Abstract
Purslane (Portulaca oleracea L.) is widely distributed and commonly utilized as an important medicinal food plant. The application of organic matter is a frequently employed strategy to enhance the quality and growth of medicinal plants. However, there is limited research on the impact of low-molecular-weight organic carbon on medicinal plants. This study evaluated the influence of the residue after evaporation (RAE) from industrial fermentation of vitamin C (L-ascorbic acid, ASA), which mainly consists of low-molecular-weight organic acids (LMWOAs), on the growth and bioactive constituents of purslane. Cultivation with different doses (2.7, 1.35, and 0.675 mL RAE per kg of soil) of RAE exhibited the highest levels of enhancement in the ASA, dopamine, total alkaloids, and total phenols content by 103.28%, 118.38%, 32.43%, and 27.64%, respectively, while promoting purslane’s growth. Furthermore, there was a dose–effect relationship between the dose of RAE and purslane’s ASA, total phenols, dopamine and total alkaloids. Metabolomic analysis revealed that the effects of RAE on pathways related to ASA synthesis, glycolysis, citrate cycle and amino acid synthesis contributed to the bioactive constituent accumulation in purslane. These findings suggest that RAE can effectively modulate the primary metabolic processes, thereby enhancing the yield and bioactive constituents of purslane. It is a valuable source of LMWOAs for the cultivation of medicinal plants. The resource utilization of RAE will enhance the production of medicinal plants, thereby contributing to satisfying the demand for bioactive natural products in the pharmaceutical, cosmetic, and food industries.
1. Introduction
Purslane (Portulaca oleracea L.), an annual plant belonging to the Portulaca family, is widely distributed across various regions and countries around the world. It is commonly utilized as a medicinal food plant. In China and the Mediterranean region, it is defined as a “power food” that can be consumed as a vegetable salad or incorporated into cooking due to its high content of ascorbic acid, unsaturated fatty acids, and remarkable antioxidant properties [1,2]. Moreover, purslane has a rich historical background as a traditional medicinal plant and is extensively utilized for disease treatment in Asia, Europe, Australia and Africa [3,4,5]. Numerous bioactive constituents present in purslane have exhibited pharmacological properties according to modern studies. For instance, purslane-derived flavonoids and phenolic compounds demonstrate potent antibacterial properties [6]. The extracts of purslane have been shown to ameliorate kidney and ulcer conditions, as well as to provide anticancer benefits [7]. Moreover, the alkaloids present in purslane possess anti-inflammatory capabilities [8,9], and certain alkaloids have also been observed to exert neuroprotective effects [10].
Medicinal food plants are botanical species utilized for the treatment and prevention of diseases, with their raw materials often serving as valuable resources in the pharmaceutical industry. The therapeutic properties of medicinal food plants rely on the synthesis of secondary metabolites (SMs) within their organisms [11]. The utilization of medicinal food plants persists as an enduring aspect of traditional medical practices in contemporary times. According to data from the World Health Organization, over 80% of the global population relies on traditional medicinal plants, including medicinal food plants, to meet their primary healthcare needs [12]. About 40% of contemporary clinical drugs are derived from active substances found in medicinal plant [13]. With advancing research on medicinal plants, diverse applications have been developed, encompassing fields such as cosmetics, agricultural chemicals, and food additives, among others [14]. Due to the extensive product consumption and raw materials demand, there is a scarcity or even depletion of medicinal plant resources [15]. Consequently, the enhancement of both the quality and the yield of medicinal plants has become a significant area of focus. SMs play a pivotal role as functional components within medicinal plants and serve as the foundation for commercial drugs [16]. This underscores the unique nature of cultivating medicinal plants, necessitating efforts to augment their natural active constituents and increase their yields.
The improvement of medicinal plant quality can be achieved through both genetic manipulation and optimization of planting conditions. The role of plant breeding in agricultural advancement is significant; however, the complexity of breeding arises from a limited understanding of the genetic background, heterozygosity, growth cycle of medicinal plants, and the intricate growth environment they inhabit [17]. Since the 20th century, optimizing medicinal plant yield and natural products through breeding played no major role [18]. Even emerging plant cell callus culture, achieving high yields of natural products such as hyoscyamine and ginsenosides, remains challenging [19]. Apart from paclitaxel [20], there have been limited reports on the commercial-scale production of drugs using plant suspension culture. Although synthetic biology has shown potential in secondary natural product production through heterologous assembly of artificial pathways, crossing species boundaries and redesigning cellular metabolism [18,21], the development of synthetic biology is restricted by the lack of biological components, chassis and supporting operating systems [22]. In contrast, the utilization of macromolecular organic matter has been well established as a common approach to enhance the bioactive constituents of medicinal plants [23]. However, agricultural waste and animal manure are currently used as sources of such organic matter, which can pose potential risks of aggravating insect pests and accumulating heavy metals and antibiotics in soil [24,25]. Therefore, it is particularly important to explore new and cleaner organic material resources for cultivating medicinal plants. Recent studies have highlighted the significance of organic acids as crucial signaling molecules in the rhizosphere micro-ecology, promoting biochemical processes surrounding plant roots and ultimately improving crop productivity [26,27]. Nevertheless, there is limited research on the impact of low-molecular-weight organic acids (LMWOAs) inputs on the bioactive constituents of medicinal plants.
Industrially, the waste fermentation residue after evaporation (RAE) generated during the production of 2-keto-L-gulonic acid (2KGA), a directly precursor of vitamin C (L-ascorbic acid, ASA) chemosynthesis, primarily consists of LMWOAs, including 2KGA (more than 20% w/v), oxalic acid, formic acid, and pentanoic acid as its main components [28]. In China, approximately 60,000 tons of waste RAE are annually discharged, and there is an urgent need for its harmless disposal and resource utilization [29]. Several studies have demonstrated that the application of RAE can improve soil’s nutrient conditions and microbial community structure, while simultaneously promoting plant growth and ASA accumulation [28,30]. Therefore, by utilizing RAE in agriculture practices, a substantial quantity of economically advantageous LMWOAs for agricultural production can be obtained at minimal cost. Furthermore, Gao et al. [31] revealed that 2KGA, the main active substance present in RAE, possesses a regulatory function in relation to ASA metabolism within plants. It significantly increases the ASA content by modulating the expression of metabolic enzymes involved in plant ASA synthesis process. Additionally, it facilitates soluble carbohydrate accumulation, thereby enhancing the salt stress resistance of plants [31]. These findings suggest that RAE has the potential to regulate plant metabolic processes; however, its impact on the growth of medicinal plants and their bioactive constituents remains unclear.
In this study, we investigated the impact of RAE application on the growth and quality of purslane, focusing on two aspects: (1) changes in the morphological characteristics and bioactive constituents, and (2) the potential mechanisms for regulating metabolic processes by RAE in purslane by using metabolomics-based analysis. The objective of this study was to assess the efficacy of RAE in improving purslane quality and provide a novel perspective on the regulation of growth and accumulation of bioactive constituents in medicinal food plants by low-molecular-weight organic carbon.
2. Materials and Methods
2.1. Experimental Design and Plant Materials
The experimental soil was collected from the foothills of Qipan Mountain, Shenyang, China (41°53′ N, 123°41′ E), with the removal of plant roots and gravel. The initial soil properties were as follows: soil pH was 7.12, soil organic carbon was 19.07 g/kg, total nitrogen was 0.96 g/kg, available phosphorus was 3.04 mg/kg, and available potassium was 107.13 mg/kg. The RAE was provided by Northeast Pharmaceutical Group Co., Ltd., located in Shenyang, China, and its characteristics are listed in Table 1.

Table 1.
The characteristics of RAE.
The experiment was divided into four groups based on the different dosages of RAE applied at one time as follows: (1) application of 2.7 mL RAE per kg of soil (R1); (2) application of 1.35 mL RAE per kg of soil (R2); (3) application of 0.675 mL RAE per kg of soil (R3); and (4) the control with only desalted water applied (CK). The dosage of the RAE application was selected with adjustments based on the study conducted by Kong et al. [30]. Additionally, a base fertilizer urea was applied at a rate of 62.5 mg per kg of the experimental soil. Prior to application, the pH of the RAE was adjusted to 6.2 ± 0.05 using KOH solution (1.0 mol/L), while a solution of KCl (pH = 6.2 ± 0.05) with a concentration of 1.0 mol/L was used to balance the K+ input in each group. Thirty seedlings were cultured in 0.36 kg of soil in a plastic culture pot (Φ × height × Φ = 10 cm × 8.5 cm × 7 cm) with six replicates per group. After the purslane plants developed their fourth leaf, RAE applications were conducted every 14 days (a total of four times). The controlled conditions in the greenhouse included light intensity of approximately 8000 lx for 14 h daily, relative humidity maintained at 50 ± 10%, and culture temperature set at 25 ± 1 °C. After a cultivation period of 56 days, intact plant samples were collected for analysis or packed into zip-lock bags before being stored at −80 °C. The soils were air-dried, placed in zip-lock bags, and stored at room temperature away from light. The test methods for the soil properties can be found in the Supplementary Material File S1.
2.2. Determination of L-Ascorbic Acid, Soluble Sugar, and Soluble Protein
The ASA content was determined using high-performance liquid chromatography (HPLC) [31]. A fresh leaf sample weighing 0.2 g was immersed in 2.0 mL of a 1.0% (w/v) metaphosphoric acid solution, protected from light, and ground on ice until no obvious tissue remained. The resulting homogenate was then centrifuged at 6000 r/min for 5.0 min, and the supernatant was collected and filtered through a filter membrane with a pore size of 0.22 µm to obtain the test solution. The HPLC (YL9100, Young In Chromass, Anyang-si, Republic of Korea) analysis was performed under the following conditions: mobile phase consisting of NaH2PO4 solution (20 mM, pH = 2.8 ± 0.05)/acetonitrile = 95:5; flow rate was set at 1.0 mL/min; UV detector wavelength was at 243 nm; column (ZORBAX Eclipse Plus C18, Agilent Technologies, Santa Clara, CA, USA) temperature was maintained at 40 °C. The content was calculated by a standard curve of 0–50 (0, 5, 10, 25, 50) μg/mL ASA. In this study, all the standard substances were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China).
The soluble sugar (SS) content was measured using the phenol-sulfuric acid method [32]. Fresh leaf samples (0.5 g) were homogenized in a centrifuge tube through a homogenizer until complete tissue disruption was achieved. Then, 5.0 mL of desalted water was added and the mixture was subjected to boiling in a water bath for 30 min, followed by cooling to room temperature with tap water and centrifugation at 6000 r/min for 10 min. Subsequently, 1.0 mL of the supernatant was transferred into a stopper test tube. To this, 0.5 mL of a 9.0% (w/v) phenol solution and 2.5 mL of concentrated sulfuric acid were added, thoroughly mixed by shaking, and then boiled again in a water bath for an additional period of 15 min. After cooling to room temperature with tap water, the absorbance was detected at 490 nm using a Multiskan Spectrum (1500, Thermo Fisher Scientific, Waltham, MA, USA). The content was calculated by a standard curve of 0–80 (0, 20, 40, 60, 80) μg/mL glucose.
The soluble protein (SP) content was determined using the Coomassie brilliant blue method [33]. After thoroughly grinding 0.5 g of fresh leaf sample using a homogenizer until no visible tissue remained, 5.0 mL of desalted water was added to the tube. Following centrifugation at 6000 r/min for 5.0 min, the supernatant was collected for further analysis. Then, 1.0 mL of the test solution and 5.0 mL of Coomassie brilliant blue solution were mixed thoroughly and the absorbance value was measured at a wavelength of 595 nm using a Multiskan Spectrum (1500, Thermo Fisher Scientific, Waltham, MA, USA). The content was calculated by a standard curve of 0–100 (0, 20, 40, 60, 80, 100) μg/mL bovine serum albumin.
2.3. Measurement of Total Phenols and Total Flavonoids Content
The total phenols content in purslane was quantified using the Folin–Ciocalteu method and appropriately modified [34]. Fresh leaf samples (0.5 g) were ground with a homogenizer and extracted with 1.0 mL methanol aqueous solution (80% v/v) under ultrasound (Ultrasonic water bath, 40 KHz, PS-30AL, Tianmeida, Shenyang, China) for 15 min, followed by centrifugation at 6000 r/min for 5.0 min to collect the supernatant. This process was repeated twice, and all the supernatants were combined and adjusted to a final volume of 5.0 mL for testing. Then, 200 μL test solution and Folin–Ciocalteu reagent (2.0 mL of 0.2 mol/L) were added to the test tube, which was shaken and incubated for 5.0 min before adding 0.75 mL of 20% (w/v) Na2CO3 solution. After shaking again and incubating for another 30 min, the absorbance value was measured at 760 nm using a Multiskan Spectrum (1500, Thermo Fisher Scientific, Waltham, MA, USA). The content was calculated by a standard curve of 0–100 (0, 10, 20, 40, 60, 80, 100) μg/mL gallic acid.
The total flavonoids content was analyzed using spectrophotometric colorimetry [35]. Fresh leaf samples (0.5 g) were ground and immersed in 5.0 mL of a 70% (v/v) ethanol aqueous solution, followed by centrifugation at 6000 r/min for 5.0 min after a water bath at 60 °C for 30 min. A supernatant volume of 1.0 mL was mixed with 0.5 mL of sodium nitrite solution (50 g/L) and thoroughly shaken. After standing for 5.0 min, 0.5 mL of aluminum nitrate solution (100 g/L) was added and shaken again, followed by standing for another 5.0 min. Subsequently, a 2.0 mL of sodium hydroxide solution (40 g/L) was added and shaken well before measuring the absorbance value at a wavelength of 508 nm using a Multiskan Spectrum (1500, Thermo Fisher Scientific, Waltham, MA, USA). The content was calculated by a standard curve of 0–100 (0, 20, 40, 60, 80, 100) μg/mL rutin.
2.4. Quantitative Analysis of Dopamine and Total Alkaloids Content
Alkaloids are also among the medicinal components found in purslane, with dopamine being one of the primary bioactive constituents within these alkaloids. The determination of the dopamine content was conducted based on the method reported by [36]. First, 0.2 g of fresh leaf sample was homogenized with a homogenizer in a centrifuge tube until no tissue fragments were visible, followed by the addition of desalted water (1.0 mL). The mixture was sonicated (Ultrasonic water bath, 40 KHz, PS-30AL, Tianmeida, Shenyang, China) for 30 min and subsequently subjected to centrifugation at 6000 r/min for 10 min. The resulting supernatant was collected and filtered using a membrane filter with a pore size of 0.22 µm. The filtrate was then analyzed by HPLC under the following conditions: mobile phase consisted of a methanol to 0.02% (v/v) formic acid solution (adjusted to pH = 5.0 ± 0.05 with ammonia water) at a ratio of 6:94; flow rate was 1.0 mL/min; column (ZORBAX Eclipse Plus C18, Agilent Technologies, Santa Clara, CA, USA) temperature was 30 °C; UV detector wavelength was set to 280 nm. The dopamine content was calculated using a standard curve. A gradient-diluted standard solution of dopamine hydrochloride (100 µg/mL) was used for detecting and constructing a standard curve.
The acid dye method was employed to determine the total alkaloids content [37]. A fresh leaf sample (0.5 g) was ground and mixed with methyl alcohol (5.0 mL). Ultrasonic extraction was performed for 30 min at 40 KHz (Ultrasonic water bath, PS-30AL, Tianmeida, Shenyang, China), followed by filtration and fixation with methyl alcohol to a final volume of 5.0 mL, which served as the test solution. Then, 0.5 mL of test solution was transferred into a test tube and combined with 10.0 mL of chloroform and 2.0 mL of 0.04% (w/v) bromocresol green buffer (prepared by adjusting pH = 4.5 ± 0.05 using a 0.02 mol/L NaOH solution in a potassium hydrogen phthalate solution at a concentration of 0.2 mol/L and adding bromocresol green to obtain buffer). After vigorous shaking for 3.0 min at 100 r/min, the mixture was allowed to stand for 30 min before taking the chloroform layer for absorbance measurement at a wavelength of 418 nm using a Multiskan Spectrum (1500, Thermo Fisher Scientific, Waltham, MA, USA). This method was used to determine the gradient-diluted standard solution of berberine hydrochloride (200 µg/mL), and a standard curve was constructed for the calculation of the total alkaloids content.
2.5. Metabolomics Analysis
The fresh leaf samples were rapidly frozen in liquid nitrogen and subsequently stored at −80 °C for the metabolomics analysis. Four independent biological replicates were included in each group. The metabolite assays were conducted by Shanghai Bioprofile Biotechnology Co., Ltd. (Shanghai, China). After thawing, 50 mg of the sample was weighed and mixed with pre-cooled 80% methanol solution. The mixture was then homogenized and disrupted using a tissue crusher, followed by sonication in an ice bath for 20 min. Subsequently, it was stored at −20 °C for one hour. The resulting supernatant was centrifuged at 16,000× g for 20 min at 4 °C and subsequently evaporated using a high-speed vacuum centrifuge. For analysis purposes, re-dissolution of the dried residue involved adding 100 μL of methanol–water solution (1:1, v/v), followed by centrifugation of the resulting supernatant at 20,000× g for 15 min at 4 °C. Finally, the supernatant obtained from this process was used as the sample for analysis. The analysis was performed using an ultra-high-performance liquid chromatography system (LC30, SHIMADZU, Kyoto, Japan) coupled with a QE Plus mass spectrometer platform (Thermo Fisher Scientific, Waltham, MA, USA). Significantly different metabolites (SDMs) were defined based on criteria of −1 ≥ log2 fold change ≥ 1 and p-value < 0.05.
2.6. Statistical Analysis
The data presented in the text represent the mean ± standard deviation of the biological replicates (n ≥ 4). The variables were compared among the groups using a one-way analysis of variance (ANOVA) and Duncan’s multiple comparisons. Principal component analysis was used to evaluate the effects of the treatment on the composition of the metabolites. The correlations between the measured variables were assessed using Spearmen’s method. All the data were statistically analyzed using GraphPad Prism software (v8.0.2) and R software (v4.0.1). The significance level was set at p < 0.05.
3. Results
3.1. Effect of RAE on Growth of Purslane
The application of RAE significantly promoted the growth of purslane (Table 2). Regarding the plant height, compared to the CK, R1, R2, and R3 exhibited increases of 39.24%, 37.35%, and 39.92%, respectively, with no significant differences observed among the different doses of RAE treatments. The leaves in the RAE treatments were significantly larger compared to those in the CK. Furthermore, each treatment (R1, R2, R3) showed a significant increase in the plant fresh weight by 175.35%, 130.28%, and 175.14%, respectively, when compared to the CK; however, there were no significant difference among the treatments. In terms of the plant dry weight, R3 demonstrated the highest increase of 276.83% among the four treatments, while both R1 and R2 significantly increased by 118.21% and 106.74%, respectively, relative to the CK. Notably, at a dosage of 0.675 mL/kg (R3), there was a significantly superior effect on increasing the plants’ dry matter accumulation compared to that of higher dose of RAE (R1 and R2).

Table 2.
Effect of RAE on the height, leaf length, leaf width, dry and fresh weight of purslane.
3.2. Effect of RAE on ASA, Soluble Sugar and Soluble Protein Contents
Figure 1a demonstrates the distinct responses of purslane’s ASA content to different doses of RAE. Compared to the CK, R1 exhibited a non-significant increase of 52.70%, while both R2 and R3 displayed significant elevations of 99.23% and 103.28%, respectively. Regarding the SS and SP, compared with the control, no significant effect on SS accumulation was observed due to the RAE treatment (Figure 1b); however, the high-dose RAE (R1) exerted detrimental effects on SP accumulation while the low-dose treatments (R2 and R3) did not significantly impact the accumulation thereof in purslane plants (Figure 1c). Nevertheless, when considering the total accumulation levels of ASA, SS and SP in the above-ground parts of purslane, all three parameters were significantly higher under the RAE treatments compared to those observed in the CK (Figure 1d–f).
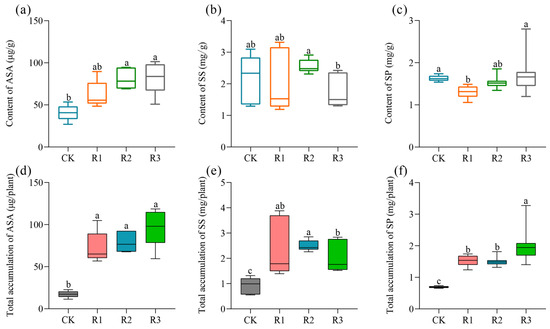
Figure 1.
Effect of RAE on the accumulation of L-ascorbic acid, soluble sugars and soluble proteins in purslane. (a) The contents of L-ascorbic acid, (b) soluble sugars, and (c) soluble proteins in the fresh samples were quantified per gram. (d) The total accumulations of L-ascorbic acid, (e) soluble sugars, and (f) soluble proteins in an individual plant. CK, control; R1, application of 2.7 mL RAE per kg of soil; R2, application of 1.35 mL RAE per kg of soil; R3, application of 0.675 mL RAE per kg of soil. ASA, L-ascorbic acid; SS, soluble sugar; SP, soluble protein. The variables were measured using fresh plant material. Different letters on the error bars (a, b, c, ab) indicate statistically significant differences (p < 0.05).
3.3. Changes in the Content of Total Phenols and Total Flavonoids
As depicted in Figure 2a, the application of a high dose of RAE (R1) significantly increased the total phenols content by 27.64% compared to the CK; however, low doses of RAE (R2 and R3) did not exhibit any significant effect on the total phenols content in purslane. Interestingly, there was a decreasing trend observed in the total phenols content as the gradient of the RAE application dose decreased. As for the total flavonoids, the content in R1 showed a significant reduction by 11.65% compared to that in the CK; nevertheless, no significant difference was observed between the R2, R3 and CK groups (Figure 2b). Despite lower levels of total flavonoids content being found in the high-dose treatment (R1), and no significant changes occurring with respect to both the total phenols and flavonoids contents at low doses (R2 and R3), all the dose groups exhibited significantly higher accumulation than that observed in the CK in the above-ground parts of purslane (Figure 2c,d).
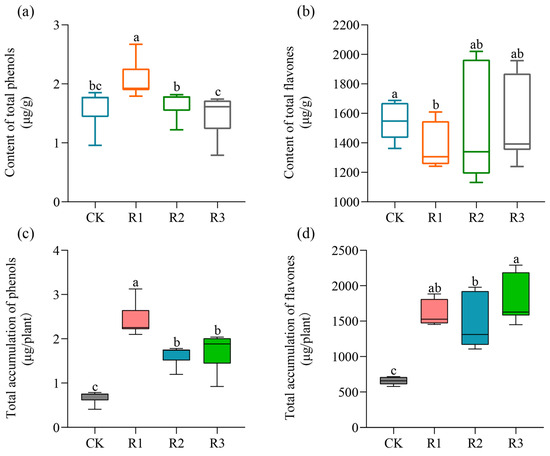
Figure 2.
Effect of RAE on the accumulation of total phenols and total flavonoids in purslane. (a) The contents of total phenols and (b) total flavones in the fresh samples were quantified per gram. (c) The total accumulations of phenols and (d) flavones in an individual plant. CK, control; R1, application of 2.7 mL RAE per kg of soil; R2, application of 1.35 mL RAE per kg of soil; R3, application of 0.675 mL RAE per kg of soil. Different letters on the error bars (a, b, c, ab, bc) indicate statistically significant differences (p < 0.05).
3.4. Dopamine and Total Alkaloids Levels in Response to RAE
The results presented in Figure 3a demonstrate a significant increase in the dopamine contents for both the R1 and R2 treatments compared to the CK, with elevations of 118.38% and 113.58%, respectively. Similarly, the total alkaloids contents of R1 and R2 were significantly elevated in comparison with that of the CK, with increases of 27.59% and 32.43%, respectively (Figure 3b). In purslane, higher application doses of RAE had a similar effect on the dopamine and total alkaloids contents, significantly increasing their levels at higher doses (R1 and R2). Furthermore, all the RAE treatments showed significantly higher accumulation of dopamine and alkaloids in the above-ground parts of purslane compared to the CK (Figure 3c,d).
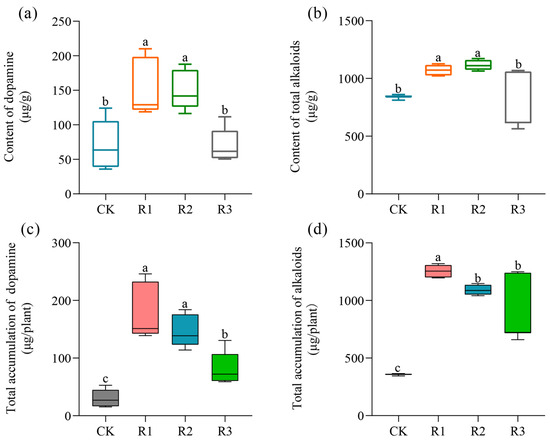
Figure 3.
Accumulation of dopamine and total alkaloids in purslane under RAE application conditions. (a) The contents of dopamine and (b) total alkaloids in the fresh samples were quantified per gram. (c) The total accumulations of dopamine and (d) alkaloids in an individual plant. CK, control; R1, application of 2.7 mL RAE per kg of soil; R2, application of 1.35 mL RAE per kg of soil; R3, application of 0.675 mL RAE per kg of soil. Different letters on the error bars (a, b, c) indicate statistically significant differences (p < 0.05).
3.5. Variable Association Analysis under the Influence of RAE
In order to investigate the potential association between the measured variables under the influence of RAE, Spearman’s rank correlation coefficient was employed for the correlation analysis. Figure 4a demonstrates significant correlations between the fluctuations in all the measured variables (except the SS and total flavonoids) and RAE. In addition, among the seven tested metabolites, except for SP, there were significant correlations with the fresh weight, dry weight or plant height of purslane; ASA presented the strongest positive correlation (r > 0.83, p < 0.01). Notably, SS showed significant positive correlations with the other six metabolites (r > 0.38, p < 0.05); dopamine and total alkaloids displayed significant positive correlations with ASA (r > 0.42, p < 0.01) as well as total phenols (r > 0.73, p < 0.01); dopamine demonstrated a highly significant positive correlation with the total alkaloids content (r > 0.95, p < 0.01). In addition, we observed significant responses (r > 0.39, p < 0.05) to varying doses of RAE for the SS, ASA, dopamine, total alkaloids, total phenols and dry weight (Figure 4b).
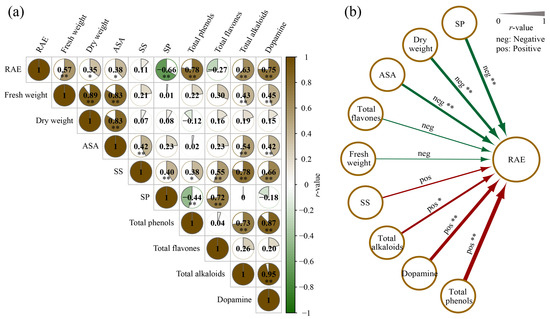
Figure 4.
Correlation analysis between RAE, metabolites, and biomass. (a) Correlation analysis based on the variable fluctuations among the CK, R1, R2, and R3 groups. (b) Response of biomass and metabolites to the dose gradient of the RAE application. Spearman’s correlation coefficient was used for the correlation analysis. *, p < 0.05; **, p < 0.01.
3.6. Metabolite Profile of Purslane Based on Metabolomics Analysis
The metabolomics analysis results revealed that the superclass classification of 1341 metabolites in purslane are lipids and lipid-like molecules (20.36%); organoheterocyclic compounds (18.79%); organic acids and derivatives (15.21%); followed by benzenoids (11.78%); organic oxygen compounds (9.02%); phenylpropanoids and polyketides (8.87%); nucleosides, nucleotides and analogues (2.54%); organic nitrogen compounds (2.16%); alkaloids and derivatives (1.57%); hydrocarbons and derivatives (0.67%); lignans, neolignans and related compounds (0.60%); organosulfur compounds (0.37%); others (4.69%); and unknown (3.36%) (Figure S1). Principal component analysis (PCA) demonstrated high reproducibility among the biological replicates while revealing distinct separation between the CK and R1 treatment (Figure S2). This indicated a strong response of purslane to RAE, resulting in notable differences in the metabolites profiles. Compared to the CK, there were 132 up-regulated SDMs and 105 down-regulated SDMs in the R1 group (Figure S3). The up-regulated SDMs were categorized into 13 classes: lipids and lipid-like molecules (count: 35); organoheterocyclic compounds (count: 24), organic acids and derivatives (count: 20), benzenoids (count: 18); nucleosides, nucleotides, and analogs (count: 10); organic oxygen compounds (count: 9); phenylpropanoids and polyketides (count: 4); alkaloids and derivatives (count: 2), organic nitrogen compounds (count: 2); homogeneous non-metal compound (count: 1); hydrocarbon derivative (count: 1); hydrocarbon (count: 1); organosulfur compound (count: 1); and unannotated compounds (count: 4) (Figure 5a,b). On the other hand, the down-regulated SDMs were divided into eight categories: lipids and lipid-like molecules (count: 25); organoheterocyclic compounds (count: 20); organic acids and derivatives (count: 18); phenylpropanoids and polyketides (count: 11); benzenoids (count: 11); organic oxygen compounds (count: 8); nucleosides, nucleotides and analogues (count: 2); lignans, neolignans and related compounds (count: 2); and unannotated compounds (count: 8) (Figure 5a,c). KEGG pathway analysis based on these SDMs revealed the top 15 enriched metabolic pathways, including the citric acid cycle; glyoxylate and dicarboxylate metabolism; glutathione metabolism; glycolysis/gluconeogenesis; pyruvate metabolism; biosynthesis of amino acids; biosynthesis of cofactor; ascorbic acid and uronate metabolism; pentose phosphate pathway; biosynthesis of isoquinoline alkaloids; and degradation of aromatic compounds (Figure 5d).
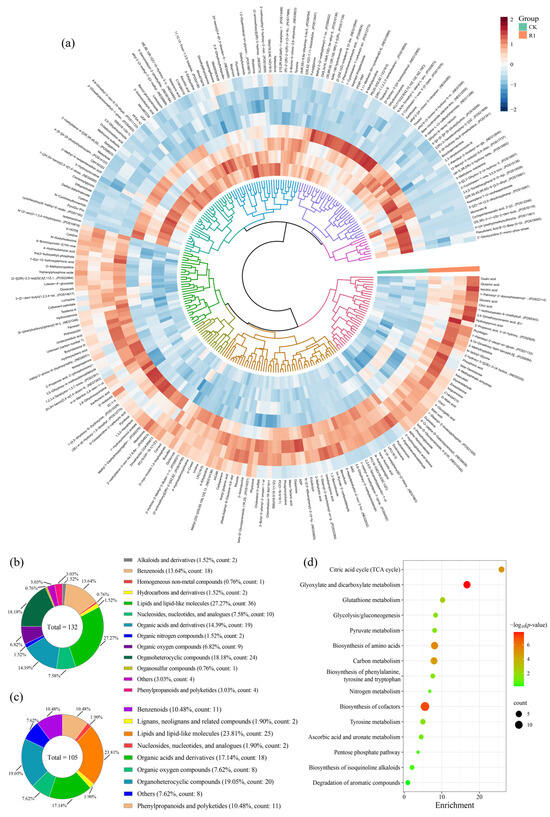
Figure 5.
Significantly different metabolites (SDMs) in the R1 group compared to the CK group. (a) Visualization of the heatmap depicting the SDMs; (b) classification of the up-regulated SDMs; (c) classification of the down-regulated SDMs; and (d) KEGG pathway enrichment analysis.
4. Discussion
The content of bioactive constituents in medicinal food plants serves as a direct indicator of the production value associated with these plants. Therefore, it holds significant research implications to ensure the maintenance of high levels of bioactive constituents while simultaneously enhancing the yield of medicinal food plants. Given its availability as a low-molecular-weight organic carbon resource, RAE plays a distinctive role in augmenting crop yields and fortifying L-ascorbic acid synthesis [28], suggesting its potential in regulating plant metabolic processes. Consequently, this study aims to investigate the effects of RAE application on purslane, a medicinal food plant renowned for its valuable bioactive constituents. In contrast to previous studies investigating the impact of RAE on the crop yield and ASA accumulation, this study emphasizes the regulatory influence of RAE on the synthesis of bioactive natural products of purslane, as well as examining the effects of RAE on purslane growth.
4.1. Contribution of RAE to the Growth of Purslane
The application of RAE significantly increased the biomass of purslane in this study (Table 2). Firstly, this can be attributed to the ability of RAE to increase the soil inorganic nutrient content. Previous studies have established a close relationship between the increase in the available nutrients in soil by RAE and crop biomass [28]. Additionally, RAE contains a variety of LMWOAs (such as 2KGA, oxalic acid), which can improve the rhizosphere environment of plants, thereby enhancing their nutrient absorption capacity [38]. Secondly, RAE contains a large amount of soluble organic carbon, which can serve as a potential source of organic carbon for plant growth [30]. This may contribute to the accumulation of certain organic acids and derivatives in purslane (Figure 5b), which are crucial for increasing dry matter accumulation and providing a carbon skeleton for synthesizing essential metabolites [28]. Thirdly, RAE increases the ASA content of the plant, thus promoting its growth. Moreover, 2KGA, the primary chemical component in RAE, plays a vital role in up-regulating the expression of L-gulono-1,4-lactone oxidase within the plant ASA synthesis pathway (myo-inositol and L-gulose pathways), thereby enhancing the conversion of L-gulono-1,4-lactone to ASA [31]. Meanwhile, it reinforces the recycling pathway of ASA to further augment ASA accumulation [31]. ASA serves as a vital antioxidant in plants by eliminating detrimental reactive oxygen free radicals and bolstering photosynthesis, consequently promoting biomass accumulation and root growth [39]. In this study, there existed a significant positive correlation between the ASA content and the biomass (Figure 4a), indicating that the increase in the ASA content (Figure 1a) was one of the key factors in the promotion of the growth of purslane by RAE. Interestingly, a lower dose of RAE treatment exhibited greater potential for increasing ASA and dry matter accumulation in purslane (Figure 4b), and this mechanism deserves further study. These findings highlight the unique function of RAE in medicinal food plant cultivation, which is not solely reliant on improving soil’s chemical properties but rather involves regulating plant metabolic activities.
4.2. Effects of Enhanced ASA Biosynthesis on Primary and Secondary Metabolism of Purslane
ASA plays a pivotal role in regulating purslane growth by RAE, while its anabolism is a crucial component of plant carbon metabolism. The intermediate metabolites involved in the synthesis pathway of ASA are associated with glycolysis, and alterations in the activity of metabolic enzymes within the ASA metabolic pathway often impact the citric acid cycle [40]. Metabolomics-based enrichment analysis of the KEGG metabolic pathways revealed significant responses to RAE in the citric acid cycle, glycolysis, as well as ascorbic acid and uronate metabolism (Figure 5d). The citric acid cycle and glycolysis are not only important energy (ATP) acquisition pathways in plants and animals but also form the core of carbon metabolism. Particularly, being the most productive metabolic pathway, the citric acid cycle generates ATP to provide energy for secondary metabolism while supplying the necessary carbon skeletons [4]. SMs serve as the primary medicinal constituents in herbal remedies and are the basis for maintaining the quality and efficacy of medicinal food plants, and the biosynthesis of secondary metabolism relies on primary metabolic pathways to provide essential energy, cofactors, and precursors such as amino acids [41,42]. In this study, we observed the significantly up-regulated levels of nucleosides, nucleotides and analogues (e.g., guanine nucleoside, NAD, UDP) in purslane after RAE application (Figure 5a,b). Concurrently, there was a notable enrichment in the biosynthesis of cofactors, along with the biosynthesis of amino acids and glutathione metabolism (Figure 5d). In addition, an elevation in the levels of glutamic acid, which acts as the primary C–N donor for synthesizing the majority of amino acids in plants, was also observed (Figure 5a). These findings suggest that RAE may modulates the ASA metabolic pathway, thereby regulating both primary and secondary metabolic processes (Figure 6) while significantly contributing to the growth and accumulation of pharmacologically active ingredients in purslane.
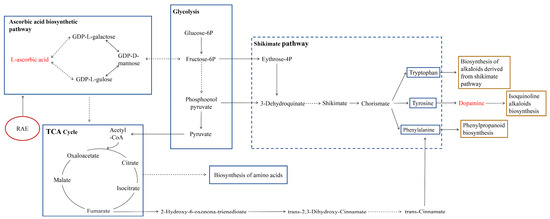
Figure 6.
Effects of RAE on primary and secondary metabolism in purslane. The metabolic pathway mapping was conducted based on the outcomes of the KEGG pathway enrichment analysis. The blue and yellow boxes indicate primary and secondary metabolism influenced by RAE, respectively. The dashed arrows indicate multiple metabolic processes. The dashed box indicates the metabolic pathways that are predicted to be influenced by RAE.
4.3. The Pathways for RAE Enhancing the Accumulation of Phenols and Alkaloids in Purslane
The shikimate pathway connects the primary and secondary metabolism in plants (Figure 6), utilizing phosphoenolpyruvate and D-erythrose-4-phosphate, which are intermediates of glycolysis and the pentose phosphate pathway, as substrates for synthesizing chorismic acid. Chorismic acid subsequently participates in the synthesis of aromatic amino acids [43]. Phenylalanine, an essential aromatic amino acid, acts as a precursor of cinnamic acid—playing a central role in phenylpropanoid metabolism [44]. The biosynthesis of phenylpropanoids is mainly accomplished through the shikimic acid pathway and malondialdehyde pathway, while phenols are an important class of compounds in phenylpropanoids [45]. In our study, we observed a positive correlation between the ASA content and the SS content (Figure 4a). Additionally, the total phenols accumulation exhibited a positive correlation with both the SS content and the RAE dose (Figure 4a,b). Based on these findings, we hypothesize that RAE affects the primary metabolic process through enhancing ASA synthesis, since the increased ASA content can feedback regulate its biosynthesis pathways [46]. Furthermore, RAE can serve as a low-molecular-weight organic carbon source for carbon metabolism and increase the available carbon skeletons for the shikimate pathway, thereby promoting the accumulation of total phenols in purslane. In higher plants, approximately 20% of the total carbon flow enters the shikimate pathway for the synthesis of aromatic amino acids [47,48]. Within this pathway, more carbon is directed toward phenylalanine synthesis [49]. Notably, our results indicate a strong response of the phenylalanine synthesis pathway to RAE in purslane (Figure 5d), which facilitates the production of certain phenylpropanoid substances (Figure 5b). Regarding the total flavonoids accumulation, SP showed a positive correlation with the total flavonoids content but a negative correlation with the total phenols content (Figure 4a). Previous studies demonstrated that protein and flavonoid syntheses compete with phenol synthesis for the limited amounts of phenylalanine [41]. This competition may explain why high doses of RAE lead to an increase in the total phenols content while causing a decrease in the SP and total flavonoids content (Figure 1c and Figure 2a,b).
Alkaloids, as nitrogen-based plant SMs synthesized through the amino acid complex path, are crucial active constituents of medicinal plants [50]. The application of RAE in this study resulted in enhanced activity of amino acid synthesis and isoquinoline alkaloids synthesis in purslane compared to the control group (Figure 5d), potentially contributing to the increase in the total alkaloids (Figure 3a). Notably, dopamine is a class catecholamine-derived alkaloid that plays a significant role not only as a neurotransmitter in the human body but also as an essential antioxidant in plants. It participates in scavenging reactive oxygen species and contributes to the normalization of photosynthesis [51]. The synthetic pathway of dopamine starts with tyrosine as a precursor: first, tyrosine decarboxylase catalyzes decarboxylation to form tyramine, followed by monoamine hydroxylase catalyzing hydroxylation of tyramine to produce dopamine [50]. Similar to phenol metabolism, tyrosine originates from the shikimate pathway [43,52], and we observed strong responsiveness of purslane’s tyrosine synthesis to RAE treatment (Figure 5d). Our results demonstrated that RAE significantly enhances the dopamine content in purslane and this increase is positively correlated with the dosage of the RAE application (Figure 3a and Figure 4b). Therefore, RAE may promote dopamine accumulation by influencing the shikimate pathway (Figure 6). Moreover, the ASA content showed a positive correlation with the dopamine levels (Figure 4a), and an elevated ASA content can reduce the redox potential in plants, which is beneficial for preventing the oxidation of dopamine [53]. This may serve as another explanation for why RAE elevated the dopamine levels.
4.4. Potential Impacts of RAE’s Soil Amelioration Ability on Bioactive Constituent Accumulation
It has been shown that the application of RAE significantly increases the utilization rate of soil carboxylic acids and carbohydrates, particularly leading to an increase in the organic carbon and total nitrogen content [30]. According to the C/N balance hypothesis, plants predominantly synthesize compounds with high nitrogen content (such as proteins) when nitrogen is readily available; however, under limited nitrogen availability, metabolism favors the synthesis of carbohydrates and nitrogen-free SMs (such as phenols) [54]. Furthermore, it has been observed that nitrogen is mainly an environmental factor affecting the alkaloid content in plants. For instance, the potato glycoalkaloids content increased with higher levels of applied ammonium nitrate in the soil [55]. In fact, LMWOAs can enhance soil’s effectiveness in terms of nitrogen through hydrogen and ionic bonding [56]. Consequently, the presence of RAE in this study led to modifications in the soil nitrogen availability (Figure S4), which is one of the environmental factors that regulate the phenols and alkaloids content in purslane. Definitely, augmenting soil organic matter is equally advantageous for the accumulation of plant alkaloids [57,58]. The variation in the RAE dosage exhibited a positive correlation with the dopamine and total alkaloids content in purslane (Figure 4b). This implies that the capacity of RAE to modify soil nutrients may contribute significantly to the dopamine and alkaloids accumulation as well. Apart from enhancing the bioaccumulation of ASA, phenols, and alkaloids, RAE exerted a significant impact on lipids, organoheterocyclic compounds, amino acids, benzenoids—all crucial primary and SMs in plants (Figure 5b,d). This suggests that RAE possesses great potential for modulating the bioactive constituents of medicinal food plants through regulation of both plant metabolism and soil nutrient dynamics.
5. Conclusions
In summary, the application of RAE effectively enhanced the biomass and increased the content of ASA as well as the bioactive constituents—phenols, alkaloids, and dopamine—in purslane. The underlying mechanism behind this enhancement process involves the regulation of key primary metabolisms, including the citric acid cycle, glycolysis, and amino acid synthesis, through promoting ASA anabolism. In addition, there was a dose–effect relationship between the applied dose of RAE and purslane’s ASA, total phenols, dopamine and total alkaloids. Our results suggest that RAE exhibits a distinctive regulatory function for growth and metabolic activities in purslane, and it is a potential low-weight-molecular organic carbon that can be used to increase the yield and improve the quality of medicinal food plants. For cultivation aimed at extracting medicinal active ingredients, a high dose (R1) of RAE is recommended, whereas for food production purposes, a lower dose (R3) of RAE is more suitable due to the increased accumulation of ASA. RAE shows us the potential value of the efficient cultivation of medicinal food plants. The resource application of RAE will not only relieve the environmental pressure of vitamin C industrial wastewater but also provide an innovative, effective and economic prospect for the fertilization of medicinal food plants.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10070683/s1, Figure S1: The superclass classification of metabolites in purslane; Figure S2: Principal component analysis of purslane’s metabolite profiles between R1 and CK groups. CK, group served as the control with only desalted water applied; R1, group received an application of 2.7 mL RAE per kg of soil; Figure S3: Significantly different metabolites in R1 compared to CK; Figure S4: Effect of RAE on the available nutrients in experimental soils. CK, control; R1, received an application of 2.7 mL RAE per kg of soil; R2, received 1.35 mL/kg RAE; R3, received 0.675 mL/kg RAE. Different letters (a, b, c, ab, bc) indicate statistically significant differences (p < 0.05). File S1: Determination of ammonia nitrogen, nitrate nitrogen, available phosphorus and available potassium in the soil [59,60,61].
Author Contributions
M.G. and Z.Z.: Investigation, methodology, data curation, validation, visualization, writing—original draft preparation; W.Y.: Formal analysis, supervision, writing—review and editing; H.S.: Software; H.X.: Conceptualization, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number 2023YFD1501200, and the Science and Technology Plan Project of Liaoning Province, grant number 2023JH/101700358.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We express our gratitude to Shuang Kong and Xiaohuan Lyu from the Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, for their invaluable experimental assistance in facilitating our research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- AlJuhaimi, F.; Mohamed Ahmed, I.A.; Özcan, M.M.; Uslu, N. Effects of fermentation, boiling, and drying methods on bioactive properties, phenolic and nutrient profiles of aerial parts of purslane (Portulaca oleracea L.) plants. Int. J. Food Sci. Technol. 2023, 58, 5809–5818. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Norman, H.A.; Gillaspy, J.E. Purslane in human nutrition and its potential for world agriculture. World Rev. Nutr. Diet. 1995, 77, 47–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, Y.; Liu, J. Determination of noradrenaline and dopamine in Chinese herbal extracts from Portulaca oleracea L. by high-performance liquid chromatography. J. Chromatogr. A 2003, 1003, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, L.; Zhou, Z.; Chen, Y.; Zhang, W.; Dai, H.; Tan, J. Homoisoflavonoids from the medicinal plant Portulaca oleracea. Phytochemistry 2012, 80, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xin, H.; Rahman, K.; Wang, S.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [PubMed]
- Budiawan, A.; Purwanto, A.; Puradewa, L.; Cahyani, E.D.; Purwaningsih, C.E. Wound healing activity and flavonoid contents of purslane (Portulaca grandiflora) of various varieties. RSC Adv. 2023, 13, 9871–9877. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, V.; Saadat, S.; Khazdair, M.R.; Gholamnezhad, Z.; El-Seedi, H.; Boskabady, M.H. Phytochemical characteristics and anti-inflammatory, immunoregulatory, and antioxidant effects of Portulaca oleracea L.: A comprehensive review. Evid. Based Complement. Altern. Med. 2023, 2023, 2075444. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, H.J.; Jang, H.; Lee, S.; Jung, K.; Lee, S.W.; Lee, S.; Rho, M. Portulaca oleracea extracts and their active compounds ameliorate inflammatory bowel diseases in vitro and in vivo by modulating TNF-α, IL-6 and IL-1β signalling. Food Res. Int. 2018, 106, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Meng, Y.; Ying, Z.; Xu, N.; Hao, D.; Gao, M.; Zhang, W.; Xu, L.; Gao, Y.; Ying, X. Three novel alkaloids from Portulaca oleracea L. and their anti-inflammatory effects. J. Agric. Food Chem. 2016, 64, 5837–5844. [Google Scholar] [CrossRef]
- Sun, H.; He, X.; Liu, C.; Li, L.; Zhou, R.; Jin, T.; Yue, S.; Feng, D.; Gong, J.; Sun, J.; et al. Effect of oleracein E, a neuroprotective tetrahydroisoquinoline, on rotenone-induced Parkinson’s disease cell and animal models. ACS Chem. Neurosci. 2016, 8, 155–164. [Google Scholar] [CrossRef]
- Farnsworth, N.R. Biological and phytochemical screening of plants. J. Pharm. Sci. 1966, 55, 225–269. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Traditional Medicine Strategy: 2014–2023; WHO Press: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/9789241506096 (accessed on 12 May 2013).
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.A.R. Medicinal plants (importance and uses). Pharm. Anal. Acta 2012, 3, 10. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and challenges in medicinal plant breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Dong, L.; Li, W.W.; Ning, Z.Y.; Liao, H.J.; Jiang, Q.; Yao, Q.S. Status and prospect of medicinal plant breeding in China. Res. Pract. Chin. Med. 2014, 28, 3–6. [Google Scholar] [CrossRef]
- Kayser, O. Ethnobotany and medicinal plant biotechnology: From tradition to modern aspects of drug development. Planta Med. 2018, 84, 834–838. [Google Scholar] [CrossRef]
- Kreis, W.; Baron, D.; Stoll, G. Biotechnologie der Arzneistoffe: Grundlagen und Anwendungen, 1st ed.; Deutscher Apotheker Verlag: Stuttgart, Germany, 2001; pp. 1–368. [Google Scholar]
- Wink, M.; Alfermann, A.W.; Franke, R.; Wetterauer, B.; Distl, M.; Windhövel, J.; Krohn, O.; Fuss, E.; Garden, H.; Mohagheghzadeh, A.; et al. Sustainable bioproduction of phytochemicals by plant in vitro cultures: Anticancer agents. Plant Genet. Resour. 2005, 3, 90–100. [Google Scholar] [CrossRef]
- Degenhardt, F.; Stehle, F.; Kayser, O. Chapter 2—The Biosynthesis of Cannabinoids. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 13–23. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Zhao, Q.; Huang, A.C. Making small molecules in plants: A chassis for synthetic biology-based production of plant natural products. J. Integr. Plant Biol. 2023, 65, 417–443. [Google Scholar] [CrossRef]
- Fallah, S.; Mouguee, S.; Rostaei, M.; Adavi, Z.; Lorigooini, Z.; Shahbazi, E. Productivity and essential oil quality of Dracocephalum kotschyi under organic and chemical fertilization conditions. J. Clean. Prod. 2020, 255, 120189. [Google Scholar] [CrossRef]
- MacLaren, C.; Labuschagne, J.; Swanepoel, P.A. Tillage practices affect weeds differently in monoculture vs. crop rotation. Soil Tillage Res. 2021, 205, 104795. [Google Scholar] [CrossRef]
- Xue, J.; Wu, J.; Hu, Y.; Sha, C.; Yao, S.; Li, P.; Lin, K.; Cui, C. Occurrence of heavy metals, antibiotics, and antibiotic resistance genes in different kinds of land-applied manure in China. Environ. Sci. Pollut. Res. 2021, 28, 40011–40021. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Yuan, J.; He, X.; Lin, Y.; Huang, Q.; Shen, Q. Enrichment of beneficial cucumber rhizosphere microbes mediated by organic acid secretion. Hortic. Res. 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lu, J.; Wang, Y.; Liu, G.; Hua, Y.; Wan, X.; Zhao, J.; Zhu, D. The abundance of nirS-type denitrifiers and anammox bacteria in rhizospheres was affected by the organic acids secreted from roots of submerged macrophytes. Chemosphere 2020, 240, 124903. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, H.; Yang, W.; Gao, M.; Zhong, X.; Zhang, L.; Chen, Z.; Xu, H. Potential utilization of vitamin C industrial effluents in agriculture: Soil fertility and bacterial community composition. Sci. Total Environ. 2022, 851, 158253. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, W.C.; Li, J.W. New progress on the second step of the mixed fermentation for vitamin C. China J. Microbiol. 2021, 41, 1–9. [Google Scholar]
- Kong, T.; Xu, H.; Wang, Z.; Sun, H.; Wang, L. Effect of a residue after evaporation from industrial vitamin C fermentation on chemical and microbial properties of alkali-saline soil. Pak. J. Pharm. Sci. 2014, 27, 1069–1074. [Google Scholar]
- Gao, M.; Sun, H.; Shi, M.; Wu, Q.; Ji, D.; Wang, B.; Zhang, L.; Liu, Y.; Han, L.; Ruan, X.; et al. 2-Keto-L-gulonic acid improved the salt stress resistance of non-heading Chinese cabbage by increasing L-ascorbic acid accumulation. Front. Plant Sci. 2021, 12, 697184. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-sulfuric acid method for total carbohydrates. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Food Science Texts Series; Springer: Boston, MA, USA, 2009; pp. 47–53. [Google Scholar] [CrossRef]
- Jones, C.G.; Hare, J.D.; Compton, S.J. Measuring plant protein with the Bradford assay. J. Chem. Ecol. 1989, 15, 979–992. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, M.; Liao, H.; Li, Y.; Han, W.; Yuan, K. Content determination of the flavonoids in the different parts and different species of Abelmoschus esculentus L. by reversed phase-high performance liquid chromatograph and colorimetric method. Pharmacogn. Mag. 2014, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Neha, S.L.; Mishra, A.K.; Rani, L.; Verma, S.P.; Sahoo, P.K. Characterization and HPLC method validation for determination of dopamine hydrochloride in prepared nano particles and pharmacokinetic application. Anal. Chem. Lett. 2022, 12, 528–541. [Google Scholar] [CrossRef]
- Li, L.; Long, W.; Wan, X.; Ding, Q.; Zhang, F.; Wan, D. Studies on quantitative determination of total alkaloids and berberine in five origins of crude medicine “Sankezhen”. J. Chromatogr. Sci. 2015, 53, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Oburger, E.; Kirk, G.J.D.; Wenzel, W.W.; Puschenreiter, M.; Jones, D.L. Interactive effects of organic acids in the rhizosphere. Soil Biol. Biochem. 2009, 41, 449–457. [Google Scholar] [CrossRef]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.Y.; Abdul Hamid, A.; Aslani, F.; Hasan, M.M.; Mohd Zainudin, M.A.; Uddin, M.K. Evaluation of antioxidant compounds, antioxidant activities, and mineral composition of 13 collected purslane (Portulaca oleracea L.) accessions. BioMed Res. Int. 2014, 2014, 296063. [Google Scholar] [CrossRef] [PubMed]
- Alhagdow, M.; Mounet, F.; Gilbert, L.; Nunes-Nesi, A.; Garcia, V.; Just, D.; Petit, J.; Beauvoit, B.; Fernie, A.R.; Rothan, C.; et al. Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007, 145, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Carbon–nitrogen ratio and in vitro assimilate partitioning patterns in Cyrtanthus guthrieae L. Plant Physiol. Biochem. 2014, 74, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Qin, Y.; Jia, Y.; Xie, X.; Li, D.; Jiang, B.; Wang, Q.; Feng, S.; Wu, Y. Transcriptomic and metabolomic data reveal key genes that are involved in the phenylpropanoid pathway and regulate the floral fragrance of Rhododendron fortunei. BMC Plant Biol. 2023, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sami, N.; Perveen, G.; Fatma, T. Biochemical characterization of novel phenylalanine ammonia-lyase from spirulina CPCC-695. Protein J. 2022, 41, 414–423. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Laing, W.A.; Martínez-Sánchez, M.; Wright, M.A.; Bulley, S.M.; Brewster, D.; Dare, A.P.; Rassam, M.; Wang, D.; Storey, R.; Macknight, R.C.; et al. An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. Plant Cell 2015, 27, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Rothe, G.M.; Maurer, W.; Mielke, C. A study on 3-deoxy-D-arabino-heptulosonic acid 7-phospate synthase in higher plants. The existence of three isoenzymes in Pisum sativum. Plant Biol. 1976, 89, 163–173. [Google Scholar] [CrossRef]
- Rubin, J.L.; Gaines, C.G.; Jensen, R.A. Enzymological basis for herbicidal action of glyphosate. Plant Physiol. 1982, 70, 833–839. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of dopamine in plants: A review. Plant Signal. Behav. 2020, 15, e1827782. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F. Superoxide as an obligatory, catalytic intermediate in photosynthetic reduction of oxygen by adrenaline and dopamine. Antioxid. Redox Signal. 2003, 5, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Young, T.E.; Ling, J.; Chang, S.; Gallie, D.R. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. USA 2003, 100, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Haukioja, E.; Ossipov, V.; Koricheva, J.; Honkanen, T.; Larsson, S.; Lempa, K. Biosynthetic origin of carbon-based secondary compounds: Cause of variable responses of woody plants to fertilization? Chemoecology 1998, 8, 133–139. [Google Scholar] [CrossRef]
- Mondy, N.I.; Munshi, C.B. Effect of nitrogen fertilization on glycoalkaloid and nitrate content of potatoes. J. Agric. Food Chem. 1990, 38, 565–567. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Wei, M.; Zeng, G.; Hou, S.; Li, D.; Xu, H. Elucidation of the mechanisms into effects of organic acids on soil fertility, cadmium speciation and ecotoxicity in contaminated soil. Chemosphere 2020, 239, 124706. [Google Scholar] [CrossRef]
- Hashemabadi, D.; Sabzevari, F.; Kaviani, B.; Ansari, M.H. Organic N-fertilizer, rhizobacterial inoculation and fungal compost improve nutrient uptake, plant growth and the levels of vindoline, ajmalicine, vinblastine, catharanthine and total alkaloids in Catharanthus roseus L. Folia Hortic. 2018, 30, 203–213. [Google Scholar] [CrossRef]
- Obidola, S.M.; Iro, I.I.; Rebecca, Z.A. Influence of organic manure and inorganic fertilizer on the growth, yield and phytochemical constituents of cabbage (Brassica oleracea). Asian J. Agric. Hortic. Res. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Shao, P.; Liang, C.; Rubert-Nason, K.; Li, X.; Xie, H.; Bao, X. Secondary successional forests undergo tightly-coupled changes in soil microbial community structure and soil organic matter. Soil Biol. Biochem. 2019, 128, 56–65. [Google Scholar] [CrossRef]
- Ma, Q.; Wen, Y.; Ma, J.; Macdonald, A.; Hill, P.W.; Chadwick, D.R.; Wu, L.; Jones, D.L. Long-term farmyard manure application affects soil organic phosphorus cycling: A combined metagenomic and 33P/14C labelling study. Soil Biol. Biochem. 2020, 149, 107959. [Google Scholar] [CrossRef]
- Pribyl, D. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).