Application of Plant Growth Regulators Mitigates Water Stress in Basil
Abstract
1. Introduction
2. Materials and Methods
2.1. Installation and Experimental Conditions
2.2. Biometric Measurements
2.3. Physiological Measurements
Chlorophyll a Fluorescence
2.4. Gas Exchange
2.5. Statistical Analysis
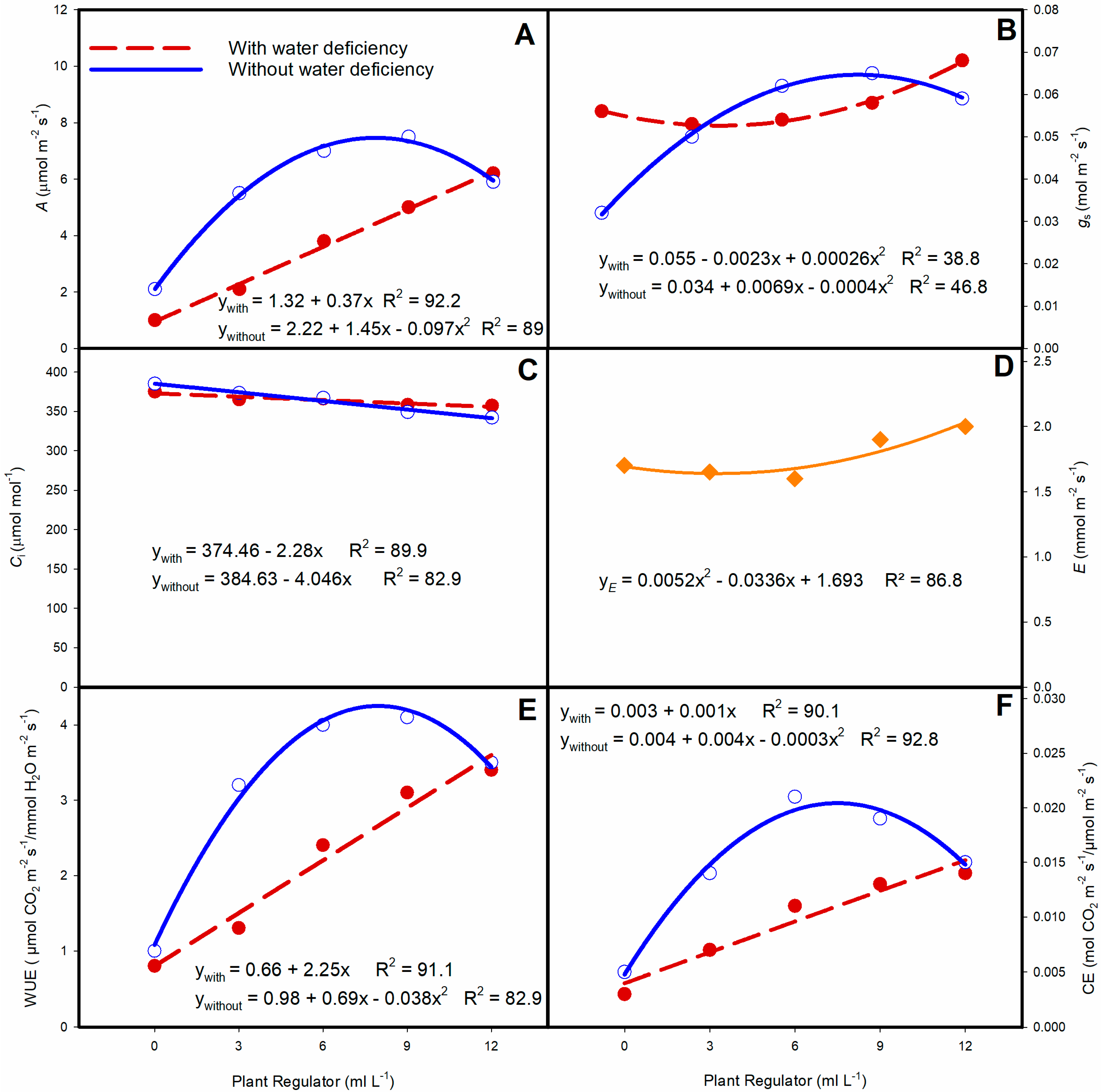
3. Results
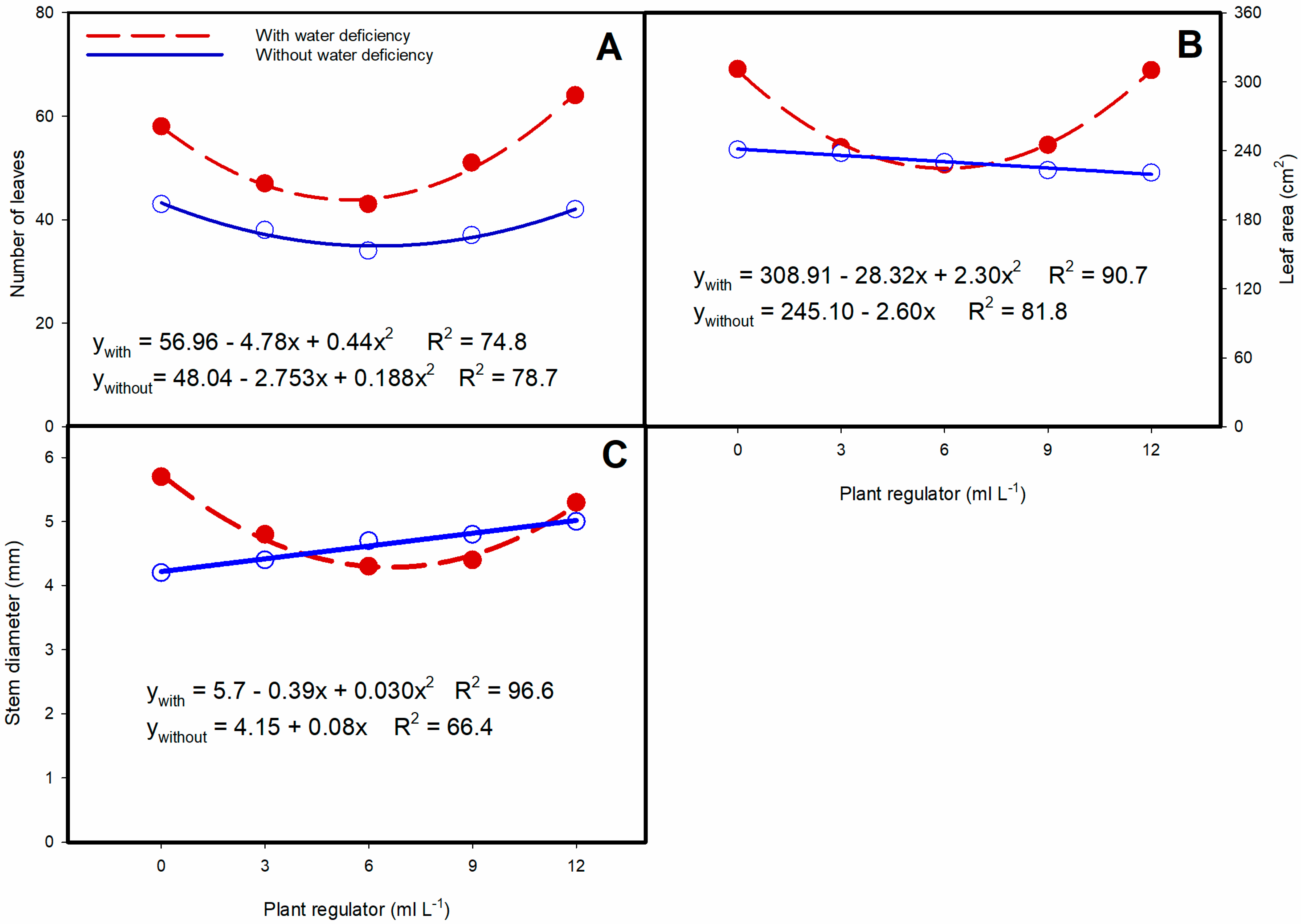
3.1. Biometric Analysis
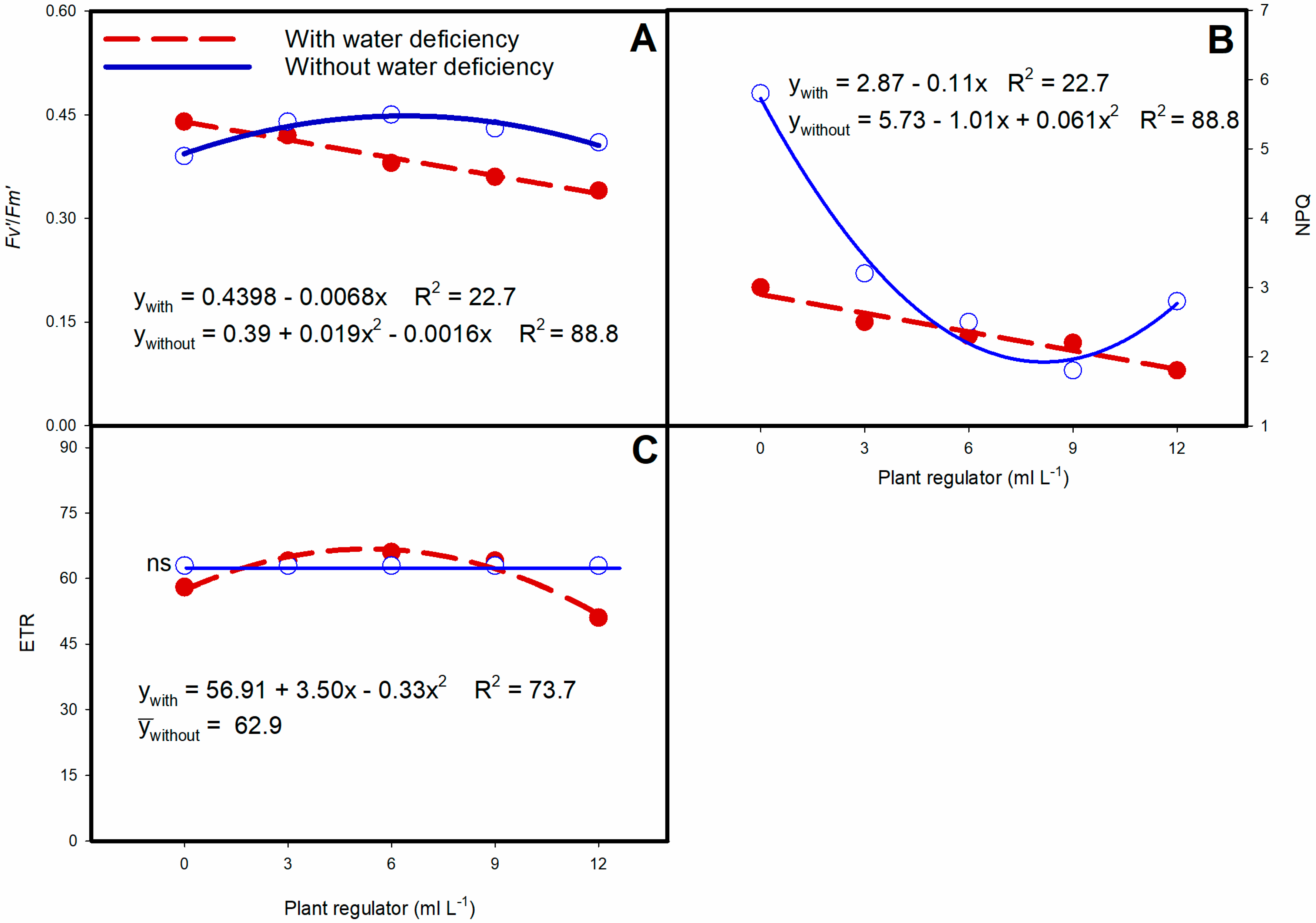
3.2. Chlorophyll a Fluorescence
4. Discussion
5. Conclusions
- (1)
- Stimulate® plant regulator effectively mitigated the effects of water deficiency in basil by significantly improving biometric parameters, chlorophyll a fluorescence, and gas exchange. These enhancements led to increased photosynthetic efficiency and better physiological responses to water stress, especially at doses of 9 and 12 mL L−1;
- (2)
- The application of the plant growth regulator mitigated the adverse effects of water deficiency and increased the tolerance of basil plants. This study not only highlights the efficacy of Stimulate® in improving resilience to water stress but also paves the way for future investigations under various environmental and genotypic conditions. Furthermore, it encourages a detailed exploration of the underlying mechanisms of bioregulator action, contributing to the optimization of agricultural practices on a global scale.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais no Brasil: Nativas e Exóticas [Medicinal Plants in Brazil: Native and Exotic], 2nd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2008. [Google Scholar]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Pre-Harvest UV-B radiation and photosynthetic photon flux density interactively affect plant photosynthesis, growth, and secondary metabolites accumulation in basil (Ocimum basilicum) plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef]
- Dorni, A.I.C.; Amalraj, A.; Gopi, S.; Varma, K.; Aanjana, S.N. Novel cosmeceuticals from plants—An industry guided review. J. Appl. Res. Med. Aromat. Plants 2017, 7, 1–26. [Google Scholar] [CrossRef]
- Bajomo, E.M.; Aing, M.S.; Ford, L.S.; Niemeyer, E.D. Chemotyping of commercially available basil (Ocimum basilicum L.) varieties: Cultivar and morphotype influence phenolic acid composition and antioxidant properties. NFS J. 2022, 26, 1–9. [Google Scholar] [CrossRef]
- Patel, R.P.; Singh, R.; Rao, B.R.R.; Singh, R.R.; Srivastava, A.; Lal, R.K. Differential response of genotype×environment on phenology, essential oil yield and quality of natural aroma chemicals of five Ocimum species. Ind. Crops Prod. 2016, 87, 210–217. [Google Scholar] [CrossRef]
- Mohamed, D.S.; Shehata, O.; Labib, M.M.; Shaban, N.S. Integrated in vivo and in silico evaluation of sweet basil oil as a protective agent against cisplatin-induced neurotoxicity in mice. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 65. [Google Scholar] [CrossRef]
- Afkari, A. Effects of drought stress and nitrogen fertilizer rate on some physiological characteristics, essential oil percentage, and yield of basil (Ocimum basilicum L.). Iran. J. Med. Aromat. Plants Res. 2018, 33, 1047–1059. [Google Scholar]
- Carvalho, B.L.; Aires, E.S.; Rodrigues, J.D.; Ono, E.O. Use of Plant Regulators for Activation of Antioxidant Enzymes in Basil Plants under Water Deficit Conditions. Stresses 2023, 3, 282–301. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal [Physiology of Plants], 6th ed.; Artmed: Porto Alegre, Brazil, 2017; 888p. [Google Scholar]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Germino, G.H.; Silva, M.A. Adaptação das plantas ao déficit hídrico [Adaptation of plants to water deficit]. Acta Iguazu 2016, 5, 15–32. [Google Scholar] [CrossRef]
- Kozlowski, T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Botan. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Fathi, S.; Bolandnazar, S.; Alizadeh-Salteh, S.; Zaare-Nahandi, F. Effects of Biological Fertilizers on Some Physiological Traits of Sweet Basil under Water Deficit Stress. J. Med. Plants By-Prod. 2023, 4, 365–374. [Google Scholar] [CrossRef]
- Batista Filho, C.G.; Marco, K.; Dallacort, R.; Santi, A.; Inoue, M.H.; Silva, E.S. Efeito do Stimulate® nas características agronômicas da soja [Effect of Stimulate® on the agronomic characteristics of soybean]. Acta Iguazu 2013, 2, 76–86. [Google Scholar] [CrossRef]
- Cavalcante, W.S.S.; Silva, N.F.; Teixeira, M.B.; Cabral Filho, F.R.; Nascimento, P.E.R.; Corrêa, F.R. Eficiência dos bioestimulantes no manejo do déficit hídrico na cultura da soja [Effectiveness of biostimulants in managing water deficit in soybean cultivation]. Irriga 2020, 25, 754–763. [Google Scholar] [CrossRef]
- Santos, R.K.A.; Cairo, P.A.R.; Barbosa, R.P.; Lacerda, J.J.; Mafra Neto, C.S.; Macedo, T.H.J. Respostas fisiológicas de plantas jovens de Eucalyptus urophylla tratadas com bioestimulante sob déficit hídrico [Physiological responses of young Eucalyptus urophylla plants treated with biostimulant under water deficit]. Ciência Florest. 2019, 29, 1072–1081. [Google Scholar] [CrossRef]
- Bulegon, L.G.; Guimarães, V.F.; Inagaki, A.M.; Battistus, A.G.; Offemann, L.C.; Souza, A.K.P. Respostas da soja ao Azospirillum brasilense e reguladores vegetais em condições de déficit hídrico [Responses of soybean to Azospirillum brasilense and plant regulators under water deficit conditions]. Rev. Bras. Ciênc. Agrár. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Raij, J.B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem Para o Estado de São Paulo [Fertilization and Liming Recommendations for the State of São Paulo], 2nd ed.; IAC: Campinas, Brazil, 1996; p. 258. [Google Scholar]
- Maxwell, K.; Johnson, G. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2010, 51, 659–668. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Demmig, B.; Bjorkman, O. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.F. Sisvar: A guide for its bootstrap procedures in multiple comparisons. Ciênc. Agrotec. 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Al-Qurainy, F.; Foolad, M.R. Drought tolerance: Roles of organic osmolytes, growth regulators, and mineral nutrients. Adv. Agron. 2011, 111, 249–296. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.R.; Marco, C.A.; Inneco, R.; Silva, T.I.; Bezerra, A.E.; Oliveira, C.W.; Freitas Júnior, S.P.; Tavares, J.F.; Chaves, J.T.L. Influência de lâminas de irrigação na biomassa, teor e composição química do óleo essencial de manjericão [Influence of irrigation levels on biomass, content, and chemical composition of basil essential oil]. Espacios 2017, 38, 21. [Google Scholar]
- Gonçalves, B.H.L.; Souza, J.M.A.; Ferraz, R.A.; Tecchio, M.A.; Leonel, S. Efeito do bioestimulante Stimulate® no desenvolvimento de mudas de maracujazeiro cv. BRS Rubi do Cerrado [Effect of the bioestimulant Stimulate® on the development of seedlings of passion fruit cv. BRS Rubi of Cerrado]. Rev. Ciênc. Agrár. 2017, 41, 147–155. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Medeiros, J.F.; Cunha, R.C.; Souza, M.W.L.; Lima, L.A. Use of biostimulants in relieving salt stress in popcorn. Rev. Ciênc. Agron. 2016, 47, 307–315. [Google Scholar] [CrossRef][Green Version]
- Souza, J.M.A.; Gonçalves, B.H.L.; Santos, A.M.F.; Ferraz, R.A.; Leonel, S. Efeito de bioestimulante no desenvolvimento inicial de plântulas do porta-enxerto cítrico tangerineira ‘Cleópatra’ [Effect of a biostimulant on the initial development of seedlings of the citrus rootstock ‘Cleopatra’ tangerine]. Sci. Plena 2013, 9, 1–8. [Google Scholar]
- Oliveira, A.; Ferreira, G.; Rodrigues, J.D.; Ferrari, T.B.; Kunz, V.L.; Primo, M.A.; Poletti, L.D. Efeito de reguladores vegetais no desenvolvimento de mudas de Passiflora alata Curtis [Effect of plant growth regulators on the development of seedlings of Passiflora alata Curtis]. Rev. Bras. Frutic. 2005, 27, 9–13. [Google Scholar] [CrossRef]
- Santos, L.P.; Barbacena, D.R.; Gonçalves, R.C.; Nascimento, C.A.C.; Carvalho, F.L.C.; França, L.C.; Adorian, G.C. Aplicação de bioestimulante e complexo de nutrientes no tratamento de sementes de soja [Application of biostimulant and nutrient complex in soybean seed treatment]. Rev. Agri-Environ. Sci. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Scalon, S.P.Q.; Lima, A.A.; Scalon Filho, H.; Vieira, M.C. Germinação de sementes e crescimento inicial de mudas de Campomanesia adamantium Camb.: Efeito da lavagem, temperatura e de bioestimulantes [Seed germination and initial seedling growth of Campomanesia adamantium Camb.: Effect of washing, temperature, and biostimulants]. Rev. Bras. Sementes 2009, 31, 96–103. [Google Scholar] [CrossRef]
- Reis, F.O.; Campostrini, E. Trocas gasosas e eficiência fotoquímica potencial em mamoeiro do grupo ‘formosa’ cultivado em condição de campo [Gas exchange and potential photochemical efficiency in papaya of the ‘Formosa’ group cultivated under field conditions]. Bragantia 2008, 67, 815–822. [Google Scholar] [CrossRef]
- Suassuna, J.F.; Melo, A.S.; Sousa, M.S.S.; Costa, F.S.; Fernandes, P.D.; Pereira, V.M.; Brito, M.E.B. Desenvolvimento e eficiência fotoquímica em mudas de híbrido de maracujazeiro sob lâminas de água [Development and photochemical efficiency in seedlings of passion fruit hybrid under water depths]. Biosci. J. 2010, 26, 566–571. [Google Scholar]
- Azevedo Neto, A.D.; Pereira, P.P.A.; Costa, D.P.; Santos, A.C.C. Fluorescência da clorofila como uma ferramenta possível para seleção de tolerância à salinidade em girassol [Chlorophyll fluorescence as a potential tool for selecting salt tolerance in sunflower]. Rev. Ciênc. Agron. 2011, 42, 893–897. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Sakamoto, A.; Nishiyama, Y.; Inaba, M.; Mjurata, N. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000, 123, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
| pH | OM * | P | S | Al3+ | H+Al3+ | K | Ca | Mg | SB * | CEC * | V% * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaC2 | g dm−3 | mg dm−3 | mmolc dm−3 | ||||||||
| 4.0 | 11 | 1 | 18 | 10 | 63 | 1.25 | 5 | 1 | 7 | 70 | 10 |
| Source of Variation | PH | NL | LA | SD |
|---|---|---|---|---|
| Water Deficiency (WD) | 80.0 ns | 88.18 ** | 21.52 ** | 7.23 * |
| Plant Regulator (PR) | 34.5 ns | 22.71 ** | 6.86 ** | 6.35 ** |
| WD × PR | 44.1 ns | 34.77 ** | 10.87 ** | 8.27 ** |
| CV (%) | 10.5 | 7.50 | 9.37 | 7.87 |
| Source of Variation | Fv/Fm | Fv′/Fm′ | qP | NPQ | ETR |
|---|---|---|---|---|---|
| Water Deficiency (WD) | 0.69 ns | 0.53 * | 1.53 ns | 115.16 ** | 5.41 * |
| Plant Regulator (PR) | 0.44 ns | 0.30 * | 0.80 ns | 187.25 ** | 5.37 * |
| WD × PR | 0.73 ns | 0.76 ** | 1.69 ns | 201.89 ** | 108.15 ** |
| CV (%) | 2.82 | 10.33 | 9.52 | 7.54 | 5.59 |
| Source of Variation | A | gs | Ci | E | WUE | CE |
|---|---|---|---|---|---|---|
| Water Deficiency (WD) | 258.20 ** | 7.52 * | 0.01 ns | 4.14 ns | 25.82 ** | 154.95 ** |
| Plant Regulator (PR) | 184.90 ** | 3.56 * | 6.37 ** | 4.09 * | 46.06 ** | 143.79 ** |
| WD × PR | 153.64 ** | 8.18 ** | 3.71 * | 1.68 ns | 25.60 ** | 117.44 ** |
| CV (%) | 8.01 | 14.78 | 4.11 | 11.19 | 17.87 | 10.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.M.R.; Marques, I.C.d.S.; Carvalho, B.L.; Aires, E.S.; Freitas Júnior, F.G.B.F.; Vargens, F.N.; Santos, V.A.Á.d.; Luz, J.H.S.d.; Souza, J.W.G.d.; Oliveira Galdino, W.d.; et al. Application of Plant Growth Regulators Mitigates Water Stress in Basil. Horticulturae 2024, 10, 729. https://doi.org/10.3390/horticulturae10070729
Silva DMR, Marques ICdS, Carvalho BL, Aires ES, Freitas Júnior FGBF, Vargens FN, Santos VAÁd, Luz JHSd, Souza JWGd, Oliveira Galdino Wd, et al. Application of Plant Growth Regulators Mitigates Water Stress in Basil. Horticulturae. 2024; 10(7):729. https://doi.org/10.3390/horticulturae10070729
Chicago/Turabian StyleSilva, Dayane Mércia Ribeiro, Isabelly Cristina da Silva Marques, Beatriz Lívero Carvalho, Eduardo Santana Aires, Francisco Gilvan Borges Ferreira Freitas Júnior, Fernanda Nery Vargens, Vinicius Alexandre Ávila dos Santos, João Henrique Silva da Luz, José Wilker Germano de Souza, Wesley de Oliveira Galdino, and et al. 2024. "Application of Plant Growth Regulators Mitigates Water Stress in Basil" Horticulturae 10, no. 7: 729. https://doi.org/10.3390/horticulturae10070729
APA StyleSilva, D. M. R., Marques, I. C. d. S., Carvalho, B. L., Aires, E. S., Freitas Júnior, F. G. B. F., Vargens, F. N., Santos, V. A. Á. d., Luz, J. H. S. d., Souza, J. W. G. d., Oliveira Galdino, W. d., Sousa, J. I. d., Melo, A. F., Silva, R. B., Barbosa, L. d. N. S., Silva, J. V., Santos, V. R. d., Góis, M. G. J. L. d., Paulino, S. S., Ono, E. O., & Rodrigues, J. D. (2024). Application of Plant Growth Regulators Mitigates Water Stress in Basil. Horticulturae, 10(7), 729. https://doi.org/10.3390/horticulturae10070729






