1. Introduction
The family Araceae constitutes a critically significant and large group of taxa among the monocotyledonous flowering plants [
1]. Within this family of more than 100 genera,
Alocasia represents a genus characterized predominantly by their perennial life cycle and presence of rhizomes or tubers. To date, taxonomic consensus recognizes the existence of 89 species within the genus
Alocasia, which are native to tropical and subtropical Asia and eastern Australia [
2].
Alocasia is vastly cultivated on a global scale, not only for its inherent botanical features but also for its horticultural appeal. This widespread cultivation is attributed to the remarkable diversity manifested in the morphological traits of its species and hybrids—specifically the variation in foliar architecture, dimensions of the lamina, leaf surface texture and coloration [
3].
Representatives of the genus
Alocasia are characterized by their substantial and expansive vegetative features, most prominently their sizeable leaves, which can be broadly classified into two types: cordate, exhibiting a heart-like shape, and sagittate, resembling an arrowhead. These leaves, notable for their expanse—typically ranging from 20 to 90 centimeters in length—are structurally supported by elongated petioles, which can significantly lift the leaf blades above the ground. In addition to their notable foliage,
Alocasia species demonstrate a well-developed vigorous vegetative proliferation. They can form large, dense, extensive stands within the forest understory, independent of their reproductive successes, indicative of well-adapted vegetative propagation mechanisms within the Araceae family [
4]. The reproductive structures of
Alocasia adhere to the floral architecture commonly associated with the Araceae. Their inflorescences consist of a spadix—a spike where the tiny flowers are concentrated—and this is enclosed in a spathe, which is a typically bract-like structure in varying shades from white to green. Often, this inflorescence arrangement is strategically positioned behind the leaf petioles, possibly as a mode of protective concealment. Furthermore, several species within the genus
Alocasia develop specialized subterranean storage organs known as corms. These corms function as reservoirs of nutrients, which facilitate the plant’s survival across seasonal variations and possess potential culinary value. Post-harvest culinary processing can render these corms edible, while ethnobotanical records attribute medicinal qualities to particular taxa within the genus, highlighting their pharmacological relevance alongside their aesthetic and nutritional values [
5].
Alocasia is also known for its ecological plasticity, thriving across a diverse range of habitats and exhibiting various vegetative forms or habits. The botanical attention and focus of this study were drawn to three distinct entities within this genus:
Alocasia melo and
Alocasia reginae, both native to the island of Borneo [
6,
7], as well as
Alocasia ×
mortfontanensis ‘Bambino’, a cultivar whose taxonomic status remains unconfirmed [
8].
Alocasia melo and
A. reginae have adapted specifically to the Bornean ecosystem, displaying characteristics typical of their native habitat. Meanwhile,
Alocasia ×
mortfontanensis ‘Bambino’ is proposed to be a horticultural selection, likely developed from
Alocasia × mortfontanensis. In the botanical nomenclature,
Alocasia ×
mortfontanensis is synonymous with
Alocasia ×
amazonica, a deliberate horticultural hybrid resulting from the crossbreeding of
Alocasia longiloba with
Alocasia sanderiana [
9].
These three Alocasia taxa are united by their ornamental value, which owes to the distinctive features in leaf morphology, coloration and variegation patterns. This is exemplified by A. reginae, especially those cultivated in the nurseries in Bogor, Indonesia; they showcase a remarkable variation in foliar pigmentations, with the adaxial (upper) surfaces of the leaves exhibiting a gradient from silver to light blue hues and abaxial (lower) surfaces ranging from silver to reddish-purple hues. The accessions of A. reginae observed in this study are noted for their prominent silver-tinted adaxial foliar surfaces.
Taxonomically, the genus
Alocasia is extensively described. However, research into their growth dynamics and leaf anatomical details remains limited. Proper understanding of morphological features and vegetative processes is crucial for its horticultural optimization. Leaf morpho-anatomy, influenced by environmental factors, plays a pivotal role in species adaptation, enhancing plant resilience, development, and fecundity across diverse ecosystems [
10].
This study focuses on quantifying the vegetative growth, foliar anatomy and physiological attributes of Alocasia melo, Alocasia reginae and the cultivar Alocasia × mortfontanensis ‘Bambino’. Investigations include assessments of the leaf chlorophyll content and stability, water content and rate of moisture losses, along with morphological and anatomical examinations. These insights could supplement advancements in Alocasia cultivation, bolster its horticultural value and provide important data for plant breeding and horticultural innovations.
2. Materials and Methods
2.1. Plant Morphology and Vegetative Growth Measurement
Specimens of Alocasia were obtained from a commercial nursery in Ciapus, West Java, Indonesia. These plants were subsequently cultivated under controlled conditions within the greenhouse facility at the Leuwikopo Experimental Farm, IPB University, Dramaga, Indonesia. The conditions within the greenhouse were maintained within a temperature range of 24–35 °C, complemented by a light intensity of 2600 lux and photosynthetic photon flux density of 47 μmol/s·m2. The growth medium comprised an equal volumetric mix of composted bamboo leaves and rice hulls (50:50 ratio). At planting, each specimen received two grams of slow-release fertilizer Osmocote (N:P:K = 14:14:14).
The morphology was described qualitatively and quantitatively. The qualitative leaf morphology was defined based on the leaf type, lobing, margin, leaf margin types, apex, base, phyllotaxy, leaf lamina color (adaxial and abaxial), leaf lamina and petiole patterns. Quantitative measurement focused on traits including shape, color, pattern, venation and textural surface attributes, conducted on a fully expanding leaf of each plant. Plant height, leaf length, leaf width and the number of leaf primary veins were determined. Plant height and leaf traits were measured once at 20 weeks after planting. Leaf size was determined by measuring each plant’s longest and widest mature, fully expanding leaf.
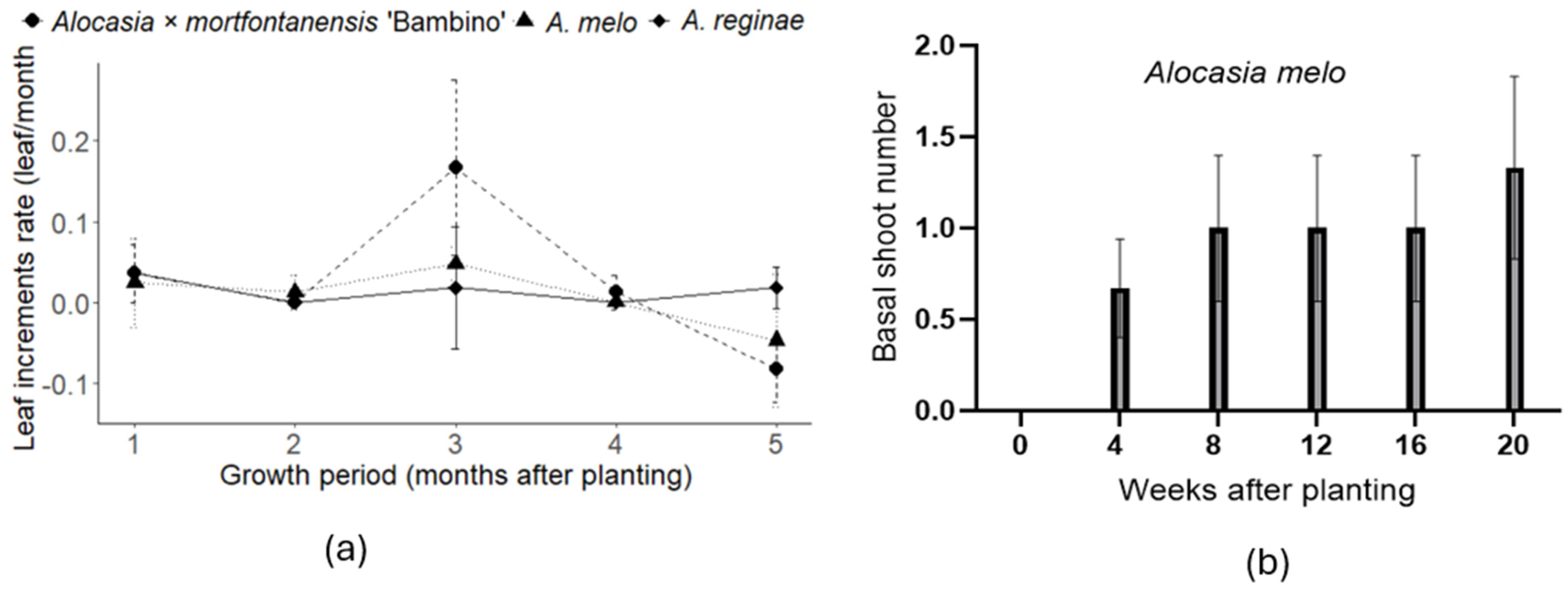
Leaf growth was recorded every two weeks. The number of new basal shoots was measured every 4 weeks for 20 weeks. The propagation rate was calculated based on how many new shoots were formed after 20 weeks. Findings for this study are presented as the leaf count quantified on a four-weekly basis, along with the monthly rate of leaf increment. Leaf increment rates were calculated using Equation (1) as follows:
2.2. Leaf Anatomy
Fresh leaves of Alocasia × mortfontanensis ‘Bambino’, A. melo and A. regina were harvested from plants reared in the greenhouse at the Leuwikopo Experimental Farm, IPB University. For leaf anatomy, fresh leaves were transversely sectioned into thin slices, mounted on glass slides and sealed with cover glasses for observation. These sections were examined with an Olympus CX23LEDRFS1 microscope (Olympus Corporation, Singapore) (at 100× magnification. Analytical measurements, comprising adaxial and abaxial epidermal thickness, mesophyll thickness and total leaf thickness, were conducted on three leaf samples per species, with measurements taken at three points and averaged for accuracy.
Stomatal observations were conducted by obtaining thin sections of the leaf’s abaxial epidermis and placing them on glass slides. Stomatal parameters such as density, type, length and width were evaluated. Stomatal density was determined by dividing the number of stomata within a microscopic field of view area of 3.461 mm
2 at 100× magnification, with calculations performed across three replicates to generate an average value. Stomatal length and width measurements were recorded for three randomly selected stomata at 400× magnification. The terminology for describing the stomata was based on [
11].
2.3. Relative Water Content, Leaf Moisture Loss and Specific Leaf Weight
Relative water content was determined following the method outlined by [
12]. Five leaves of each
Alocasia species were cut into squares (5 × 5 cm
2) using a scalpel and immediately weighed to obtain their fresh weight (FW). These leaves were then immersed in deionized water maintained at a temperature of 25 °C for a duration of four hours to achieve full turgidity and the turgid weight (TW). Subsequently, the samples were dried in a hot air oven at 60 °C for 72 h to obtain the dry weight (DW). Relative water content was calculated using Equation (2) as follows:
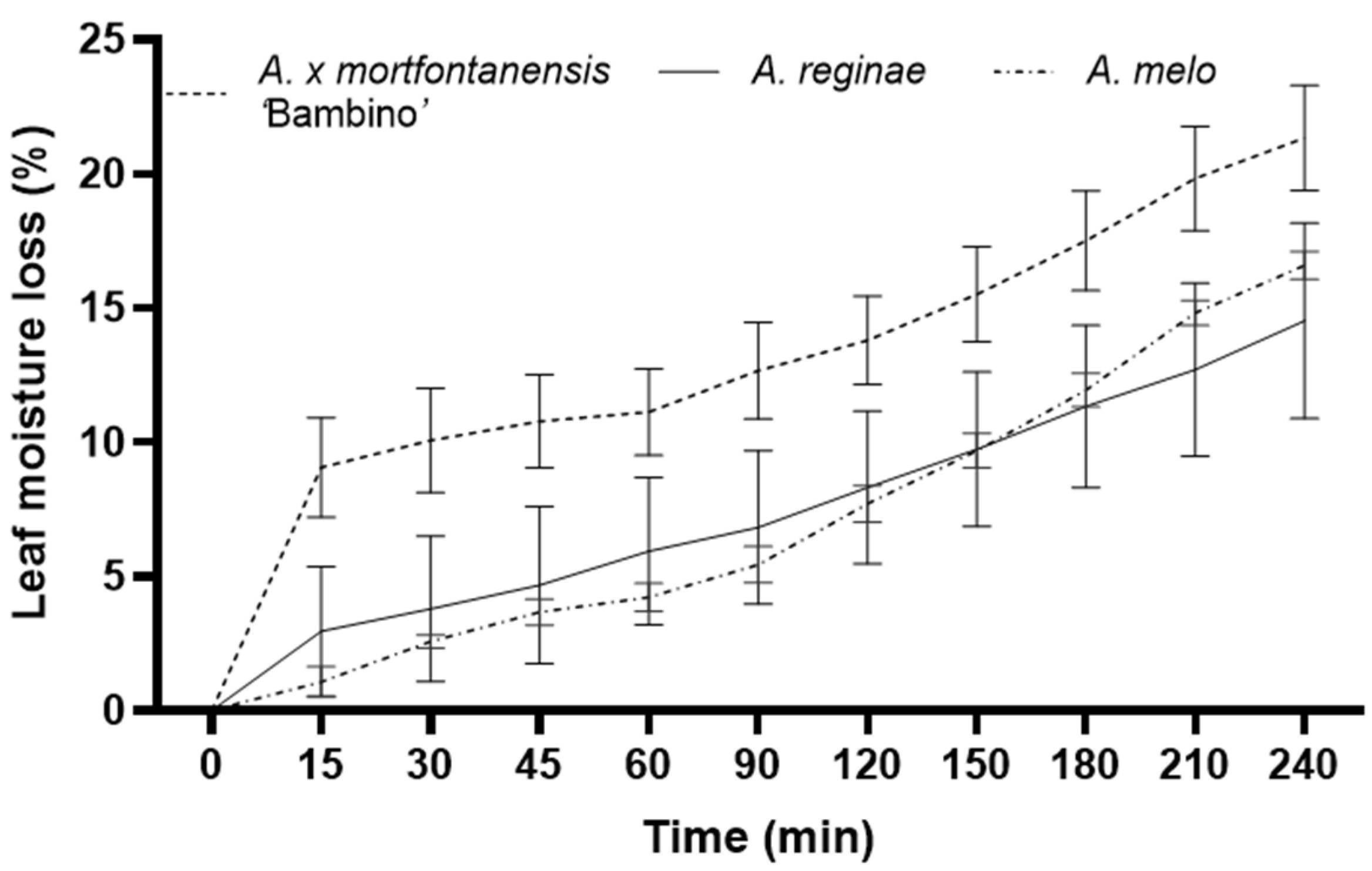
Leaf moisture loss was determined following the established methodology of [
13]. Leaf samples were placed in a laminar airflow cabinet at a controlled ambient temperature of 25 °C. The leaf weight of all samples was measured at 15 min intervals for 240 min using a precision electronic balance (Adam Equipment, Milton Keynes, UK).
Specific leaf weight (SLW) is defined as the ratio of a leaf’s dry mass to its corresponding leaf area [
14], while specific leaf area (SLA) is the ratio of leaf area to leaf dry mass [
15]. Triplicate biological samples were collected for analysis to calculate SLW.
2.4. Pigment Content and Chlorophyll Stability Index
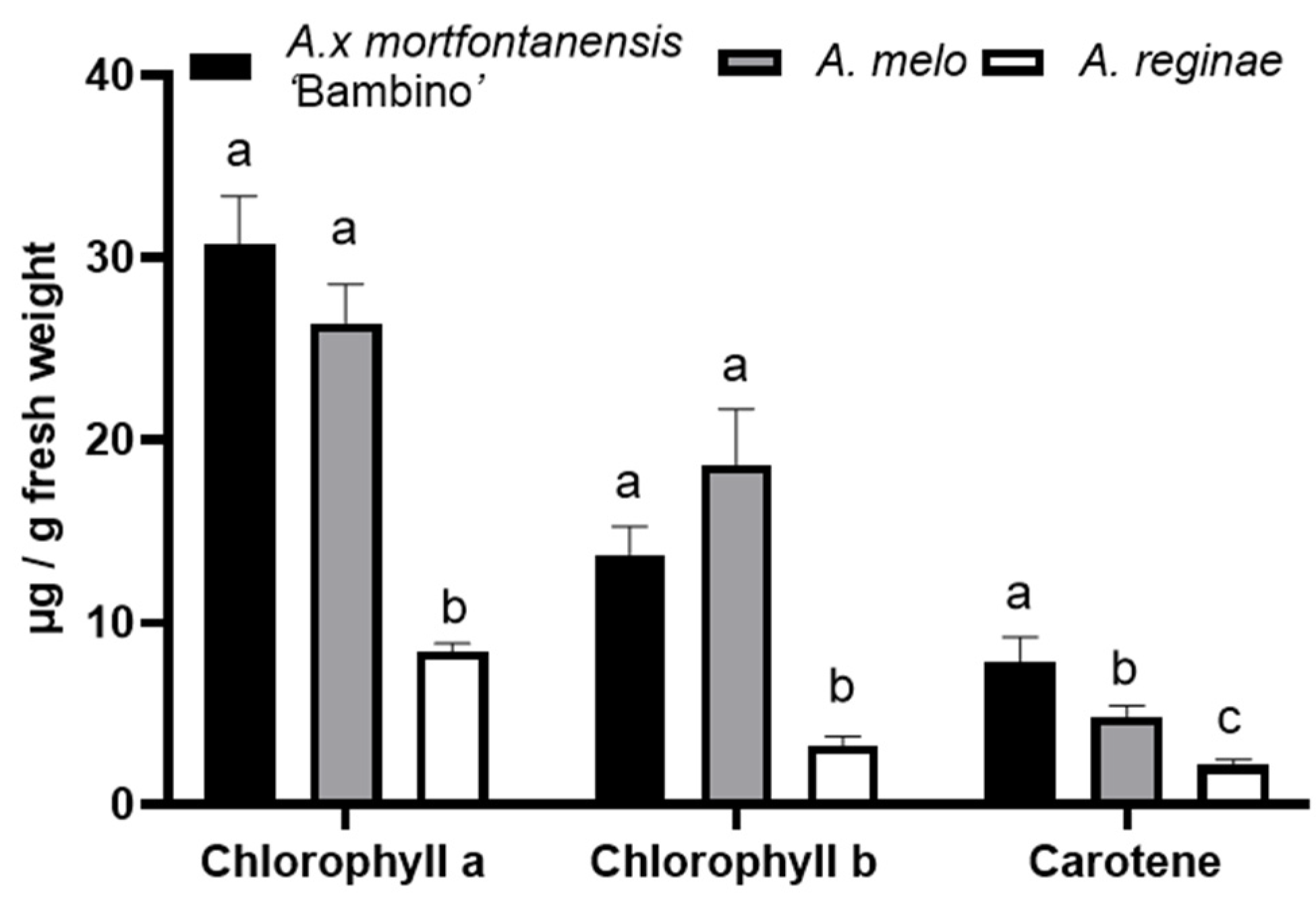
Leaf chlorophyll and leaf carotene content were determined according to [
16]; leaf samples were cut into fine pieces of about 25 mm
2 following extraction using 100% acetone. Solutions were stored for 48 h in a −20 °C fridge until the leaves turned white, which indicated that the pigments had been fully dissolved. The analytical determination was performed with a spectrophotometer Genesys 10 s UV-Vis (Thermo Scientific, Madison, WI, USA) at the 662 and 645 wavelengths for chlorophyll a and b, and 470 nm for carotene [
16].
The chlorophyll stability index (CSI) of the three
Alocasia species was determined based on the methodology established by [
17]. Fresh leaf samples were collected and cut into 5 × 5 cm
2 pieces. To assess the stability of the photosynthetic pigments, the leaf pieces were immersed in a water bath containing deionized water at 56 °C for 30 min, while control leaf samples were immersed in deionized water at room temperature (25 °C). Quantification of chlorophyll content for both the hot-water-treated and control leaf samples was performed using a SPAD-502 chlorophyll meter (Minolta Corp, Romsey, NJ, USA). Eleven readings were logged for each treatment at intervals of 0, 15, 30, 45, 60, 90, 120, 150, 180, 210 and 240 min for each species. The CSI was according to [
17].
2.5. Scanning Electron Microscopy
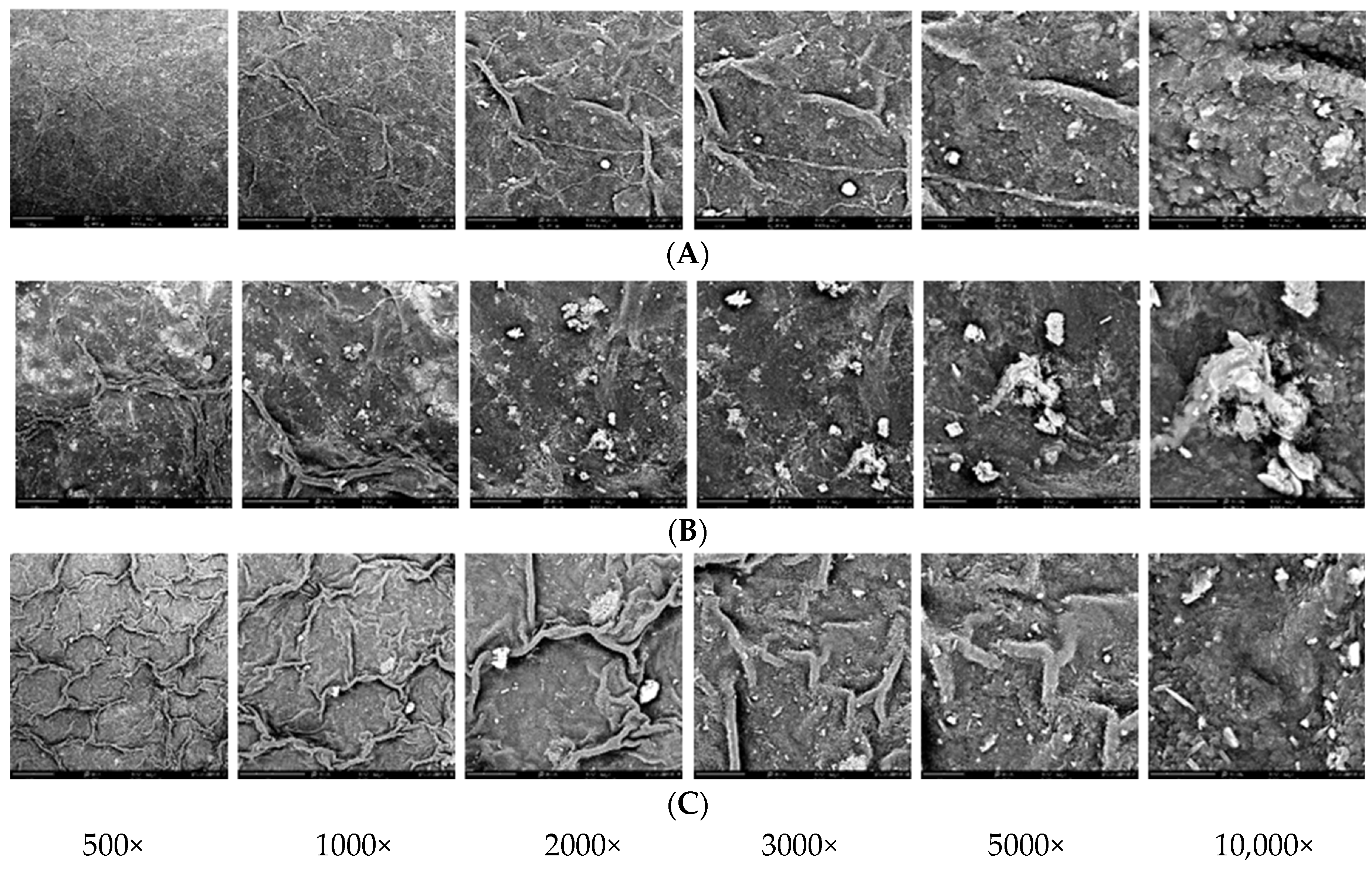
Scanning electron microscopy (SEM) was performed to analyze the microstructure of the fresh leaves of the three Alocasia species. One leaf sample of each species was cross-sectioned using a scalpel, mounted on holders and coated with Au–Pd (gold–palladium) particles using a Quorum sputter coater. Leaf surface microscopic visualization was performed using a scanning electron microscope (Thermo Scientific Phenom ProX, Eindhoven, The Netherlands) at 1000×, 2000×, 3000×, 5000× and 10,000× magnifications. The elemental composition of the leaf surface was analyzed using SEM with an energy-dispersive X-ray (EDX) system to determine the elemental content on the sample surface. Simultaneously, mapping analysis was conducted to determine the distribution of elements on the leaf sample surface. The weight percentage of an element is the weight of that element measured in the sample divided by the weight of all elements in the sample multiplied by 100.
4. Discussion
Our study demonstrated the leaf morphological characteristics that can be used to identify the three
Alocasia species.
Alocasia × mortfontanensis ‘Bambino’ exhibits the fastest leaf growth (
Figure 1), alongside the greatest ratio of chlorophyll a to chlorophyll b and carotene content (
Figure 4). However, this taxon also presented the lowest chlorophyll stability (
Table 6) and the fastest leaf moisture loss (
Figure 3). A higher stability index indicates better resilience of chlorophyll to stress, while a lower stability index may suggest damage or degradation of chlorophyll molecules. Accordingly,
A. melo and
A. reginae leaves maintain a higher stability index than
Alocasia × mortfontanensis ‘Bambino,’ inferring broader adaptability to temperature variations, as proposed by [
18].
It was observed that
Alocasia × mortfontanensis ‘Bambino’ possesses the thinnest leaves (
Table 4), and this physiognomy potentially facilitates its highest water loss compared to
A. reginae and
A. melo. The correlation analysis between specific leaf weight and leaf moisture loss suggests a negative relationship (−0.297,
Supplementary Figure S1); this is consistent with previous findings [
18] that thicker leaves, especially those with a more substantial cuticular layer, can mitigate desiccation.
Alocasia melo and
A. reginae display similarities in their leaf growth rates (
Figure 1), leaf thickness (
Table 4), rate of moisture loss (
Figure 3) and chlorophyll stability (
Table 6). The chlorophyll stability index is an indicative measure of the endurance of chlorophyll molecules under varying environmental stresses [
17].
Leaf quality is important for tropical ornamentals, with moisture loss being a crucial factor influencing this aspect. To the best of our knowledge, apart from
A. longiloba and
A. mycorrhiza [
19], no information is available on the edibility of the tubers and leaves of the three
Alocasia involved in this study. Interestingly,
A. melo is the only species that produces basal shoots, whereas the other two species only show leaf increments. This information provides insights into the propagation capabilities of the three Alocasia, which will be useful for commercial production practices and postproduction handling of these ornamental plants.
Variations in the ratio of chlorophyll a to chlorophyll b may reflect the presence of additional pigments, such as anthocyanins or carotenoids, which are contributors to the red or purple hues in leaf tissues [
20,
21], as exemplified by the leaf blade in
A. melo (
Figure 2). Moreover, variations in pigment composition alongside leaf structure and genetic factors can influence the observed color diversity in both wild and cultivated plants.
Leaf thickness and leaf water content can offer valuable insights into the plant’s water utilization, including its tolerance to drought and salinity conditions [
22].
Alocasia × mortfontanensis ‘Bambino’ showed the highest moisture loss compared to the other two species, likely due to its thinner leaf structure and potentially higher stomatal density, facilitating transpiration [
23]. This characteristic was not observed in
A. melo and
A. reginae, which possess relatively thicker leaves. These findings may provide information for optimal cultivation practices, including irrigation strategies and the selection of suitable planting media [
24].
All three
Alocasia taxa used in this study showed a lower stomatal density (
Table 4) compared to other Indonesian species of
Alocasia, such as
A. alba, which exhibits stomatal densities ranging from 25 to 55 stomata per mm
2 on the adaxial surface and 80–106 stomata per mm
2 on the abaxial surface [
25]. Other leaf characters were consistent with other species of
Alocasia. However,
A. melo and
A. reginae have much thicker leaves than
A. alba, likely due to a thick spongy mesophyll layer, as shown in
Table 4. Thick layers of spongy mesophyll have also been reported in other species of
Alocasia [
25]. The contributory role of abaxial gas exchange in photosynthesis, accounting for approximately 50% of the overall activity, emphasizes its importance in overall leaf gas exchange processes [
23].
Specific leaf weight fluctuates in response to environmental changes and can vary at different times of the day. Studies on alfalfa have shown that the specific leaf weight is at its lowest in the morning and increases in the afternoon due to the production of photosynthates during the day [
26]. As such, specific leaf weight can affect dry matter production and the efficiency with which photosynthates are translocated within the plant, providing valuable information for cultivation and production methods.
Morphological and anatomical investigations are relevant in elucidating diversity levels and provide taxonomic information within the genus
Alocasia. SEM EDX analysis demonstrated relatively high concentrations of potassium, calcium, chloride and silicon on the
A. melo leaf surface as compared to
A. reginae and
Alocasia × mortfontanensis ‘Bambino’ (
Figure 5).
Alocasia melo contains rhodium, which is not detectable in
A. reginae and
Alocasia × mortfontanensis ‘Bambino’ (
Table 7). Potassium (K) is involved in stomatal regulation [
27], but its interaction with other measured parameters was not explored within the context of this study.
Araceae, including
Alocasia and
Colocasia, are characterized by the presence of calcium oxalate (Ca(COO)
2) in all parts of the plants, which may lead to skin irritation [
28].
Our study demonstrated that
Alocasia melo leaves contain about 6% Ca, the highest of the three
Alocasia. While not essential for plant development, silicon offers advantages for plants under stressful conditions. It has been recognized for mitigating abiotic and biotic stressors in plants [
29,
30,
31].
Typically, plants contain trace amounts of rhodium, estimated to be in the range of 1–2 ppb [
10], yet we found an unusually high (2%) proportion of rhodium on the
A. melo leaves. Rhodium is a rare non-radioactive metal and does not play a known biological role. It occurs uncombined in nature and with other platinum metals in river sands [
32].
Our study detected the presence of magnesium in
A. reginae and
Alocasia × mortfontanensis ‘Bambino’ leaves (
Table 7). Mg is an essential component of chlorophyll pigments in the light-capturing complex of chloroplasts; therefore, it is involved in photosynthetic CO
2 assimilation [
33].