Abstract
Heterodera schachtii (Schmidt, 1871) (Nematoda: Heteroderidae) is one of the most widespread plant-parasitic nematodes (PPNs) associated with cabbages, which cause severe yield losses in cruciferous vegetables. This study aimed to improve the current understanding of the prevalence and detection of H. schachtii in the cabbage-growing areas of Niğde Province, Türkiye. Field surveys were conducted between November and December 2021, and 100 soil samples were collected immediately after cabbage harvesting. Heterodera schachtii populations were identified by morphological and morphometric methods along with Internal Transcribed Spacer of the ribosomal region (ITS-rDNA) and Cytochrome Oxidase Subunit (COI-mtDNA) sequencing. The mean body length of H. schachtii was 463 ± 7 μm, while stylet and hyaline length ranged between 20.7–27.8 μm and 20.1–32.1 μm, respectively. Nearly half of the surveyed areas were infested with H. schachtii with a 41% incidence rate. However, the Merkez District had the highest proportion of infested fields with an over 51% incidence rate. The population density was determined in 41 samples with a mean of 79.5 cysts per 250 g of soil. These results will help to determine the control and management strategies of H. schachtii.
1. Introduction
Cabbage, Brassica oleracea var. capitata (L.) (Brassicales: Brassicaceae) is one of the most important vegetable crops widely produced and consumed worldwide. Türkiye, with an 860 kt annual production capacity, is one of the leading cabbage producers and exporters in the world [1]. Among the cabbage production centers of Türkiye, Niğde province is the second-largest producer of cabbages due to favorable climatic conditions [2]. However, numerous pests and pathogens reduce the yield and marketable value of cabbages, including sugar beet cyst nematodes, Heterodera schachtii (Schmidt 1871) (Nematoda: Heteroderidae). Heterodera schachtii is a common PPN worldwide [3]. However, H. schachtii tends to occur more frequently in temperate climates, and less commonly in subtropical–tropical climates [4]. The nematode has been recorded in Africa, Australia, Europe, the Middle East, China, Türkiye, and America [4,5,6,7,8]. Even though H. schachtii is primarily a sugar beet pathogen and called the beet cyst nematode (BCN), it can reproduce on more than 200 plant species including brassica crops (e.g., Brassica napus, Brassica oleracea, Brassica rapa, Raphanus sativus and Sinapis alba (Brassicales: Brassicaceae), chenopod crops (e.g., Spinacia oleracea), and amaranth crops (e.g., Beta vulgaris) (Caryophyllales: Amaranthaceae) [9,10,11]. Heterodera schachtii can inflict considerable damage to Brassica crops, causing yield losses of up to 50% [4,9].
The symptoms of H. schachtii on crops are generally stunted, yellow-colored leaves, a reduction in size, and the more intense formation of fibrous root systems [3]. While the impact of H. schachtii on cabbage production has not been fully defined, Abawi and Mai [9] reported that the nematode can reduce the marketable head weight of cabbage from 42 to 54%. For efficient management of H. schachtii, knowing the phylogenetic relationships of populations has potential importance in management. The availability of molecular analyses, such as Internal Transcribed Spacer of the ribosomal region (ITS-rDNA) and Cytochrome Oxidase Subunit (COI-mtDNA) sequencing, has enabled the determination of phylogenetic links and reconstruction cyst nematode phylogenetic trees [12,13]. In addition, H. schachtii, which is under the schachtii group, can be separated from the closely related species [Heterodera trifolii, (Goffart, 1932), Heterodera glycines (Ichinohe, 1952), Heterodera betae (Wouts Rumpenhorst and Sturhani, 2001) (Nematoda: Heteroderidae) and others] by morphological and morphometric characteristics. This study is aimed at determining the distribution, prevalence and population density of H. schachtii and to identify and investigate the phylogenetic connections of the H. schachtii populations in the Niğde province, the second-largest cabbage-growing area in Türkiye.
2. Materials and Methods
2.1. Nematode Population Collection
In total, 100 soil samples were collected from the cabbage fields in Merkez, Bor, and Ulukışla districts of Niğde Province after the harvest of the crops in 2021. Sampling consisted of a randomized collection of soil (soil weight of 250 g) from each field (Figure 1). Five 50 g subsamples were taken in a zigzag pattern across the field, with about 5–8 m between the sampling points. Cysts were extracted from 250 g soil samples using a modified sieving–decanting method [14]. Cysts were collected under a stereomicroscope (Zeiss, Munich, Germany) for further observations and measurements.
Figure 1.
The map of the sampling areas.
2.2. Morphological Studies
For morphological characterization, the under-bridge shape of the fenestra area and bullae were used as a reference. The fenestra length, semi-fenestra width, vulval bridge width, and vulval slit length were used for morphometric identification of the cysts. The morphometric ratios (a, b, and c) published by Handoo [15] and Subbotin et al. [16] were used as a standard reference. Heterodera schachtii J2s and cysts were compared with descriptions and identification keys of the previously published ones by Handoo [15], Franklin [17], and Mulvey and Golden [18]. A Leica light microscope model DM5500 B (Leica, Wetzlar, Germany) equipped with a Leica DFC295 optic camera was used to examine and photograph the samples. Leica software version 4.1.0 was used to record and analyze all measurements.
2.3. Molecular Identification
For DNA extraction, a single full cyst was randomly selected from each of 12 H. schachtii representative samples from three districts (Merkez, Bor, and Ulukışla), and the DNA of each population was extracted according to Holterman et al. [19]. The ITS1-5.8S-ITS2 of rRNA was amplified using the forward primer TW81 (5′-GTT TCC GTA GGT GAA CCT GC-3′) and the reverse primer AB28 (5′-ATA TGC TTA AGT TCA GCG GGT-3′) [20]. For amplification of the partial mtDNA-COI gene, the forward JB3 (5′-TTT GGG CAT CCT GAG GTT TAT-3′) and reverse JB5 (5′-AGC ACC TAA ACT TAA AAC ATA ATG AAA ATG-3′) primers were used [21,22]. The polymerase chain reaction (PCR) program was set for the ITS primers as follows: 95 °C for 5 min; 35 cycles at 94 °C for 60 s, 55 °C for 45 s, and 72 °C for 120 s; and a final extension at 72 °C for 10 min. For COI primers, the program was set at 95 °C for 10 min; 40 cycles at 95 °C for 60 s, 41 °C for 45 s, and 72 °C for 120 s; and a final extension step at 72 °C for 10 min.
Products of the PCR were visualized through agarose gel electrophoresis, following a typical protocol, and sequenced by a commercial sequencing company (Macrogen Inc., Seoul, South Korea). The obtained sequences of the COI and ITS were blasted in NCBI GeneBank to identify the nematodes (www.ncbi.com) (accessed on 12 March 2024). MEGA software (version 7) was used to perform phylogenetic analysis on the obtained sequences, as well as the reference sequences and nucleotides from various countries available in GenBank [23]. The sequences of OP028952, OP028953, OP028954, OP028955, OP028956, OP028957, OP028958, OP028959, and OP028960 were included in the ITS analysis, whereas sequences of OP036613, OP036614, OP036615, OP036616, OP036617, OP036618, OP036619, OP036620, OP036621, OP036622, OP036623, and OP036624 were used in the COI locus analysis, respectively. Tamura and Nei [24]’s model was used to construct a tree by the neighbor-joining method with 1000 bootstrap replicates.
3. Results
The H. schachtii cysts were detected in 41 out of the 100 soil samples collected from the surveyed cabbage fields (Table 1). The prevalence in Merkez, Bor, and Ulukışla districts were 51%, 33%, and 42%, respectively. The determined infested samples with H. schachtii showed an average of 79.5 cysts per 250 g of soil (Table S1).

Table 1.
The prevalence and density of Heterodera schachtii in cabbage fields sampled in three districts of Niğde Province, Türkiye in 2021.
3.1. Morphological Identification
All the cysts of H. schachtii that were examined and identified were lemon-shaped, light to dark brown, and had zigzag ridges (Figure 2). The morphological characteristics and morphometric measurements of the J2s and cysts of H. schachtii are shown in (Figure 2 and Figure 3). Thirty cysts and J2s were used in morphological studies. The cyst length and width of H. schachtii populations varied from 603–987 μm to 405–689 μm, respectively. The fenestra length and width were 31–56 μm and 21–25 μm, respectively. The cysts vulval bridge width varied from 4.3 to 8.8 μm. The morphometric measurements of the cysts were consistent with the previous studies and are given in Table 2.
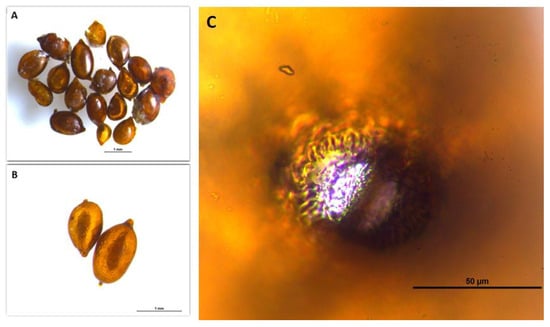
Figure 2.
Microscope images of Heterodera schachtii cysts obtained from Niğde Province: (A,B): cysts of H. schachtii; (C): fenestra region cysts of H. schachtii.
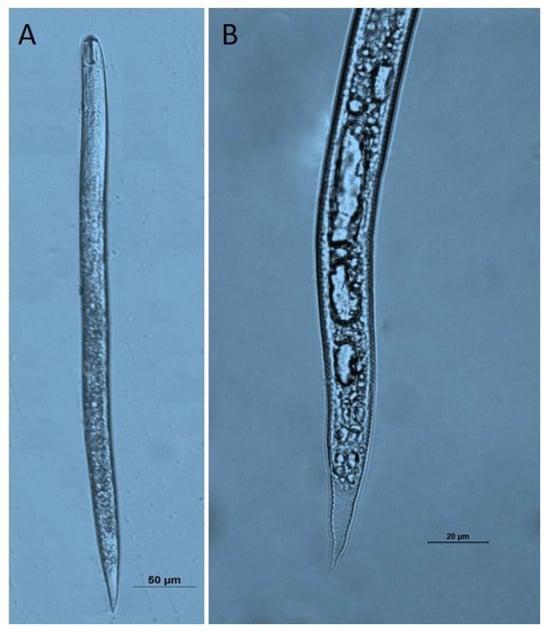
Figure 3.
Microscope images of Heterodera schachtii second-stage juveniles obtained from Niğde Province. (A): second-stage juveniles; (B): tail region.

Table 2.
Morphometric characteristics (Mean ± S.E.) of Heterodera schachtii cyst populations extracted from cabbage fields that were sampled in Niğde Province, Türkiye.
The body length and body width of H. schachtii J2s ranged between 391–517 and 18–27, respectively. Body length (mean = 463.22 ± 7.41 and range = 390.8–516.6 μm); stylet knob width (mean = 3.63 ± 0.28 and range = 1.2–5.8 μm); stylet length (mean = 24.45 ± 0.37 and range = 20.7–27.8 μm), median bulb length (mean = 15.26 ± 0.74 and range = 9.3–21.1 μm); dorsal gland orifice to stylet (DGO) (mean = 4.29 ± 0.17 and range = 2.8–5.7 μm); tail (mean = 47.14 ± 0.8 and range = 40.2–53.3 μm); body diameter at the anus (mean = 13.02 ± 0.46 and range = 9.8–17.1 μm); hyaline (mean = 26.2 ± 0.66 and range = 20.1–32.1 μm). The morphometric measurements of the cysts were consistent with previous studies and were given in Table 3.

Table 3.
Selected morphometric characters (Mean ± S.E.) of second-stage juveniles of Heterodera schachtii populations recovered from cabbage fields in Niğde Province, Türkiye.
3.2. Nucleotide Sequence and Phylogenetic Analysis
Twelve representative H. schachtii populations were selected from the samples collected from each district and identified using the COI region. All the species were also confirmed by the rDNA-ITS region, except for three populations (Table 4). The DNA of all the randomly selected cysts were successfully extracted and amplified using ITS and COI primer sets according to Holterman et al. [19]. PCR products were detected via a standard gel electrophoresis protocol and the observed band sizes were 1028 and 470 bp for the ITS and the COI regions, respectively.

Table 4.
Accession numbers for populations of Heterodera schachtii obtained from the cabbage fields of Niğde, Türkiye.
After sequencing, the BLAST analysis performed on 12 samples showed a 99.81–100% similarity to the H. schachtii sequences from Algeria (MG800690), China (MW856650), France (EF611103), Germany (EF611115.1), Morocco (EF611108), South Africa (MF754150.1), South Korea (MF043911), and the USA (AY590282), whereas the COI nucleotide results showed a 99.81–100% similarity index to the H. schachtii sequences from Iran (MG598807), Japan 135 (LC421661), Mexico (MK134702), Poland (KC172918), South Korea (KY775597), and the USA (KY884308). Phylogenetic trees constructed from Turkish populations of H. schachtii nucleotides, including GenBank samples from various countries, had Turkish populations grouped under the same branches and the other Heterodera species separated from each group, as expected. Genetic variation in the COI sequences obtained from the Turkish population was very low compared with the ITS sequences. The high bootstrap values support these findings (Figure 4).
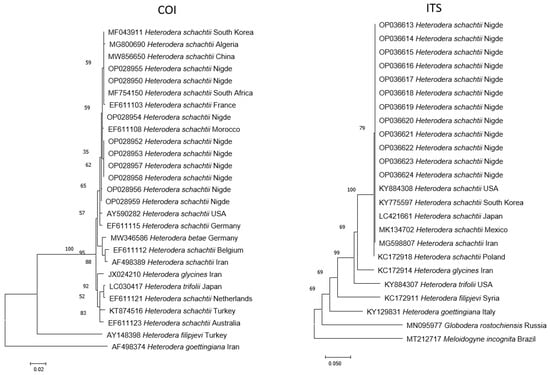
Figure 4.
The phylogenetic analysis of Heterodera schachtii populations based on ITS rDNA and cytochrome oxidase II gene sequences.
4. Discussion
Various researchers have described the structure and morphological characteristics of cyst nematodes. The earliest description of the top-cone structures was by Oostenbrink and Den Ouden [29]. Despite Cooper [30]’s disagreement with Oostenbrink and Den Ouden [29] that H. schachtii and H. trifolii can be distinguished by the measurement of the fenestra length, Oostenbrink and Den Ouden’s [29] method of distinguishing for cyst nematodes was followed. Both researchers stated that H. schachtii and H. trifolii had an average fenestra length of between 32.1 and 45.6 μm. In addition, Mulvey [31] noted that there was no significant relationship between the cyst size and fenestra length of H. schachtii. Also, the anal bullae present in several cysts are important for distinguishing H. schachtii [31]. Heterodera schachtii, for example, has a noticeably high cone, whereas Heterodera cruciferae [18], (Rhabditia: Hoplolaimidae) has a considerably lower cone [31]. Heterodera schachtii has been reported in the previous studies in the sugar beet fields of the Kırklareli [32,33,34], Eskişehir and Adapazarı [35,36], Konya [37], and Şanlıurfa provinces in Türkiye [28]. Similar to the aforementioned studies, in the present study, the morphological and morphometric characteristics of cysts from Niğde populations were measured and recorded. In the current study, low magnification separation of specimens was made based on bullae and the presence of an under-bridge. However, when identifying cyst nematode species, J2s are also convenient [38].
Differentiating between various Heterodera species may be possible through the study of the morphological characteristics of the juvenile stylet. For H. schachtii, the most reliable juvenile characteristics are the stylet length and the hyaline portion of the tail [26]. Based on the morphological characteristics of cysts and J2s, including the morphometric measurements, the present study identified 41 H. schachtii populations. Previous studies have also used morphological and morphometric identification methods of cysts and J2s to identify H. schachtii populations in the Southeast Anatolian Region of Türkiye [28]. The cysts and J2s morphological and morphometric characteristics from Niğde H. schachtii populations were highly identical to those of South Korea [26,27], Türkiye [28], and Xijiang, China populations [25].
According to morphological and morphometric characteristics, H. schachtii which is a member of the schachtii group, may be distinguished from closely similar species (H. trifolii, H. glycines, H. betae) [38,39,40]. Molecularly, a species may be distinguished from other members of the schachtii group by the sequences of the PCR ITS-RFLP, ITS-rRNA, and COI regions [41,42]. Sequences of the ITS ribosomal DNA can be used to assume the phylogeny of Heterodera species [41]. The mtDNA gene COI would alternatively provide a strong candidate for more reliable species definition and ultimately, successful DNA barcoding for cyst nematode species [42].
Accordingly, 9 of the specimens collected from two districts, Kaynarca and Sazlıca, of the province of Niğde were identified as H. schachtii by ITS primers, while 12 of the specimens collected from the same two districts of Niğde Province were identified as H. schachtii by COI primers. After that, populations of H. schachtii from other countries were compared to the studies samples, and the characteristics of the J2s and cysts populations of Niğde were found to be quite similar. The population of Niğde in the same branch with other populations of H. schachtii, and the sequence showed high similarity of 99.81–100%, according to the molecular analyses of the rDNA-ITS and mtDNA COI gene sequences.
During this study, the following symptoms were observed in the infested cabbage crops: yellowing of leaves on plants, plants that are dwarfed, plants with signs of wilting and young plants that are dead. The roots had an unusual quantity of highly branched roots; cysts that were white within the early stage later became brown on the root. The study’s findings are in line with the expected symptoms of field infestation with H. schachtii [16]. Heterodera schachtii has a major impact on cabbage production and the previous reports showed that the marketable head weight of transplanted cabbage was reduced by 25, 31, 34, and 42%, and that of seed-sown cabbage by 21, 28, 46, and 54%, respectively, at the initial population densities of 9, 18, 34, and 68 eggs and larvae per g of soil, respectively [9]. Alternatively, according to the reports of Abawi and Mai [9] and Dababat et al. [28], the present study has determined the prevalence of cyst nematodes in the surveyed area and found it to be 51, 33, and 42% in Merkez, Bor, and Ulukşla, respectively, with an average cyst density of 79.5 cysts per 250 g of soil.
5. Conclusions
These findings suggest that it is necessary to determine H. schachtii prevalence in cabbage production areas to avoid large economic losses in production. With the high prevalence and yield-limiting population densities reported in cabbage fields in Niğde, farmers will need to have the status of their fields analyzed to determine the presence and density of H. schachtii to apply suitable control and management strategies. The present study managed to establish a phylogenetic connection among H. schachtii populations collected from the cabbage-growing areas of Niğde Province, Türkiye. Also, this study identified the cyst populations in the cabbage fields in Niğde Province, Türkiye, based on morphological and molecular characteristics. This is the first definitive finding of H. schachtii in the Niğde cabbage fields, which can be scaled up to a national level to have a more efficient management strategy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10060635/s1, Table S1. Basic information about the study area.
Author Contributions
Conceptualization, G.B.A., E.Y., E.E., A.D., M.İ., R.B. and H.T.; methodology, G.B.A., E.Y., E.E., M.İ., R.B. and H.T.; validation, M.İ., R.B., A.D. and H.T.; formal analysis, G.B.A., M.İ., R.B. and H.T.; investigation, G.B.A., M.İ., R.B. and H.T.; data curation, G.B.A., M.İ., R.B. and H.T.; writing—original draft preparation, G.B.A., E.Y., E.E., A.D., M.İ., R.B. and H.T.; writing—review and editing, E.Y., E.E., A.D., M.İ., R.B. and H.T.; project administration, A.D., M.İ., R.B. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This study is a part of Gülsüm Badel Akyol’s thesis at the Graduate School of Natural and Applied Sciences of Niğde Ömer Halisdemir University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization of the United Nations, FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 21 May 2023).
- Turkish Statistical Institute, TUIK. Available online: https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr (accessed on 24 April 2023).
- Daub, M. Root and Tuber Crops. In Integrated Nematode Management: State of the Art and Visions for the Future; Sikora, R.A., Desaeger, J., Molendijk, L., Eds.; CABI Publishing: Wallingford, UK, 2021; pp. 317–394. [Google Scholar]
- Moens, M.; Perry, R.N.; Jones, J.T. Cyst Nematodes-Life Cycle and Economic Importance. In Cyst Nematodes; Perry, R.N., Moens, M., Jones, J.T., Eds.; CAB International: Wallingford, UK, 2018; pp. 1–18. [Google Scholar]
- Baldwin, J.G.; Mundo-Ocampo, M. Heteroderinae, Cyst-and Non. Manual of agricultural nematology. In Manual of Agricultural Nematology; Nickle, W.R., Ed.; CRC Press: New York, NY, USA, 1991; pp. 275–362. [Google Scholar]
- Evans, K.; Rowe, J.A. Distribution and economic importance. In The Cyst Nematodes; Sharma, S.B., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 1–30. [Google Scholar]
- Güvercin, B.; Akyazı, F. Occurrence and distribution of cyst nematodes, Heterodera spp. (Tylenchida: Heteroderidae) associated with black cabbage, Brassica oleracea var. acephala L.(Brassicales: Brassicaceae) in the Eastern Black Sea Region of Türkiye. Turk. J. Entomol. 2024, 48, 139–154. [Google Scholar]
- Kwon, S.B.; Park, D.K.; Won, H.S.; Moon, Y.G.; Lee, J.H.; Kim, Y.B.; Choi, B.G.; Seo, H.T.; Ko, H.R.; Lee, J.K.; et al. Spread of cyst nematodes in highland Chinese cabbage field in Gangwon-do. Korean J. Appl. Entomol. 2018, 57, 339–345. [Google Scholar]
- Abawi, G.S.; Mai, W.F. Effect of initial population densities of Heterodera schachtii on yield of cabbage and table beets in New York State. Phytopathol. Res. 1980, 70, 481–484. [Google Scholar] [CrossRef]
- Muller, J. The economic importance of Heterodera schachtii in Europe. Helminthology 1999, 36, 205–213. [Google Scholar]
- Haida, A.M.; Al-Assas, K.M.; Dawabah, A.A. Prevalence, distribution and intraspecific variation of Heterodera schachtii populations from semiarid environment. Saudi. J. Biol. Sci. 2016, 23, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Handoo, Z.A.; Subbotin, S.A. Taxonomy, identification and principal species. In Cyst Nematodes; Perry, R.N., Moens, M., Jones, J.T., Eds.; CAB International: Wallingford, UK, 2018; pp. 365–398. [Google Scholar]
- Subbotin, S.A.; Toumi, F.; Elekçioğlu, I.H.; Waeyenberge, L.; Maafi, Z.T. DNA barcoding, phylogeny and phylogeography of the cyst nematode species of the Avenae group from the genus Heterodera (Tylenchida: Heteroderidae). Nematology 2018, 20, 671–702. [Google Scholar] [CrossRef]
- Fenwick, D.W. Methods for the recovery and counting of cysts of Heterodera schachtii from soil. J. Helminthol. 1940, 18, 155–172. [Google Scholar] [CrossRef]
- Handoo, Z.A. A key and compendium to species of the Heterodera avenae group (Nematoda: Heteroderidae). J. Nematol. 2002, 34, 250–262. [Google Scholar]
- Subbotin, S.A.; Mundo-Ocampo, M.; Baldwin, J.G. Systematics of cyst nematodes (Nematoda: Heteroderinae). In Nematology Monographs and Perspectives 8B; Hunt, D.J., Perry, R.N., Eds.; Brill: Leiden, The Netherlands, 2010. [Google Scholar]
- Franklin, M.T. Heterodera latipons n. sp., a cereal cyst nematode from the Mediterranean region. Nematology 1969, 15, 535–542. [Google Scholar] [CrossRef]
- Mulvey, R.H.; Golden, A.M. An illustrated key to the cyst-forming genera and species of Heteroderidae in the western hemisphere with species morphometrics and distribution. J. Nematol. 1983, 15, 1–59. [Google Scholar]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, O.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Boil. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Burnell, A.M.; Powers, T.O. Characterization of Heterorhabditis isolates by PCR amplification of segments of mtDNA and rDNA genes. J. Nematol. 1994, 26, 260. [Google Scholar] [PubMed]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Derycke, S.; Remerie, T.; Vierstraete, A.; Backeljau, T.; Vanfleteren, J.; Vincx, M.; Moens, T. Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Mar. Ecol. Prog. Ser. 2005, 300, 91–103. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Boil. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Boil. Evol. 1993, 10, 512–526. [Google Scholar]
- Huan, P.; Hui, L.; Li, G.; Jian, R.; Li, G.K.; Gao, H.F.; Peng, D.L. Identification of Heterodera schachtii on sugar beet in Xinjiang Uygur Autonomous Region of China. J. Integr. Agri. 2022, 21, 1694–1702. [Google Scholar] [CrossRef]
- Mwamula, A.O.; Ko, H.R.; Kim, Y.; Kim, Y.H.; Lee, J.K.; Lee, D.W. Morphological and Molecular Characterization of Heterodera schachtii and the Newly Recorded Cyst Nematode, H. trifolii Associated with Chinese Cabbage in Korea. Plant. Pathol. J. 2019, 35, 90. [Google Scholar] [CrossRef]
- Kang, H.; Ko, H.; Park, B.; Choi, I. Characterization of Heterodera sojae Virulence Phenotypes in Korea. Plant Pathol. J. 2022, 38, 366. [Google Scholar] [CrossRef] [PubMed]
- Dababat, A.A.; Elekçioğlu, H.; Imren, M.; Orakci, G.E.; Cui, J.; Peng, D.; Huang, W.; Liu, S.; Qiao, F. First report of sugar beet nematode, Heterodera schachtii Schmidt, 1871 (Nemata: Heteroderidae) in sugar beet growing areas of Sanliurfa, Turkey. Turk. Entomol. Derg. 2016, 40, 303–314. [Google Scholar]
- Oostenbrink, I.M.; den Ouden, I.H. With a summary: The structure of the cone top as a taxonomical character in Heterodera species with lemon-shaped cysts. Tijdschr. Over Plantenziekten 1954, 60, 146–151. [Google Scholar]
- Cooper, B.A. A preliminary key to British species of Heterodera for use in soil examination. In Soil Zoology; Kevan, D.K., Ed.; Butterworths Scientific Publications: London, UK, 1995; pp. 269–280. [Google Scholar]
- Mulvey, R.H. Identification of Heterodera cysts by terminal and cone top structures. Can. J. Zool. 1972, 50, 1277–1292. [Google Scholar] [CrossRef]
- Turner, S.J.; Subbotin, S.A. Cyst Nematodes. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CAB International: Wallingford, UK, 2013; pp. 10–143. [Google Scholar]
- Diker, T. Nebat parazit nematodları. Turk. Şeker Fabr. Neşriyatı 1959, 70, 98. [Google Scholar]
- Tokmakoğlu, O. Şeker Pancarı Hastalık ve Zararlıları Atlası; Turkish Sugar Factories Publishing: Ankara, Turkey, 1974. [Google Scholar]
- Susurluk, İ.A.; Ökten, M.E. Investigations on distribution of Heterodera schachtii Schmitd, 1871 (Tylenchida: Heteroderidae) in sugarbeet cultivation areas of Eskișehir district. Turk. Entomol. Derg. 1999, 23, 143–147. [Google Scholar]
- Tan, A.N.; Ökten, E. Adapazarı İli ve Çevresi Şekerpancarı Ekiliş Alanlarında Heterodera Schachtii Schmidt, 1871 (Tylenchida: Heteroderidae)’ in Yayılışı Üzerine Araştırmalar. Uludağ Üniversitesi Ziraat Fakültesi Derg. 2008, 22, 1–8. [Google Scholar]
- Ertürk, S.; Ökten, M. Investigations on the distribution of Heterodera schachtii Schmidt, 1871 (Tylenchida: Heteroderidae) in sugar beet cultivation areas of Konya sugar factory. Plant. Prot. Bull. 2013, 53, 77–84. [Google Scholar]
- Golden, A.M. Morphology and identification of cyst nematodes. In Cyst Nematodes; Lamberti, F., Taylor, C.E., Eds.; Springer: Boston, MA, USA, 1986; pp. 23–45. [Google Scholar]
- Steele, A.E.; Whitehand, L. Comparative morphometrics of eggs and second-stage juveniles of Heterodera schachtii and a race of H. trifolii parasitic on sugarbeet in The Netherlands. J. Nematol. 1984, 16, 171. [Google Scholar] [PubMed]
- Maafi, Z.T.; Sturhan, D.; Handoo, Z.; Mor, M.; Moens, M.; Subbotin, S. Morphological and molecular studies on Heterodera sacchari, H. goldeni and H. leuceilyma (Nematoda: Heteroderidae). Nematology 2007, 9, 483–497. [Google Scholar] [CrossRef]
- Madani, M.; Vovlas, N.; Castillo, P.; Subbotin, S.A.; Moens, M. Molecular characterization of cyst nematode Species (Heterodera spp.) from the Mediterranean Basin using RFLPs and sequences of ITS-rDNA. J. Phytopathol. 2004, 152, 229–234. [Google Scholar] [CrossRef]
- Waeyenberge, L. Biochemical and Molecular Identification. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CAB International: Wallingford, UK, 2018; pp. 419–436. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).