The Accumulation and Physiological Responses of Camellia sinensis to Heavy Metals
Abstract
1. Introduction
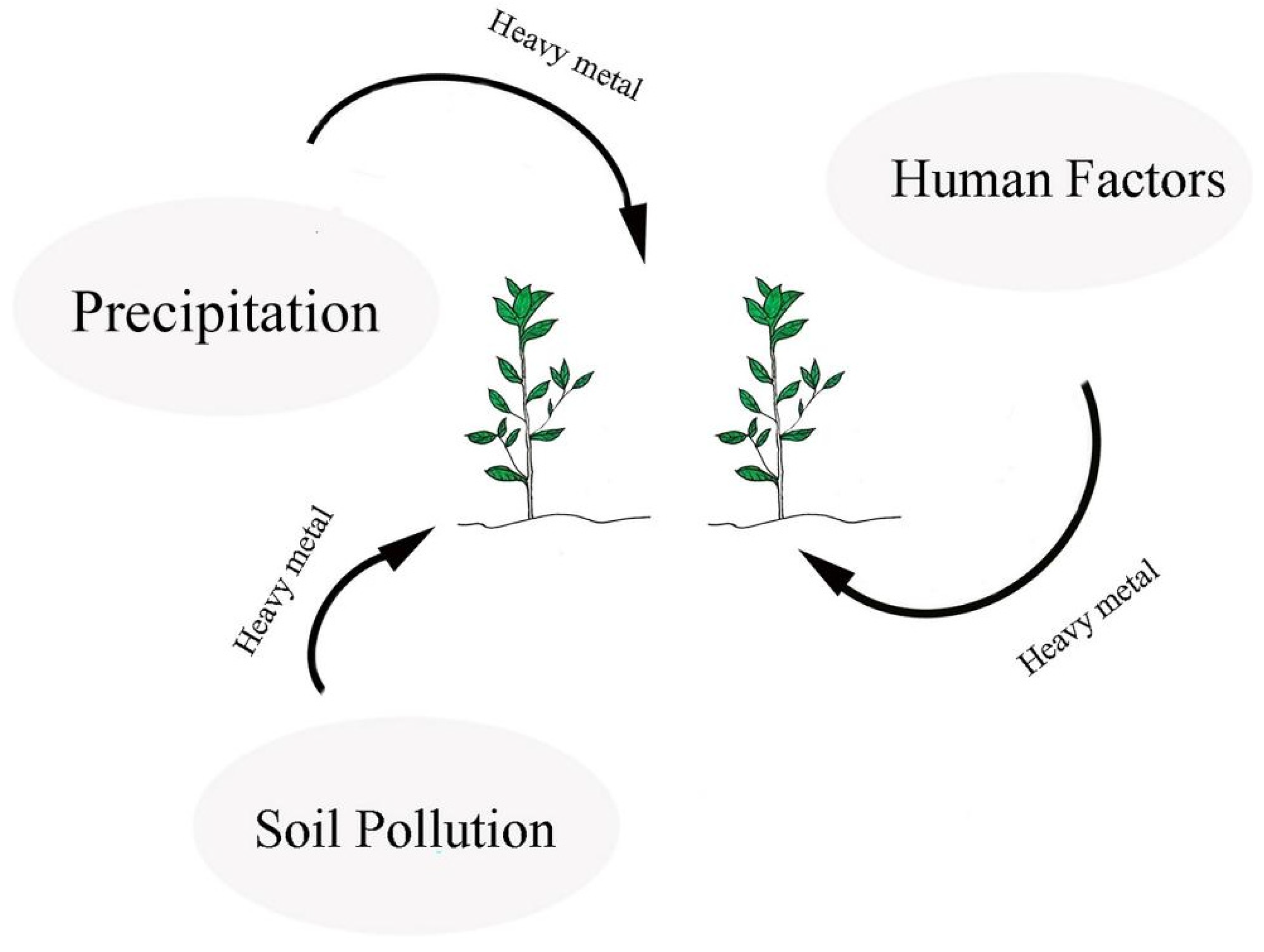
2. Sources of Heavy Metals in Camellia sinensis
2.1. Soil
2.2. Water
2.3. Human Factors
3. Differences in the Enrichment Abilities of Camellia sinensis for Heavy Metals
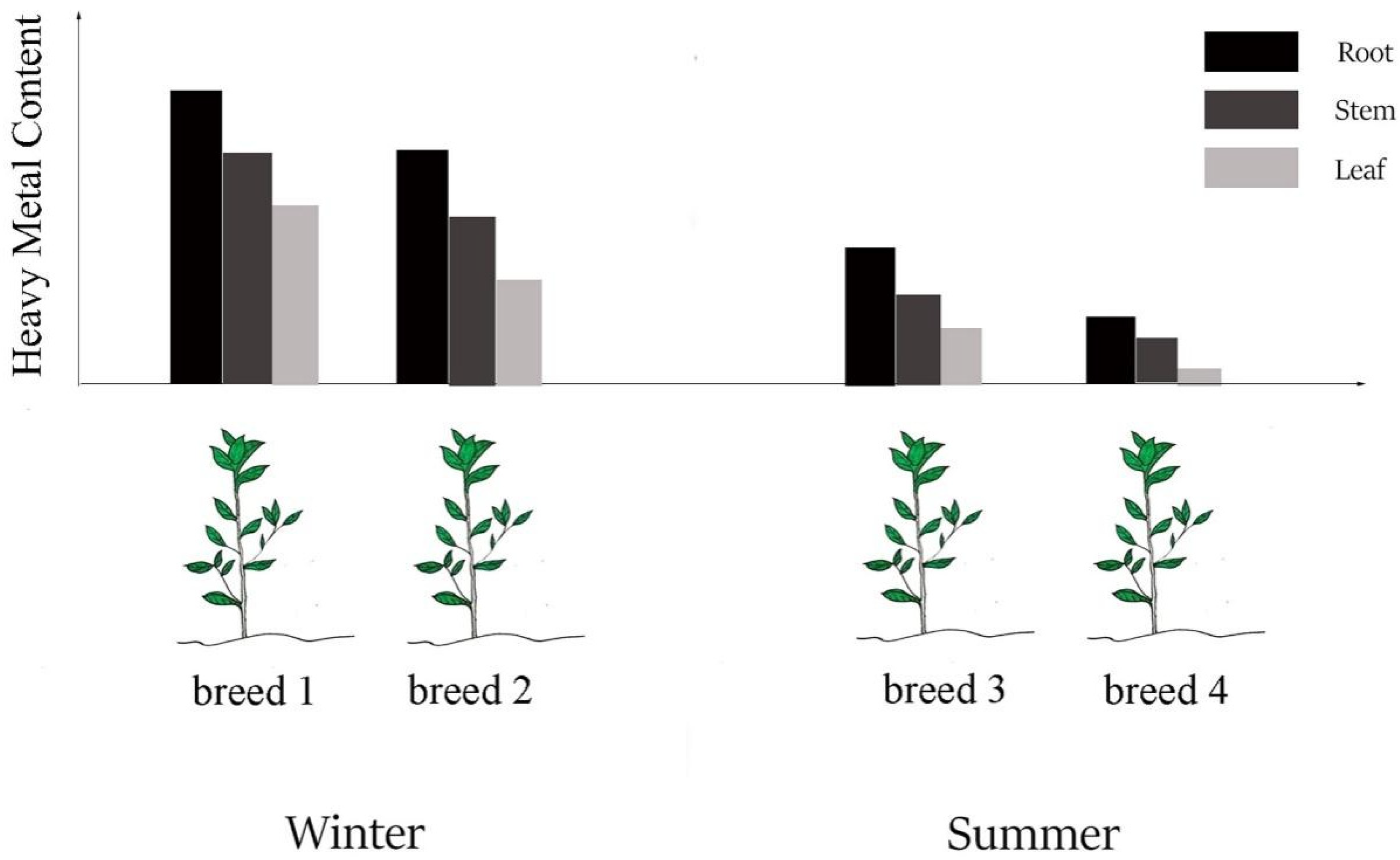
3.1. Different Parts (Organs) Have Different Enrichment Abilities for Heavy Metals
3.2. Different Breeds Have Different Enrichment Abilities for Heavy Metals
3.3. Different Seasons Have Different Enrichment Abilities for Heavy Metals
4. Physiological Effects of Heavy Metal Stress on Camellia sinensis
4.1. Effects of Heavy Metal Stress on Growth and Development
4.2. Effects of Heavy Metal Stress on the Antioxidant System
4.3. Effects of Heavy Metal Stress on Physiological Metabolism
5. Tolerance of Camellia sinensis to Heavy Metals and Its Adaptive Mechanisms to Heavy Metal Stress
6. Discussion and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Yu, X.H.; Yu, S.P.; Wu, R.M.; Yan, P.; Han, W.Y.; Fu, J.Y.; Li, X. Research progress on the effects of heavy metal stresses on tea plants and regulation technology. China Tea 2021, 43, 14–18. [Google Scholar]
- Chen, N.C.; Zheng, Y.J.; He, X.F.; Li, X.F.; Zhang, X.X. National soil pollution survey bulletin. China Environ. Prot. Ind. 2014, 5, 10–11. [Google Scholar]
- Zhang, X.L.; Wang, W.M.; Dai, W.Q.; Wang, X.L.; Zhang, X.W.; Wu, D.S.; Huang, T. Accumulation characteristics and ecological risk of sediment heavy metals in the marginal area of Poyang Lake. Earth Environ. 2023, 3, 1–10. [Google Scholar]
- Li, M.; Wang, C.C.; Bi, J.; Qin, Y.S.; Wang, K.; Xiang, P. Human health risk assessment of heavy metals in food: A review. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2021, 50, 1–9. [Google Scholar]
- Huang, Y.; Yuan, H.; Huang, Z.J.; Lu, Y. Progress in research on environmental exposure and health hazards of heavy metals in China. Chin. J. Public Health 2016, 32, 1113–1116. [Google Scholar]
- Sun, C.Y. The situation and control strategies of heavy metal health hazards. Chin. J. Ind. Med. 2014, 27, 243. [Google Scholar]
- Lei, Y.; Su, X.Q.; Wang, F.; Li, Z.; Xiao, L.Z. Research on heavy metal stress on tea. Food Nutr. China 2010, 5, 19–23. [Google Scholar]
- Tao, H.Z. Studies on the diurnal variation of the photosynthesis tea plant (Camellia sinensis). Acta Agron. Sin. 1991, 17, 9. [Google Scholar]
- Yang, Y.J.; Yu, F.L.; Chen, L.; Zeng, J.M.; Yang, S.J.; Li, S.F.; Shu, A.M.; Zhang, Z.F.; Wang, Y.S.; Wang, H.S.; et al. Elite germplasm evaluation and genetic stability of tea plants. J. Tea Sci. 2003, 23, 8. [Google Scholar]
- Handbook of Tea Plants Physiology and Tea Biochemistry Experiments Institute of Tea Research; Chinese Academy of Agricultural Sciences, Agricultural Publishing House: Beijing, China, 1983.
- Chen, L.; Yang, Y.J.; Yu, F.L. Specification and Data Standards for Describing Tea Germplasm Resources; China Agricultural Press: Beijing, China, 2005. [Google Scholar]
- Yin, J.F.; Wang, F.; Shen, D.Y.; Yuan, H.B.; Chen, J.X.; Xu, Y.Q. Analysis of the types and composition of tea beverage products in Hangzhou market. China Tea 2006, 28, 26–27. [Google Scholar]
- Dong, X.Y.; Zhang, Y.X. The overseas dissemination of Chinese tea. Newsl. Seric. Tea 2008, 5, 40. [Google Scholar]
- Chen, Z.M.; Lin, Z. Tea and human health: Biomedical functions of tea active components and current issues. J. Zhejiang Univ.-Sci. B 2015, 16, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.H.; Huang, C.M. Pathogenic risk assessment of Chinese Drinking-Tea-Type Endemic Fluorine Disease. Chin. J. Control Endem. Dis. 2006, 21, 132–135. [Google Scholar]
- Gong, Q.L.; Yang, J.Z.; Wang, Z.L.; Yan, H. Migration of heavy metals in the soil-tea plant system and health risks of drinking tea: A case study of Qiongzhong County, Hainan Province. Geophys. Geochem. Explor. 2023, 47, 826–834. [Google Scholar]
- Liu, C.L.; Zhang, J.; Peng, Y.S.; Ni, X.R.; Yang, R.D. Contents and health risks assessment of heavy metals in soil and tea in Leishan, Guizhou Province. Acta Agric. Zhejiangensis 2020, 32, 1049–1059. [Google Scholar]
- Jin, C.W.; Zheng, S.J.; He, Y.F.; Di Zhou, G.; Zhou, Z.X. Lead contamination in tea garden soils and factors affecting its bioavailability. Chemosphere 2005, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, S.S.; Pan, R.Y.; Gao, S.L. Research progress on soil acidification of tea garden. J. Tea 2019, 45, 17–23. [Google Scholar]
- Shi, Y.Z.; Ruan, J.Y.; Ma, L.F.; Han, W.Y.; Wang, F. Accumulation and distribution of Arsenic and Cadmium by tea plants. J. Zhejiang Univ.-Sci. B 2008, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W. Analysis of the effects of heavy metal chemicals enrichment in tea plants. Tea Fujian 2017, 39, 12–13. [Google Scholar]
- Wang, Y.; Li, B.G.; Zhang, M.K. Effect of atmospheric deposition on heavy metal accumulation in tea leaves. Sci. Technol. Rev. 2011, 29, 55–59. [Google Scholar]
- Kupper, H.; Mijovilovich, A.; Meyer-Klaucke, W.; Kroneck, P.M. Tissue- and age-dependent differences in the complexation of Cadmium and Zinc in the Cadmium/Zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 2004, 134, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bao, J.Q.; Yu, M.G.; Chen, Y.X. Lead adsorption by the root cell wall of tea plant. Chin. J. Appl. Ecol. 2014, 25, 427–432. [Google Scholar]
- Mukhopadyay, M.; Bantawa, P.; Das, A.; Sarkar, B.; Bera, B.; Ghosh, P.; Mondal, T.K. Changes of growth, photosynthesis and alteration of leaf anti oxidative defense system of tea [Camellia sinensis (L.) O. Kuntze] seedlings under Aluminum stress. Biometals 2012, 25, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Z. Study on the Absorption and Accumulation Characteristics of Lead Elements in Tea Plants and Their Pollution Sources. Master’s Thesis, Zhejiang University, Hangzhou, China, 2003. [Google Scholar]
- Qin, C. Study on the Occurrence Forms and Potential Migration Risks of Heavy Metals in the Soil around the Water Source Area. Master’s Thesis, Jilin University, Changchun, China, 2022. [Google Scholar]
- Zhang, B.Y.; Fen, Q.Y.; Xue, Y.; Liu, D.X.; Zhao, Z.H.; Yan, S.J.; Li, J. Accumulation characteristics, seasonal changes, and source analysis of heavy metals in major greening tree species on common highways in Fuzhou, China. Chin. J. Appl. Environ. Biol. 2022, 28, 1199–1208. [Google Scholar]
- Shi, Y.Z.; Ruan, J.Y.; Ma, L.F.; Han, W.Y.; Wang, F. Absorption and accumulation of As and Cd in tea. J. Ecol. Rural. Environ. 2006, 22, 70–75. [Google Scholar]
- Lan, H.X. Study on the Physiological and Ecological Effects of Pb, Cd and Composite Pollution on Tea Plants. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2008. [Google Scholar]
- Zhu, X.Y. Study on the Effects of As and Cd on the Growth of Tea Plants and Their Absorption and Accumulation Characteristics in Tea Plants. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2008. [Google Scholar]
- Kang, M.L.; Luo, Y.P. A study on properties of uptake and accumulation of lead by tea plant. J. Tea 2004, 30, 88–90. [Google Scholar]
- Wang, X.P.; Ma, Y.J.; Xu, Y.C. Studies on contents of Arsenic, Selenium, Mercury and Bismuth in tea samples collected from different regions by Atomic Fluorescence Spectrometry. Spectrosc. Spectr. Anal. 2008, 28, 1653–1657. [Google Scholar]
- Fang, Y.S.; Wang, Y.M.; Zhao, J.W.; Jiang, Q.H. The content distribution of five heavy metals in soil and tea leaves in different tea gardens. J. Kunming Univ. 2019, 41, 37–41. [Google Scholar]
- Dai, S.J. A Study on the Safety of Tea Production in Soil Polluted by Heavy Metals (Cd, Pb, Hg, As). Master’s Thesis, Hunan Agricultural University, Changsha, China, 2017. [Google Scholar]
- Qin, Y.; Shi, P.; Wang, Y.; Lan, W.; Luo, Q.; Lu, Z.; Wen, L. Enrichment characteristics of Selenium, Mercury and Arsenic in different tea varieties. Southwest China J. Agric. Sci. 2017, 30, 1396–1401. [Google Scholar]
- Liu, S.C.; Luo, X.Y.; Zhao, Z.Q.; Yu, Y.C.; Zhao, H.F.; Wei, J.; Lv, J. Study on uptake and accumulation of Pb, Cd and Cu in tea plant. Southwest China J. Agric. Sci. 2011, 24, 1805–1812. [Google Scholar]
- Cui, S.Z.; Shen, B.H.; Yang, W.Q. Distribution characteristics of heavy metal content in tea leaves in different seasons from Fengqing Tea Plantation in Yunnan. J. Kunming Univ. 2021, 43, 38–40. [Google Scholar]
- He, Y.J.; Wei, G.X.; Lu, L.Y.; Weng, Y.Y.; Zhang, Z.F. Determination of four heavy metals content in Kudingcha in different seasons by Microwave Assisted Flame Atomic Absorption Spectrometry. Guangdong Chem. Ind. 2020, 47, 170–171+162. [Google Scholar]
- Zhang, Q.; Wei, S.H.; Dai, H.P.; Jia, G. The alleviating effects of Selenium on Cadmium-induced toxicity in tea leaves. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2020, 44, 200–204. [Google Scholar]
- Tang, J.M.; Xu, J.Y.; Wu, Y.S.; Li, Y.; Tang, Q. Effects of high concentration of chromium stress on physiological and biochemical characters and accumulation of chromium in tea plant (Camellia sinensis L.). Afr. J. Biotechnol. 2012, 11, 2248–2255. [Google Scholar]
- Wu, Y.S.; Liang, Q.H.; Tang, Q. Effect of Pb on growth, accumulation and quality component of tea plant. Procedia Eng. 2011, 18, 214–219. [Google Scholar]
- Xia, J.G.; Lan, H.X.; Wu, D.Y. Lead stress on growth of tea trees and physiological index in leaves of tea. J. Agro-Environ. Sci. 2010, 29, 43–48. [Google Scholar]
- Fan, S.S. Characterization of Pb after Accumulated and Localized in Tissues and Cells of Hydroponic Tea. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2012. [Google Scholar]
- Xu, J. Study on the Absorption, Accumulation, and Tolerance Mechanism of Lead in Tea Plants (Camellia sinensis L.). Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2011. [Google Scholar]
- Li, X.; Ahammed, G.J.; Zhang, X.-N.; Zhang, L.; Yan, P.; Zhang, L.-P.; Fu, J.-Y.; Han, W.-Y. Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 2021, 403, 123922. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Jia, X.L.; Chen, X.T.; Lin, S.; Li, Y.; Wang, F.; Hu, Y.; Wang, H. Physiological response and subcellular distribution in different tea plants under Pb stress. J. Agric. Sci. Technol. 2017, 19, 92–99. [Google Scholar]
- Luo, L.; Xie, Z.L.; Liu, P.; Xu, G.D.; Luo, H. Physiological response of tea plant to Aluminum toxicity. J. Agro-Environ. Sci. 2006, 25, 305–308. [Google Scholar]
- Zagoskina, N.V.; Goncharuk, E.A.; Alyavina, A.K. Effect of cadmium on the phenolic compounds formation in the callus cultures derived from various organs of the tea plant. Russ. J. Plant Physiol. 2007, 54, 237–243. [Google Scholar] [CrossRef]
- Mao, P.S.; Li, Y.S.; Zhang, B. The relationship between heavy metals and tea trees and tea leaves. Newsl. Seric. Tea 2021, 2, 31–33. [Google Scholar]
- Liang, Q.H. Study on the Physiological Characteristics of Tea Leaves Caused by Combined Pollution of Cr, As, Cd, and Pb. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2010. [Google Scholar]
- Li, X.L. Study on the Effects of Lead and Chromium on the Growth of Tea Trees and Their Absorption and Accumulation Characteristics in Tea Plants. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2008. [Google Scholar]
- Yu, M.G. The regulatory mechanism of tea polyphenols on the bioavailability of lead in tea plants. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2010. [Google Scholar]
- Tomsett, A.B.; Thurman, D.A. Molecular biology of metal tolerances of plants. Plant Cell Environ. 1988, 11, 383–394. [Google Scholar] [CrossRef]
- Lambinon, J.; Auquier, P. La flore et la végétation des terrains calaminaires de la Wallonie septentrionale et de la Rhénanie aixoise. Types chorologiques et groupes écologiques. Nat. Mosana 1963, 16, 113–131. [Google Scholar]
- Liu, Z.Q.; Yang, J.G.; Wu, Z.H.; Li, Z.F.; Song, B.L.; Feng, R.W. Factors restraining uptake/translocation of heavy metals (metalloids) related with plant roots and its mechanisms. Res. Agric. Mod. 2022, 42, 284–293. [Google Scholar]
- Hammer, D.; Keller, C. Changes in the rhizosphere of metalaccumulating plants evidenced by chemical extractants. J. Environ. Qual. 2002, 31, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.D.; Berti, W.R.; Huang, J.W. Phytoremediation of contaminated soils. Trends Biotechnol. 1995, 13, 393–397. [Google Scholar] [CrossRef]
- Yang, X.E.; Long, X.X.; Ni, W.Z. Physiological and molecular mechanisms of heavy metal uptake by hyperaccumulting plants. Plant Nutr. Fertil. Sci. 2002, 8, 8–15. [Google Scholar]
- Mei, L.; Li, L.; Daud, M.K.; Chen, J.H.; He, Q.L.; Zhu, S.J. Advances on response and resistance to heavy metal stress in cotton. Cotton Sci. 2018, 30, 102–110. [Google Scholar]
- Liu, L.; Sun, H.; Chen, J.; Zhang, Y.; Li, D.; Li, C. Effects of Cadmium stress on growth and Cadmium accumulation in cotton (Gossypium hirsutum L.) seedlings. Cotton Sci. 2014, 26, 466–470. [Google Scholar]
- Li, L.; Chen, J.H.; He, Q.L.; Zhu, S.J. Accumulation, transportation, and bioconcentration of Cadmium in three upland cotton plants under Cadmium stress. Cotton Sci. 2012, 24, 535–540. [Google Scholar]
- Jia, Y.H. The Study of Three Plants to Two Heavy Metals Resistance and Recover Potentials. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2008. [Google Scholar]
- Liu, G.Y.; Chai, T.Y.; Sun, T. Heavy metal absorption, transportation, and accumulation in hyperaccumulator Thlaspi caerulescens. Chin. J. Biotechnol. 2010, 26, 561–568. [Google Scholar]
- Shen, H.L.; He, Z.Y. Advance of the mechanisms of Arsenic hyperaccumulation in Pteris vittata L. and applications for Arsenic-remediation. Plant Physiol. J. 2014, 50, 591–598. [Google Scholar]
- Wei, Z.Y.; Chen, T.B.; Huang, Z.C.; Zhang, X. Cretan Brake (Pteris cretica L.): An arsenic-accumulating plant. Acta Ecol. Sin. 2002, 5, 777–778. [Google Scholar]
- Yao, S.Y. Cd Repairing Performance and Strengthening Measures of Celosia argentea Linn. for Phytoremediation of Cd-Contaminated Soil; Guilin University of Technology: Guilin, China, 2018; Volume 2, pp. 1–75. [Google Scholar]
- Fu, X.P. Mechanisms of Cadmium Uptake and Tolerance in Phytolacca americana L. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2011. [Google Scholar]
- Baker AJ, M. Metal tolerance. New Phytol. 1987, 106, 93–111. [Google Scholar] [CrossRef]
- Han, W.Y.; Xu, Y.W. The growth and physiological effects of Zinc on tea trees. J. Tea Sci. 2005, 5, 22–23. [Google Scholar]
- Li, P.W. Resistance Characteristics and Ultrastructural Localization Characterization of Four Heavy Metal Ions Absorbed and Accumulated by Tea Trees. Ph.D. Thesis, Sichuan Agricultural University, Chengdu, China, 2015. [Google Scholar]
- Ge, H.Y. Screening of Aluminum Resistant Rhizosphere Bacteria and Their Effects on Aluminum Tolerance Behavior of Tea Trees. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2013. [Google Scholar]
- GB15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment: Beijing, China, 2018.
- Cui, H.; Zhao, Y.; Hu, K.; Xia, R.; Zhou, J.; Zhou, J. Impacts of atmospheric deposition on the heavy metal mobilization and bioavailability in soils amended by lime. Sci. Total Environ. 2024, 914, 170082. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.G.; Jiang, Z.L.; Luo, Q. The accumulation and distribution of heavy metals in teas on both sides of highway. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2002, 26, 39–42. [Google Scholar]
- Wan, J.M.; Qi, J.S.; Li, T.Z.; Zhang, L.Y.; Tian, M. Impact of leaf absorption of atmospheric heavy metals on plants: A review. Plant Sci. J. 2023, 41, 694–704. [Google Scholar]
- Xu, W.W.; Teng, Y.T.; Ren, J.H.; Zhang, Y.P.; Long, Z. Effect of different activators on the remediation of Cd and Pb single and compound contaminated soils. Environ. Pollut. Control 2019, 41, 882–886+890. [Google Scholar]
- Zhou, Y.C.; Li, M.S. Heavy metal contamination and transportation in Soil-Tea Leaf-Tea Liquor System in two tea gardens of Guangxi. J. Agro-Environ. Sci. 2008, 27, 2151–2157. [Google Scholar]
- Cao, D.; Ma, L.L.; Jin, X.F.; Gong, Z.M. Research advance of resistance to abiotic stresses of tea. Hunan Agric. Sci. 2015, 10, 152–154. [Google Scholar]
- Xu, Y.Q.; Hu, Z.; Jiang, C.J.; Hu, Y.M.; Li, J.; Li, Y.Y. Effect of cross adaptation of drought-low temperature on cold resistance of tea plants (Camellia sinensis). J. Anhui Agric. Univ. 2020, 47, 1–6. [Google Scholar]
- Xu, Y.Q. Study on Cross Adaptation of Drought Induced to Chilling Stress in Tea Seedlings; Anhui Agricultural University: Hefei, China, 2022; Volume 6, pp. 1–70. [Google Scholar]
- Cao, D.; Jin, X.F.; Ma, L.L.; Liu, Y.L. Effect of exogenous nitric oxide on physiological characteristics of tea plants under drought stress. Acta Tea Sin. 2016, 57, 76–79. [Google Scholar]
- He, J.Z. Effects of Exogenous Nitric Oxide on Seeds Germination and Seedlings Physiological Characteristicsof Tea (Camellia sinensis) under Osmotic Stress. Ph.D. Thesis, Anhui Agricultural University, Hefei, China, 2017. [Google Scholar]
- Macnair, M.R. Tolerance of higher plants to toxic materials. In Genetic Consequences of Man-Made Charge; Bishop, J.A., Cook, L.M., Eds.; Academic Press: London, UK; New York, NY, USA, 1981; pp. 177–207. [Google Scholar]
- Yu, L.; Tang, S.; Kang, J.; Korpelainen, H.; Li, C. Responses of dioecious Populus to heavy metals: A meta-analysis. For. Res. 2023, 3, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Xiao, J.; Wu, C.; Yu, L. The Accumulation and Physiological Responses of Camellia sinensis to Heavy Metals. Horticulturae 2024, 10, 680. https://doi.org/10.3390/horticulturae10070680
Dai H, Xiao J, Wu C, Yu L. The Accumulation and Physiological Responses of Camellia sinensis to Heavy Metals. Horticulturae. 2024; 10(7):680. https://doi.org/10.3390/horticulturae10070680
Chicago/Turabian StyleDai, Haixiang, Juan Xiao, Chuansheng Wu, and Lei Yu. 2024. "The Accumulation and Physiological Responses of Camellia sinensis to Heavy Metals" Horticulturae 10, no. 7: 680. https://doi.org/10.3390/horticulturae10070680
APA StyleDai, H., Xiao, J., Wu, C., & Yu, L. (2024). The Accumulation and Physiological Responses of Camellia sinensis to Heavy Metals. Horticulturae, 10(7), 680. https://doi.org/10.3390/horticulturae10070680





