Impact of Twig-Tip Dieback on Leaf Nutrient Status and Resorption Efficiency of Mango (Mangifera indica L.) Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Twig-Tip Dieback Evaluation
2.3. Sample Collection
2.4. Sample Processing and Analysis
2.5. Sufficiency Levels for the Nutrient Status of the Leaves
2.6. Deviation from the Optimum Percentage (DOP) Index
2.7. Nutrient RE
2.8. Statistical Analysis
3. Results
3.1. Soil and Leaf Nutrient Analysis
3.2. The DOP Index
3.3. Nutrient Resorption Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NT (Northern Territory) Farmers. NT Mangoes. Available online: https://ntfarmers.org.au/commodities/mangoes/ (accessed on 2 November 2023).
- Umar, M.; McConchie, C.; Tran-Nguyen, L.; Asis, C.A.; Eyles, A.; Stanley, R.; Gracie, A. Perception of Australian consumers on resin canal discouloration (RCD) in mango. Acta Hortic. 2020, 1299, 13–18. [Google Scholar] [CrossRef]
- Grice, K.R.E.; Bally, I.S.E.; Wright, C.L.; Maddox, C.; Ali, A.; Dillon, N.L. Mango germplasm screening for the identification of sources of tolerance to anthracnose. Australas. Plant Pathol. 2023, 52, 27–41. [Google Scholar] [CrossRef]
- Giblin, F.R.; Tan, Y.P.; Mitchell, R.; Coates, L.M.; Irwin, J.A.G.; Shivas, R.G. Colletotrichum species associated with pre-and post-harvest diseases of avocado and mango in eastern Australia. Australas. Plant Pathol. 2018, 47, 269–276. [Google Scholar] [CrossRef]
- Johnson, G.I.; Cooke, A.W.; Mead, A.J.; Wells, I.A. Stem end rot of mango in Australia: Causes and control. Acta Hortic. 1991, 291, 288–295. [Google Scholar] [CrossRef]
- Asis, C.A.; Meschiari, L.; McConchie, C. Ionome balance analysis of mango fruit from orchard with and without resin canal discolouration. Acta Hortic. 2019, 1244, 221–228. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Ray, J.D.; Lanoiselet, V.; Hardy, G.E.S.J.; Burges, T.I. Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley Region of Western Australia. Eur. J. Plant Pathol. 2011, 130, 379–391. [Google Scholar] [CrossRef]
- McTaggart, A.R.; Grice, K.R.; Shivas, R.G. First report of Vialaea minutella in Australia, its association with mango branch dieback and systematic placement of Vialaea in the Xylariales. Australas. Plant Dis. Notes 2013, 8, 63–66. [Google Scholar] [CrossRef]
- Umar, U.D.; Ahmed, N.; Zafar, M.Z.; Rehman, A.; Naqvi, S.A.H.; Zulfiqar, M.A.; Malik, M.T.; Ali, B.; Saleem, M.H.; Marc, R.A. Micronutrients foliar and drench application mitigate mango sudden decline disorder and impact fruit yield. Agronomy 2022, 12, 2449. [Google Scholar] [CrossRef]
- Saeed, A.; Shad, M.A.; Nawaz, H.; Shafqat, M.N.; Muneer, Z.; Shaheen, A.; Shah, S.T.A. Quick decline disease disturbs the levels of important phytochemicals and minerals in the stem bark of mango (Mangifera indica). Int. J. Agron. 2016, 2016, 8219356. [Google Scholar] [CrossRef]
- Saeed, E.E.; Sham, A.; AbuZarqa, A.; Al Shurafa, K.A.; Al Naqbi, T.S.; Iratni, R.; El-Tarabily, K.; AbuQamar, S.F. Detection and management of mango dieback disease in the United Arab Emirates. Int. J. Mol. Sci. 2017, 18, 2086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamil, F.H.; Saeed, E.E.; El-Tarabily, K.A.; Abu Qamar, S.F. Biological control of mango dieback disease caused by Lasiodiplodia theobromae using stretomycete and non-streptomycete Actinobacteria in the United Arab Emirates. Front. Microb. 2018, 9, 829. [Google Scholar] [CrossRef]
- NT Rural Review. Mango Common Dieback. 2021. Available online: https://industry.nt.gov.au/publications/primary-industry-publications/newsletters/regional-newsletters/rural-review/nt-rural-review-november-2021/mango-common-dieback (accessed on 5 November 2023).
- Kazmi, M.R.; Fateh, F.S.; Majeed, K.; Muhammad, A.; Kashkhely, I.H.; Ahmad, I.; Jabeen, A. Incidence and etiology of mango sudden death phenomenon. Pak. J. Phytopathol. 2005, 17, 154–158. [Google Scholar]
- Shukla, P.K.; Tahseen, F. Dieback: The great constraint in perennial fruit crops. In Precision Agriculture and Sustainable Crop Production; Chourasia, H.K., Acharya, K., Singh, V.K., Eds.; Today and Tomorrow’s Printers and Publishers: New Delhi, India, 2020; pp. 197–211. [Google Scholar]
- NT Rural Review. Mango Twig Tip Dieback. 2021. Available online: https://industry.nt.gov.au/publications/primary-industry-publications/newsletters/regional-newsletters/rural-review/nt-rural-review-november-2021/mango-twig-tip-dieback (accessed on 5 November 2023).
- Ram, R.A.; Rahim, M.A.; Alam, M.S. Diagnosis and management of nutrient constraints in mango. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 12, pp. 629–650. [Google Scholar] [CrossRef]
- Tripathi, R.; Terwai, R.; Sing, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V. Physiological disorders in perennial woody tropical and subtropical fruit crops: A review. Indian J. Agric. Sci. 2016, 86, 703–717. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Gupta, N.; Debnath, S.; Sharma, S.; Sharma, P.; Purohit, J. Role of nutrients in controlling plant diseases in sustainable agriculture. In Agriculturally Important Microbes for Sustainable Agriculture; Meena, V.S., Mishra, P., Bisht, J., Pattanayak, A., Eds.; Springer: Singapore, 2017; Volume 2, pp. 217–262. [Google Scholar] [CrossRef]
- Gonzalez de Andrés, E.; Gazol, A.; Querejeta, J.I.; Igual, J.M.; Colangelo, M.; Sánchez-Salguero, R.; Linares, J.C.; Camarero, J.J. The role of nutritional impairment in carbon-water balance of silver fir drought-induced dieback. Glob. Chang. Biol. 2022, 28, 4439–4458. [Google Scholar] [CrossRef]
- Weinmann, M.; Bradáčová, K.; Nikolic, M. Relationship between mineral nutrition, plant diseases, and pests. In Marschner’s Mineral Nutrition of Higher Plants, 4th ed.; Marschner, P., Ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2023; Chapter 10; pp. 445–476. [Google Scholar] [CrossRef]
- Bal, T.L.; Storer, A.J.; Jurgensen, M.F.; Doskey, P.V.; Amacher, M.C. Nutrient stress predisposes and contributes to sugar maple dieback across its northern ranges: A review. Forestry 2015, 18, 64–83. [Google Scholar] [CrossRef]
- Akillioglu, A. Micronutrient levels of cherry orchards in the Aegean region of Turkey. Acta Hortic. 1997, 448, 199–202. [Google Scholar] [CrossRef]
- Nageli, P.; Grant, J.; Nichols, J.D.; Sheil, D.; Horton, B. Bell miner associated dieback: Nutrient cycling and herbivore crown damage in Eucalytus propinqua. Australas. For. 2016, 79, 74–82. [Google Scholar] [CrossRef]
- Gerrish, G.; Dombois, D.M.; Bridges, K.W. Nutrient limitation and Metrosideros forest dieback in Hawai’i. Ecology 1988, 69, 723–727. [Google Scholar] [CrossRef]
- Cao, J.; Cheng, C.; Yang, J.; Wang, Q. Pathogen infection drives patterns of nutrient resorption in citrus plants. Sci. Rep. 2015, 5, 14675. [Google Scholar] [CrossRef]
- Huang, C.; Xu, C.; Ma, Y.; Song, T.; Xu, Z.; Li, S.; Liang, J.; Zhang, L. Nutritional diagnosis of the mineral elements in Tainong mango leaves during flowering in karst areas. Land 2022, 11, 1311. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Shahkoomahally, S.; Asis, C.; McConchie, C. Influence of rootstocks on scion leaf mineral content in mango tree (Mangifera indica L.). Hortic. Environ. Biotechnol. 2021, 62, 725–735. [Google Scholar] [CrossRef]
- Ganeshamurthy, A.N.; Reddy, Y.T.N. Fitness of mango for colonization in low fertility soils and dry lands: Examination of leaf life-span, leaf nutrient resorption, and nutrient use efficiency in elite mango varieties. Agric. Res. 2015, 4, 254–260. [Google Scholar] [CrossRef]
- Du, B.; Ji, H.; Liu, S.; Kang, H.; Yin, S.; Liu, C. Nutrient resorption strategies of three oak tree species in response to interannual climate variability. For. Ecosys. 2021, 8, 70. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods—Australasia; CSIRO Publishing: Clayton, Australia, 2011; pp. 110–111. [Google Scholar]
- McQuaker, N.R.; Brown, D.F.; Kluckner, P.D. Digestion of environmental materials for analysis by inductively coupled plasma-atomic emission spectrometry. Anal. Chem. 1979, 51, 1082–1084. [Google Scholar] [CrossRef]
- Reuter, D.J.; Robinson, J.B. Plant Analysis, an Interpretation Manual, 2nd ed.; CSIRO Publishing: Clayton, Australia, 1997; pp. 366–367. [Google Scholar]
- Heras, L.; Montañés, L. Desviación del óptimo porcentual (DOP): Nuevo índice para la interpretación del análisis vegetal. An. Estac. Exp. Aula Dei 1991, 20, 93–108. [Google Scholar]
- Kumar, P.; Sharma, S.K.; Kumar, A. Foliar nutritive fluids affect generative potential of apples: Multilocation DOP indexing and PCA studies under dry temperate agro-climatic conditions of north-west Himalaya. Sci. Hortic. 2017, 218, 265–274. [Google Scholar] [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Gómez-Aparisi, J.; Betrán, J.; Moreno, M. Influence of almond× peach hybrids rootstocks on flower and leaf mineral concentration, yield and vigour of two peach cultivars. Sci. Hortic. 2005, 106, 502–514. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Wei, X.; Ni, X.; Wu, F. Monthly dynamical patterns of nitrogen and phosphorus resorption efficiencies and C: N: P stoichiometric ratios in Castanopsis carlesii (Hemsl.) Hayata and Cunninghamia lanceolata (Lamb.) Hook. Plantations. Forests 2022, 13, 1458. [Google Scholar] [CrossRef]
- Montañés, L.; Heras, L.; Abadía, J.; Sanz, M. Plant analysis interpretation based on a new index: Deviation from optimum percentage (DOP). J. Plant Nutr. 1993, 16, 1289–1308. [Google Scholar] [CrossRef]
- Tadayon, M.S.; Sadeghi, S. The relation between compositional nutrient diagnosis indices and susceptibility of Valencia orange to citrus decline. J. Plant Nutr. 2023, 46, 261–274. [Google Scholar] [CrossRef]
- Milošević, T.; Moreno, M.Á.; Milošević, N.; Milinković, M. Regulation of yield, fruit size, and leaf mineral nutrients of the ‘Šumadinka’ sour cherry cultivar with help of rootstocks. J. Plant Growth Regul. 2023, 42, 5587–5599. [Google Scholar] [CrossRef]
- Spann, T.M.; Schumann, A.W. The role of plant nutrients in disease development with emphasis on citrus and huanglongbing. Proc. Fla. State Hort. Soc. 2009, 22, 169–171. Available online: https://swfrec.ifas.ufl.edu/hlb/database/pdf/00001871.pdf (accessed on 5 December 2023).
- Azim Nejad, Z.; Badehian, Z.; Rezaei Nejad, A.; Bazot, S. Do soil properties and ecophysiological responses of aak (Quercus brantii Lindl.) correlate with the rate of dieback? Trees 2021, 35, 1639–1650. [Google Scholar] [CrossRef]
- Pustika, A.B.; Subandiyah, S.; Holford, P.; Beattie, G.A.C.; Iwanami, T.; Masaoka, Y. Interactions between plant nutrition and symptom expression in mandarin trees infected with the disease huanglongbing. Australas. Plant Dis. Notes 2008, 3, 112–115. [Google Scholar] [CrossRef]
- Mathew, J.; Haris, A.A.; Bhat, R.; Kumar, V.K.; Muralidharan, K.; John, K.S.; Surendran, U. A comparative assessment of nutrient partitioning in healthy and root (wilt) disease affected coconut palms grown in an Entisol of humid tropical Kerala. Trees 2021, 35, 621–635. [Google Scholar] [CrossRef]
- Sousa Filho, H.R.; Jesus, R.M.D.; Bezerra, M.A.; da Silva, V.H.; da Silva, A.L., Jr.; Alves, J.P.; Santana, G.; Souza, J.O.D., Jr. Mineral nutrients and plant-fungal interaction in cocoa trees (Theobroma cacao L.). J. Braz. Chem. Soc. 2021, 32, 337–346. [Google Scholar] [CrossRef]
- Soares, T.P.; Pozza, E.A.; Pozza, A.A.; Mafia, R.G.; Ferreira, M.A. Calcium and potassium imbalance favours leaf blight and defoliation caused by Calonectria pteridis in Eucalyptus plants. Forests 2018, 9, 782. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, Y.T.; Wu, Y.Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.L. Plant disease resistance-related signaling pathways: Recent progress and future prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- Pervez, H.; Ashraf, M.; Makhdum, M.I.; Mahmood, T. Potassium nutrition of cotton (Gossypium hirsutum L.) in relation to cotton leaf curl virus disease in aridisols. Pak. J. Bot. 2007, 39, 529–539. Available online: https://www.pakbs.org/pjbot/PDFs/39(2)/PJB39(2)529.pdf (accessed on 5 December 2023).
- Graça, R.N.; Alfenas, A.C.; Maffia, L.A.; Titon, M.; Alfenas, R.F.; Lau, D.; Rocabado, J.M.A. Factors influencing infection of eucalypts by Cylindrocladium pteridis. Plant Pathol. 2009, 58, 971–981. [Google Scholar] [CrossRef]
- Maity, A.; Sharma, J.; Sarkar, A.; More, A.K.; Pal, R.K. Nutrient imbalance indices are closely related with susceptibility of pomegranate to bacterial blight disease. Sci. Hortic. 2016, 211, 79–86. [Google Scholar] [CrossRef]
- Gopi, R.; Madhavi, G.B.; Kapoor, C.; Raj, C.; Singh, S.; Ramprakash, T. Role of mineral nutrients in the management of plant diseases. In Plant Disease Management Strategies; Nehra, S., Trivedi, P.C., Eds.; Agrobios Research: Rajasthan, India, 2021; Chapter 4; pp. 87–117. [Google Scholar]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan: Leaf traits of Australian sclerophyll species. Funct. Ecol. 2003, 17, 10–19. [Google Scholar] [CrossRef]
- Prieto, I.; Querejeta, J.I. Simulated climate change decreases nutrient resorption from senescing leaves. Glob. Chang. Biol. 2020, 26, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Yao, X.; Li, J.; Deng, Q. Co-evaluation of plant leaf nutrient concentrations and resorption in response to fertilization under different nutrient-limited conditions. Diversity 2022, 14, 385. [Google Scholar] [CrossRef]
- Smith, S.; Hill, J. Supporting sustainable development–risks and impacts of plant industries on soil condition. Tech. Bull. 2011, 340, 1–27. Available online: https://industry.nt.gov.au/__data/assets/pdf_file/0005/233258/tb340.pdf (accessed on 23 November 2023).
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.M.; Yvin, J.C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef]
- Etienne, P.; Diquelou, S.; Prudent, M.; Salon, C.; Maillard, A.; Ourry, A. Macro and micronutrient storage in plants and their remobilization when facing scarcity: The case of drought. Agriculture 2018, 8, 14. [Google Scholar] [CrossRef]
- Achat, D.L.; Pousse, N.; Nicolas, M.; Augusto, L. Nutrient remobilization in tree foliage as affected by soil nutrients and leaf life span. Ecol. Monogr. 2018, 88, 408–428. [Google Scholar] [CrossRef]
- McIntire, C.D.; Huggett, B.A.; Dunn, E.; Munck, I.A.; Vadeboncoeur, M.A.; Asbjornsen, H. Pathogen-induced defoliation impacts on transpiration, leaf gas exchange, and non-structural carbohydrate allocation in eastern white pine (Pinus strobus). Trees 2021, 35, 357–373. [Google Scholar] [CrossRef]
- Gortari, F.; Guiamet, J.J.; Cortizo, S.C.; Graciano, C. Poplar leaf rust reduces dry mass accumulation and internal nitrogen recycling more markedly under low soil nitrogen availability, and decreases growth in the following spring. Tree Physiol. 2019, 39, 19–30. [Google Scholar] [CrossRef]
- Rahman, A.; Albadrani, G.M.; Waraich, E.A.; Awan, T.H.; Yavaş, İ.; Hussain, S. Plant secondary metabolites and abiotic stress tolerance: Overview and implications. In Plant Abiotic Stress Responses and Tolerance Mechanisms; Hussain, S., Hussain Awan, T., Ahmad Waraich, E., Iqbal Awan, M., Eds.; IntechOpen: Rijeka, Croatia, 2023; Chapter 3; pp. 133–206. [Google Scholar] [CrossRef]
- McMahon, P. Effect of nutrition and soil function on pathogens of tropical tree crops. In Plant Pathology; Cumagon, C.J.R., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 10; pp. 241–272. Available online: https://www.intechopen.com/chapters/34847 (accessed on 5 December 2023).
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Faisal Hayat, F.; Deng, L.; Narejo, M.; Bozdar, B.; et al. Micronutrients and their effects on horticultural crop quality, productivity and sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]




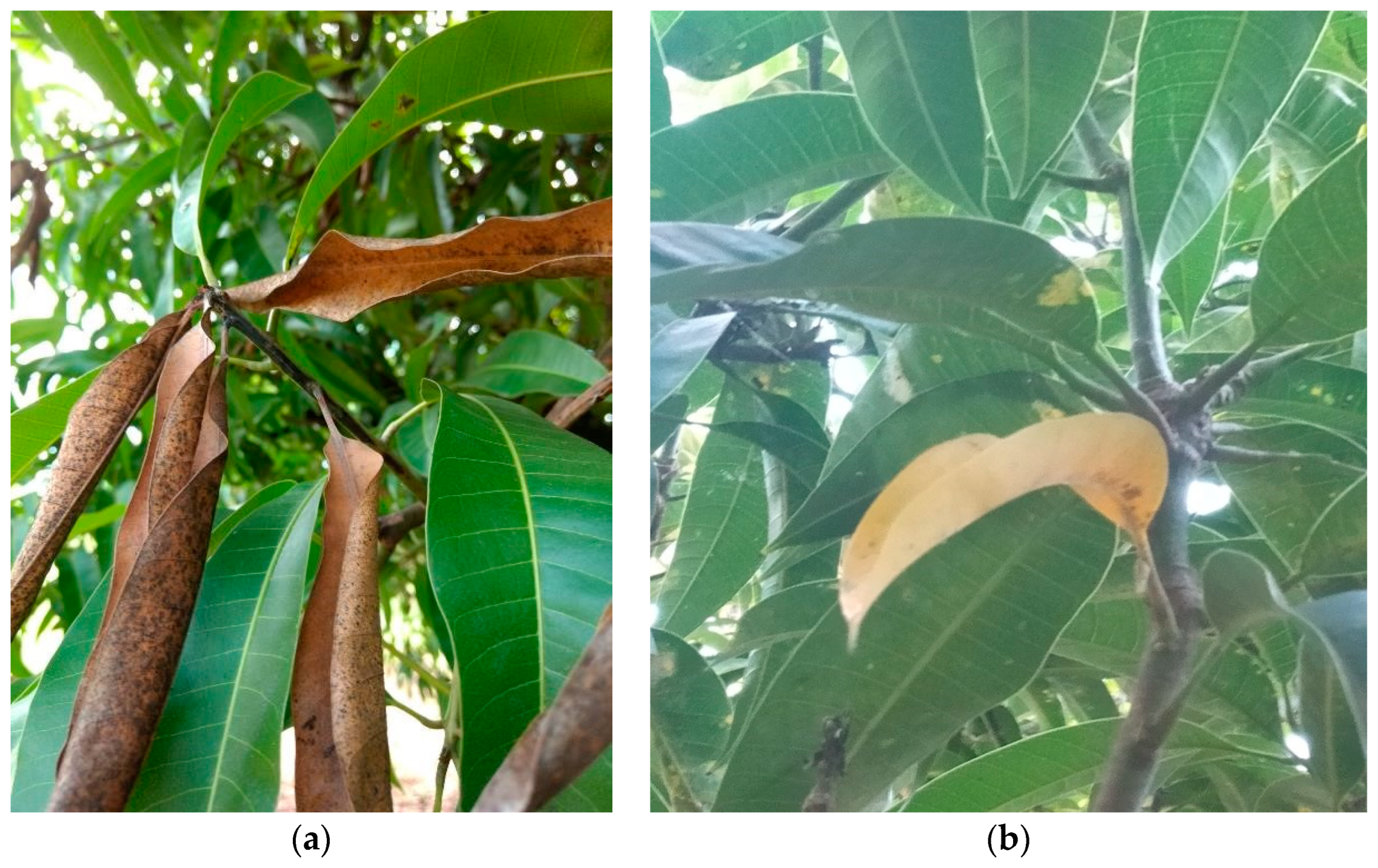
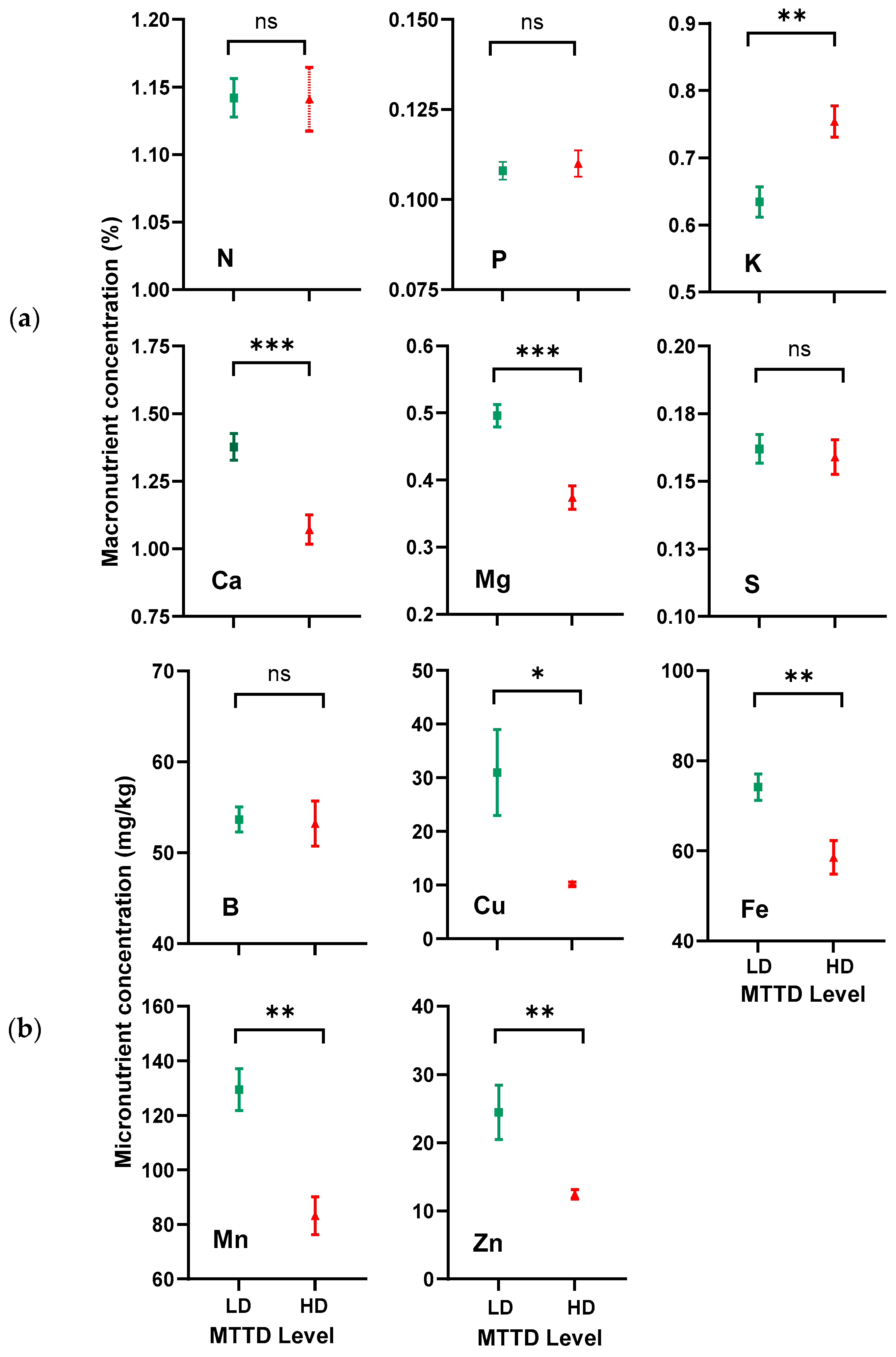
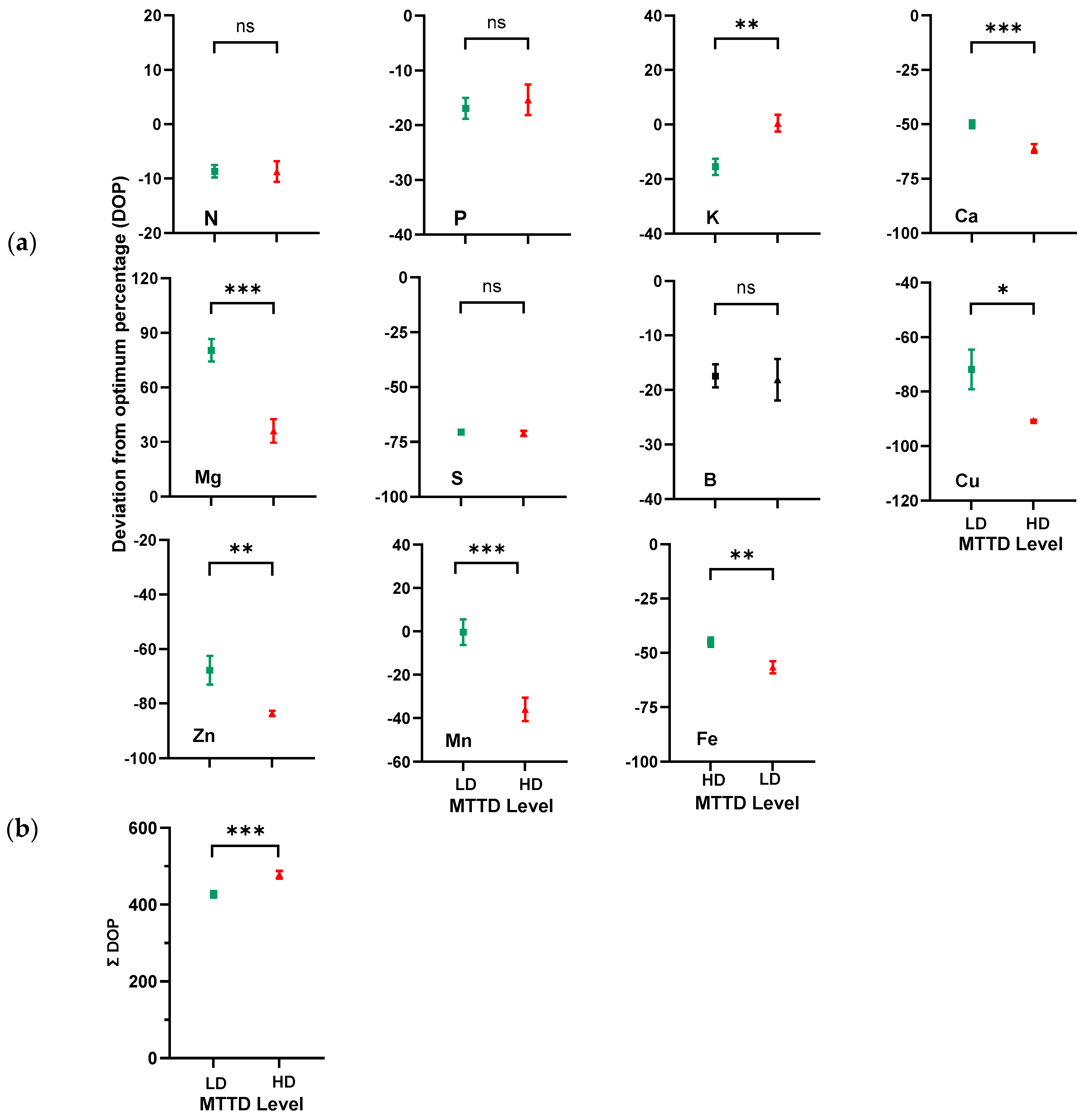
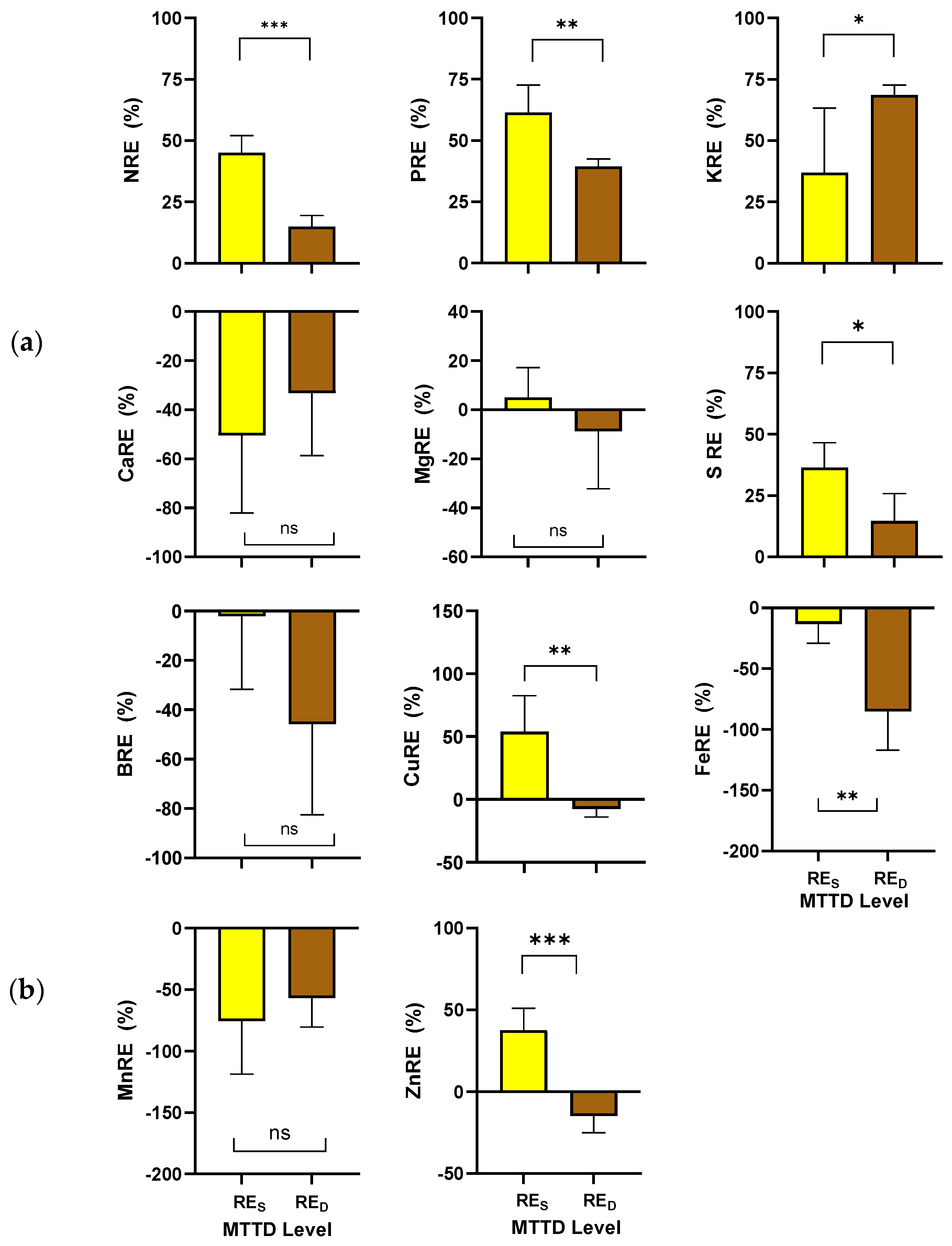
| Soil Properties | Dieback Level | p-Value | |
|---|---|---|---|
| LD | HD | ||
| Soil pH (H2O) | 7.36 ± 0.13 | 7.26 ± 0.05 | 0.49 ns |
| Organic C (%) | 1.06 ± 0.11 | 0.93 ± 0.04 | 0.33 ns |
| EC (dS/m) | 0.04 ± 0.008 | 0.03 ± 0.003 | 0.46 ns |
| Total available N (mg/kg) | 1.23 ± 0.36 | 2.42 ± 0.39 | 0.06 ns |
| Available P (mg/kg) | 10.80 ± 1.93 | 12.6 ± 2.44 | 0.57 ns |
| Available S (mg/kg) | 4.64 ± 1.71 | 5.22 ± 1.42 | 0.80 ns |
| Exchangeable K (cmol(+)/kg) | 0.16 ± 0.04 | 0.11 ± 0.01 | 0.23 ns |
| Exchangeable Ca (cmol(+)/kg) | 4.01 ± 0.55 | 3.66 ± 0.31 | 0.59 ns |
| Exchangeable Mg (cmol(+)/kg) | 2.70 ± 0.52 | 2.45 ± 0.27 | 0.68 ns |
| Extractable B (mg/kg) | 0.22 ± 0.02 | 0.21 ± 0.01 | 0.94 ns |
| Extractable Cu (mg/kg) | 2.19 ± 0.20 | 2.44 ± 0.31 | 0.54 ns |
| Extractable Fe (mg/kg) | 14.84 ± 0.75 | 16.26 ± 0.74 | 0.21 ns |
| Extractable Mn (mg/kg) | 6.74 ± 2.38 | 4.97 ± 0.66 | 0.49 ns |
| Extractable Zn (mg/kg) | 8.26 ± 4.37 | 0.93 ± 0.04 | 0.33 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asis, C.A.; Niscioli, A. Impact of Twig-Tip Dieback on Leaf Nutrient Status and Resorption Efficiency of Mango (Mangifera indica L.) Trees. Horticulturae 2024, 10, 678. https://doi.org/10.3390/horticulturae10070678
Asis CA, Niscioli A. Impact of Twig-Tip Dieback on Leaf Nutrient Status and Resorption Efficiency of Mango (Mangifera indica L.) Trees. Horticulturae. 2024; 10(7):678. https://doi.org/10.3390/horticulturae10070678
Chicago/Turabian StyleAsis, Constancio A., and Alan Niscioli. 2024. "Impact of Twig-Tip Dieback on Leaf Nutrient Status and Resorption Efficiency of Mango (Mangifera indica L.) Trees" Horticulturae 10, no. 7: 678. https://doi.org/10.3390/horticulturae10070678
APA StyleAsis, C. A., & Niscioli, A. (2024). Impact of Twig-Tip Dieback on Leaf Nutrient Status and Resorption Efficiency of Mango (Mangifera indica L.) Trees. Horticulturae, 10(7), 678. https://doi.org/10.3390/horticulturae10070678






