 2024; 10(8):818. https://doi.org/10.3390/horticulturae10080818
Chicago/Turabian StyleKara, Ecem, Hatıra Taşkın, Şenay Karabıyık, İlknur Solmaz, Nebahat Sarı, Tolga Karaköy, and Gökhan Baktemur. 2024. "Are Cytological and Morphological Analyses Sufficient in Ploidy Determination of Watermelon Haploid Plants?" Horticulturae 10, no. 8: 818. https://doi.org/10.3390/horticulturae10080818
APA StyleKara, E., Taşkın, H., Karabıyık, Ş., Solmaz, İ., Sarı, N., Karaköy, T., & Baktemur, G. (2024). Are Cytological and Morphological Analyses Sufficient in Ploidy Determination of Watermelon Haploid Plants? Horticulturae, 10(8), 818. https://doi.org/10.3390/horticulturae10080818






