Influence of Culture Conditions on Growth and Daidzein and Genistein Production in Hairy Root Cultures of Pueraria candollei var. mirifica
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bacterial Strain and Culture Conditions
2.3. Plant Material and Preparation of Sterilized Plantlets
2.4. Induction of Hairy Roots Using Cocultivation Techniques
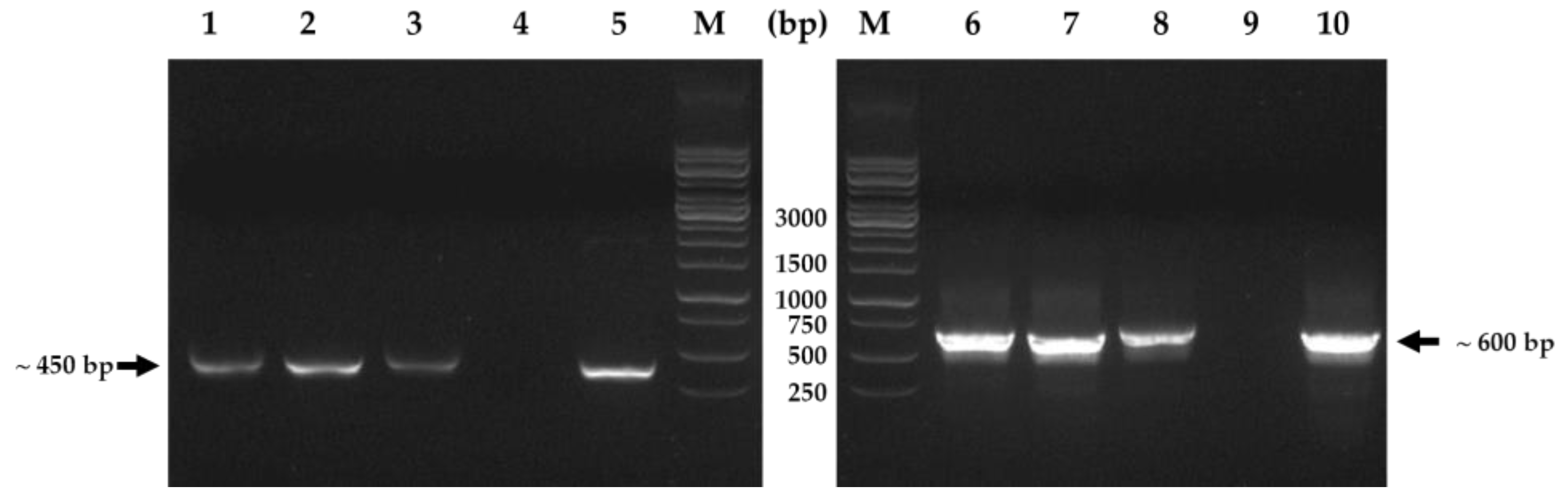
2.5. Confirmation of Transformed Roots Using Polymerase Chain Reaction (PCR)
2.6. Effect of Culture Conditions on Growth and Daidzein and Genistein Production in Hairy Root Culture of P. mirifica
2.7. Analytical Procedure
2.8. Statistical Analysis
3. Results and Discussion
3.1. Induction of Hairy Roots from P. mirifica by A. rhizogenes ATCC15834
3.2. Confirmation of Hairy Roots Using PCR
3.3. Effect of Culture Conditions on Growth and Daidzein and Genistein Production in Hairy Root Cultures of P. mirifica
3.3.1. Effect of Culture Medium on Growth and Daidzein and Genistein Production

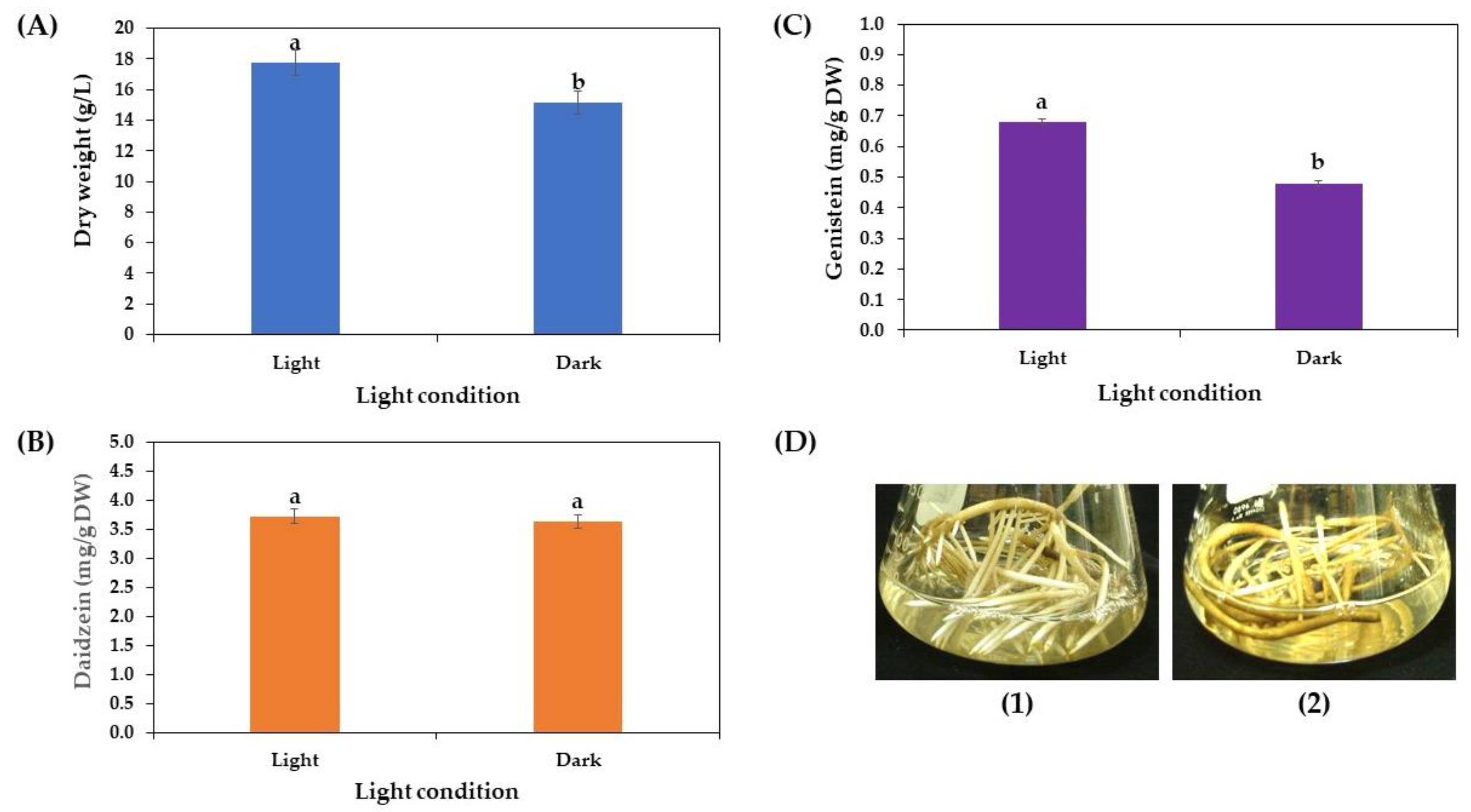
3.3.2. Effects of Light Conditions on Growth and Daidzein and Genistein Production
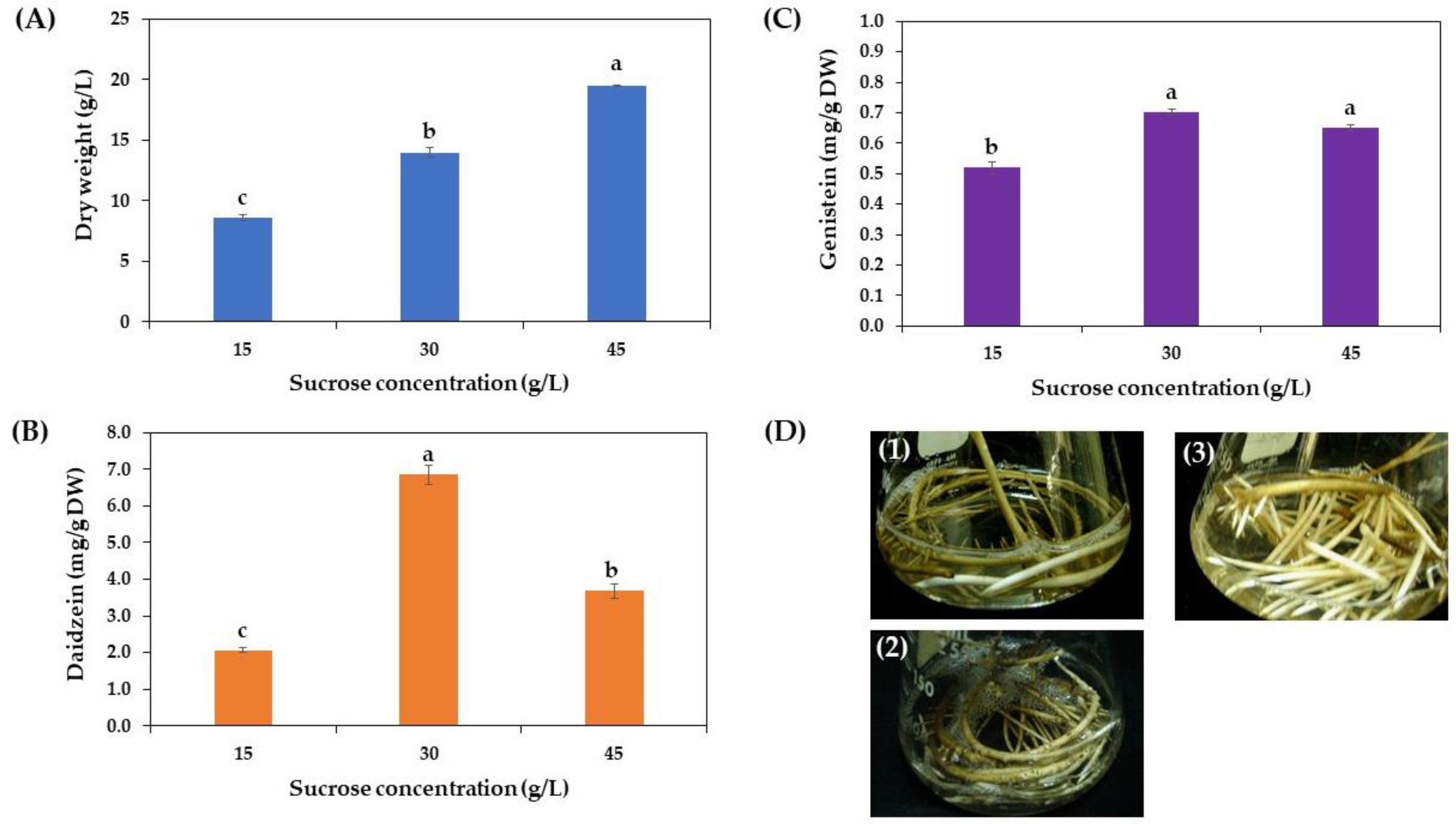
3.3.3. Effect of the Sucrose Concentration on Growth and Daidzein and Genistein Production
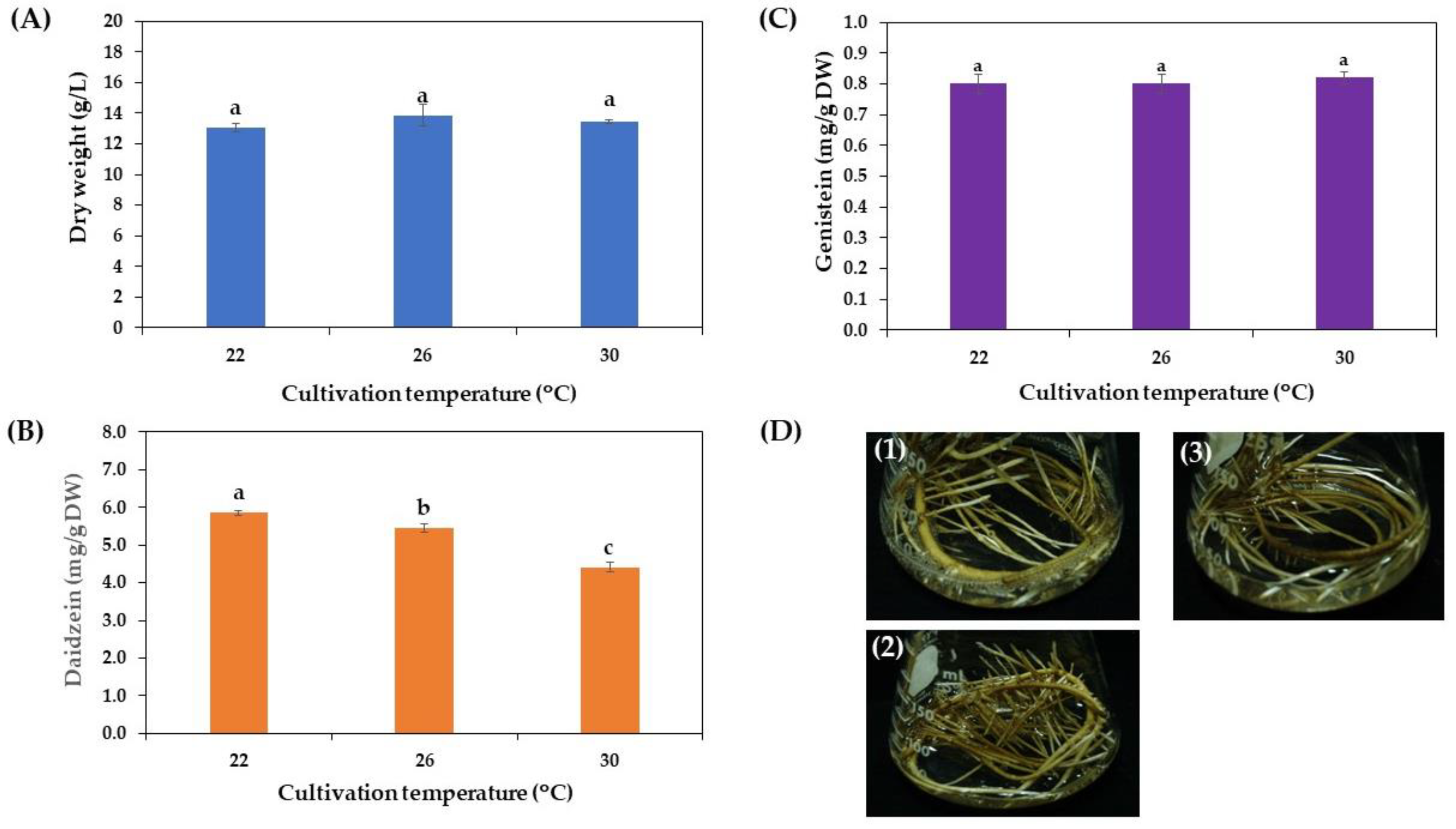
3.3.4. Effect of Incubation Temperature on Growth and Daidzein and Genistein Production
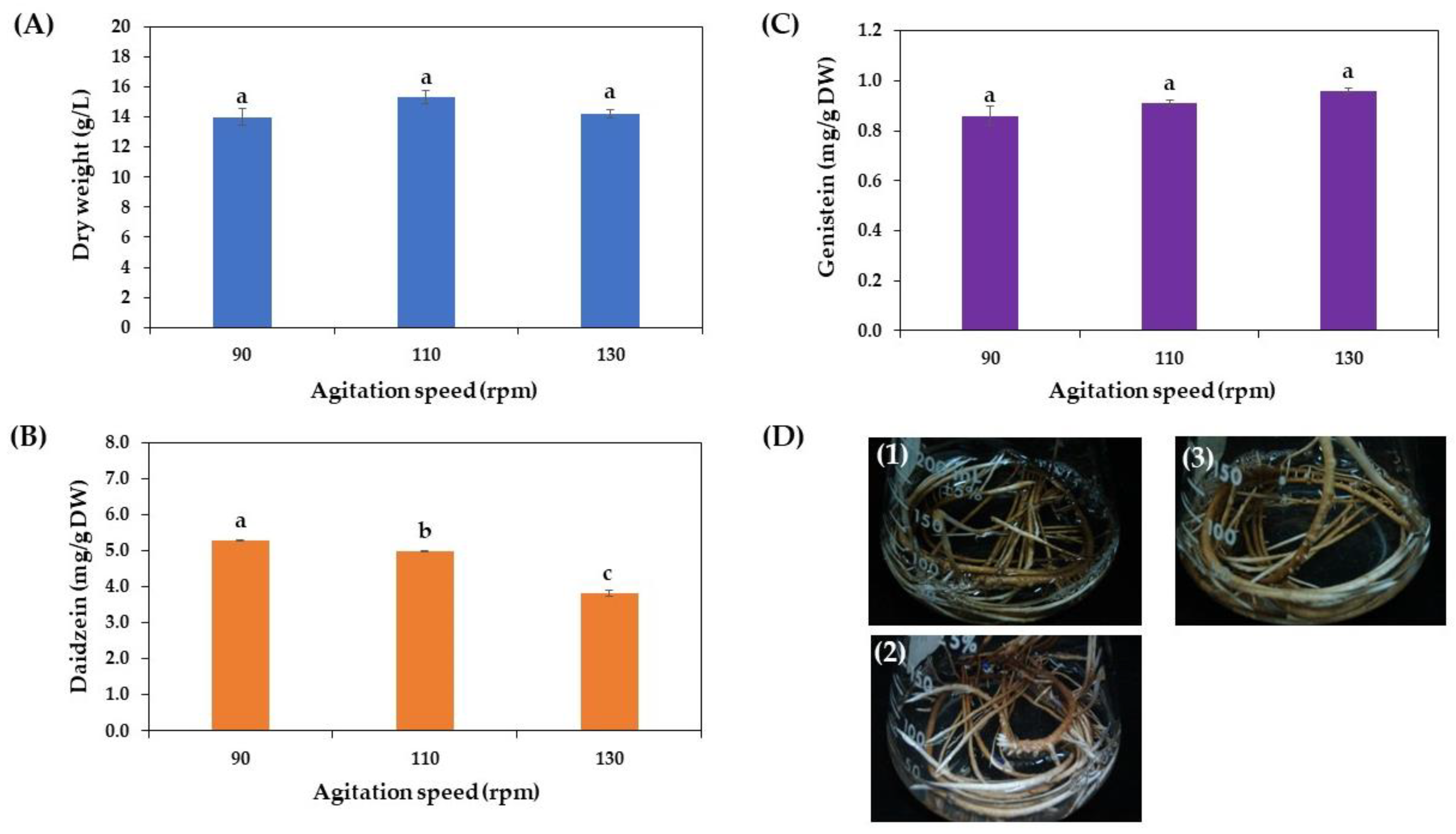
3.3.5. Effect of Agitation Speed on Growth and Daidzein and Genistein Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherdshewasart, W.; Kitsamai, Y.; Malaivijitnond, S. Evaluation of the estrogenic activity of the wild Pueraria mirifica by vaginal cornification assay. J. Reprod. Dev. 2007, 53, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Niyomdham, C. Notes on Thai and Indo-Chinese Phaseoleae (Leguminosae-Papilionoideae). Nord. J. Bot. 1992, 12, 339–346. [Google Scholar] [CrossRef]
- Malaivijitnond, S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Front. Med. 2012, 6, 8–21. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inoue, M.; Otani, T.; Sasazuki, S.; Kurahashi, N.; Miura, T.; Yamamoto, S.; Tsugane, S. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: A nested case-control study from the Japan public health center-based prospective study group. J. Clin. Oncol. 2008, 26, 1677. [Google Scholar] [CrossRef]
- Rani, D.; Kobtrakul, K.; Vimolmangkang, S. Pueraria candollei var. mirifica: A precious source of pharmaceuticals and cosmeceuticals. Thai J. Pharm. Sci. 2022, 46, 1–10. [Google Scholar] [CrossRef]
- Sirisa-Ard, P.; Peerakam, N.; Huy, N.Q.; On, T.; Long, P.T.; Intharuksa, A. Development of anti-wrinkle cream from Pueraria candollei var. mirifica (Airy Shaw & Suvat.) Niyomdham, “Kwao Krua Kao” for menopausal women. Int. J. Pharm. Pharm. Sci. 2018, 10, 16–21. [Google Scholar]
- Intharuksa, A.; Kitamura, M.; Peerakam, N.; Charoensup, W.; Ando, H.; Sasaki, Y.; Sirisa-Ard, P. Evaluation of white Kwao Krua (Pueraria candollei Grah. ex Benth.) products sold in Thailand by molecular, chemical, and microscopic analysis. J. Nat. Med. 2020, 74, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Cherdshewasart, W.; Subtang, S.; Dahlan, W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria labata. J. Pharm. Biomed. Anal. 2007, 43, 428–434. [Google Scholar] [CrossRef]
- Dixon, R.A.; Canovas, P.; Guo, Z.T.; He, X.Z.; Lamb, C.; McAlister, F. Molecular controls for isoflavonoid biosynthesis in relation to plant and human health. In Phytochemicals in Human Health Protection, Nutrition, and Plant Defense: Recent Advances in Phytochemistry; Romeo, J.T., Ed.; Plenum Publisher: New York, NY, USA, 1999. [Google Scholar]
- Hsieh, C.Y.; Santell, R.C.; Haslam, S.Z.; Helferich, W.G. Estrogenic effects of genistein on the growth of estrogen receptor positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar] [PubMed]
- Ren, M.Q.; Kuhn, G.; Wegner, J.; Chen, J. Isoflavones, substances with multi-biological and clinical properties. Eur. J. Nutr. 2001, 40, 135–146. [Google Scholar] [CrossRef]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef] [PubMed]
- Thanonkeo, S.; Panichajakul, S. Production of isoflavones, daidzein and genistein in callus cultures of Pueraria candollei Wall. ex Benth. var. mirifica. Songklanakarin J. Sci. Technol. 2006, 28, 45–53. [Google Scholar]
- Amir, M.; Aqil, M.; Ismail, M.V.; Akhtar, M.; Khan, A.H.; Mujeeb, M. Effect of carbon source and incubation temperature on total content of secondary metabolites of callus culture of Solanum nigrum. World J. Pharmaceu. Res. 2017, 6, 905–922. [Google Scholar]
- Vazquez-Marquez, A.M.; Zepeda-Gómez, C.; Burrola-Aguilar, C.; Bernabé-Antonio, A.; Nieto-Trujillo, A.; Cruz-Sosa, F.; Rodriguez-Monroy, M.; Estrada-Zúñiga, M.E. Effect of stirring speed on the production of phenolic secondary metabolites and growth of Buddleja cordata cell cultured in mechanically agitated bioreactor. Plant Cell Tiss. Organ Cult. 2019, 139, 155–166. [Google Scholar] [CrossRef]
- Sae-Foo, W.; Yusakul, G.; Putalun, W. Enhancement of isoflavonoid production and release in Pueraria candollei cell suspension culture using elicitors for improving pharmacological activities. Plant Cell Tiss. Organ Cult. 2024, 156, 99. [Google Scholar] [CrossRef]
- Shohael, A.M.; Ali, M.B.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos. Plant Cell Tiss. Cult. 2006, 85, 219–228. [Google Scholar] [CrossRef]
- Hussain, M.J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Root cultures, a boon for the production of valuable compounds: A comparative review. Plants 2022, 11, 439. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Näkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.M.; Ritala, A.; Cardon, F. Hairy root cultures-a versatile tool with multiple applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Morey, K.J.; Peebles, C.A.M. Hairy roots: An untapped potential for production of plant products. Front. Plant Sci. 2022, 13, 937095. [Google Scholar] [CrossRef]
- Udomsuk, L.; Jarukamjorn, K.; Tanaka, H.; Putalun, W. Isoflavonoid production in a hairy roots culture of Pueraria candollei. Z. Naturforsch. 2009, 64, 687–691. [Google Scholar] [CrossRef]
- Kim, S.; Cha, M.S.; Lee, E.; Kim, I.; Kwon, J.E.; Kang, S.C.; Park, T.H. In vitro induction of hairy root from isoflavones-producing Korean wild arrowroot Pueraria labata. J. Plant Biotechnol. 2012, 39, 205–211. [Google Scholar] [CrossRef]
- Habibi, P.; Piri, K.; Deljo, A.; Moghadam, Y.A.; Ghiasvand, T. Increasing scopolamine content in hairy roots of Atropa belladonna using bioreactor. Braz. Arch. Biol. Technol. 2015, 58, 166–174. [Google Scholar] [CrossRef]
- Verma, P.C.; Singh, H.; Negi, A.S.; Saxena, G.; Rahman, L.; Banerjee, S. Yield enhancement strategies for the production of picroliv from hairy root culture of Picrorhiza kurroa Royle ex Benth. Plant Signal. Behav. 2015, 10, e1023976. [Google Scholar] [CrossRef] [PubMed]
- Skala, E.; Olszewska, M.A.; Makowczyńska, J.; Kicel, A. Effect of sucrose concentration on Rhaponticum carthamoides (Willd.) Iljin transformed root biomass, caffeoylquinic acid derivative, and flavonoid production. Int. J. Mol. Sci. 2022, 23, 13848. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Fattahi, M.; Nazemiyeh, H. Optimization of induction and hairy root culture establishment in two mullein species, Verbascum erianthum and Verbascum stachydiforme. Sci. Rep. 2024, 14, 5636. [Google Scholar] [CrossRef] [PubMed]
- Thimmaraju, R.; Bhagyalakshmi, N.; Narayan, M.S.; Ravishankar, G.A. Kinetics of pigment release from hairy root cultures of Beta vulgaris under the influence of pH, sonication, temperature and oxygen stress. Process Biochem. 2003, 38, 1069–1076. [Google Scholar] [CrossRef]
- Jamloki, A.; Bhattacharyya, M.; Nautiyal, M.C.; Patni, B. Elucidating the relevance of high temperature and elevated CO2 in plant secondary metabolites (PSMs) production. Heliyon 2021, 7, e07709. [Google Scholar] [CrossRef] [PubMed]
- Habibi, P.; Grossi de Sa, M.F.; Lopes da Silva, A.L.; Makhzoum, A.; da Luz Costa, J.; Borghetti, I.A.; Soccol, C.R. Efficient genetic transformation and regeneration system from hairy root of Origanum vulgare. Physiol. Mol. Biol. Plants 2016, 22, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.F.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Springs Harbor Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Furner, I.J.; Huffman, G.A.; Amasino, R.M.; Garfinkel, D.J.; Gordon, M.P.; Nester, E.W. An Agrobacterium transformation in the evolution of the genus Nicotiana. Nature 1986, 319, 422–427. [Google Scholar] [CrossRef]
- Georgev, M.I.; Agostini, E.; Ludwig-Műller, J.; Xu, J. Genetically transformed roots: From plant disease to biotechnological resource. Trends Biotechnol. 2012, 30, 528–537. [Google Scholar] [CrossRef]
- Lopes da Silva, A.L.; Oliveira, Y.; Procopiuk, C.; Mudry, C.S.; Brondani, G.E.; Costa, J.L.; Scheidt, G.N. Transient expression of uidA gene in leaf explants of Eucalyptus saligna Sm. Transformed via Agrobacterium tumefaciens. Biosci. J. 2013, 29, 1–7. [Google Scholar]
- Liu, C.; Guo, C.; Wang, Y.; Ouyang, F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua L. Process Biochem. 2002, 38, 581–585. [Google Scholar] [CrossRef]
- Zobayed, S.; Saxena, P.K. In vitro regeneration of Echinacea purpurea L.: Enhancement of somatic embryogenesis by indolebutyric acid and dark pre-incubation. In Vitro Cell. Dev. Biol. Plant 2003, 39, 605–612. [Google Scholar]
- Luczkiewicz, M.; Zarate, R.; Migas, D.W.; Migas, P.; Verpoote, R. Production of pulchelin E in hairy roots, callus and suspension cultures of Rudbeckia hirta L. Plant Sci. 2002, 163, 91–100. [Google Scholar] [CrossRef]
- Caillot, S.; Rosiau, E.; Laplace, C.; Thomasset, B. Influence of light intensity and selection scheme on regeneration time of transgenic flax plants. Plant Cell Rep. 2009, 28, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Sidik, N.J.; Awal, A. Optimization of sucrose concentration and light treatment in cell suspension culture establishment of Barringtonia racemosa L. Indian J. Sci. Technol. 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Tognetti, J.A.; Pontis, H.G.; Martinez-Noel, G.M.A. Sucrose signaling in plants: A world yet to be explored. Plant Signal. Behav. 2013, 8, e23316. [Google Scholar] [PubMed]
- Naik, P.M.; Al-Khayri, J.M. Impact of abiotic elicitors on in vitro production of plant secondary metabolites: A review. J. Adv. Res. Biotechnol. 2016, 1, 7. [Google Scholar]
- Xu, H.; Park, J.H.; Kim, Y.K.; Park, N.I.; Lee, S.Y.; Park, S.U. Optimization of growth and pyranocoumarins production in hairy root culture of Angelica gigas Nakai. J. Med. Plants Res. 2009, 3, 978–981. [Google Scholar]
- Kochan, E.; Szymańska, G.; Szymczyk, P. Effect of sugar concentration on ginsenoside biosynthesis in hairy root cultures of Panax quinquefolium cultivated in shake flasks and nutrient sprinkle bioreactor. Acta Physiol. Plant. 2014, 36, 613–619. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, Y.S.; Li, X.; Kim, H.H.; Arasu, M.V.; Al-Dhabi, N.A.; Lee, S.Y.; Park, S.U. Influence of different carbohydrates on flavonoid accumulation in hairy root cultures of Scutellaria baicalensis. Nat. Prod. Commun. 2016, 11, 799–802. [Google Scholar] [CrossRef]
- Lourenço, P.M.L.; de Castro, S.; Martins, T.M.; Clemente, A.; Domingos, A. Growth and proteolytic activity of hairy roots from Centaurea calcitrapa: Effect of nitrogen and sucrose. Enzyme Microb. Technol. 2002, 31, 242–249. [Google Scholar] [CrossRef]
- Petrova, M.; Zayova, E.; Dincheva, I.; Badjakov, I.; Vlahova, M. Influence of carbon sources on growth and GC-MS based metabolite profiling of Arnica montana L. hairy roots. Turk. J. Biol. 2015, 39, 469–478. [Google Scholar] [CrossRef]
- Beigmohamadi, M.; Movafeghi, A.; Jafari, S.; Sharafi, A. Potential of the genetically transformed root cultures of Plumbago europaea for biomass and plumbagin production. Biotechnol. Prog. 2019, 36, e2905. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Oxidative stress in plants. Antioxidants 2020, 9, 481. [Google Scholar] [CrossRef]
- Sumaryono, B.; Muslihatin, W.; Ratnadewi, D. Effect of carbohydrate source on growth and performance of in vitro sago palm (Metroxylon sagu Rottb.) plantlets. HAYATI J. Biosci. 2012, 19, 88–92. [Google Scholar] [CrossRef]
- Makowczyńska, J.; Kalemba, D.; Skała, E. Establishment of Codonopsis pilosula (Franch.) Nannf. transformed roots, influence of the culture conditions on root growth and production of essential oil. Ind. Crops Prod. 2021, 165, 113446. [Google Scholar] [CrossRef]
- Li, H.; Tang, M.; Tan, Y.; Ma, D.; Wang, Y.; Zhang, H. Improved production of chlorogenic acid from cell suspension cultures of Lonicera macranthoids. Trop. J. Pharm. Res. 2016, 15, 919–927. [Google Scholar] [CrossRef][Green Version]
- Baque, M.A.; Elgirban, A.; Lee, E.J.; Paek, K.Y. Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiol. Plant. 2012, 34, 405–415. [Google Scholar] [CrossRef]
- Noviyanti, R.; Sari, R.L.K.; Kristanti, A.N.; Yachya, A.; Manuhara, Y.S.W. Biomass and flavonoid production of Gynura procumbens adventitious roots induced by sucrose, phenylalanine and tyrosine. Biosci. Res. 2017, 14, 934–941. [Google Scholar]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Li, H.; Huang, W.; Wang, G.L.; Wu, Z.J.; Zhuang, J. Expression profile analysis of ascorbic acid-related genes in response to temperature stress in the tea plant, Camellia sinensis (L.). O. Kuntze. Genet. Mol. Res. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Chan, L.K.; Koay, S.S.; Boey, P.L.; Bhatt, A. Effects of abiotic stress on biomass and anthocyanin production in cell cultures of Melastoma malabathricum. Biol. Res. 2010, 43, 127–135. [Google Scholar] [CrossRef]
- Singh, M.; Chaturvedi, R. Evaluation of nutrient uptake and physical parameters on cell biomass growth and production of spilanthol in suspension cultures of Spilanthes acmella Murr. Bioprocess Biosyst. Eng. 2012, 35, 943–951. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R.; Naidu, R.T. Establishment of in vitro cell suspension culture, kinetics of cell growth, pH, nutrient uptake and production of 2-hydroxy-4-methoxybenzaldehyde from the germinated root of Decalepis hamiltonii Wight & Arn.-an endangered plant. Curr. Appl. Sci. Technol. 2022, 22, 1–12. [Google Scholar]
- Jiyeon, C.; Jeong, W.J.; Kim, J.S.; Lim, J.; Park, C.S.; Kwon, D.Y.; Choi, I.; Kim, J.H. Hydrolysis of isoflavone glucosides in soymilk fermented with single or mixed cultures of Lactobacillus paraplantarum KM, Weissella sp. 33, and Enterococcus faecium 35 isolated from humans. J. Microbiol. Biotechnol. 2008, 18, 573–578. [Google Scholar]
- El-Shazly, A.I.; Gamal, A.A.; El-Dein, A.N.; Mettwally, W.S.A.; Farid, M.A. Production of isoflavones-enriched soy yogurt through soymilk fermentation using probiotic bacteria. Egypt. Pharm. J. 2021, 20, 42–50. [Google Scholar]
- Shin, K.C.; Kang, S.H.; Oh, D.K.; Kim, D.W.; Kim, S.H.; Na, C.S.; Kim, Y.S. Production of daidzein and genistein from seed and root extracts of Korean wild soybean (Glycine soja) by thermostable β-galactosidase from Thermoproteus uzoniensis. Appl. Sci. 2022, 12, 3481. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Li, G.; Savolainen, O.; Chen, Y.; Nielsen, J. De novo biosynthesis of bioactive isoflavonoids by engineered yeast cell factories. Nat. Commun. 2021, 12, 6085. [Google Scholar] [CrossRef]
- Singhvi, M.; Kim, M.; Kim, B.S. Production of therapeutically significant genistein and daidzein compounds from soybean glycosides using magnetic nanocatalyst: A novel approach. Catalysts 2022, 12, 1107. [Google Scholar] [CrossRef]
- Suntichaikamolkul, N.; Tantisuwanichkul, K.; Prombutara, P.; Kobtrakul, K.; Zumsteg, J.; Wannachart, S.; Schaller, H.; Yamazaki, M.; Saito, K.; De-eknamkul, W.; et al. Transcriptome analysis of Pueraria candollei var. mirifica for gene discovery in the biosynthesis of isoflavones and miroestrol. BMC Plant Biol. 2019, 19, 581. [Google Scholar] [CrossRef]
- Chansakaow, S.; Ishikawa, T.; Sekin, K.; Okada, M.; Higuchi, Y.; Kudo, M.; Chaichantipyuth, C. Isoflavonoids from Pueraria mirifica and their estrogenic activity. Planta Med. 2000, 66, 572–575. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanonkeo, S.; Palee, T.; Thanonkeo, P.; Klanrit, P. Influence of Culture Conditions on Growth and Daidzein and Genistein Production in Hairy Root Cultures of Pueraria candollei var. mirifica. Horticulturae 2024, 10, 788. https://doi.org/10.3390/horticulturae10080788
Thanonkeo S, Palee T, Thanonkeo P, Klanrit P. Influence of Culture Conditions on Growth and Daidzein and Genistein Production in Hairy Root Cultures of Pueraria candollei var. mirifica. Horticulturae. 2024; 10(8):788. https://doi.org/10.3390/horticulturae10080788
Chicago/Turabian StyleThanonkeo, Sudarat, Tipawan Palee, Pornthap Thanonkeo, and Preekamol Klanrit. 2024. "Influence of Culture Conditions on Growth and Daidzein and Genistein Production in Hairy Root Cultures of Pueraria candollei var. mirifica" Horticulturae 10, no. 8: 788. https://doi.org/10.3390/horticulturae10080788
APA StyleThanonkeo, S., Palee, T., Thanonkeo, P., & Klanrit, P. (2024). Influence of Culture Conditions on Growth and Daidzein and Genistein Production in Hairy Root Cultures of Pueraria candollei var. mirifica. Horticulturae, 10(8), 788. https://doi.org/10.3390/horticulturae10080788





