Biogenic Nano-Fertilizers as a Sustainable Approach to Alleviate Nitrate Accumulation and Enrich Quality Traits of Vegetable Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental Conditions
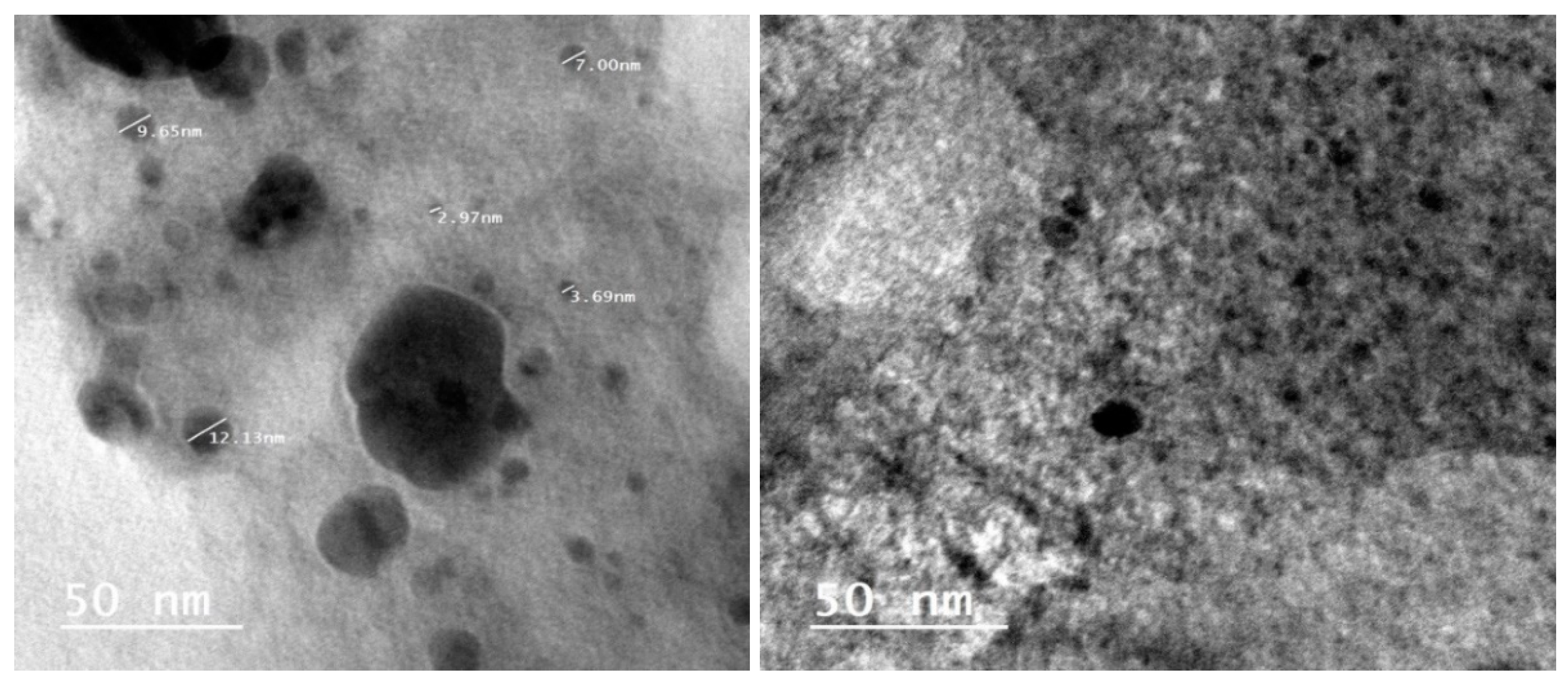
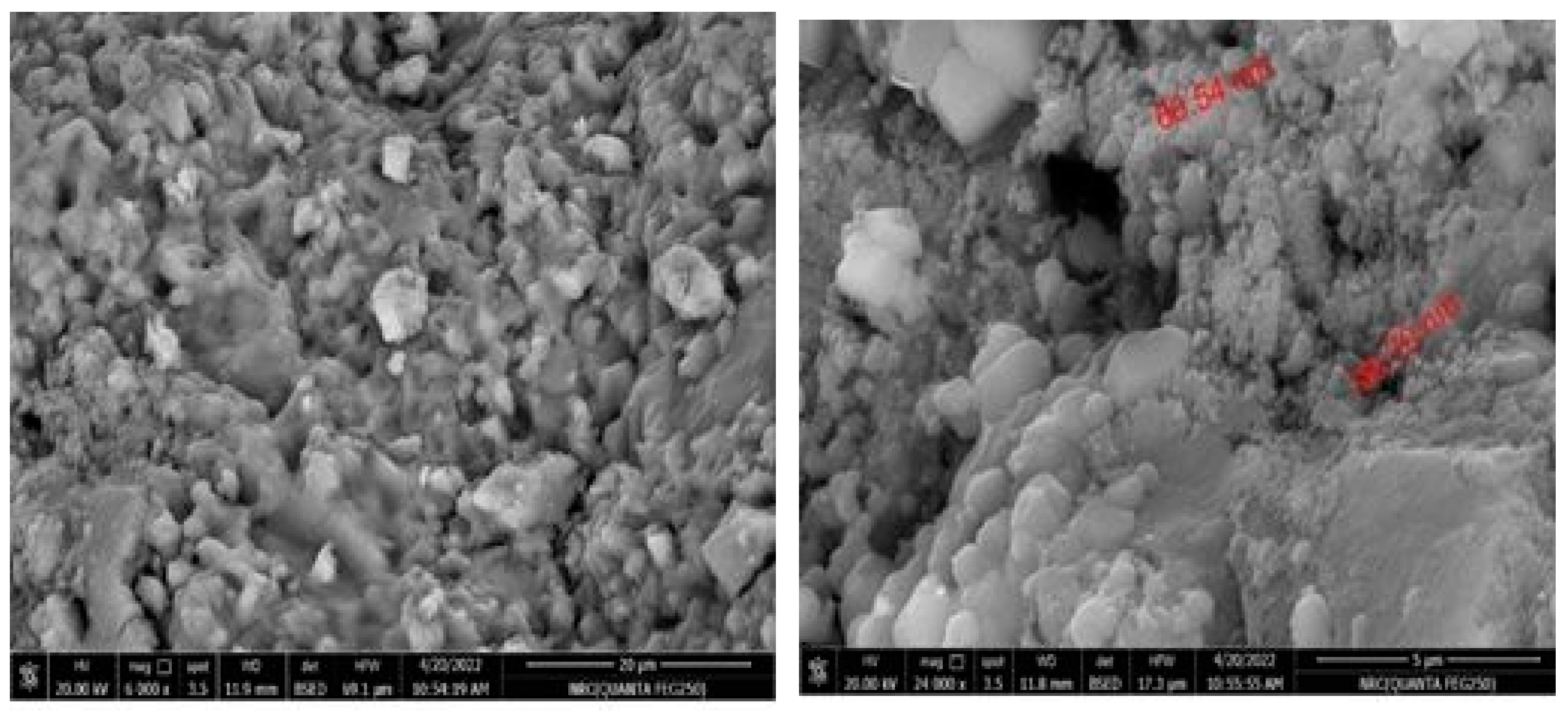
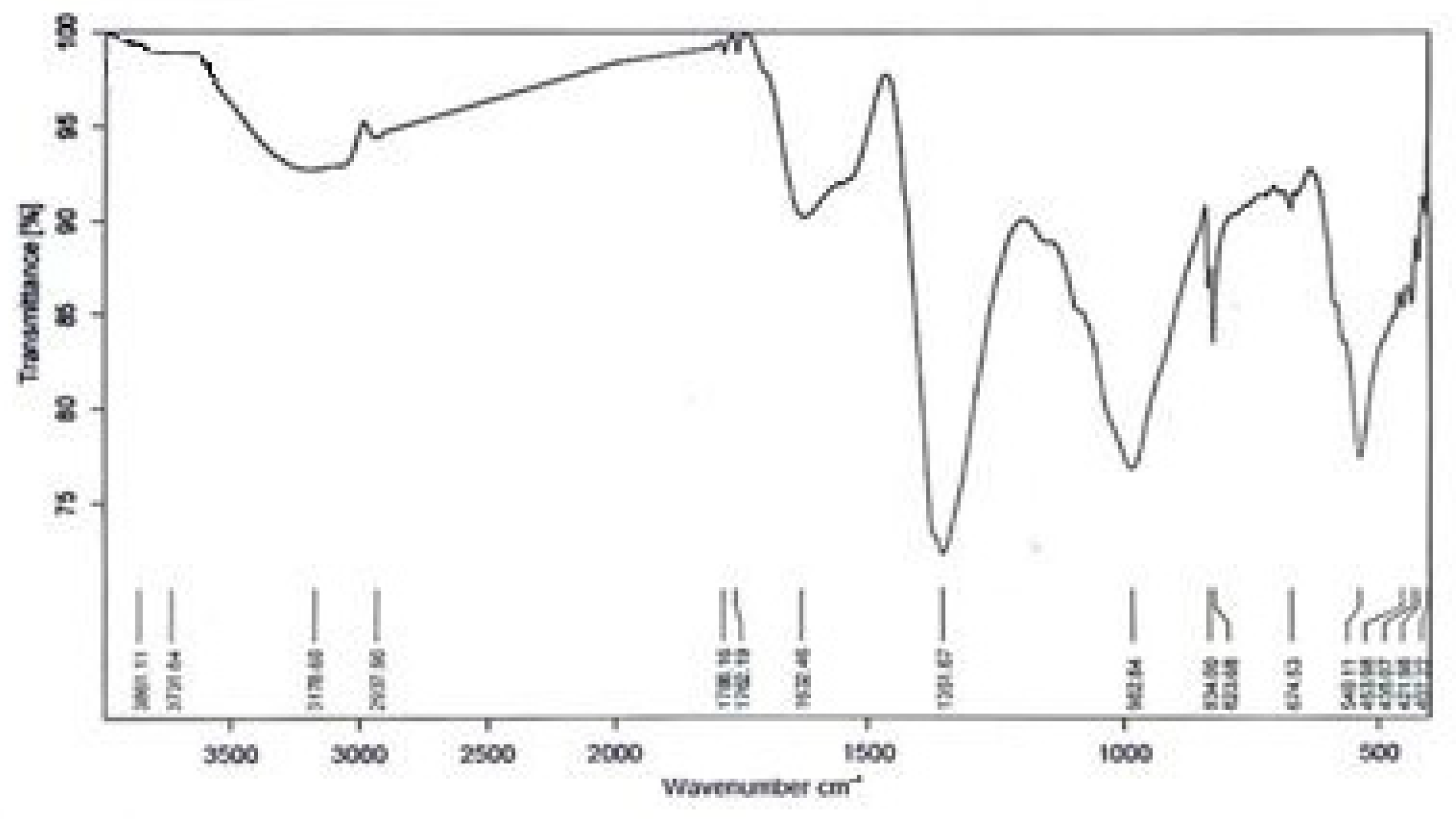
2.2. Nanoparticles Synthesis
2.3. Nanoparticles Measurement Techniques
2.4. Plant Growth Measurements
2.5. Photosynthetic Pigments
2.6. Vitamin C and Nitrate Contents
2.7. Macro-Nutrient Contents
2.8. Determination of Total Phenolic, Total Flavonoids, and Antioxidant Capacity
2.9. Statistical Analyses
3. Results
3.1. Plant Growth Traits
3.2. Photosynthetic Pigments
3.3. Yield and Its Quality
3.4. Macro-Nutrient Uptake
3.5. Antioxidant Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bijay-Singh, C.E.; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Jha, A.; Pathania, D.; Damathia, B.; Raizada, P.; Rustagi, S.; Singh, P.; Rani, G.M.; Chaudhary, V. Panorama of Biogenic Nano-Fertilizers: A Road to Sustainable Agriculture. Environ. Res. 2023, 235, 116456. [Google Scholar] [CrossRef]
- Ma, G.; Cheng, S.; He, W.; Dong, Y.; Qi, S.; Tu, N.; Tao, W. Effects of Organic and Inorganic Fertilizers on Soil Nutrient Conditions in Rice Fields with Varying Soil Fertility. Land 2023, 12, 1026. [Google Scholar] [CrossRef]
- Abdelkader, M.; Voronina, L.; Shelepova, O.; Puchkov, M.; Loktionova, E.; Zhanbyrshina, N.; Yelnazarkyzy, R.; Tleppayeva, A.; Ksenofontov, A. Monitoring Role of Exogenous Amino Acids on the Proteinogenic and Ionic Responses of Lettuce Plants under Salinity Stress Conditions. Horticulturae 2023, 9, 626. [Google Scholar] [CrossRef]
- Abdelkader, M.; Voronina, L.; Baratova, L.; Shelepova, O.; Zargar, M.; Puchkov, M.; Loktionova, E.; Amantayev, B.; Kipshakbaeva, A.; Arinov, B. Biostimulants-Based Amino Acids Augment Physio-Biochemical Responses and Promote Salinity Tolerance of Lettuce Plants (Lactuca Sativa L.). Horticulturae 2023, 9, 807. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, F.; Chen, X.; Miao, Y.; Li, J.; Shi, L.; Xu, J.; Ye, Y.; Liu, C.; Yang, Z. On-Farm Evaluation of an in-Season Nitrogen Management Strategy Based on Soil Nmin Test. Field Crops Res. 2008, 105, 48–55. [Google Scholar] [CrossRef]
- Cárdenas-Navarro, R.; Adamowicz, S.; Robin, P. Nitrate Accumulation in Plants: A Role for Water. J. Exp. Bot. 1999, 50, 613–624. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.; Li, J.; Ahmad, M.I.; Sher, A.; Rashid, A.; Ali, W. Evaluation of Wheat Varietal Performance under Different Nitrogen Sources. Am. J. Plant Sci. 2017, 8, 561. [Google Scholar] [CrossRef][Green Version]
- Vidican, R.; Păcurar, F.; Vâtcă, S.D.; Pleșa, A.; Stoian, V. Arbuscular Mycorrhizas Traits and Yield of Winter Wheat Profiled by Mineral Fertilization. Agronomy 2020, 10, 846. [Google Scholar] [CrossRef]
- Abdelkader, M.; Zargar, M.; Murtazova, K.M.-S.; Nakhaev, M.R. Life Cycle Assessment of the Cultivation Processes for the Main Vegetable Crops in Southern Egypt. Agronomy 2022, 12, 1527. [Google Scholar] [CrossRef]
- Lucarini, M.; D’Evoli, L.; Tufi, S.; Gabrielli, P.; Paoletti, S.; Di Ferdinando, S.; Lombardi-Boccia, G. Influence of Growing System on Nitrate Accumulation in Two Varieties of Lettuce and Red Radicchio of Treviso. J. Sci. Food Agric. 2012, 92, 2796–2799. [Google Scholar] [CrossRef] [PubMed]
- Boink, A.; Speijers, G. Health Effects of Nitrates and Nitrites, a Review. In Proceedings of the International Conference on Environmental Problems Associated with Nitrogen Fertilisation of Field Grown Vegetable Crops, Potsdam, Germany, 30 August–1 September 1999; Volume 563, pp. 29–36. [Google Scholar]
- European Food Safety Authority. Nitrate in vegetable. EFSA J. 2008, 689, 1–79. Available online: https://www.google.com/search?q=13.+European+Food+Safety+Authority.+Nitrate+in+vegetables+EFSA+Journal.+2008%3B68%3A91%E2%80%9379.&rlz=1C1CHBF_enEG1103EG1103&oq=13.+European+Food+Safety+Authority.+Nitrate+in+vegetables+EFSA+Journal.+2008%3B68%3A91%E2%80%9379.+&gs_lcrp=EgZjaHJvbWUyBggAEEUYOdIBCDQyMzRqMGo0qAIAsAIB&sourceid=chrome&ie=UTF-8 (accessed on 29 April 2024).
- Iammarino, M.; Di Taranto, A.; Cristino, M. Monitoring of Nitrites and Nitrates Levels in Leafy Vegetables (Spinach and Lettuce): A Contribution to Risk Assessment. J. Sci. Food Agric. 2014, 94, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M. Nitrate Accumulation in Plants, Factors Affecting the Process, and Human Health Implications. A Review. In Agronomy for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Gundimeda, U.; Naidu, A.N.; Krishnaswamy, K. Dietary Intake of Nitrate in India. J. Food Compos. Anal. 1993, 6, 242–249. [Google Scholar] [CrossRef]
- Temme, E.H.M.; Vandevijvere, S.; Vinkx, C.; Huybrechts, I.; Goeyens, L.; Van Oyen, H. Average Daily Nitrate and Nitrite Intake in the Belgian Population Older than 15 Years. Food Addit. Contam. Part A 2011, 28, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Brkić, D.; Bošnir, J.; Bevardi, M.; Bošković, A.G.; Miloš, S.; Lasić, D.; Krivohlavek, A.; Racz, A.; Mojsović–Ćuić, A.; Trstenjak, N.U. Nitrate in Leafy Green Vegetables and Estimated Intake. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Khairy, A.M.; Tohamy, M.R.; Zayed, M.A.; Mahmoud, S.F.; El-Tahan, A.M.; El-Saadony, M.T.; Mesiha, P.K. Eco-Friendly Application of Nano-Chitosan for Controlling Potato and Tomato Bacterial Wilt. Saudi J. Biol. Sci. 2022, 29, 2199–2209. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; ALmoshadak, A.S.; Shafi, M.E.; Albaqami, N.M.; Saad, A.M.; El-Tahan, A.M.; Desoky, E.-S.M.; Elnahal, A.S.; Almakas, A.; Abd El-Mageed, T.A. Vital Roles of Sustainable Nano-Fertilizers in Improving Plant Quality and Quantity-an Updated Review. Saudi J. Biol. Sci. 2021, 28, 7349–7359. [Google Scholar] [CrossRef]
- Al-Juthery, H.W.; Habeeb, K.H.; Altaee, F.J.K.; AL-Taey, D.K.; Al-Tawaha, A.R.M. Effect of Foliar Application of Different Sources of Nano-Fertilizers on Growth and Yield of Wheat. Biosci. Res. 2018, 4, 3976–3985. [Google Scholar]
- Abdelkader, M.; Geioushy, R.A.; Fouad, O.A.; Khaled, A.G. Investigation the Activities of Photosynthetic Pigments, Antioxidant Enzymes and Inducing Genotoxicity of Cucumber Seedling Exposed to Copper Oxides Nanoparticles Stress. Sci. Hortic. 2022, 305, 111364. [Google Scholar] [CrossRef]
- Easwaran, C.; Moorthy, G.; Christopher, S.R.; Mohan, P.; Marimuthu, R.; Koothan, V.; Nallusamy, S. Nano Hybrid Fertilizers: A Review on the State of the Art in Sustainable Agriculture. Sci. Total Environ. 2024, 929, 172533. [Google Scholar] [CrossRef]
- Al-Juthery, H.W.; Lahmod, N.R.; Al-Taee, R.A. Intelligent, Nano-Fertilizers: A New Technology for Improvement Nutrient Use Efficiency (Article Review). In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 735, p. 012086. [Google Scholar]
- Abdelkader, M.M.; Gaplaev, M.S.; Terekbaev, A.A.; Puchkov, M.Y. The Influence of Biostimulants on Tomato Plants Cultivated under Hydroponic Systems. J. Hortic. Res. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Brennan, G.; Tennant, M.; Blomsma, F. Business and Production Solutions: Closing Loops and the Circular Economy. In Sustainability; Routledge: London, UK, 2015; pp. 219–239. [Google Scholar]
- Bora, K.A.; Hashmi, S.; Zulfiqar, F.; Abideen, Z.; Ali, H.; Siddiqui, Z.S.; Siddique, K.H. Recent Progress in Bio-Mediated Synthesis and Applications of Engineered Nanomaterials for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 999505. [Google Scholar] [CrossRef]
- Gauri, M.; Ali, S.J.; Khan, M.S. A Review of Apium Graveolens (Karafs) with Special Reference to Unani Medicine. Int. Arch. Integr. Med. 2015, 2, 131. [Google Scholar]
- Kooti, W.; Ghasemiboroon, M.; Asadi-Samani, M.; Ahangarpoor, A.; Abadi, A.; Afrisham, R.; Dashti, N. The Effects of Hydro-Alcoholic Extract of Celery on Lipid Profile of Rats Fed a High Fat Diet. Adv. Environ. Biol. 2014, 8, 325–330. [Google Scholar]
- Thomas, E.; Rathore, I.; Tarafdar, J.C. Bio-Inspired Synthesis of Nitrogen Nanoparticles and Its Application on Pearl Millet (Pennisetum Americanum L) Cv. HHB 67. J. Bionanosci. 2016, 10, 300–306. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Rathore, I. Microbial Synthesis of Nanoparticles for Use in Agriculture Ecosystem. In Microbes for Plant Stress Management; New India Publishing Agency: Delhi, India, 2016; pp. 105–118. [Google Scholar]
- Abdelghany, T.M.; Al-Rajhi, A.M.; Yahya, R.; Bakri, M.M.; Al Abboud, M.A.; Yahya, R.; Qanash, H.; Bazaid, A.S.; Salem, S.S. Phytofabrication of Zinc Oxide Nanoparticles with Advanced Characterization and Its Antioxidant, Anticancer, and Antimicrobial Activity against Pathogenic Microorganisms. Biomass Convers. Biorefinery 2023, 13, 417–430. [Google Scholar] [CrossRef]
- Kundu, D.; Hazra, C.; Chatterjee, A.; Chaudhari, A.; Mishra, S. Extracellular Biosynthesis of Zinc Oxide Nanoparticles Using Rhodococcus Pyridinivorans NT2: Multifunctional Textile Finishing, Biosafety Evaluation and in Vitro Drug Delivery in Colon Carcinoma. J. Photochem. Photobiol. B Biol. 2014, 140, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.M.; Elsayed, H.M.A. Biodiversity of Photosynthetic Pigments, Macronutrients Uptake and Fruit Quality of Tomato Genotypes. Russ. J. Plant Physiol. 2022, 69, 50. [Google Scholar] [CrossRef]
- Abdelkader, M.; Elkhawaga, F.A.; Suliman, A.A.; Puchkov, M.; Kuranova, K.N.; Mahmoud, M.H.; Abdelkader, M.F. Understanding the Regular Biological Mechanism of Susceptibility of Tomato Plants to Low Incidences of Blossom-End Rot. Horticulturae 2024, 10, 648. [Google Scholar] [CrossRef]
- AYDOĞMUŞ, Z.; ÇETİN, S.; ÖZGÜR, M.Ü. Determination of Ascorbic Acid in Vegetables by Derivative Spectrophotometry. Turk. J. Chem. 2002, 26, 697–704. [Google Scholar]
- Alt, D.; Füll, A.-M. Control of the Nitrogen Status of Lettuce by Nitrate Analysis of Plant Sap. In Proceedings of the Symposium on the Fertilization of Vegetables under Protected Cultivation, Naaldwijk, The Netherlands, 6–10 April 1987; Volume 222, pp. 23–28. [Google Scholar]
- Szwonek, E. Evaluation of Plant Nutrition Status by Fresh Index Part or Sap Analysis. In Proceedings of the Symposium on the Fertilization of Vegetables under Protected Cultivation, Naaldwijk, The Netherlands, 6–10 April 1987; Volume 222, pp. 201–206. [Google Scholar]
- Hanafy Ahmed, A.H.; Kheir, N.F.; Talaat, N.B. Physiological Studies on Reducing the Accumulation of Nitrate in Jew’s Mallow (Corchorus Olitorius L) and Radish (Raphanus Sativus L) Plants. Bull.-Fac. Agric. Univ. Cairo 1997, 48, 25–64. [Google Scholar]
- Cottenie, A.; Verloo, M.; Kiekens, L.; Velghe, G.; Camrbynek, R. Chemical Analysis of Plant and Soil. Lab. Agroch. State Univ. 1982, 63, 44–45. [Google Scholar]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Suliman, A.A.; Elkhawaga, F.A.; Zargar, M.; Bayat, M.; Pakina, E.; Abdelkader, M. Boosting Resilience and Efficiency of Tomato Fields to Heat Stress Tolerance Using Cytokinin (6-Benzylaminopurine). Horticulturae 2024, 10, 170. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Do Thi, N. Effects of Extraction and Processing Methods on Antioxidant Compound Contents and Radical Scavenging Activities of Laver (Porphyra Tenera). Prev. Nutr. Food Sci. 2014, 19, 40. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- McKersie, B.D.; Bowley, S.R.; Harjanto, E.; Leprince, O. Water-Deficit Tolerance and Field Performance of Transgenic Alfalfa Overexpressing Superoxide Dismutase. Plant Physiol. 1996, 111, 1177–1181. [Google Scholar] [CrossRef]
- Bellaire, B.A.; Carmody, J.; Braud, J.; Gossett, D.R.; Banks, S.W.; Cranlucas, M.; Fowler, T.E. Involvement of Abscisic Acid-Dependent and—Independent Pathways in the Upregulation of Antioxidant Enzyme Activity during NaCl Stress in Cotton Callus Tissue. Free. Radic. Res. 2000, 33, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Pengelly, A. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicine; Routledge: London, UK, 2020. [Google Scholar]
- Lu, L.; Zhang, Y.; Li, L.; Yi, N.; Liu, Y.; Qaseem, M.F.; Li, H.; Wu, A.-M. Physiological and Transcriptomic Responses to Nitrogen Deficiency in Neolamarckia Cadamba. Front. Plant Sci. 2021, 12, 747121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; (Max) Lu, G.Q. Magnetic Nanocomposites with Mesoporous Structures: Synthesis and Applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Cluster Bean (Cyamopsis Tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Fortin, D.; Beveridge, T.J. From Biology to Biotechnology and Medical Applications. In Biomineralization; Wiley: Hoboken, NJ, USA, 2000; pp. 7–22. [Google Scholar]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-Mediated Biosynthesis and Characterization of TiO2 Nanoparticles and Their Activity against Pathogenic Bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 Million Species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Sastry, M.; Ahmad, A.; Khan, M.I.; Kumar, R. Biosynthesis of Metal Nanoparticles Using Fungi and Actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Waskela, P.; Naruka, I.S.; Shaktawat, R.P.S. Effect of Row Spacing and Level of NPK on Growth and Yield of Fennel (Foeniculum Vulgare). J. Krishi Vigyan 2017, 6, 78–82. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.A. Effect of Nitrogen Fixing Bacteria and Moringa Leaf Extract on Fruit Yield, Estragole Content and Total Phenols of Organic Fennel. Sci. Hortic. 2020, 265, 109209. [Google Scholar] [CrossRef]
- Opera, C.N.; Asigbu, J.E. Nutrient Content of Poultry Manures and the Optimum Role for Egyptian Fruit Yield in a Weathered Tropical Ultisol Bid. Agric. Hort. 1996, 13, 341–350. [Google Scholar] [CrossRef]
- Rashid, M.; Hussain, K.; Malik, A.A.; Narayan, S.; Nazir, G.; Mazahir, S. Impact of Climate Change on Vegetable Crops and Its Mitigation. Int. J. Curr. Microbiol. Appl. Sci. Spec. 2020, 11, 2429–2445. [Google Scholar]
- Shaimaa, M.E.S.; Glala, A.A.; Adam, S.M. Response of Two Celery Cultivars to Partial or Complete Organic Nitrogen Alternation Strategies. Aust. J. Basic Appl. Sci. 2011, 5, 22–29. [Google Scholar]
- Dufault, R.J. Use of Slow-Release Nitrogen and Phosphorus Fertilizers in Celery Transplant Production. HortScience 1987, 22, 1268–1270. [Google Scholar] [CrossRef]
- Li HuiHe, L.H.; Wang ZhengYin, W.Z.; Zhang Hao, Z.H.; Li BaoZhen, L.B. Effects of Organic Manures on the Nutritional Quality of Foliage Vegetables in Soilless Culture. J. Southwest Agric. Univ. 2003, 25, 66–69. [Google Scholar]
- Mantelin, S.; Touraine, B. Plant Growth-Promoting Bacteria and Nitrate Availability: Impacts on Root Development and Nitrate Uptake. J. Exp. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.M.; Glala, A.A.; Ezzo, M.I. Influence of Plant Growth Promotion Rhizosphere-Bacteria “Pgpr” Enrichment and Some Alternative Nitrogen Organic Sources on Tomato. In Proceedings of the IV International Symposium on Ecologically Sound Fertilization Strategies for Field Vegetable Production, Alnarp, Sweden, 22–29 September 2008; Volume 852, pp. 131–138. [Google Scholar]
- El-Magd, A.; Zaki, M.M.F.; Abou-Hussein, S.D. Effect of Organic Manure and Different Levels of Saline Irrigation Water on Growth, Green Yield and Chemical Content of Sweet Fennel. Aust. J. Basic Appl. Sci. 2008, 2, 90–98. [Google Scholar]
- El-Sheshtawy, A.A.; Hager, M.A.; Shawer, S.S. Effect of Bio-Fertilizer, Phosphorus Source and Humic Substances on Yield, Yield Components and Nutrients Uptake by Barley Plant. J. Biol. Chem. Environ. Sci 2019, 14, 279–300. [Google Scholar]
- Eisa, E.A. Effect of Some Different Sourses of Organic Fertilizers and Seaweed Extract on Growth and Essential Oil of Sweet Fennel (Foeniculum Vulgare Mill.) Plants. J. Plant Prod. 2016, 7, 575–584. [Google Scholar] [CrossRef]
- Youssef, M.A.; AL-Huqail, A.A.; Ali, E.F.; Majrashi, A. Organic Amendment and Mulching Enhanced the Growth and Fruit Quality of Squash Plants (Cucurbita Pepo L.) Grown on Silty Loam Soils. Horticulturae 2021, 7, 269. [Google Scholar] [CrossRef]
- Helaly, A.A.; Mady, E.; Salem, E.A.; Randhir, T.O. Stimulatory Effects of Growth-Promoting Bacteria on Growth, Nutritional Composition, and Yield of Kale Plants. J. Plant Nutr. 2022, 45, 2465–2477. [Google Scholar] [CrossRef]
- Nada, R.S. Influence of Bio and Nano Fertilization on Growth, Yield and Active Ingredient in Matricaria Chamomilla L. Plant. Ph.D. Thesis, Al-Azhar University, Cairo, Egypt, 2019. [Google Scholar]
- Helaly, M.N.; El-Metwally, M.A.; El-Hoseiny, H.; Omar, S.A.; El-Sheery, N.I. Effect of Nanoparticles on Biological Contamination of in Vitro Cultures and Organogenic Regeneration of Banana. Aust. J. Crop Sci. 2014, 8, 612–624. [Google Scholar]
- Machado, R.M.; Alves-Pereira, I.; Faty, Y.; Perdigão, S.; Ferreira, R. Influence of Nitrogen Sources Applied by Fertigation to an Enriched Soil with Organic Compost on Growth, Mineral Nutrition, and Phytochemicals Content of Coriander (Coriandrum Sativum L.) in Two Successive Harvests. Plants 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, T.; Mohammadi, S.; Ghahremani, Z. Effect of Nitrogen and Potassium Fertilizer on Growth, Yield and Chemical Composition of Sweet Fennel. J. Plant Nutr. 2020, 43, 1189–1204. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-Biofertilizers as Bio-Emerging Strategies for Sustainable Agriculture Development: Potentiality and Their Limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef]
| Physical Properties | Soil Depth (cm) | Chemical Properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Texture | Sand% | Silt% | Clay% | pH | EC, dSm−1 | OM, % | HCO3 | Cl | Na | K | Ca | |
| meq/L | ||||||||||||
| Sandy-loam | 54.28 | 30.0 | 14.94 | 0–25 | 8.1 | 4.42 | 0.4 | 3.1 | 4.0 | 8.5 | 1.3 | 1.0 |
| 55.19 | 29.59 | 13.43 | 25–50 | 8.1 | 5.13 | 0.3 | 0.4 | 3.4 | 6.8 | 1.1 | 1.8 | |
| 57.71 | 30.92 | 11.37 | 50–75 | 8.3 | 7.85 | 0.3 | 2.0 | 6.2 | 8.0 | 0.6 | 2.2 | |
| Cultivars | Nitrogen Source and Concentration | First Season | |||
|---|---|---|---|---|---|
| Plant Height (cm) | Number of Leaves/Plant | Fresh Weight (g/plant) | Dry Weight (g/plant) | ||
| Local Egyptian Balady | Mineral 100 kg | 55.3 ± 0.24 cd | 73.3 ± 2.72 e | 566.3 ± 13.44 d | 54.4 ± 0.62 d |
| Bio-NPs 20 ppm | 56.2 ± 0.21 c | 73.9 ± 4.02 e | 509.4 ± 9.41 f | 48.9 ± 0.51 f | |
| Bio-NPs 30 ppm | 59.6 ± 0.17 bc | 82.6 ± 3.92 cd | 560.3 ± 11.27 d | 53.1 ± 0.68 de | |
| Bio-NPs 40 ppm | 61.1 ± 0.19 b | 82.7 ± 3.04 cd | 520.5 ± 13.51 e | 50.6 ± 0.41 e | |
| Tall Utah 52–75 | Mineral 100 kg | 58.1 ± 0.26 c | 99.1 ± 2.02 ab | 1122.4 ± 11.03 a | 83.9 ± 53 b |
| Bio-NPs 20 ppm | 67.9 ± 0.21 ab | 98.8 ± 2.13 ab | 989.5 ± 8.93 c | 83.2 ± 0.74 b | |
| Bio-NPs 30 ppm | 69.8 ± 0.32 a | 102.2 ± 2.92 a | 1121.4 ± 11.12 a | 83.2 ± 1.04 a | |
| Bio-NPs 40 ppm | 69.5 ± 0.22 a | 99.2 ± 2.01 a | 1014.4 ± 9.38 b | 82.5 ± 0.92 a | |
| Second season | |||||
| Local Egyptian Balady | Mineral 100 kg | 44.3 ± 04.7 d | 84.8 ± 2.54 e | 579.3 ± 3.28 d | 54.8 ± 0.73 d |
| Bio-NPs 20 ppm | 53.3 ± 0.37 c | 82.7 ± 1.09 ef | 529.5 ± 7.28 de | 50.4 ± 1.02 e | |
| Bio-NPs 30 ppm | 54.2 ± 0.35 bc | 87.6 ± 2.38 d | 572.1 ± 9.26 bc | 53.9 ± 0.94 de | |
| Bio-NPs 40 ppm | 56.9 ± 0.41 b | 88.1 ± 2.15 cd | 556.9 ± 7.37 de | 52.6 ± 0.61 de | |
| Tall Utah 52–75 | Mineral 100 kg | 53.9 ± 0.33 c | 99.9 ± 3.01 bc | 1018.3 ± 8.42 c | 95.6 ± 1.05 ab |
| Bio-NPs 20 ppm | 54.3 ± 0.27 bc | 98.4 ± 2.02 bc | 1008.2 ± 7.32 c | 93.2 ± 0.83 b | |
| Bio-NPs 30 ppm | 59.8 ± 0.49 a | 107.1 ± 2.84 a | 1116.5 ± 8.53 a | 95.1 ± 0.62 a | |
| Bio-NPs 40 ppm | 59.9 ± 0.52 a | 106.7 ± 2.94 a | 1013.5 ± 6.29 b | 93.8 ± 0.81 b | |
| Genotype | Nitrogen Source and Concentration | First Season | |||
|---|---|---|---|---|---|
| Chlorophyll A (mg/g) | Chlorophyll B (mg/g) | Total Chlorophyll (mg/g) | Carotenoids (mg/g) | ||
| Local Egyptian Balady | Mineral 100 kg | 1.58 ± 0.04 a | 0.66 ± 0.01 a | 2.25 ± 0.01 a | 0.42 ± 0.02 c |
| Bio-NPs 20 ppm | 1.41 ± 0.03 b | 0.61 ± 0.01 b | 2.03 ± 0.02 d | 0.44 ± 0.02 b | |
| Bio-NPs 30 ppm | 1.36 ± 0.02 c | 0.59 ± 0.03 c | 1.97 ± 0.01 a | 0.47 ± 0.01 a | |
| Bio-NPs 40 ppm | 1.33 ± 0.02 d | 0.59 ± 0.01 d | 1.92 ± 0.01 c | 0.45 ± 0.03 b | |
| Tall Utah 52–75 | Mineral 100 kg | 1.34 ± 0.03 cd | 0.61 ± 0.02 b | 1.96 ± 0.01 d | 0.41 ± 0.02 c |
| Bio-NPs 20 ppm | 1.28 ± 0.01 d | 0.57 ± 0.01 d | 1.87 ± 0.03 c | 0.39 ± 0.02 d | |
| Bio-NPs 30 ppm | 1.18 ± 0.02 e | 0.51 ± 0.01 fe | 1.70 ± 0.01 c | 0.39 ± 0.01 d | |
| Bio-NPs 40 ppm | 1.21 ± 0.01 de | 0.55 ± 0.03 de | 1.75 ± 0.01 bc | 0.40 ± 0.02 cd | |
| Second season | |||||
| Local Egyptian Balady | Mineral 100 kg | 1.52 ± 0.02 a | 0.75 ± 0.03 a | 2.29 ± 0.02 a | 0.49 ± 0.02 a |
| Bio-NPs 20 ppm | 1.48 ± 0.02 b | 0.73 ± 0.01 b | 2.23 ± 0.01 b | 0.43 ± 0.01 c | |
| Bio-NPs 30 ppm | 1.32 ± 0.01 c | 0.69 ± 0.02 c | 2.03 ± 0.01 d | 0.45 ± 0.02 b | |
| Bio-NPs 40 ppm | 1.42 ± 0.02 d | 0.58 ± 0.01 cd | 2.00 ± 0.01 b | 0.43 ± 0.02 c | |
| Tall Utah 52–75 | Mineral 100 kg | 1.51 ± 0.01 a | 0.47 ± 0.01 d | 1.98 ± 0.01 d | 0.44 ± 0.01 b |
| Bio-NPs 20 ppm | 1.38 ± 0.03 bc | 0.51 ± 0.01 e | 1.91 ± 0.02 cd | 0.38 ± 0.02 d | |
| Bio-NPs 30 ppm | 1.25 ± 0.02 d | 0.51 ± 0.02 e | 1.76 ± 0.01 c | 0.39 ± 0.02 d | |
| Bio-NPs 40 ppm | 1.41 ± 0.02 c | 0.48 ± 0.01 cd | 1.90 ± 0.02 d | 0.36 ± 0.02 e | |
| Genotype | Nitrogen Source and Concentration | First Season | |||
|---|---|---|---|---|---|
| Fresh Yield (ton/Acre) | Dry Yield (Kg/Acre) | Vitamin C Content (mg/100 g) | NO3–N ppm | ||
| Local Egyptian Balady | Mineral 100 kg | 5.1 ± 0.03 e | 521.3 ± 5.27 g | 5.1 ± 0.03 c | 342.5 ± 9.04 a |
| Bio-NPs 20 ppm | 4.2 ± 0.01 f | 523.6 ± 4.84 g | 5.3 ± 0.02 b | 237.9 ± 7.37 c | |
| Bio-NPs 30 ppm | 5.2 ± 0.06 e | 571.9 ± 6.04 fg | 5.6 ± 0.02 a | 132.1 ± 9.25 de | |
| Bio-NPs 40 ppm | 5.1 ± 0.02 e | 563.7 ± 3.02 fg | 5.4 ± 0.05 ab | 174.3 ± 5.93 d | |
| Tall Utah 52–75 | Mineral 100 kg | 8.5 ± 0.02 c | 783.5 ± 7.24 ef | 5.1 ± 0.03 c | 311.2 ± 8.59 b |
| Bio-NPs 20 ppm | 10.3 ± 0.03 b | 924.1 ± 6.73 c | 5.3 ± 0.01 b | 121.5 ± 7.47 e | |
| Bio-NPs 30 ppm | 11.2 ± 0.02 a | 1002.5 ± 7.14 a | 5.6 ± 0.02 a | 108.5 ± 8.35 f | |
| Bio-NPs 40 ppm | 10.4 ± 0.02 ab | 982.4 ± 5.93 b | 5.4 ± 0.03 ab | 116.4 ± 7.28 f | |
| Second season | |||||
| Local Egyptian Balady | Mineral 100 kg | 5.7 ± 0.06 de | 592.4 ± 7.04 d | 5.2 ± 0.05 bc | 378.3 ± 6.84 a |
| Bio-NPs 20 ppm | 5.2 ± 0.04 e | 567.7 ± 9.32 e | 5.2 ± 0.03 bc | 243.6 ± 5.93 b | |
| Bio-NPs 30 ppm | 6.1 ± 0.08 d | 603.1 ± 11.32 d | 5.4 ± 0.05 a | 201.4 ± 7.37 c | |
| Bio-NPs 40 ppm | 5.8 ± 0.03 de | 556.2 ± 9.73 f | 5.1 ± 0.03 c | 198.5 ± 9.46 c | |
| Tall Utah 52–75 | Mineral 100 kg | 9.7 ± 0.05 b | 893.5 ± 8.04 cd | 5.2 ± 0.03 bc | 312.4 ± 11.04 a |
| Bio-NPs 20 ppm | 9.2 ± 0.02 bc | 1018.3 ± 9.47 bc | 5.2 ± 0.04 bc | 213.6 ± 8.64 bc | |
| Bio-NPs 30 ppm | 11.7 ± 0.03 a | 1173.8 ± 7.62 a | 5.3 ± 0.02 ab | 187.4 ± 9.27 d | |
| Bio-NPs 40 ppm | 11.3 ± 0.02 a | 1043.6 ± 9.51 b | 5.2 ± 0.05 bc | 99.2 ± 7.47 d | |
| Genotype | Nitrogen Source and Concentration | First Season | |||
|---|---|---|---|---|---|
| Nitrogen, % | Phosphorus, % | Potassium, % | Calcium, % | ||
| Local Egyptian Balady | Mineral 100 kg | 5.3 ± 0.02 a | 0.5 ± 0.02 b | 2.2 ± 0.02 c | 2.1 ± 0.04 d |
| Bio-NPs 20 ppm | 4.1 ± 0.01 b | 0.5 ± 0.01 b | 2.4 ± 0.02 b | 2.1 ± 0.07 d | |
| Bio-NPs 30 ppm | 3.7 ± 0.02 c | 0.6 ± 0.01 a | 2.7 ± 0.01 a | 2.3 ± 0.0 b | |
| Bio-NPs 40 ppm | 3.6 ± 0.01 cd | 0.6 ± 0.02 a | 2.6 ± 0.02 ab | 2.3 ± 0.03 b | |
| Tall Utah 52–75 | Mineral 100 kg | 5.2 ± 0.02 a | 0.45 ± 0.01 b | 2.2 ± 0.02 c | 2.2 ± 0.09 c |
| Bio-NPs 20 ppm | 4.1 ± 0.03 b | 0.4 ± 0.02 c | 2.6 ± 0.01 ab | 2.3 ± 0.07 b | |
| Bio-NPs 30 ppm | 2.1 ± 0.02 de | 0.5 ± 0.02 b | 2.6 ± 0.01 a | 2.4 ± 0.05 a | |
| Bio-NPs 40 ppm | 2.2 ± 0.02 d | 0.5 ± 0.02 a | 2.6 ± 0.02 ab | 2.4 ± 0.08 a | |
| Second season | |||||
| Local Egyptian Balady | Mineral 100 kg | 5.1 ± 0.03 a | 0.5 ± 0.01 b | 2.2 ± 0.01 c | 2.2 ± 0.04 c |
| Bio-NPs 20 ppm | 3.2 ± 0.02 bc | 0.4 ± 0.01 c | 2.4 ± 0.01 b | 2.1 ± 0.03 d | |
| Bio-NPs 30 ppm | 2.1 ± 0.02 de | 0.6 ± 0.02 a | 2.6 ± 0.02 a | 2.4 ± 0.07 a | |
| Bio-NPs 40 ppm | 2.2 ± 0.02 de | 0.6 ± 0.01 b | 2.6 ± 0.01 a | 2.4 ± 0.06 a | |
| Tall Utah 52–75 | Mineral 100 kg | 5.1 ± 0.03 a | 0.5 ± 0.01 b | 2.3 ± 0.02 d | 2.2 ± 0.04 a |
| Bio-NPs 20 ppm | 3.6 ± 0.01 bc | 0.4 ± 0.02 a | 2.6 ± 0.02 a | 2.3 ± 0.04 b | |
| Bio-NPs 30 ppm | 2.4 ± 0.02 de | 0.5 ± 0.01 a | 2.7 ± 0.01 a | 2.3 ± 0.07 b | |
| Bio-NPs 40 ppm | 2.1 ± 0.02 e | 0.5 ± 0.01 b | 2.6 ± 0.02 a | 2.2 ± 0.07 c | |
| Genotype | Nitrogen Source and Concentration | First Season | |||
|---|---|---|---|---|---|
| Total Phenols (GAE/mL) | Total Flavonoids (C.E./mL) | DPPH (T.E./mL) | ABTS (T.E./mL) | ||
| Local Egyptian Balady | Mineral 100 kg | 0.21 ± 0.01 c | 0.17 ± 0.02 b | 0.58 ± 0.07 c | 4.3 ± 0.12 c |
| Bio-NPs 20 ppm | 0.24 ± 0.01 cd | 0.17 ± 0.01 b | 0.62 ± 0.05 bc | 4.6 ± 0.09 b | |
| Bio-NPs 30 ppm | 0.35 ± 0.02 a | 0.21 ± 0.01 a | 0.76 ± 0.07 a | 5.0 ± 0.21 a | |
| Bio-NPs 40 ppm | 0.35 ± 0.02 a | 0.19 ± 0.02 ab | 0.75 ± 0.04 a | 5.1 ± 0.09 a | |
| Tall Utah 52–75 | Mineral 100 kg | 0.17 ± 0.01 d | 0.15 ± 0.01 c | 0.47 ± 0.04 e | 3.7 ± 0.13 d |
| Bio-NPs 20 ppm | 0.21 ± 0.04 c | 0.16 ± 0.02 bc | 0.51 ± 0.02 d | 3.6 ± 0.11 d | |
| Bio-NPs 30 ppm | 0.26 ± 0.05 b | 0.18 ± 0.02 b | 0.59 ± 0.02 c | 4.2 ± 0.16 c | |
| Bio-NPs 40 ppm | 0.26 ± 0.03 b | 0.17 ± 0.02 b | 0.57 ± 0.05 c | 4.4 ± 0.11 bc | |
| Second season | |||||
| Local Egyptian Balady | Mineral 100 kg | 0.21 ± 0.03 d | 0.17 ± 0.01 bc | 0.56 ± 0.03 c | 3.7 ± 0.18 c |
| Bio-NPs 20 ppm | 0.25 ± 0.02 c | 0.19 ± 0.01 ab | 0.54 ± 0.06 cd | 3.9 ± 0.13 b | |
| Bio-NPs 30 ppm | 0.31 ± 0.02 a | 0.22 ± 0.02 a | 0.73 ± 0.06 a | 5.7 ± 0.21 a | |
| Bio-NPs 40 ppm | 0.32 ± 0.02 a | 0.18 ± 0.01 b | 0.73 ± 0.03 a | 5.3 ± 0.17 a | |
| Tall Utah 52–75 | Mineral 100 kg | 0.19 ± 0.03 cd | 0.15 ± 0.01 c | 0.51 ± 0.07 d | 3.1 ± 0.16 e |
| Bio-NPs 20 ppm | 0.22 ± 0.01 cd | 0.17 ± 0.02 bc | 0.53 ± 0.03 d | 3.4 ± 0.13 d | |
| Bio-NPs 30 ppm | 0.26 ± 0.02 bc | 0.19 ± 0.01 ab | 0.61 ± 0.03 b | 3.9 ± 0.17 b | |
| Bio-NPs 40 ppm | 0.28 ± 0.02 b | 0.18 ± 0.01 b | 0.59 ± 0.07 b | 3.8 ± 0.09 bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkader, M.; Zargar, M.; Bayat, M.; Pakina, E.; Shehata, A.S.A.; Suliman, A.A. Biogenic Nano-Fertilizers as a Sustainable Approach to Alleviate Nitrate Accumulation and Enrich Quality Traits of Vegetable Crops. Horticulturae 2024, 10, 789. https://doi.org/10.3390/horticulturae10080789
Abdelkader M, Zargar M, Bayat M, Pakina E, Shehata ASA, Suliman AA. Biogenic Nano-Fertilizers as a Sustainable Approach to Alleviate Nitrate Accumulation and Enrich Quality Traits of Vegetable Crops. Horticulturae. 2024; 10(8):789. https://doi.org/10.3390/horticulturae10080789
Chicago/Turabian StyleAbdelkader, Mostafa, Meisam Zargar, Maryam Bayat, Elena Pakina, Ahmed S. A. Shehata, and Ahmed A. Suliman. 2024. "Biogenic Nano-Fertilizers as a Sustainable Approach to Alleviate Nitrate Accumulation and Enrich Quality Traits of Vegetable Crops" Horticulturae 10, no. 8: 789. https://doi.org/10.3390/horticulturae10080789
APA StyleAbdelkader, M., Zargar, M., Bayat, M., Pakina, E., Shehata, A. S. A., & Suliman, A. A. (2024). Biogenic Nano-Fertilizers as a Sustainable Approach to Alleviate Nitrate Accumulation and Enrich Quality Traits of Vegetable Crops. Horticulturae, 10(8), 789. https://doi.org/10.3390/horticulturae10080789










