1. Introduction
To achieve higher-demand food production goals, agriculture will inevitably expand further into marginal, degraded lands, which are commonly characterized by poor soil structure, low fertility, and often soil salinity. Soil salinization has become a significant global environmental issue and is expected to worsen with projected climate change. The nature of soils and irrigation practice, crop type and phenology, climate-type and seasonal weather, and the duration of crop exposure together determine the extent and impact of salinity on plant growth and crop productivity, involving complex, multi-faceted plant tolerance traits. Soil is generally considered salt-affected when its electrical conductivity (EC) exceeds 4 dS·m
−1. At the plant level, salt toxicity occurs when Na
+ and Cl
– accumulate above the concentrations required [
1].
In higher plants, salinity can trigger hyperosmotic and hyperionic stress, leading to important morphological, physiological and biochemical alterations that, over time, may contribute to the eventual demise of the plant [
2]. The overaccumulation of Na+ results in an increase in the soil’s osmotic pressure, a decrease in water potential, and a reduction in root water uptake, consequently diminishing water availability and resulting in a slower rate of plant growth [
3,
4,
5]. The ion imbalance occurs when harmful amounts of salt accumulate in mature and older leaves, subsequently entering the xylem transpiration stream essential for maintaining the plant’s water balance. Excessive Na
+ accumulation can compete with the uptake of other major cations, such as K
+ and Ca
2+ [
6]. In susceptible species unable to efficiently regulate Na
+ transport, the ionic effect outweighs the osmotic one, reducing photosynthetic capacity and impairing the carbohydrate supply required by young growing tissues, further decreasing plant growth rate [
7]. Salinity can also cause a dramatic reduction in stomatal aperture and induce a stress-related decline in photosystem II (PSII) photochemistry, leading to PSII photoinhibition and/or photodamage [
8]. Additionally, reduced leaf expansion can result in a buildup of unused photosynthate in growing tissues, generating signals to downregulate photosynthesis. To cope with salinity, plants have evolved complex mechanisms to modulate ion homeostasis, ion compartmentalization and export, and osmolyte biosynthesis. Frequently observed metabolites with osmolyte functions include sugars, sugar alcohols, and complex sugars, whose biosynthesis and accumulation play important roles in maintaining homeostasis, osmotic adjustment, and cellular redox balance [
2].
Tomato (
Solanum lycopersicum L., formerly
Lycopersicon esculentum Mill.) is the most-consumed berry fruit worldwide, both as fresh and processed food, and is a key component of the Mediterranean diet [
9]. It is an excellent source of nutrients and bioactive compounds beneficial for human health and chronic diseases, including lycopene, a potent liposoluble antioxidant and anti-inflammatory agent. Native to western South America, the tomato’s wild relatives have adapted to severely saline coastal regions, which has contributed to the selection of genetic or physiological traits that ensure high fitness and stress tolerance. However, during domestication, modern varieties have lost much of their stress resistance. As a result, most commercial tomato varieties are classified as moderately salt-tolerant, with seed germination, plant growth, and fruit development being drastically affected by high salinity levels. This loss of stress resistance is due to varietal improvement programs that primarily focused on yield, market demands for shelf-life, fruit size, and organoleptic quality, often at the expense of relevant traits, such as stress tolerance/resistance [
10].
Fortunately, potential tolerance traits have been identified within ancestral tomato-related germplasm and landraces. Solanum pimpinellifolium L. (SP), the closest wild relative of the cultivated tomato, is a bushy plant with small red fruits about 1.5 cm in diameter and is found in the dry coastal regions of South America. In these areas, it frequently encounters brackish groundwater, salt-laden mist, and other harsh environmental conditions. Having evolved under such challenging conditions, SP demonstrates phenotypic robustness that was lost during the domestication of cultivated tomatoes. Furthermore, numerous quantitative trait loci have been identified in wild tomatoes, including those for biotic stress (tomato yellow leaf curl virus), abiotic stress (salinity), fruit quality, and other agronomic traits. As a result, SP is considered an important source of genes that can confer favorable stress tolerance to cultivated tomatoes.
Landraces are recognized as local community heritage [
11], with these diverse plant populations having been selected and maintained over time by traditional farmers to meet their social, economic, cultural and ecological needs. Moreover, they are often suited to low-input or organic farming due to their adaptability to local agroclimatic conditions [
12], and they currently play a key role in agrobiodiversity conservation. Thus, there is a growing emphasis on incentives and measures supporting the ex situ and in situ conservation and sustainable use of landrace germplasm to develop plants capable of counteracting future climate change [
13]. Ciettaicale (CE) is a landrace cultivated in the Basilicata region of southern Italy, where it faces intense drought fluctuations during summer and brackish water from aquifers. Despite these harsh environmental conditions, CE demonstrates a notable capacity for adaptation. Compared to other commercial tomato genotypes, this landrace has shown interesting tolerance to salt at germination and post-germination stages [
14] and the early vegetative stage [
15] and has achieved a tolerable balance between yield and fruit quality under moderate salinity stress [
16].
Given that the cultivation of food crops is encouraged on marginal, degraded lands abandoned by agricultural users, it becomes imperative to select species based on their ability to thrive under unfavorable conditions. Therefore, this study aimed to scrutinize the biometric and physiological performance of fully mature tomato plants belonging to a commercial variety, an ancestral wild relative, and a landrace. We assessed their responses to short-term exposure of sodium chloride (NaCl) and examined their capacity for recovery upon cessation of stress.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
In the present study, a standard tomato variety, a traditional southern Italian landrace, and a wild species were used as genetic material. Specifically, the following genotypes were selected: the standard commercial variety “Moneymaker” (Thompson and Morgan, Ipswich, UK), an indeterminate type of tomato that can grow up to 2 m tall and produces rounded, vibrant red fruits; the landrace “Ciettaicale” (De Angelis S.r.l., Tolve, Italy), hailing from the Basilicata region, which is an indeterminate growth habit tomato with pear-shaped or globose fruits; and Solanum pimpinellifolium L. LA1579 (Tomato Genetics Resource Center, Department of Plant Sciences, University of California-Davis, CA, USA), chosen as the closest wild relative of the cultivated tomato. This wild species has a smaller stature and produces diminutive, round fruits. Its ability to hybridize with cultivated tomato varieties makes it a valuable source of resistance traits within breeding initiatives.
The research was carried out at the Department of Agriculture, Food, and Environment, University of Pisa, Italy. Seeds were germinated in rockwool propagation cube (Grodan
®, Roermond, Netherlands, Ø 25 mm) receiving a basal nutrient solution, as described in Kiferle et al. [
17]. The EC and pH of the nutrient solution were mantained between 2.3–2.6 dS m
−1 and 5.5–5.8 (adjusted with diluted H
2SO
4), respectively. After 10 days, selected uniform seedlings were transferred to larger rockwool cubes (Grodan
®, 133 × 133 × 160 mm; 2 rockwool cubes per plant) within an open hydroponic system. Sixty-four seedlings of each genotype were arranged in a randomized block design on benches and fertigated with the basal nutrient solution for three weeks. Throughout the experiment, the pots were rotated within their blocks at one-week intervals to minimize the confounding effects of external factors. Irrigation was supplied to the plants for 1 min 4 times per day. No shoot pruning or inflorescence thinning was performed during the entire experiment.
After five weeks, plants were divided into four groups (16 plants for each genotype × treatment). The nutrient solution system encompassed a non-treated control (S0) and three increasing salinity levels, with NaCl concentrations of 120 (S120), 240 (S240), and 360 mM (S360). These concentrations represented moderate to severe salinity stress for the species based on preliminary observations. Salinity stress was incrementally increased by approximately 6.3 mS cm−1 (equivalent to 60 mM NaCl) daily to prevent osmotic shock, reaching the final concentrations within two, four, and six days, respectively. Once the highest salt concentration was achieved, the plants were subjected to salt stress for one week. Following this period, all rockwool cubes underwent a 10 min irrigation with tap water to wash off previous nutrient solutions and then were fertilized with the basal nutrient solution for an additional recovery week. Climatic parameters were continuously monitored using a weather station inside the glasshouse. The mean values for air temperature, relative humidity and daily global radiation were 21.7 °C, 72.6%, and 21.8 MJ m−2, respectively.
2.2. Biometric and Cations Analyses
Biometric traits were evaluated at the end of the treatment (7 days after treatment, DAT) through destructive measurements. For each treatment, 10 plants were harvested, and the epigeal fraction was separated and washed with water to remove dust and debris. Fresh weight (FW) was recorded, after which the samples were dried for at least 72 h at 60 °C and then weighed to determine dry weight (DW). Additional sets of plants, not used for biometric trait testing, were collected and immediately processed or ground in liquid nitrogen, then stored at −80 °C for further molecular and biochemical analyses.
The content of cations, including Na
+, K
+, Ca
2+, Mg
2+, Fe
2+, Zn
2+, Cu
2+, and Mn
2+, were determined as described by Moles et al. [
15].
2.3. Chlorophyll a Fluorescence
Chlorophyll
a fluorescence was measured using a pulse-amplitude modulated fluorometer (Mini-PAM; Heinz Walz GmbH, Effeltrich, Germany), as previously described [
18]. Briefly, measurements were taken at growing light intensity of 1500 μmol m
−2 s
−1 on fully expanded leaves to monitor the effects of salinity during the application of stress (1, 4 and 7 DAT) and after 7d of recovery. The actual photochemical efficiency of photosystem II in the light (Φ
PSII) was determined as Φ
PSII = (Fm’ − F’)/Fm’ at steady state, where Fm’ is the maximum fluorescence yield emitted by the leaves after a saturating light flash during actinic light exposure, and F’ is the fluorescence yield emitted under actinic illumination.
2.4. Relative Water Content and Leaf Water Potential
Relative water content (RWC) was determined at 4 and 7 DAT according to Barrs and Weatherley [
19] using mid-leaf section of fully expanded leaves from at least nine replicates. Tissue FW was recorded, and leaf samples were transferred to tubes with de-ionized water and kept overnight in the dark at 4 °C. On the second day, after carefully removing excess water from the leaf surfaces, turgid weight (TW) was recorded. The leaves were then dried at 80 °C for 24 h, after which they were reweighed to determine DW. RWC was calculated using the following equation: RWC (%) = (FW − DW)/(TW − DW) × 100.
Leaf water potential (ΨW) was measured before dawn (pre-dawn, pd) on three fully expanded leaves of similar age situated on the median portion of stems and well exposed to light using a Scholander-type pressure chamber.
2.5. Analysis of Pigments
Pigments were extracted by incubating tissues (~100 mg) in 1.5 mL 80% acetone for 1 week at 4 °C in darkness. The absorbance of extracts was measured spectrophotometrically at 470.0, 663.2, and 646.8 nm. These absorbance values were used for calculation of chlorophyll
a (Chl
a), chlorophyll
b (Chl
b), and total carotenoids (Car) contents [
20].
2.6. Soluble Carbohydrate Quantification
Leaf samples of 100 mg FW were ground to a powder in liquid nitrogen and then extracted and assayed using coupled enzymatic assay methods to ascertain the increase in A
340, as described by Pompeiano et al. [
18]. The method’s accuracy was verified using known amounts of standards.
Extraction performance was evaluated through a recovery experiment. Glucose (Glc), fructose (Fru), and sucrose (Suc) were added twice as standards to the sample before extraction. Depending on the sugar, the recovery percentage ranged between 96% and 103%. The amount of soluble carbohydrates was adjusted based on the recovery results and expressed as µmol hexose equivalents per g FW.
2.7. Statistical Analysis
After performing the Shapiro–Wilk test to diagnose normality assumptions, linear mixed-effects models were used to control the effects of experimental runs and blocks (i.e., random variables) while testing the effects of genotype, treatment, exposure and/or recovery time, as well as their interactions, on all response variables. To achieve this, the “lmer” function implemented in the
lme4 R package [
21] was used. The
lmerTest package was employed to estimate
p-values for each factor in the model, applying the Satterthwaite approximation for the denominator degrees of freedom or the
F-statistic [
22]. Statistically different means in the other response variables were identified by Tukey’s HSD via the
multcomp package [
23], with probability levels lower than 0.05 considered significant.
To identify relationships among the experimental conditions (species by treatments) based on biometric and physiological data recorded during the time-course experiment, multiple factorial analysis (MFA) was utilized, implemented in the
FactoMineR R package [
24]. MFA was performed in two steps, as described by Pompeiano et al. [
20]. The individual data sets were then projected onto the global analysis to examine communalities and discrepancies. Traits (biomass, cations, PSII efficiency, RWC and LWP, nonstructural carbohydrates, and pigments) that significantly contributed to MFA dimensions were used to explain differences among experimental conditions (
α = 0.05). All computations were performed with R 4.3.2 [
25], and the R package
ggplot2 [
26] was used for data visualization.
4. Discussion
Tomato stress tolerance is recognized as a developmentally regulated, state-specific phenomenon. For instance, stress tolerance during tomato germination appears to be distinct from stress tolerance during vegetative growth stages. Building upon evaluations of biometric and eco-physiological performances during germination and post-germination stages [
14], early growth stages [
15], and characteristics related to yield and fruit quality [
16], this study investigated the response of three distinct tomato genotypes to escalating salinity levels. Our focus was on assessing biometric changes and time-dependent alterations in physiological traits during fully mature stage.
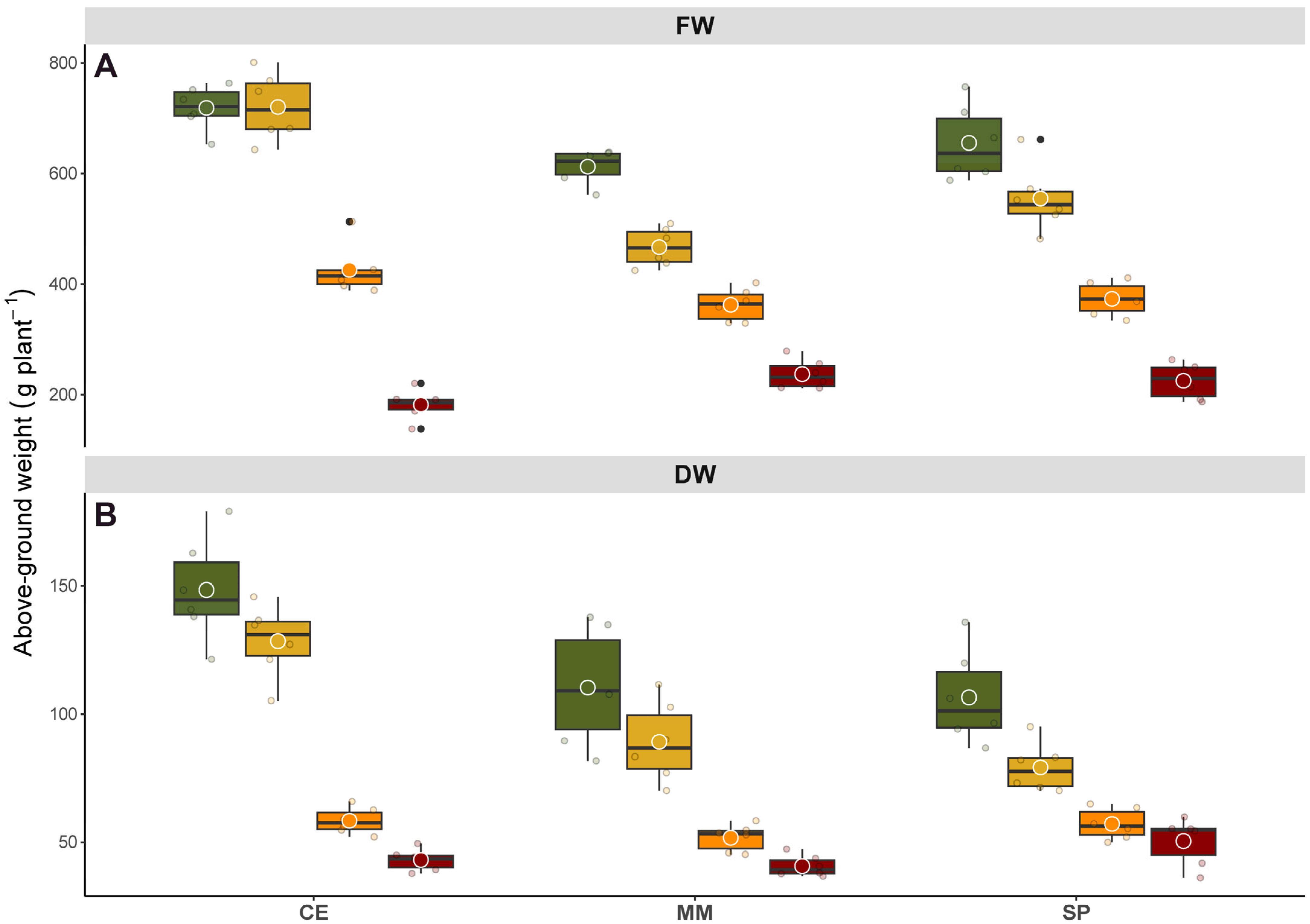
Our data underscore the tomato clade’s capacity to withstand short-term exposure to low and intermediate salinity stress, as well as its ability to recover once stress is removed, albeit inter- and intraspecific variations in morphological and physiological responses have been clearly observed. It is important to note that direct comparisons with prior research, particularly studies characterizing species exposed to salinity across different growth and developmental stages under controlled environmental conditions, may not be feasible due to the unique parameters of our experiment. In a previous study examining early tomato growth stages [
15], irrigation with 300 mM NaCl was found to stimulate dry matter production compared to untreated plants, with more pronounced effect observed in CE over a commercial variety. In contrast, each salinity level in our study negatively impacted biomass production, even over a short period (
Figure 1). Notably, even the lowest salt concentration (120 mM NaCl) led to a significant reduction in biomass after prolonged exposure, likely due to the initial and transient osmotic stress following salt shock. Interestingly, CE appeared less affected, displaying a characteristic response observed in halophyte species under salt stress and drought conditions. Under moderate salinity stress, enhanced synthesis of root cytokinins has been reported to alter the hormonal balance in tomato shoots [
27], resulting in a higher root-to-shoot biomass ratio due to restricted aboveground growth and a greater allocation of photosynthates to belowground organs to enhance scavenging capacity and alleviate osmotic pressure [
28]. This adaptive root system architecture modulation, driven by negative halotropism under abiotic stress, representing a crucial strategy for enhancing plant water usage and stress resilience [
29]. This may elucidate the severe reduction in above-ground biomass observed across all genotypes when exposed to 240 mM NaCl. At the highest salinity levels, as ion concentrations surpass a threshold, the plant’s exclusion mechanism fails, leading to stunted growth and eventual decline due to induced low osmotic potential, nutritional imbalance, specific ion effect, or a combination thereof [
30].
Ion exclusion serves as a crucial mechanism for plants to adapt to saline stress, primarily by preventing the accumulation of Na
+ ions in the shoots and leaves to toxic levels. Under control conditions, SP and CE exhibited lower ions levels compared to MM (
Table 1). Interestingly, these concentrations remained relatively stable even under low stress conditions, indicating the enhanced ability of these genotypes to maintain ionic balance. This resilience could be attributed to improvements in cellular uptake, sequestration, and both ion inclusion and exclusion mechanisms. A similar trend was observed in the K
+/Na
+ ratio, a key indicator of salt tolerance closely linked to efficient compartmentalization and/or exclusion of ions [
31,
32]. This underscores the sensitivity of MM to salinity stress. Furthermore, the higher concentration of Ca
2+ and Ca
2+/Na
+ ratio in CE under low salinity levels stood out its superior salt tolerance. Given that Ca
2+ plays a pivotal role in determining a plant’s salt sensitivity and the associated ratio reflects the plant’s ability to survive osmotic stress [
33], these findings highlight the robustness of CE in adverse conditions.
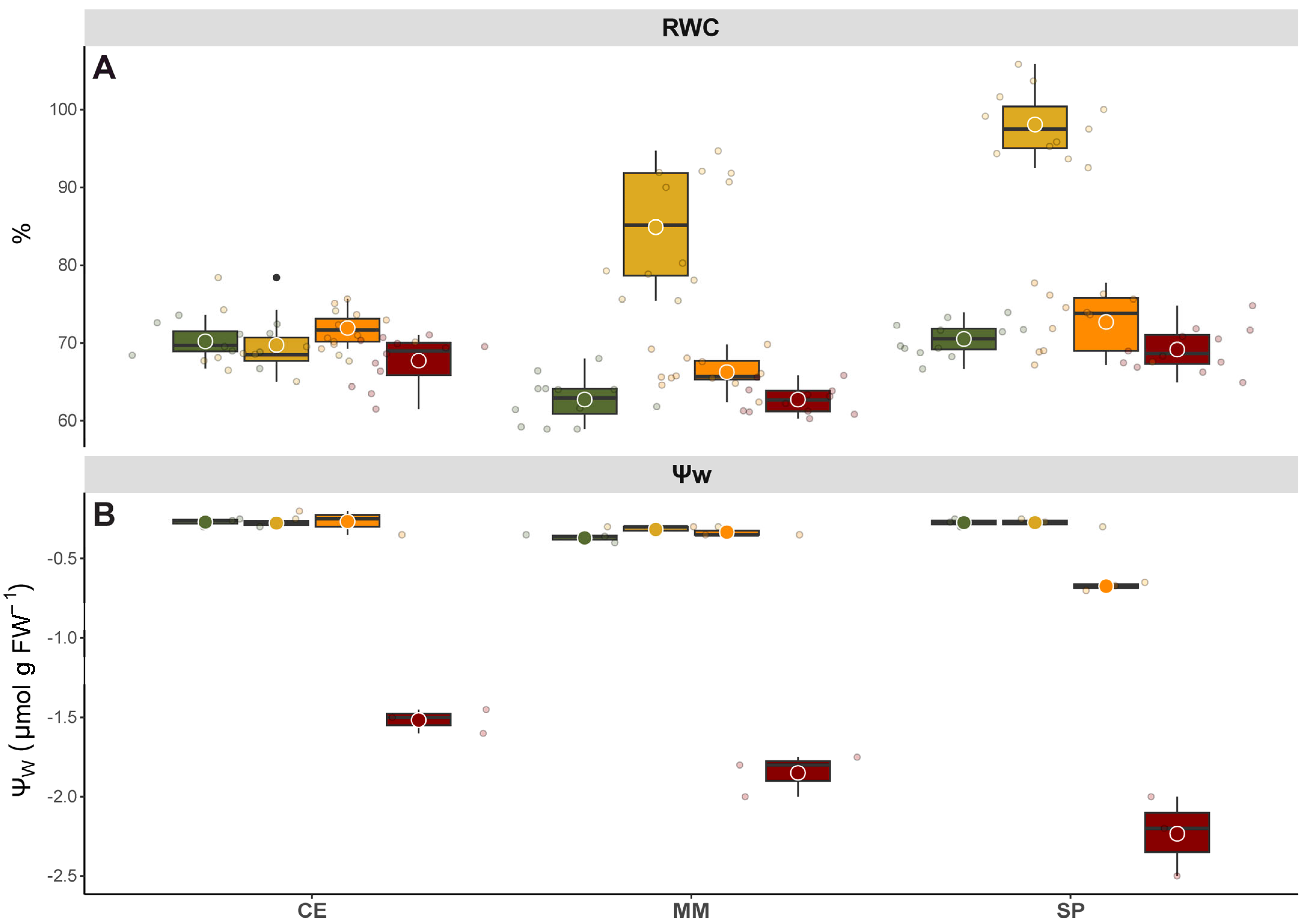
Relative water content serves as a valuable indicator of a plant’s water status, offering insights into leaf survival and metabolic activity, making it a key parameter for comparing plants’ sensitivity and tolerance to salinity stress [
34]. In this study, it was observed that MM and SP exhibited increased RWC only under low salinity levels, suggesting the activation of specific pathways geared towards enhancing salinity stress tolerance (
Figure 3). Conversely, RWC in CE remained unaffected by any treatment, implying that its unique salt tolerance might stem from environmental adaptations developed in response to saline soils in the Mediterranean region. Furthermore, the consistent RWC in CE could be attributed to its superior K
+/Na
+ ratio under stress conditions compared to other genotypes.
Unlike other water status indicators, Ψ
W provides a genuine measure of soil–plant–atmosphere balance, offering valuable insights into plant water relationships. In this study, Ψ
W data revealed markedly differences among genotypes, suggesting different strategies to cope with stress (
Figure 3). Specifically, SP exhibited higher salt sensibility, with a decrease in Ψ
W observed from intermediate stress levels onwards, likely as a mechanism to alleviate osmotic imbalances and maintain cell turgor. In contrast, halophytes and salt-tolerant species synthesize organic solutes for osmotic adjustment within the vacuole, representing a more energy-efficient strategy [
35]. Additionally, the sustained high Ψ
W observed in CE under the highest salinity level may be attributed to dehydration avoidance mechanisms [
30].
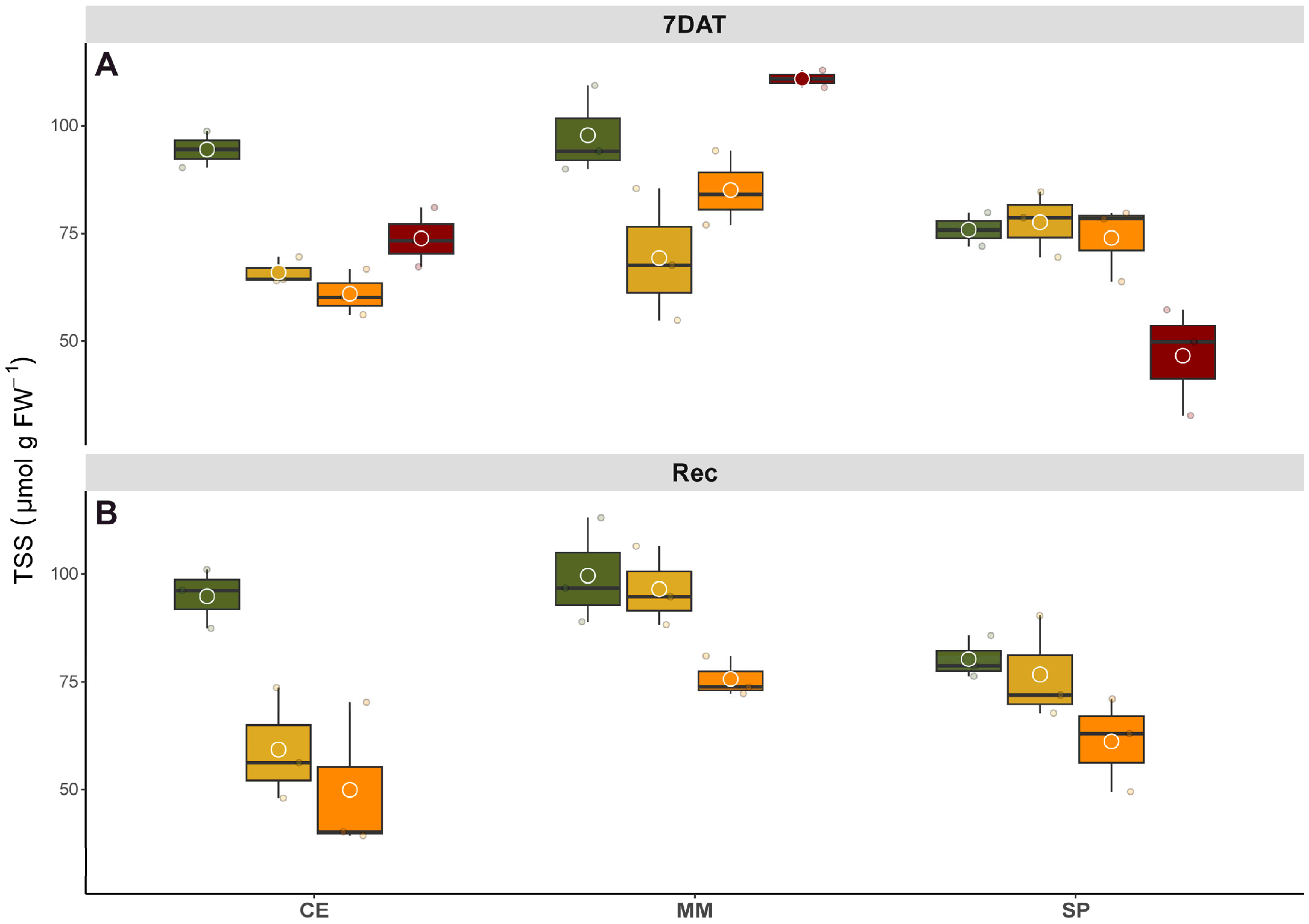
In this study, we investigated the levels of carbohydrate in leaves both at the conclusion of the salinity treatment and during the recovery phase, aiming to provide a comprehensive understanding of the plants’ condition. Consistent with previous research, changes in carbohydrate metabolism appear to be a widespread response, potentially enhancing tolerance through enhanced catabolism of compatible osmolytes (
Figure 4). Soluble carbohydrates may interact with membrane phospholipids and proteins to stabilize their structures, thereby mitigating desiccation caused by salinity, drought, and freezing stress [
20], while also stimulating the activity of antioxidant enzymes [
36,
37]. Here, the heightened TSS catabolism observed in CE and MM at the onset of the stress may be indicative of efficient physiological and metabolic plasticity. Conversely, the increase in TSS recorded in MM from the intermediate salinity levels onwards and in CE at highest salinity level could be attributed to sink-limitation consequences, as previously observed in stressed
Paspalum vaginatum (Swartz) plants [
20]. Concerning SP’s response to increasing salinity, this genotype exhibited an opposing adaptation strategy, maintaining control of sugar homeostasis in the source tissues until intermediate salinity levels. However, exposure to the highest salt treatment led to dramatic changes in plant metabolism for SP. Upon removal of the stress, MM and SP under low salinity treatment had fully recovered their TSS levels within a week, underscoring the prompt osmotic adjustment ability of these genotypes. Conversely, CE subjected to salt treatment still exhibited distressed TSS levels after the recovery period, suggesting ongoing carbohydrate translocation and metabolism.
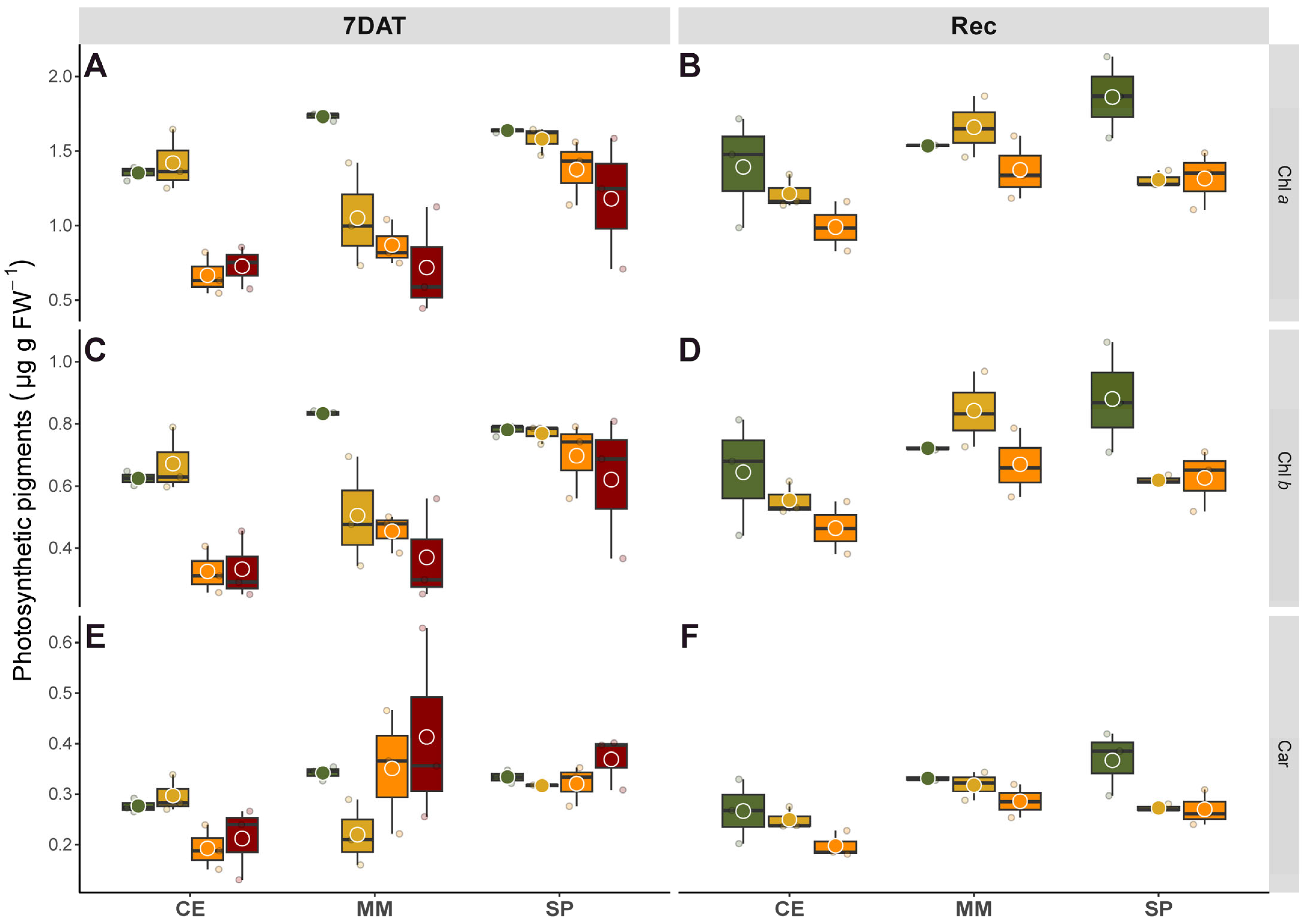
The response of pigments to salinity markedly differed among genotypes, revealing distinct defense mechanisms (
Figure 5). Typically, photosynthetic pigments in tomato gradually decrease in response to increasing salinity, correlating with oxidative damage that induces chlorosis [
38,
39]. Both ionic and oxidative stresses contribute to Chls degradation due to ROS-induced higher chlorophyllase activity under salinity conditions [
40]. Overall, we observed reductions in Chl contents parallel to the decay of both Chl
a and
b, with the latter generally exhibiting more stability in plants tolerant to salinity stress [
41]. Consistent with previous findings [
15], the landrace CE showed a gradual reduction in Chl
a and
b along to salt gradient, albeit less pronounced than in the commercial MM. In fact, the decline in Chls content in MM was quite abrupt even with moderate salt levels, correlating with its higher Na
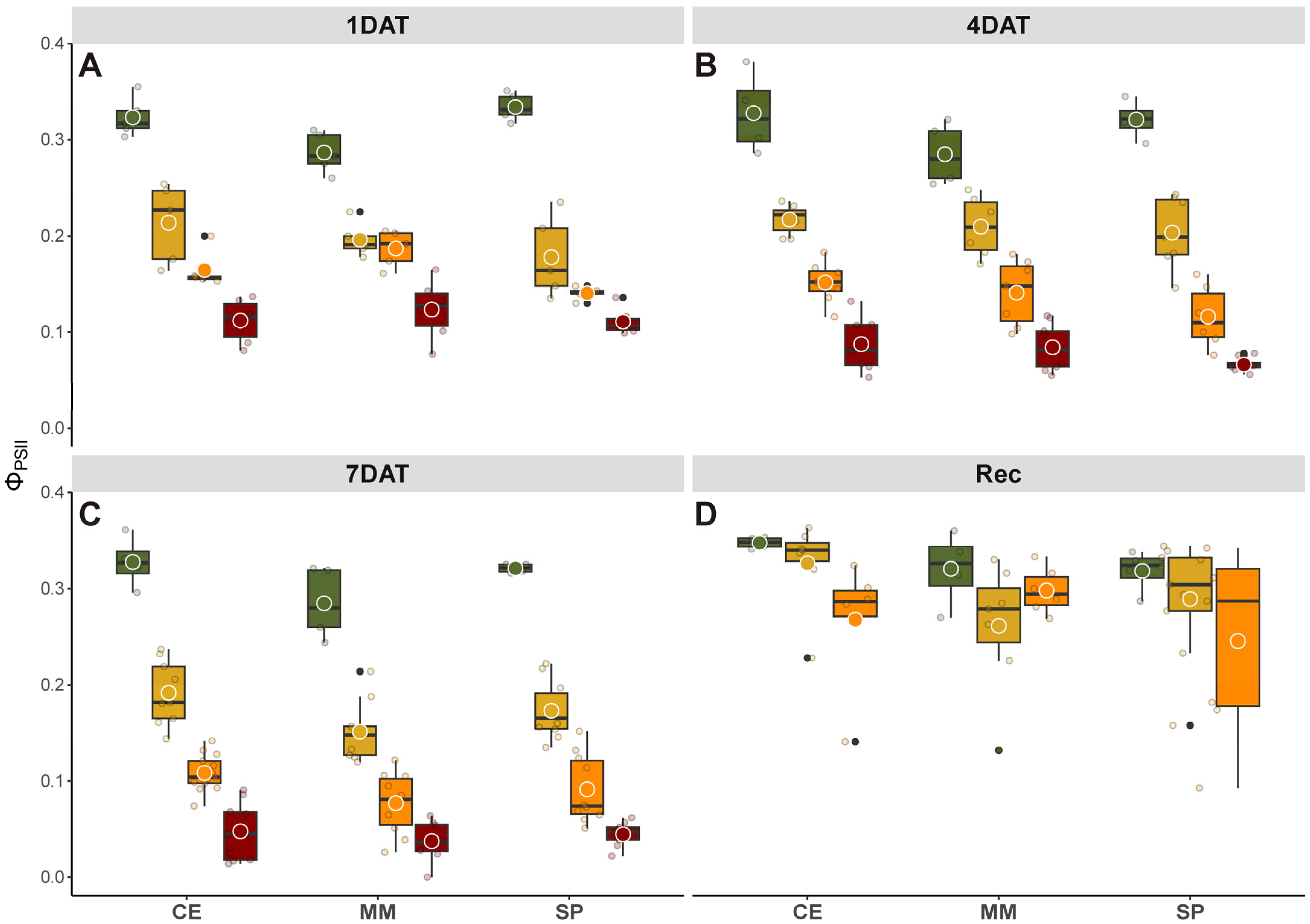
+ accumulation compared to CE and SP, highlighting MM’s salt-hypersensitive behavior. Changes in Chls content, whether due to slow synthesis or fast breakdown, are normally part of a defense photoprotection mechanism aimed at limiting light absorbance and thereby affecting photosynthesis. Correspondingly, post-recovery, salinity affected Φ
PSII with variations between genotypes, where CE and SP displayed similar photosynthetic performance but better than MM under salt stress (
Figure 2). Carotenoids act as antioxidants, scavenging ROS, and function in photosynthesis as collectors and quenchers, favoring the dissipation of excess radiant energy and preserving PSII activity [
42]. Moreover, changes in the ratio between pigments might reflect rearrangements in the stoichiometry of PSII core and its associated light harvesting complexes (LHCII), promoting excessive energy dissipation [
20], thus serving as stress indicators. The pronounced salt-induced increase in Car/Chls observed in MM (+71% on average under intermediate and higher salinity treatments compared to the other two genotypes, data derived from
Figure 5) confirmed its hypersensitivity to salinity. In contrast, the lower ratios in CE and SP suggested greater salt tolerance in these genotypes, with more efficient antioxidant mechanisms protecting photosynthesis.
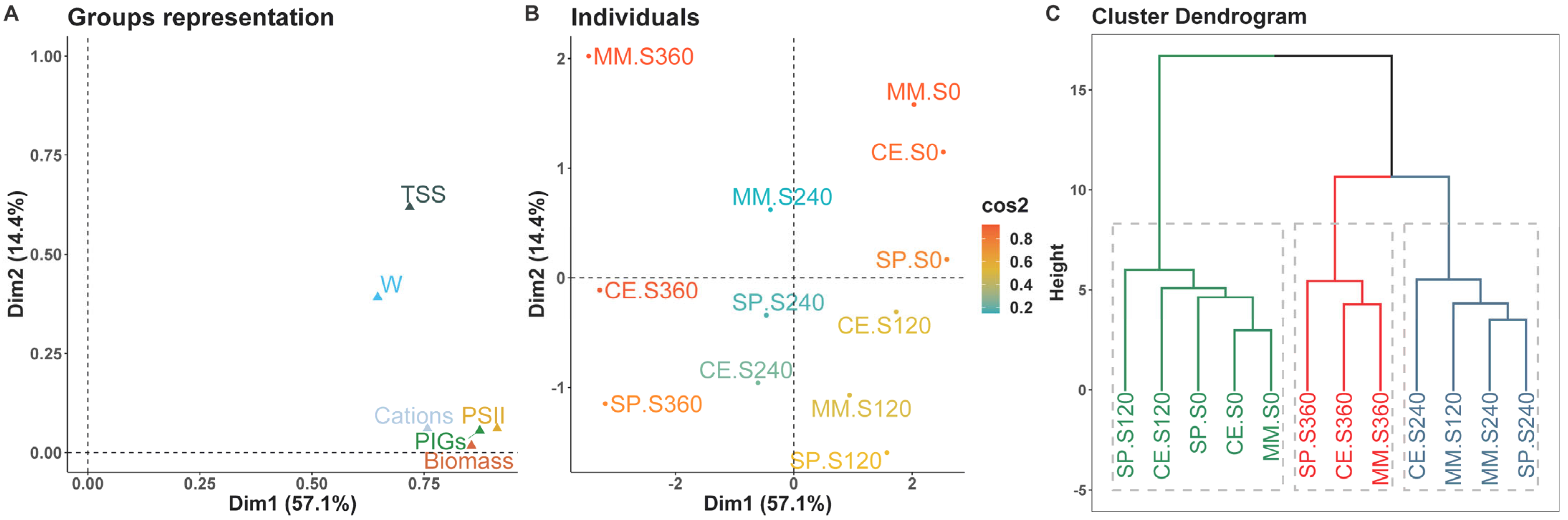
MFA facilitated the examination of observations based on biometric and physiological data within a unified framework, providing a cohesive representation of the associations between variables recorded throughout the time-course experiment (
Figure 6). The use of the multicanonical analysis emphasized a general equilibrium among the genotypes under control and low salinity level, with the exception of the commercial variety, wherein the complexity of salt stress response hindered the introgression of tolerance traits. Particularly under high salinity levels, MM shared fewer similarities with the other two genotypes.
Our previous studies, conducted at various growth stages of the species, suggest that utilizing tomato landrace germplasm can be an effective strategy to counteract detrimental environmental factors, including salinity. While genetic variation for salt tolerance in wild germplasm and landrace populations remains largely unexploited, the present research underscores that improved salt tolerant genotypes can lead to substantial, positive impacts on horticultural production. The salt tolerance mechanism of domesticated tomato (exhibiting a glycophytic-like response) demonstrates efficiency under mild stress conditions but proves inadequate at higher salinity levels.