Abstract
The increasing invasion of Rugulopteryx okamurae algae along the coast of southeastern Spain has prompted efforts to explore its potential as a resource. Consequently, composting this alga is considered a viable solution for agricultural applications. This study investigates the composting process of mixtures of R. okamurae seaweed with plant residues and characterizes the final compost for its use as a substrate and/or source of nutrients to determine the most effective composition of the mixture. The composting process was conducted using varying proportions of seaweed (100%, 30–35%, and 15%) combined with plant residues (from vegetable plants, fruits, and gardens) and included both washed and unwashed seaweed. The first trials revealed challenges associated with Rugulopteryx okamurae, such as a low C/N ratio and algae washing. Consequently, a second trial was conducted to optimize the mixtures, aiming for a C/N ratio close to 30. Additionally, it was decided not to wash the algae to reduce the electrical conductivity (EC) in the mixtures. The findings indicate that the composting process remains unaffected by high electrical conductivity when algae are unwashed. However, washing the algae before composting did affect the compost quality, as the composts with washed algae with garden waste (SwP 34.0 dS m−1) had a lower electrical conductivity (EC) than did the unwashed composts with garden waste (SP 51.6 dS m−1 and SFP 64.9 dS m−1). On the other hand, the compost-only horticultural and garden waste (FHP 43.7 dS m−1) had a high EC; therefore, the EC was not increased with low proportions of unwashed algae, as was seen with the compost with 15% unwashed algae (SFHP 47.6 dS m−1). The other quality parameters were not affected by the absence of algae washing at the beginning of the composting process.
1. Introduction
Globally, non-indigenous macroalgae have impacted at least 30% of native ecosystems [1] by dominating space and severely reducing the abundance and diversity of local species, demonstrating their invasive nature. The brown macroalga Rugulopteryx okamurae (Dawson) I.K.Hwang, W.J.Lee, and H.S.Kim (Dictyotales, Ochrophyta) has been added to the list of invasive species due to its invasion of the coasts of the Alboran Sea in the western Mediterranean since 2016 [2,3]. It is also beginning to invade areas along the coast of Provence in France [4] and the Portuguese coast [5]. Originating from East Asia (specifically China, Japan, and Korea, as reported by DeClerck et al. [6]), R. okamurae was first detected in the Mediterranean in 2002. Since 2015, it has extensively colonized the rocky shores along the southern and northern coasts of the Strait of Gibraltar. Copious amounts of the plant were first observed in July 2016 [7], and by 2017, they had grown to occupy almost 100% of the coastal habitats in Cádiz (Spain), reaching depths of up to 5 and 30 m [8]. As a result, the significant growth of R. okamurae [9] coincided with notable changes in the biodiversity of native communities just two years after the first observation of this species. Subsequently, massive deposits of this species were sighted at points beyond the limits of the northern coasts of Cádiz in Granada and Almeria (Spain) [8,10,11,12]. Furthermore, models predicting suitable areas for the establishment of R. okamurae have shown a risk to nearby areas along the southeastern Iberian Peninsula [8].
The impact of this species is still significant today, as its stems are frequently washed ashore in the northern Alboran Sea, requiring clean-up and disrupting tourist activities. Invasive macroalgae reduce the attractiveness of beaches and affect shellfish and aquaculture areas [13,14]. According to Spanish coastal legislation (Law 22/1988), management is the responsibility of the municipality. Data recorded in 2015–2016 show that municipal cleaning machines had to remove more than 5000 t of detached biomass from the tourist beaches of Ceuta, North Africa [9,15,16]. This entails a high cost, as already seen in the “control strategy for Rugulopteryx okamurae” carried out by the Spanish government; five municipalities participated in this strategy and stated that during the period of January–September 2019, the cost was EUR 400,000 and a total of 10,000 t of algal blooms were removed [17]. The production of algal biomass also involves the disposal of countless tons in landfills or via incineration [18]. Both problems require improvements in waste management to reduce disposal costs and to find more environmentally sustainable alternatives [19]. Therefore, composting Rugulopteryx okamurae algae is suggested as a solution that would allow for localized management. It can be managed in the same way as other seaweeds, such as Posidonia oceanica [20], where composting has been a solution for recycling.
Compost is used as a means of improving the chemical, physical, and biological properties of soils as a cultivation substrate and biofertilizer, as well as a potting medium in agricultural applications [21,22,23]. Currently, the deterioration of soils has led to the promotion of soilless cultivation [24]. This method of cultivation involves the use of a substrate that does not contain soil as a support for the roots of the plants [25]. In comparison to traditional soil cultivation, soilless cultivation is demonstrably more profitable [26]. Therefore, a substrate is sought that is porous and allows the plant to have access to nutrients [25]. Given the lack of an ideal substrate, soilless growing media are often a mixture of different substrates that provide the physical characteristics needed by the plants based on yield and economic benefits [27]. In addition to the economic benefits, the search for sustainable materials that help reduce waste also plays a role [26]. Compost represents a cost-effective and environmentally friendly alternative to peat, which is experiencing increasing environmental costs and economic constraints [23].
The soil improvements produced using compost can enhance fertility, soil structure, water, and chemical conservation properties [28,29,30]. In this sense, for centuries, seaweed has been used as a fertilizer in coastal areas around the world [31]. Seaweed is particularly beneficial due to its high potassium and micronutrient content, as well as its growth activators and alginates, which can improve soil structure [32]. Algae can be used for a variety of purposes, but as it is also the base of the food chain, its sustainable use is critical. Seaweed waste can be turned into an innovative organic compost additive. Seaweed is rich in organic chemical compounds, including proteins, amino acids, laminarin, specific sulfated polysaccharides, and alginate [33,34]. Organic fertilizers from seaweed residues have been found to enhance plant growth and improve plant resistance to various stresses [35,36]. However, it is necessary to consider the problems that often accompany the use of some seaweeds for agriculture. Recently, a phytochemical study identified the compound dilkamural, which may be responsible for the deterrence and toxic effect of macroalgae against herbivores [37]. On the other hand, R. okamurae is highly toxic due to its high concentration of sesquiterpenes, which are not present in other algae [38]. Likewise, the high terpene content of these algae, which constitutes the largest fraction of their volatile organic compound (VOC) content, makes their degradation difficult [39].
Another problem is that some marine algae species have high salt contents and low carbon/nitrogen ratios [38]. This issue is of interest as it creates uncertainty regarding the success of the final compost, especially if the high salt content of the seaweed compost can have negative effects on the crop, both in the plant and in the harvest [40]. Several studies have shown that the addition of around 10 and 20% seaweed to compost has no adverse effects [41]. There is also evidence that seaweed extracts have bioactive properties at low rates [42], but at high rates, they may affect plant growth because of salinity [43]. It is also important to consider washing algae used for composting, as the high salinity of algae can interfere with crop development [44]. Therefore, supplements such as forest residues with high carbon levels or small amounts of fishery could be added to the compost to compensate for the deficiencies found [14]. Because R. okamurae is rich in nitrogenous biomass (C/N ratio 10 to 15 [45]), it is necessary to add C-rich but N-poor materials [46] to improve the utilization of R. okamurae. To ensure the success of the process, it is recommended that the mixture of materials to be composted have a C/N ratio of 30 [47]. Specifically, Madejón et al. [48] propose co-composting with other plant remains to compensate for deficiencies.
In this context, the southeastern region of Spain stands out as a significant producer of horticultural crops, resulting in substantial quantities of vegetable waste [22]. This waste can serve as a valuable source of carbon in the composting process for algae. By incorporating this waste, it becomes possible to enhance crucial properties such as the C/N ratio, ultimately leading to the production of high-quality compost. Moreover, the utilization of this compost within the production system itself presents an opportunity to decrease reliance on mineral or synthetic fertilizers [49,50,51]. Furthermore, it aligns with the circular economy requirements mandated by European regulations [52,53].
To achieve this, mixtures were formulated using R. okamurae and local plant waste (horticultural crops and garden waste) in the pursuit of producing high-quality compost. The primary objective of this study is to assess the evolution of the composting process based on various mixtures and to characterize the final compost for its potential utilization as both a substrate and a nutrient source.
2. Materials and Methods
2.1. Experiment Location and Period of Study
To establish the pre-treatment and composting process (Figure 1), a 200 m2 plot of land was prepared at the IFAPA experimental farm in La Cañada (SE Spain 36°49′58.860″ N, 2°24′7.351″ W). For experiment 1 (2020–2021), the treatments were divided into three piles, and for experiment 2 (2021–2022), into four piles, on a concrete surface with the infrastructure illustrated in Figure 1. In the algae washing process used during experiment 1, a washing pond and open-air drying tables were utilized (see Figure 2).

Figure 1.
Scheme and compositions of composting piles. Experiment 1 (2020–2021) (A) and experiment 2 (2021–2022) (B). The photos were taken at the end of both composting processes. Horticultural waste: only stems and leaves of pepper plants; fruit: zucchini, pepper, cucumber; and gardening pruning: palm tree and rubber fig in the first experiment and the second experiment.

Figure 2.
Scheme and compositions of area for washing (A,B) and airing out (A,C) algae for Experiment 1.
Experiment 1 was conducted from November 2020 to May 2021, while Experiment 2 was carried out from September 2021 to April 2022. The mean temperatures and total monthly precipitation recorded during Experiment 1 and Experiment 2 are presented in Table 1. On days when it rained, the compost was covered to avoid excess moisture from the inclement weather. According to the Koppen–Geiger climate classification [54], the weather pattern during this period can be classified as BSh, denoting a hot semi-arid climate.

Table 1.
The mean temperatures (Tm) and total monthly rainfall (Pt) recorded during Experiments 1 and 2 at the nearest weather station, the airport (Almeria), are presented below.
2.2. Origin of the Materials and Characterization
The study materials used for the composting mixtures comprised R. okamurae algae and remnants of local vegetation, including the remains of horticultural crops (stems and leaves of pepper plants and zucchini, pepper, and cucumber fruit) and garden waste (from palm trees and rubber figs). Given the abundance of horticultural waste in the study area, it was considered for use in the composting mix, along with garden waste, as a potential alternative. In both trials, the industrial composting plant “Servicios Ambientales Las Chozas”, located in El Ejido, Almería, provided the shredded vegetable and garden waste. This waste was cut to a length of about 5 cm.
The drift seaweed was collected from a stony coastal area and mechanically shaken to reduce the sand content as much as possible. This method was effective because the percentage of sand in this biomass measured via sieving (0.5 mm mesh size) was less than 5%, which meant that no desanding process had to be carried out in the plant, thus speeding up and reducing the cost of pre-treatment.
In order to address the problem of high conductivity in algae, two different mechanisms have been proposed for the purpose of reducing conductivity. In Experiment 1, an attempt is made to reduce the conductivity of the initial material through washing. Nevertheless, in Experiment 2, an alternative method for reducing conductivity is employed through the mixing of materials. The following procedures were employed:
- -
- Experiment 1 (2020–2021): The R. okamurae seaweed was collected from the coast near the town of Tarifa (Cadiz, Spain) in August 2020. It weighed 1730 kg and filled 5 m3, with a density of 0.346 kg L−1 and a moisture content of 75%. The seaweed drifts were subjected to a water treatment with a conductivity of 0.6 dS·m−1, which resulted in a reduction of their high electrical conductivity (EC) from 84 dS m−1 to levels of 13 dS·m−1 (Figure 2). The ratio used to wash the biomass algae was 1:1.4 (volume/volume), and each batch had an average volume of 941 L (295 kg). As the algae sample to be composted was 1730 kg, it was divided into 6 batches for washing. The salt washing time for each batch was 48 h, which was determined via EC measurements of the wash water and biomass at 24 and 48 h. After 48 h, the water was removed, and the biomass was transferred to drying tables, where it remained for 3 to 10 days, depending on the weather. After drying, eight subsamples were taken from each batch to determine the percentage of moisture, dry matter, and EC of the saturated paste extract. The mean EC of the six batches was 22 dS m−1, where batch 1 went from 85.7 dS m−1 to 9.9 dS m−1, batch 2 from 72.9 dS m−1 to 16.2 dS m−1, batch 3 from 75.5 dS m−1 to 31.6 dS m−1, batch 4 from 84.2 dS m−1 to 28.0 dS m−1, batch 5 from 87.1 dS m−1 to 35.0 dS m−1, and batch 6 from 99.8 dS m−1 to 27.1 dS m−1. Given the lower EC of the first two batches, it was deemed necessary to conduct a second washing, combining batches 3 + 4 and 5 + 6, obtaining an average final EC of 12.4 dS m−1 for all batches. The same procedure was performed with a biomass/water ratio (1:1.4). The water consumption was approximately 12 m3 for the algae used in experiment 1, with a final biomass/water ratio of 1:2.17.
- -
- Experiment 2 (2021–2022): In this experiment, 5 m3 of R. okamurae algae was collected, which is the same amount as in the previous experiment. The source of the algae was the Guadalmesí beach in Tarifa (Cadiz, Spain), a coastal area with mostly pebbles. The use of pebbles was intended to reduce the amount of sand in the algae collection, which could reduce the quality of the compost. The above material was received in August 2021 and stored at the “Servicios Medioambientales Las Chozas” industrial composting plant in El Ejido, Almeria (Spain). It was left to dry out in the open during the same month. One solution to avoid having to wash the algae before composting is to mix it with other materials [55]. Some authors, such as Illera et al. [56], demonstrated that it is possible to lower the EC for a few hours in an organic substrate as compost for soilless culture with water (15 h) or using a nutritive solution for a long period of time of cultive (60 days).
2.3. Treatment Composition
Prior to mixing, the starting materials were evaluated to determine the ratios used in the compost piles (Table 2). Experimental mixtures, including 100% algae, were used as a starting point in experiment 1. Experiment 2 aimed to improve the mixtures, with specific attention paid to the C/N ratio. For each sample, pH and EC were measured in saturated paste extract. The compost’s pH was determined via potentiometry, and the EC was determined in a water suspension via electrometry. The dry matter, organic matter, and ash contents were determined via calcination at 550 °C for 24 h. The total organic carbon content was calculated using the conversion factor via Waksman’s coefficient with organic matter. Nitrogen (N) was determined via the method of Kjeldahl [57], and the C/N ratio was calculated accordingly. Macronutrients such as potassium (K), calcium (Ca), magnesium (Mg), and sodium (Na) were determined via Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Phosphorus (P), sulfur (S), and chloride (Cl) were determined using ionic chromatography.

Table 2.
Physical–chemical analysis of raw materials: seaweed, horticultural waste (only stems and leaves of pepper plants), fruit (zucchini, pepper, and cucumber), and gardening pruning (palm tree and rubber fig) in different experiments (1 and 2).
The proportion of each type of organic matter in different treatments was determined based on the C/N ratio, as shown in Table 3. The experiment produced a final C/N ratio of approximately 20 for the first trial and 25–30 for the second one. The piles had a pyramidal shape with a base length of 2.5 m, a height of 1 m, and a top length of 1 m, which is equivalent to a volume of roughly 2.5 m3 for each pile in the first experiment. In the second trial, the piles had a pyramidal shape with a base length of 2.5 m, a height of 1.5 m, and a top length of 1.5 m, resulting in a proximal volume of 5 m3 for each pile.

Table 3.
The composition of different compost piles in fresh percentage in experiments 1 and 2.
2.4. Parameter Evaluation
2.4.1. Compost Control Parameter Monitoring
To promote aeration and thus aerobic processes throughout the composting process, periodic turning was carried out every 7–15 days (Figure 3A), followed by irrigation to maintain the humidity of the material (Figure 3B). Both of these measures are recommended to avoid anaerobic conditions and promote the activity of microorganisms [58]. The irrigation of the piles was carried out according to the measurement of the moisture percentage of the samples taken every 10–15 days in order to maintain moisture levels of 30–60% on a fresh mass basis. The moisture percentage fluctuated between 30% and 70% (rain), with the optimum value close to 60%. Moisture plays a fundamental role in the transport of dissolved nutrients, which are necessary for the metabolic activity of the strains present in the compost [59].

Figure 3.
Monitoring of the compost process: (A) turning of the piles; (B) irrigation of the piles; and (C,D) periodic sample collection.
During the composting process, the temperature of the materials was monitored using analog thermometers(Gesa Termómetros S.L., Vizcaya, Spain) fixed in the piles at a depth of 0.4 m. Samples were taken periodically (Figure 3C,D). In experiment 1, samples were taken at intervals over time, totaling 12 samples initially spaced 10 days apart and, subsequently, after stabilization, between 15 and 20 days apart. In experiment 2, a total of 8 samples were taken with an initial periodicity of 15 days and, subsequently, after stabilization, with intervals between 20 and 25 days. Each sample consists of eight subsamples taken randomly in the central zone of the pile. Moisture content and dry weight were determined using desiccation in a forced-air oven at 65 °C (24–48 h). The dried samples were crushed and sieved (1 or 2 mm mesh size for analyses). For each sample, pH and EC were measured in the saturated paste extract. The compost’s pH was determined using potentiometry. The EC was determined in a water suspension via electrometry. The dry matter, organic matter, and ash contents were determined via calcination at 550 °C for 24 h. Nitrogen (N) was determined using the method of Kjeldahl [57]. The total organic carbon content was calculated using the conversion factor via Waksman’s coefficient with the organic matter, and the C/N ratio was calculated accordingly.
2.4.2. Quality of Compost
Physical–Chemical Parameters of Compost
At the conclusion of the experiment, the mature compost was subjected to a 12-mm mesh sieving process in order to remove any plastic or large uncomposted materials. Samples were extracted (using the same methodology employed during the composting process) for physical and chemical characterization analysis. Each sample comprised eight randomly selected subsamples obtained during the trial. For each sample, the pH and EC values of the saturated paste extract were determined. The pH of the compost was determined via potentiometry. The EC was determined in a water suspension using electrometry. The moisture content and dry weight were determined via desiccation in a forced-air oven at 65 °C (24–48 h). The real density was determined according to the method of de Boodt et al. [59], which states that the real density of organic materials (OMs) is 1.45 g cm−3 and that of ash as mineral material (MM) is g cm−3. Therefore, the real density (RD) of the substrate is as follows:
To determine the bulk density (or apparent density) of the compost, although the most commonly used method is the official method by de Boodt and Verdonck [59], as well as that by Raviv et al. [60], another quicker and simpler method for measuring bulk density was employed in this study. The applied method is based on the one described by the British Standard of Measurement modified by Ansorena [61], with a precision of ±1 g. Therefore, the bulk density (bd) was calculated using the following expression:
Additionally, a test was conducted using the TMECC method [62]. The estimation of total porosity (TP) was carried out based on the determinations of real and bulk density using the following expression:
The dry matter and organic matter were determined via calcination. The total organic carbon content was calculated using the conversion factor via Waksman’s coefficient with organic matter. Nitrogen (N) was determined using the method of Kjeldahl [58], and the C/N ratio was calculated accordingly for nitrogen and organic carbon. Macronutrients such as potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), and heavy metals were determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Phosphorus pentoxide (P), sulfur trioxide (S), and chloride (Cl) were determined using ionic chromatography.
Germination Bioassays
Germination bioassays are used to evaluate the salinity, toxic substances, soil pathogens, and some physical and chemical properties of compost [63]. The germination index was measured using the method of Zucconi et al. [64]. For each of the composts, a 1/10 dilution of the saturated extract with distilled water was used. For the control, distilled water was used with three replicates for one of two species. Subsequently, three replicates were carried out for each of the treatments in Petri dishes with filter paper and with 20 seeds of two species: cress and lettuce. At 48 h after sowing (or incubation), the number of germinated seeds and root length were assessed, and the average root length and germination percentage were calculated for three replicates. The root length was measured using a ruler. These measurement values were used to calculate the germination index (GI) [64]. The GI is a rapid and reliable method to test the phytotoxicity of compost, quantifying the effect of the water extract on plant growth. Compost is not phytotoxic when the germination index exceeds 60 [65]. The formula was as follows:
2.5. Statistical Analysis
The statistical analysis of the results was conducted using the Statgraphics 19 for Windows software package, and an analysis of variance (one-way ANOVA) test was performed. Subsequently, multiple range tests were conducted to ascertain the significance level using Fisher’s least significant difference (LSD) test at p ≤ 0.05.
3. Results and Discussion
3.1. Assessment of the Composting Process
3.1.1. Temperature
Figure 4 displays the temperature evolution of the composting material. The dates were based on mean values retrieved using an analog thermometer at three locations of the pile with a depth of 0.4 m. The four stages of the process are classified according to the temperature attained. The mesophilic stage, referred to as Figure 4A in experiment 1, starts a few days after the onset and exhibits temperatures near 30 °C until it increases to about 40 °C. The process of composting progresses into a thermophilic stage from day 8 to day 45, which is characterized by varying temperature peaks that always surpass the mesophilic temperature range. The maximum values recorded during this stage were 53 °C, 48 °C, and 38 °C in the SwP, SwH, and Sw piles, respectively. Figure 4A illustrates that the Sw treatment did not reach the minimum temperature of around 45 °C required for effective sanitization during this phase [66]. It is evident that algae alone cannot facilitate composting, and addicional materials are essential to achieve the required C/N ratio and stimulate microorganisms throughout the composting process. After 45 days, the cooling phase begins and lasts until day 63, after which the maturation phase ensues. The procedure for composting utilizing washed seaweed culminates on day 176.
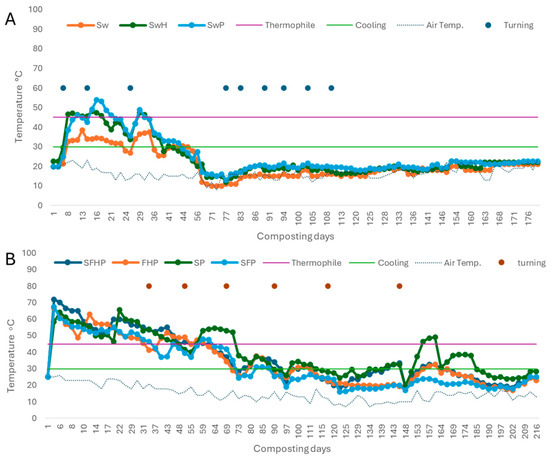
Figure 4.
Changes in the mean values of temperature using washed seaweed (A) and unwashed seaweed (B) during the composting process. Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 4 0% gardening pruning; SP: 35% seaweed + 65% gardening pruning; and SFP: 30% seaweed + 20% fruit + 50% gardening pruning. Thermophile: minimum thermophile phase (45 °C); cooling: minimum cooling phase (30 °C).
In experiment 2 using unwashed seaweed (see Figure 4B), the temperature measurements for the treatments fell within the expected range for a composting process. Nonetheless, there were variations in recorded temperature values among treatments. The composting material piles were started at the ambient temperature of the season at the time of the experiments, which was 24 °C. Following the microorganisms’ activity, the temperature quickly started to rise from mesophilic levels during the initial days of the process, reaching approximately 45 °C. This initial temperature increase continued until day 5 of the process. The phase of elevated temperatures, known as the thermophilic phase, occurred from day 6 to day 57 during the process. The temperature levels reached during this phase were higher than the mesophilic temperature range, with maximum values of 72 °C, 67.5 °C, 66.5 °C, and 65.5 °C, in the SFHP, SFP, FHP, and SP stacks, respectively. The treatments reached the minimum temperature range required for sanitizing the material at this stage, which falls between 45 and 60 °C, as suggested by Moreno and Mormeneo [67]. This stage is crucial for successful sanitization. A temperature drop below 45 °C initiates the mesophilic cooling phase, which varies in duration according to the treatment. Thus, the SFHP and FHP treatments commence this phase on day 59, with temperatures of 44.5 °C and 43.25 °C, respectively. The SP therapy initiates this phase on the 71st day, with a temperature of 38 °C, although this procedure entails alternating stages of cooling and temperature elevation beyond 45 °C (from days 153 to 157). In relation to the SFP treatment, this phase commences on the 62nd day of the process, with a temperature of 43.5 °C. At 175 days, a distinct cooling–maturing phase is evident. The lower temperature stabilization compared with previous phases is attributed to reduced microbial activity. This is because less recalcitrant compounds have already undergone degradation in the earlier phases, leaving behind the more complex compounds for consumption [67].
When comparing the temperature of the composting process between washed and unwashed seaweed, it becomes apparent that the crucial factor is the material mixture ratio required to achieve a C/N ratio of approximately 30 (Table 3). Processes with a lower C/N ratio struggle to reach the necessary temperature values for successful composting. It has been noted that as the amount of algae increases, the time it takes to reach the temperature required to degrade the material also takes longer due to the recalcitrant capacity of the R. okamurae algae, as has been noted by other authors [48]. Regarding temperature increases, it was observed that each time the pile is turned, it promotes a rise in temperature. These temperature increases are encouraged by turning the pile, which promotes oxygenation of the compost and exposes new areas of the material to the action of microorganisms. These microorganisms continue to work on degradation, indicating that the composting process is ongoing [67].
3.1.2. Moisture
The initial moisture content of the materials before the mixer was approximately 5% in Experiment 1 and 70% in the unwashed and undried algae, 93% in the fruit, and less than 10% in the remaining materials in Experiment 2 (see Table 2). This moisture content is comparable to that reported by other researchers who have used algae [45]. During the composting process, both the aeration and moisture content of the treatments were regulated. Moisture is one of the most important factors in good composting, and it is necessary for the survival of microorganisms in the compost [68].
An optimal humidity level (30–60%) was maintained, and turning and irrigation were carried out whenever necessary. Overall, the moisture percentage in all treatments fell between 30 and 70% (Figure 5). During the interval between 70 days and 100 days of Experiment 1, the moisture content was maintained at 65% to 70% due to rainfall. This range is permissible since these values are not surpassed or maintained over time.
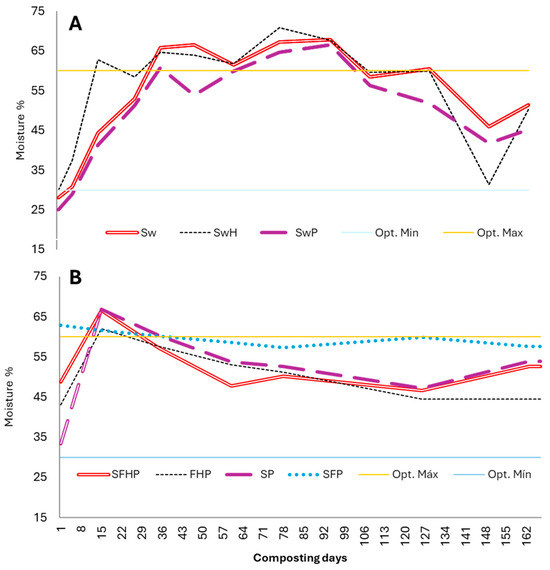
Figure 5.
Changes in the mean values of moisture with washed seaweed (A) and unwashed seaweed (B) during the composting process. Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 40% gardening pruning; SP: 35% seaweed + 65% gardening pruning; SFP: 30% seaweed + 20% fruit + 50% gardening pruning. Opt. Max.: optimum maximum moisture (60%); Opt. Min.: optimum minimum moisture (30%).
A moisture content of 30–60% by weight is desirable for a compost pile; moisture levels of 65–70% inhibit airflow and create anaerobic conditions that produce odor [66]. Nutrients are also washed away when piles become too wet. The addition of dry carbon material and more frequent turning can help remove excess moisture and even reduce salinity [69]. To conserve the required nutrients or to achieve other specific goals, such as a higher rate of sanitization (pathogen reduction), turning frequency should be optimized. No measurements of the lixiviates were taken, as the irrigation attempts did not result in any drainage.
3.1.3. Parameters Monitored: Organic Matter, Total Nitrogen, C/N Ratio, and pH and EC
Compost samples were collected periodically, with each consisting of eight random subsamples from the pile. The saturated extract was measured using these samples, and the following results were obtained: The change in pH is an indicator of the degradation of organic matter and the mineralization of proteins and derivatives [70]. The graphs in Figure 6 show that washed algae (A) demonstrated similar behavior to the unwashed treatments (B). The pH of the compost oscillates between 7 and 8 throughout the process, leading to an alkaline pH (8) that is safe for agricultural use [48]. The activity of microorganisms needs to be between 7 and 8.5 for optimum microbial activity [71].
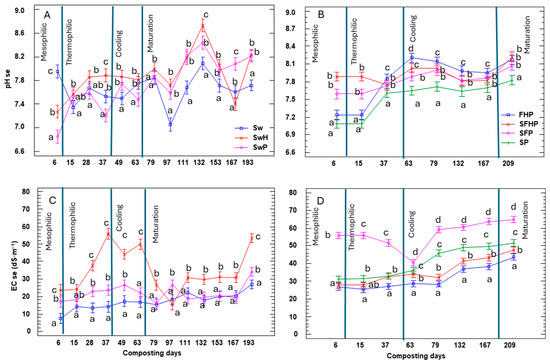
Figure 6.
Mean values of pH (A,B) and EC (C,D) with washed seaweed and unwashed seaweed during the composting process. All parameters show significant differences, as their values were p < 0.05 using the one-way ANOVA test. The letters between the same day of composting correspond to the multiple range test and LSD test at the 95% confidence level. The bars in the graph represent the standard deviation. Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 40% gardening pruning; SP: 35% seaweed + 65% gardening pruning; and SFP: 30% seaweed + 20% fruit + 50% gardening pruning.
The electrical conductivity (EC, dS m−1) fluctuates and rises steadily during the process (refer to Figure 6C,D). With a consistent trend among all composts, the differences between them are very marked. The compost that exhibited a lower EC value at the outset continued to demonstrate a lower EC value throughout the study period. In contrast, the compost that exhibited a higher EC value at the outset continued to demonstrate the highest salt content, as did SwH and SFP. This is attributed to the mineralization of organic matter, which enhances the nutrient concentration, consequently leading to an upsurge in salt levels. The ultimate compost contains a high salt concentration, thus posing a significant challenge for composting process development and the quality of the end product. High salinity can result in osmotic stress, which has a negative impact on the microbial populations responsible for composting and can restrict plant growth and productivity when high-salinity compost is added as an amendment [48]. The final values in Table 4 revealed elevated levels, particularly in treatments involving a high percentage of unwashed seaweed (SFP and SP). Similarly, the treatment involving horticultural waste with washed algae also exhibited high concentrations. As soils with an electrical conductivity greater than 4 dS m−1 exhibit growth inhibition [61,72], a phytotoxicity test was carried out on the compost (Section 3.2.2), which showed that the high levels of algae in the compost could be osmotically stressful to plants.
In the analysis of the assay of the compost composed solely of 100% washed seaweed, it was noted that the initial C/N ratio was inadequate (refer to Figure 7E). It was challenging to attain the ideal composting conditions, resulting in nitrogen losses (refer to Figure 7C) due to seaweed’s tendency to release nitrogen [73]. The remaining treatments show nitrogen fluctuations, ultimately resulting in a rise from the initial value (Figure 7C,D). Therefore, it is essential to utilize addicional materials to improve the carbon/nitrogen ratio alongside lignocellulosic materials [74]. The most efficient co-composting method, as proven by Madejón et al. [48], requires the combination of seaweed and garden waste in ratios of 1:2–1:1. Another successful approach is to mix organic hay, grass, sawdust, and spent mushrooms, as demonstrated by Michalak et al. [75]. Throughout the remaining treatments, the organic matter underwent successful degradation, leading to a reduction in the percentage of organic carbon during the composting process (as shown in Figure 7A,B). All composts attained a C/N ratio of less than 18 (as illustrated in Figure 7E,F), indicating their maturity. It is worth noting that even those with ratios below 14, such as Sw, SwH, SwP, and SFHP, which were identified by Dang et al. [66], fall within the ideal range for compost.
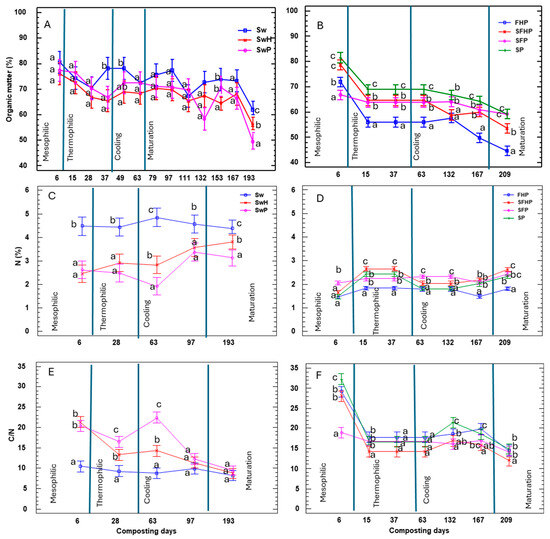
Figure 7.
Mean values of organic matter (A,B), total nitrogen (C,D) and relation C/N (E,F) with washed seaweed and unwashed seaweed during the composting process. All parameters show significant differences, as their values were p < 0.05 using the one-way ANOVA test. The letters between the same day of composting correspond to the multiple range test and LSD test at the 95% confidence level. The bars in the graph represent the standard deviation. Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 40% gardening pruning; SP: 35% seaweed + 65% gardening pruning; and SFP: 30% seaweed + 20% fruit + 50% gardening pruning.
3.2. Quality of Compost
3.2.1. Physical–Chemical Parameters of Compost
At the end of the ripening period, sieving was carried out. The final product was then characterized by analyzing moisture, real density, bulk density, porosity, saturation, dry matter, organic matter, total nitrogen, total organic carbon and C/N ratio, pH, and EC in the extract of saturated paste, available nutrients, and heavy metals (Table 4, Table 5 and Table 6).
In the final characteristics (Table 4) of the resulting products, it was observed that the total organic matter complied with the requirements (>40% in dry matter) of Spanish legislation [51,76]. However, in terms of the moisture content, the compost has an excess and does not comply with RD 999/2017 [51] on fertilizer products. The moisture content of the compost can be reduced by increasing the drying time in the open air and by turning the compost before storage [77].
The pH (Table 4) of the compost samples was higher than the parametric reference values for the use of compost as a substrate, which range from 5 to 7 [78,79]. However, as a fertilizer, the compost has an alkaline pH, which is in line with the average pH for this type of material [23,44,48]. The EC is high, and if the compost were to be used 100% as a substrate, it would be highly saline and phytotoxic [64,65]. If the compost is to be used as a substitute for peat, it should be used in low proportions, as has been shown in other studies [80]. However, as a soil amendment, the EC is very similar in some cases, such as Sw 26.9 dS m−1 and SwP 34.0 dS m−1, and even higher than that of manure [81] (Table 4). On the other hand, the highest values are recorded for composts with the highest content of unwashed seaweed (SwH 53.4 dS m−1, SFHP 47.6 dS m−1, FHP 43.7 dS m−1, SP 51.6 dS m−1, and SFP 64.9 dS m−1). These composts have almost twice the EC of commercial fertilizers such as manure [81], which can be a problem. The high EC renders the use of 100% as a substrate inappropriate. However, this does not preclude the possibility of employing it in smaller proportions. Some authors [23,82] have demonstrated that 20–50% of compost can be substituted for conventional substrate. Even compost with a high EC and no prior washing exhibited a higher germination rate [82].
The bulk density (Table 4) of the compost samples varies between 0.2 g cm−3 (Sw) and 0.5 g cm−3 (FHJ), which meets the criteria established by Abad et al. [78] for the use of compost as a substrate (>0.15 and <0.75 g cm−3). The ideal porosity for a substrate for soilless culture is around 70-85% [80]. In the case of the different composts, it can be seen that except for Sw, the rest are in this interval. However, according to Abad et al. [78], the optimal value of an ideal substrate is >85%, which would be within the ranges of composts such as Sw 90.4% and SwH 85.9%. However, the rest of the composts (SwP 83.9%, SFHP 78.7%, FHP 74.7%, SP 83%, and 79.44%) have values very close to the porosity of the ideal substrate when other materials are used as a substrate.
The C/N ratio (Table 5) is lower because these materials, unlike blond peat, have undergone a greater biotransformation process [83]. The nutrient load of the composts varies according to their composition, but they all comply with the values established by MARM [79]. The Sw compost has the highest concentration of total nitrogen (4.4%), SwH has the highest amount of P (3%), and FHP has the highest proportion of K (3.6%). Regarding Ca content, the composts have similar values (11.5–14.3%) except for Sw (6.5%). On the other hand, the unwashed compost has a higher content (2.7–3%). The cation exchange capacity (CEC) ranges from 99.1 meq·(100 g−1) (FHP) to 164.9 meq·(100 g−1) (SwH), which is in line with the reference values for the use of compost as a substrate [78].
As required by Spanish legislation on fertilizers, the levels of Escherichia coli and Salmonella in the compost were determined using the recommended methods (RD 506/2013) [84]; the material was sanitized and was determined to meet the requirements for Salmonella and E. coli.
Regarding the concentration of heavy metals (Table 6), the composts would be included in Category A [51,76] of the Spanish legislation, except for SwP, whose Cu exceeds the limit of Category A and is included in Category B. When comparing the composts obtained with a similar SwP content to that of Madejón et al. [48], it is evident that they have comparable heavy metal levels (refer to Table 6).
In comparison to other composts that employ the co-composting of manure and plant debris, these have been observed to exhibit higher levels of Fe and Zn (8879 mg kg−1 and 605 mg kg−1) [70]. The average limits for heavy metals in biowaste compost in European countries are 1.4, 93, 143, 1.0, 47, 121, 416, and 23 mg kg−1 for Cd (cadmium), Cr (chromium), Cu (copper), Hg (mercury), Ni (nickel), Pb (lead), Zn (zinc), and As (arsenic) [85]. These values are higher than those reported for composts made from RO, with the exception of SwP, which exceeds the limits for Cu.

Table 4.
Physical characterization of final compost.
Table 4.
Physical characterization of final compost.
| Experiment 1 | Experiment 2 | Ideal Substrate [76] | Manure [81] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Sw | SwH | SwP | SFHP | FHP | SP | SFP | ||
| pH se | 7.7 a | 8.2 c | 8.2 bc | 8.2 c | 8.2 bc | 7.8 a | 8.1 b | 5.0–7.0 | 8.9 | |
| EC se | dS m−1 | 26.9 a | 53.4 b | 34.0 b | 47.6 d | 43.7 c | 51.6 e | 64.9 f | <3.5 | 29.8 |
| Moisture | % | 39.4 bc | 44.8 d | 43.5 d | 41.0 c | 39.0 b | 37.3 a | 48.2 e | 43.7 | |
| Real density | g cm−3 | 1.8 a | 1.8 a | 1.9 b | 1.8 a | 1.9 b | 1.8 a | 1.8 a | ||
| Bulk density | g cm−3 | 0.2 a | 0.2 a | 0.3 b | 0.4 c | 0.5 d | 0.3 b | 0.4 c | <0.75 | |
| Porosity | % | 90.4 c | 86.9 bc | 83.9 b | 78.7a | 74.7 a | 83.0 b | 79.44 a | >85 | |
| Saturation | % | 330.0 d | 200.0 c | 180.0 b | 174.7 b | 155.7 a | 211.8 c | 183.0 b | ||
| Dry matter | % | 60.6 cd | 55.2 b | 56.5 b | 59.0 c | 61.0 d | 62.7 e | 51.8 a | ||
se—saturation extract. All values with letters (left to right) indicate significant differences in the ANOVA test among the composts for this parameter. The letters correspond to the multiple range test and the LSD test at the 95% confidence level. Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 40% gardening pruning; SP: 35% seaweed + 65% gardening pruning; and SFP: 30% seaweed + 20% fruit + 50% gardening pruning.

Table 5.
Chemical characterization of the final compost with plant-available nutrients.
Table 5.
Chemical characterization of the final compost with plant-available nutrients.
| Experiment 1 | Experiment 2 | Compost [48] | Manure [81] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Sw | SwH | SwP | SFHP | FHP | SP | SFP | ||
| Organic matter | % | 54.8 b | 57.1 bc | 47.1 a | 54.25 b | 44.65 a | 59.25 c | 59.15 c | - | 56 |
| N | % | 4.4 e | 3.8 d | 3.1 c | 2.6 b | 1.8 a | 2.3 b | 2.5 b | 3 | 1.8 |
| Organic carbon | % | 36.0 c | 32.6 bc | 28.8 a | 31.2 b | 25.98 a | 34.4 c | 34.38 c | 63.7 | 32.2 |
| C/N | 8.2 a | 8.7 ab | 9.4 b | 12.0 c | 14.4 d | 14.9 d | 14.1 d | 21.2 | 17.9 | |
| P | % | 0.46 a | 3.0 c | 0.9 ab | 1.4 bc | 1.1 ab | 0.7 ab | 1.4 bc | 0.2 * | 1.1 |
| K | % | 0.24 a | 1.68 c | 0.6 b | 2.5 d | 3.6 f | 2.9 e | 2.5 d | 0.4 * | 2.4 |
| Ca | % | 6.5 a | 12.45 bc | 14.3 c | 13.71 c | 14.1 c | 11.5 b | 12.4 bc | 13.6 * | 11.2 |
| Mg | % | 1.1 a | 1.8 a | 1.3 a | 2.8 b | 3.0 b | 2.7 b | 2.9 b | 0.6 * | 1.6 |
| Na | % | 1.5 c | 1.2 b | 1.1 a | 2.2 d | 2.7 e | 4.1 g | 3.5 f | 0.4 * | |
| S | % | 0.1 a | 0.3 c | 0.2 b | 0.2 b | 0.2 b | 0.4 d | 0.4 d | 0.1 * | |
| ∑cations (CEC) | meq·(100 g−1) | 125.4 c | 164.9 e | 111.7 b | 125.3 c | 99.1 a | 145.7 d | 159.1 e | ||
* Units % (weight/weight) adjusted to g·kg−1; all values with letters (left to right) indicate significant differences in the ANOVA test among the composts for this parameter. The letters correspond to the multiple range test and the LSD test at the 95% confidence level. Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 40% gardening pruning; SP: 35% seaweed + 65% gardening pruning; and SFP: 30% seaweed + 20% fruit + 50% gardening pruning. Horticultural waste: only stems and leaves of pepper plants; fruit: zucchini, pepper, and cucumber; and gardening pruning: palm tree and rubber fig.

Table 6.
The concentration of heavy metals in the compost.
Table 6.
The concentration of heavy metals in the compost.
| Heavy Metals | Experiment 1 | Experiment 2 | Spanish Legislation [76,84] | Compost [48] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | Sw | SwH | SwP | SFHP | FHP | SP | SFP | Cat. A | Cat. B | SwP |
| Cd | 0.25 c | 0.15 a | 0.33 d | 0.21 b | 0.23 c | 0.16 a | 0.23 c | <0.7 | <2 | 0.04 |
| Cu | 11.4 a | 22.45 b | 157.9 d | 33.5 c | 36.45 c | 25.00 b | 32.95 c | <70 | <300 | 12.1 |
| Cr | 6.9 a | 10.6 ab | 14.8 bc | 22.5 d | 49.5 e | 16.2 bcd | 18.2 cd | <70 | <250 | 11.4 |
| Hg | <0.050 a | <0.050 a | <0.050 a | <0.050 a | 0.13 c | 0.102 bc | 0.057 ab | <0.4 | <1.5 | - |
| Ni | 9.2 ab | 8.1 a | 8.25 a | 9.0 ab | 15.15 c | 9.15 ab | 11.05 b | <25 | <90 | 6.13 |
| Pb | 4.2 a | 8.0 cd | 4.25 a | 5.75 b | 7.4 c | 5.25 ab | 8.85 d | <45 | <150 | 7.04 |
| Zn | 91.1 b | 85.5 ab | 159.4 d | 62.9 a | 124.05 c | 79.15 ab | 66.5 a | <200 | <500 | 28.1 |
| Fe | 3309.3 a | 4423.7 b | 3122.9 a | 4223.8 b | 5839.4 d | 4640.5 bc | 4975.3 c | 4400 | ||
| Mn | 42.3 a | 91. b8 | 180.8 e | 91.0 b | 119.6 d | 96.6 bc | 102.2 c | 166 | ||
All values with letters (left to right) indicate significant differences in the ANOVA test among the composts for this parameter. The letters correspond to the multiple range test and the LSD test at the 95% confidence level. Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 40% gardening pruning; SP: 35% seaweed + 65% gardening pruning; and SFP: 30% seaweed + 20% fruit + 50% gardening pruning. Horticultural waste: only stems and leaves of pepper plants; fruit: zucchini, pepper, and cucumber; and gardening pruning: palm tree and rubber fig.
3.2.2. Phytotoxicity Test of Compost
One of the most important criteria for assessing the suitability of composts for agronomic use is the germination index, which must be determined prior to the return of the composts to agricultural land in order to prevent environmental hazards [86]. The cause of phytotoxicity is that unripe compost induces high microbial activity, which reduces the oxygen concentration in the soil and blocks the available nitrogen [64].
To ascertain the maturity of the compost samples and their potential to induce phytotoxicity, as well as to investigate the impact of salinity, a germination bioassay study was conducted on watercress (Figure 8) and lettuce (Figure 9). The germination index (GI) of cress seeds (Figure 8) was higher than 60%, which shows that the compost was mature [65], indicating the disappearance of phytotoxic problems such as tolerance to excess salinity in the compost [64]. For all the treatments, there were no statistical differences, given the diversity of the data.
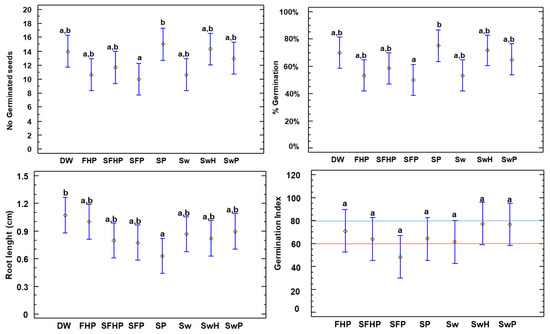
Figure 8.
Parameters measured in cress (Nasturtium officinale): No. of germinated seeds, root length (cm), germination percentage, and germination index. All values with different letters (left to right) indicate significant differences in the ANOVA test among the composts for this parameter. The letters correspond to the multiple range test and the LSD test at the 95% confidence level. The red line describes the 60 threshold, below which composting is considered immature. The blue line indicates the 80 threshold; values above this threshold indicate the absence of excess salinity. DW: distilled water—control; Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 40% gardening pruning; SP: 35% seaweed + 65% gardening pruning; and SFP: 30% seaweed + 20% fruit + 50% gardening pruning.
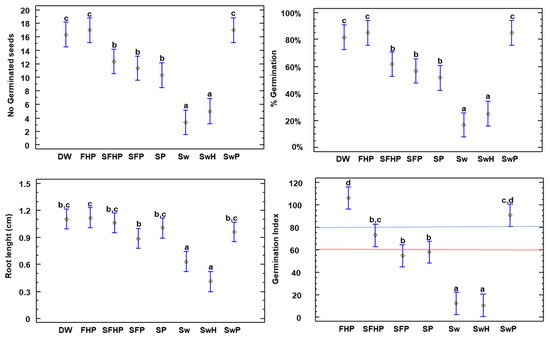
Figure 9.
Parameters measured in lettuce (Lactuca sativa): No. of germinated seeds, root length (cm), germination percentage, and germination index. All values with letters (left to right) indicate significant differences in the ANOVA test among the composts for this parameter. The letters correspond to the multiple range test and the LSD test at the 95% confidence level. The red line describes the 60 threshold, below which composting is considered immature. The blue line indicates the 80 threshold; values above this threshold indicate the absence of excess salinity. DW: distilled water—control; Sw: 100% washed seaweed; SwH: 33% washed seaweed + 67% horticultural waste; SwP: 33% washed seaweed + 67% gardening pruning; SFHP: 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning; FHP: 20% fruit + 40% horticultural waste + 40% gardening pruning; SP: 35% seaweed + 65% gardening pruning; and SFP: 30% seaweed + 20% fruit + 50% gardening pruning.
In the study with lettuce (Figure 9), significant differences were observed. The compost with high proportions of washed seaweed, Sw and SwH, did not exceed 60, meaning that these were not considered mature composts. However, the other composts had values higher than 60. This may be due to the fact that the initial C/N ratio was low, and these composts did not reach the most suitable temperatures for decomposition (see C/N ratio). The composts that did not reach 80 of the GI are SFP and SP, together with those mentioned above. The reason for these composts (SFP and SP) not exceeding the values is related to their high concentration of salts and NaCl, as can be seen in the final characterizations (Table 4), since the rest of the values are similar to the other treatments.
4. Conclusions
Based on the results of the two composting processes, conclusions were formulated about the composting process and the quality of the composts.
Composting process:
The salt content of unwashed seaweed does not affect the temperature during the composting process; therefore, there is no need to use water for washing to ensure that the material is composted.
Among the parameters measured in this study, the low degradation and composting of the 100% washed algae treatment (Sw) stand out. The results of this study show the inability of this compost to compost effectively without the addition of addicional materials. The low C/N ratio (10) is highlighted as one of the factors responsible for limiting degradation, as it hinders the ability of the compost to reach optimal temperatures during the composting process. This characteristic sets it apart from the rest of the treatments, which had an initial C/N ratio of >20.
Compost characterization:
All composts, regardless of whether or not they were washed and the proportion of algae used, satisfied the parameters of pH, bulk density, and porosity. However, their high electrical conductivity (EC) may be an obstacle to their use in enormous quantities as a substitute for peat.
The observed nutrient load of the compost is similar to that in other types of compost and even to that in manure. However, this load varies according to the initial composition of the materials used. On the other hand, the composts with horticultural waste (FHP 43.7 dS m−1), those with horticultural waste with washed seaweed (SwH 53.4 dS m−1), and those with unwashed seaweed (SFHP 47.6 dS m−1, SP 51.6 dS m−1, and SFP 64.9 dS m−1) had a high EC exceeding that of manure (29.8 dS m−1).
In conclusion, the composting of Rugulopteryx okamurae seaweed in various proportions (100%, 30–35%, and 15%) with plant residues (stems and leaves of vegetable plants, fruit, and garden waste) is possible when the mixtures reach a C/N ratio close to 30, regardless of whether the algae have been previously washed or not. According to Spanish legislation [51,76,84], the compost does not meet the humidity percentage requirement; however, this is a physical parameter that can be easily corrected.
Regarding the remaining parameters, the tested composts did not present any impediment to meeting the requirements established by Spanish legislation. As a recommendation, it would be interesting to continue researching ways to counteract the salinity of the compost once the composting process is completed.
Author Contributions
Conceptualization, A.C.-B., M.d.C.S.-S. and M.L.S.-P.; methodology A.C.-B.; software, A.C.-B.; validation, A.C.-B., M.d.C.S.-S. and M.L.S.-P.; formal analysis, A.C.-B., M.d.C.S.-S. and M.L.S.-P.; investigation, A.C.-B., F.B., M.d.C.S.-S., and M.L.S.-P.; resources, M.d.C.S.-S. and M.L.S.-P.; data curation, A.C.-B. and F.B.; writing—original draft preparation, A.C.-B., F.B., and M.d.C.S.-S.; writing—review and editing, A.C.-B., M.d.C.S.-S., and M.L.S.-P.; visualization, A.C.-B., M.d.C.S.-S. and M.L.S.-P.; supervision, M.d.C.S.-S. and M.L.S.-P.; project administration, M.d.C.S.-S. and M.L.S.-P.; funding acquisition, M.d.C.S.-S. and M.L.S.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Maritime and Fisheries Fund, project ‘Estudio de caracterización del alga invasora Rugulopteryx okamurae y posible valorización de su biomasa en las costas de Andalucía’ [FEM.DIP2019.001].
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davidson, A.D.; Campbell, M.L.; Hewitt, C.L.; Schaffelke, B. Assessing the impacts of non indigenous marine macrogalgae: An update of current knowledge. Bot. Mar. 2015, 58, 55–79. [Google Scholar] [CrossRef]
- El Aamri, F.; Idhalla, M.; Tamsouri, M.N. Ocurrence of the invasive brown seaweed Rugulopteryx okamurae (EY Dawson) IK Wang, WJ Lee and HS Kim (Dictyotales, Phaeophyta) in Morocco (Mediterranean Sea). MedFAR 2018, 1, 92–96. [Google Scholar]
- García-Gómez, J.C.; González, A.R.; Maestre, M.J.; Espinosa, F. Detect coasta disturbances and climate change effects in coralligenous community through sentinel stations. PLoS ONE 2020, 15, e0231641. [Google Scholar] [CrossRef]
- Ruitton, S.; Blanfuné, A.; Boudouresque, C.F.; Guillemain, D.; Michotey, V.; Roblet, S.; Thibault, D.; Thibaut, T.; Verlaque, M. Rapid Spread of the Invasive Brown Alga Rugulopteryx okamurae in a National Park in Provence (France, Mediterranean Sea). Water 2021, 13, 2306. [Google Scholar] [CrossRef]
- Faria, J.; Prestes, A.C.L.; Moreu, I.; Cacabelos, E.; Martins, G.M. Dramatic changes in the structure of shallow-water marine benthic communities following the invasion by Rugulopteryx okamurae (Dictyotales, Ochrophyta) in Azores (NE Atlantic). Mar. Pollut. Bull. 2022, 175, 113358. [Google Scholar] [CrossRef] [PubMed]
- DeClerck, L.; Leliaert, F.; Verbruggen, H.; Lane, C.E.; DePaula, J.C.; Payo, D.I.; Coppejans, E. A revised classification of the dictyoteae (Dictyotales, Phaeophyceae) based on rbcL and 26S ribosomal DNA sequence data analyses. J. Phycol. 2006, 42, 1271–1288. [Google Scholar] [CrossRef]
- Altamirano-Jeschke, M.; De la Rosa Álamos, J.; Martínez Medina, F.J. Arribazones de la especia exótica Rugulopteryx okamurae (EY Dawson) en el Estrecho de Gibraltar. 2016. Repositorio Universidad de Málaga. Available online: http://hdl.handle.net/10630/12433 (accessed on 5 October 2023).
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Rey Díaz de Rada, J.; Donázar-Aramendía, I.; Chacón, M.; Quintero, J.J.; Magariño, S.; Megina, C. Monitoring Extreme Impacts of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in El Estrecho Natural Park (Biosphere Reserve). Showing Radical Changes in the Underwater Seascape. Front. Ecol. Evol. 2021, 9, 639161. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Sempere-Valverde, J.; Megina, C. The invasive macroalga Rugulopteryx okamurae: Substrata plasticity and spatial colonization pressure on resident macroalgae. Front. Ecol. Evol. 2021, 9, 631754. [Google Scholar] [CrossRef]
- Altamirano, M.; De la Rosa, J.D.; Carmona, R.; Zanolla, M.; Muñoz, A.R. Macroalgas invasoras en las costas andaluzas. Algas 2019, 55e, 10–13. [Google Scholar]
- CAGPyDS Resultados de Los Trabajos con Rugulopteryx Okamurae en la ZEC y PN del Estrecho en el Marco del Convenio Suscrito Entre Amaya, Agapa, y Ocean Cleaner Technology S.L.; Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible de la Junta de Andalucía; Agencia de Medio Ambiente y Agua de Andalucía: Seville, Spain, 2020.
- Figueroa, F.L.; Vega, J.; Gómez-Valderrama, M.; Korbee, N.; Mercado, J.M.; Bañares, E. Invasión de la especie exótica Rugulopteryx okamurae en Andalucía I: Estudios preliminares de la actividad fotosintética. Algas 2020, 56, 35. [Google Scholar]
- Morand, P.; Briand, X. Excessive growth of macroalgae: A symptom of environmental disturbance. Bot. Mar. 1996, 39, 491–516. [Google Scholar] [CrossRef]
- Illera-Vives, M.; Labandeira, S.S.; Lopez-Mosquera, M.E. Production of compost from marine waste: Evaluation of the product for use in ecological agriculture. J. Appl. Phycol. 2013, 25, 1395–1403. [Google Scholar] [CrossRef]
- Ocaña, O.; Afonso-Carrillo J y Ballesteros, E. Proliferación masiva de una especie dictiotálica (Phaeophyceae, Ochrophyta) por el Estrecho de Gibraltar (Nota de investigación). Rev. Acad. Cañar. Ciencia. 2016, 28, 165–170. [Google Scholar]
- El Aamri, F.; Idhalla M y Tamsouri, M.N. Presencia del alga parda invasora Rugulopteryx okamurae (EY Dawson) IK Hwang, WJ Lee and HS Kim (Dictyotales, Phaeophyta) en Marruecos (Mar Mediterráneo). MedFAR 2018, 1, 92–96. [Google Scholar]
- MITECO. 2021–2022—Estrategia de Control de Rugulopteryx okamurae. Enlace. Available online: https://www.miteco.gob.es/content/dam/miteco/es/biodiversidad/publicaciones/estrategias/estrategia_rokamurae_cs_28072022_tcm30-543560.pdf (accessed on 24 January 2023).
- Castaldi, P.; Melis, P. Growth and yield characteristics and heavy metal content on tomatoes grown in different growing media. Commun. Soil Sci. Plant Anal. 2004, 35, 85–98. [Google Scholar] [CrossRef]
- Abdool-Ghany, A.A.; Pollier, C.G.; Oehlert, A.M.; Swart, P.K.; Blare, T.; Moore, K.; Solo-Gabriele, H.M. Assessing quality and beneficial uses of Sargassum compost. Waste Manag. 2023, 171, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Guillén, J.; MartínezVidal, J.; Triviño, A.; Soler, G.; Fages, E.; Torre, L. Guía de Buenas Prácticas Para la Gestión, Recogida y Tratamiento de Los Arribazones de Algas y Plantas Marinas en Las Costas; Proyecto Seamatter LIFE11 ENV/ES/000600; Instituto de Ecología Litoral: El Campello, Spain, 2014; 24p. [Google Scholar]
- Ruiz, J.L.; Salas Sanjuan, M.D.C. The use of plant growth promoting bacteria for biofertigation; effects on concentrations of nutrients in inoculated aqueous vermicompost extract and on the yield and quality of tomatoes. Biol. Agric. Hortic. 2022, 38, 145–161. [Google Scholar] [CrossRef]
- Carricondo-Martínez, I.; Falcone, D.; Berti, F.; Orsini, F.; Salas-Sanjuan MD, C. Use of Agro-Waste as a Source of Crop Nutrients in Intensive Horticulture System. Agronomy 2022, 12, 447. [Google Scholar] [CrossRef]
- Berti, F.; Salas-Sanjuán MD, C.; Hernández-López, F.; Correa-Bustos, A.; Segura-Pérez, M.L. Use of Compost Based on Invasive Algae Rugulopteryx okamurae as a Peat Alternative in Nursery Growing Media. Agronomy 2023, 13, 948. [Google Scholar] [CrossRef]
- Hussain, A.; Iqbal, K.; Aziem, S.; Mahato, P.; Negi, A.K. A review on the science of growing crops without soil (soilless culture)—A novel alternative for growing crops. Int. J. Agric. Crop Sci. 2014, 7, 833. [Google Scholar]
- Savvas, D.; Gianquinto, G.; Tuzel, Y.; Gruda, N. Soilless Culture. Good Agricultural Practices for Greenhouse Vegetable Crops, Principles for Mediterranean Climate Areas. 217. FAO Plant Production and Protection Paper. 2013; pp. 303–354. Available online: https://www.fao.org/3/a-i3284e.pdf (accessed on 10 January 2024).
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems–A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, N.P.; y Carrión, C. Los sustratos en los cultivos sin suelo. In Tratado de Cultivo Sin Suelo; Urrestarazu, M., Ed.; Mundi-Prensa: Madrid, Spain, 2004; pp. 113–158. Available online: https://www.researchgate.net/publication/259286675_Tratado_de_cultivo_sin_suelo (accessed on 30 March 2024).
- Shang, X.C.; Zhang, M.; Zhang, Y.; Hou, X.; Yang, L. Waste seaweed compost and rhizosphere bacteria Pseudomonas koreensis promote tomato seedlings growth by benefiting properties, enzyme activities and rhizosphere bacterial community in coastal saline soil of Yellow River Delta, China. Waste Manag. 2023, 172, 33–42. [Google Scholar] [CrossRef]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, L.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, N.; Lin, Y.; Zhan, Y.; Ding, X.; Liu, Y.; Zhang, A.; Ding, G.; Xu, T.; Li, J. Recycling of nutrients from organic waste by advanced compost technology—A case study. Bioresour. Technol. 2021, 337, 125411. [Google Scholar] [CrossRef]
- Zemke-White, W.L.; Ohno, M. World seaweed utilisation: An end-of-century summary. J. Appl. Phycol. 1999, 11, 369–376. [Google Scholar] [CrossRef]
- Blunden, G. Agricultural uses of seaweed and seaweed products. In European Seaweed Resources: Uses and Potential; Guiry, M.D., Blunden, G., Eds.; Wiley: Chichester, England, 1991; pp. 65–81. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 27, 270–279. [Google Scholar] [CrossRef]
- Gibilisco, P.E.; Negrin, V.L.; Idaszkin, Y.L. Assessing the use of two halophytes species and seaweed composting in Cu-pollution remediation strategies. Mar. Pollut. Bull. 2022, 176, 113413. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Wang, M.P.; Chen, L.; Li, Y.T.; Chen, L.; Liu, Z.Y.; Wang, X.J.; Yan, P.S.; Qin, S. Responses of soil microbial communities to a short-term application of seaweed fertilizer revealed by deep amplicon sequencing. Appl. Soil Ecol. 2018, 125, 288–296. [Google Scholar] [CrossRef]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A novel chemical weapon involved in the invasive capacity of the alga Rugulopteryx okamurae in the Strait of Gibraltar. Estuar. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar] [CrossRef]
- Barcellos, L.; Pham, C.K.; Menezes, G.; Bettencourt, R.; Rocha, N.; Carvalho, M.; Felgueiras, H.P. A concise review on the potential applications of Rugulopteryx okamurae macroalgae. Mar. Drugs 2023, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Patón, D.; García-Gómez, J.C.; Loring, J.; Torres, A. Composting the invasive toxic seaweed Rugulopteryx okamurae using five invertebrate species, and a mini-review on composting Macroalgae. Waste Biomass Valorization 2023, 14, 167–184. [Google Scholar] [CrossRef]
- Cole, A.J.; Roberts, D.A.; Garside, A.L.; de Nys, R.; Paul, N.A. Seaweed compost for agricultural crop production. J. Appl. Phycol. 2016, 28, 629–642. [Google Scholar] [CrossRef]
- Winberg, P.; de Mestre, C.; Willis, S. Evaluating Microdictyon umbilicatum bloom biomass as a compost conditioner for Australian, native coastal plants, Rhagodia candoleana and Banksia integrifolia. Compost. Sci. Util. 2013, 21, 64–74. [Google Scholar]
- Crouch, I.J.; Van Staden, J. Evidence for the Presence of Plant Growth Regulators in Commercial Seaweed Products. Plant Growth Regul. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- Maze, J.; Morand, P.; Potoky, P. Stabilization of Green Tides Ulva by a Method of Composting with a View to Pollution Limitation. J. Appl. Phycol. 1993, 5, 183–190. [Google Scholar] [CrossRef]
- González Henríquez, M.N.; Jaizme-Vega MCAlcoverro Pedrola, T.R.; Haroun Tabraue, J.; Socorro Monzón, A.R. Aprovechamiento de Arribazones Naturales y Residuos Vegetales de Jardinería Como Fuente de Materia Orgánica Para la Elaboración del compost. 2011. Available online: https://www.icia.es/icia/index.php?option=com_content&view=article&id=1188:aprovechamiento-de-arribazones-naturales-y-residuos-vegetales-de-jardineria-como-fuente-de-materia-o&catid=71&Itemid=100060 (accessed on 3 March 2024).
- Geider, R.; La Roche, J. Redfeld revisited: Variability of C:N: P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- De la Lama-Calvente, D.; Fernandez-Rodriguez, M.; Llanos, J.; Mancilla-Leyton, J.; Borja, R. Enhancing methane production from the invasive macroalga Rugulopteryx okamurae through anaerobic co-digestion with olive mil solid waste: Process performance and kinetic analysis. J. Appl. Phycol. 2021, 33, 4113–4124. [Google Scholar] [CrossRef]
- Han, W.; Clarke, W.; Pratt, S. Composting of waste algae: A review. Waste Manag. 2014, 34, 1148–1155. [Google Scholar] [CrossRef]
- Madejón, E.; Panettieri, M.; Madejón, P.; Perez-de-Mora, A. Composting as sustainable managing option for seaweed blooms on recreational beaches. Waste Biomass Valorization 2022, 13, 863–875. [Google Scholar] [CrossRef]
- Reglamento (UE) 2019/1009 del Parlamento Europeo y del Consejo, de 5 de junio de 2019, Por el Que se Establecen Disposiciones Relativas a la Puesta a Disposición en el Mercado de los Productos Fertilizantes UE y se Modifican los Reglamentos (CE) n.o 1069/2009 y (CE) n.o 1107/2009 y se Deroga el Reglamento (CE) n.o 2003/2003 (Texto Pertinente a Efectos del EEE). Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 29 February 2024).
- Carricondo-Martínez, I.; Berti., F.; Salas-Sanjuán., M.d.C. Different Organic Fertilisation Systems Modify Tomato Quality: An Opportunity for Circular Fertilisation in Intensive Horticulture. Agronomy 2022, 12, 174. [Google Scholar] [CrossRef]
- Real Decreto 999/2017, de 24 de Noviembre, Sobre Productos Fertilizantes. BOE-A-2017-14332. Available online: https://www.boe.es/eli/es/rd/2017/11/24/999 (accessed on 23 January 2024).
- Murray, M.; Skene, K.; Haynes, K. The Circular Economy: An Interdisciplinary Exploration of the Concept and Application in a Global Context. J. Bus. Ethics 2015, 140, 369–380. [Google Scholar] [CrossRef]
- Ley 3/2023, de 30 de marzo, de Economía Circular de Andalucía. Available online: https://www.boe.es/eli/es-an/l/2023/03/30/3 (accessed on 23 January 2024).
- Arnfield, A.J. “Köppen Climate Classification”. Encyclopedia Britannica. 16 October 2023. Available online: https://www.britannica.com/science/Koppen-climate-classification (accessed on 17 January 2024).
- Chong, C. Experiences with wastes and composts in nursery substrates. HortRechnology 2005, 15, 739–747. [Google Scholar] [CrossRef]
- Vives, M.I.; Mosquera ME, L.; Fabal, A.L.; del Carmen Salas-Sanjuan, M. Acondicionamiento de un compost salino para su uso como sustrato de cultivo. Recur. Rurais 2012, 18, 13–19. [Google Scholar]
- Hesse, P.R. A Textbook of Soil Chemical Analysis; John Murray: London, UK, 1971. [Google Scholar]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- de Boodt, M.; Verdonck, O. The physical properties of the substrates in horticulture. Acta Hort. 1972, 26, 37–44. [Google Scholar] [CrossRef]
- Raviv, M.; Wallach, R.; Silber, A.; Bar-Tal, A. Substrates and their analysis. In Hydroponic Production of Vegetables and Ornamentals; Embryo Publications: Athens, Greece, 2002; Chapter 2; pp. 25–102. ISBN 9789608002128. [Google Scholar]
- Ansorena Miner, J. Sustratos. In Propiedades y Caracterización; Mundi-Prensa: Madrid, Spain, 1994; 172p. [Google Scholar]
- TMECC, 2001. Test Methods for the Examination of Composting and Compost. US Composting Council. 2001. Available online: https://www.compostingcouncil.org/page/tmecc (accessed on 29 February 2024).
- Zucconi, F.M.; Monaco, A.; Forte, M. Phytotoxins during the stabilization of organic matter. In Composting of Agricultural and Other Wastes; Gasser, J.K.R., Ed.; Elsevier Applied Science Publishers: New York, NY, USA, 1985; pp. 73–86. [Google Scholar]
- Zucconi, F.M.; Pera, A.; Forte, M.; De Bertoldi, M. Evaluating toxicity of immature compost. BioCycle 1981, 22, 5457. [Google Scholar]
- Zucconi, F.; De Bertoldi, M. Production and characterization of compost. BioCycle 1987, 28, 56–61. [Google Scholar]
- Dang, B.T.; Ramaraj, R.; Le, M.V.; Tomoaki, I.; Pham, T.T.; Le Na, P.T.; Tran, D.P. Current application of seaweed waste for composting and biochar: A review. Bioresour. Technol. 2023, 375, 128830. [Google Scholar] [CrossRef]
- Moreno-Casco, J.; Mormeneo-Bernat. Microbiología y bioquímica del proceso de compostaje. In Compostaje; Moreno, J., Moral, R., Eds.; Ediciones Mundi-Prensa: Madrid, Spain, 2005. [Google Scholar]
- Kim, E.; Lee, D.H.; Won, S.; Ahn, H. Evaluation of optimum moisture content for composting of beef manure and bedding material mixtures using oxygen uptake measurement. Asian-Australas. J. Anim. Sci. 2016, 29, 753–758. [Google Scholar] [CrossRef]
- Sembera, J.A.; Meier, E.J.; Waliczek, T.M. Composting as an alternative management strategy for sargassum drifts on coastlines. Horttechnology 2018, 28, 80–84. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, S.; Li, X.; Rong, K.; Li, J.; Jiang, L. Effects of microbial inoculant and additives on pile composting of cow manure. Front. Microbiol. 2023, 13, 1084171. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, F. Effects of composts obtained from hazelnut wastes on the cultivation of pepper (Capsicum annuum) seedlings. Sci. Rep. 2024, 14, 3019. [Google Scholar] [CrossRef] [PubMed]
- Amacher, J.K.; Koenig, R.; Kitchen, B. Salinity and plant tolerance. AG-SO 2000, 3, 1–8. [Google Scholar]
- Al-Dulaimi, O.; Rateb, M.E.; Hursthouse, A.S.; Thomson, G.; Yaseen, M. The brown seaweeds of Scotland, their importance and applications. Environments 2021, 8, 59. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal compost–toward sustainable fertilization. Rev. Inorg. Chem. 2013, 33, 161–172. [Google Scholar] [CrossRef]
- Michalak, I.; Tuhy, L.; Chojnacka, K. Co-Composting of Algae and Efect of the Compost on Germination and Growth of Lepidium sativum. Pol. J. Environ. Stud. 2013, 25, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Real Decreto 865/2010, de 2 de Julio, Sobre Sustratos de Cultivo. BOE-A-2010-11153. Available online: https://www.boe.es/eli/es/rd/2010/07/02/865 (accessed on 29 February 2024).
- Mapama—Ministerio De Medio Ambiente Y Medio Ruraly Marino Gobierno, D. E. Obtain of Manual de Compostaje. 2008. Available online: https://www.miteco.gob.es/content/dam/miteco/es/calidad-y-evaluacion-ambiental/temas/prevencion-y-gestion-residuos/Manual%20de%20compostaje%202011%20PAGINAS%201-24_tcm30-185556.pdf (accessed on 15 January 2024).
- Abad, M.; Noguera, P.; Noguera, V.; y Segura, M.L. Los sustratos para el semillero hortícola. Planteles. Compend. Hortic. 1999, 13, 59–68. [Google Scholar]
- MARM, 2010—Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid (España). Guía Práctica de la Fertilización Racional de los Cultivos en España. Available online: https://www.mapa.gob.es/es/agricultura/publicaciones/Publicaciones-fertilizantes.aspx (accessed on 9 February 2024).
- Cabrera, R.I. Propiedades, uso y manejo de sustratos de cultivo para la producción de plantas en maceta. Rev. Chapingo Ser. Hortic. 1999, 5, 5–11. [Google Scholar] [CrossRef]
- Segura, M.L. Fertirrigación de Cultivos Hortícolas en Condiciones Salinas con Sistema Enarenado y Sustratos Alternativos. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 1995. [Google Scholar]
- Illera, M. Reducción de la salinidad en un compost: ¿Lavado o mezcla? In Libro de Resúmenes de las III Jornadas de Compostaje de la REC; 2012; pp. 127–130. ISBN 978-84-8408-788-5. Available online: https://www.researchgate.net/publication/272089824_REDUCCION_DE_LA_SALINIDAD_EN_UN_COMPOST_LAVADO_O_MEZCLA (accessed on 15 January 2024).
- Lopez, M.J.; Masaguer, A.; Paredes, C.; Perez, L.; Muñoz, M.; Salas, M.C.; Hernandez, R. De Resíduos a Recursos: El Camino hacia la Sostenibilidad; Mundi-Prensa: Madrid, Spain, 2015; pp. 91–121. ISBN 9781512938319. [Google Scholar]
- Real Decreto 506/2013, de 28 de Junio, Sobre Productos Fertilizantes. Available online: https://www.boe.es/eli/es/rd/2013/06/28/506/con (accessed on 29 February 2024).
- EU. Heavy Metals and Organic Compounds from Wastes Used as Organic Fertilisers. Available online: https://ec.europa.eu/environment/pdf/waste/compost/hm_finalreport.pdf (accessed on 4 April 2024).
- Tiquia, S.M.; Tam NF, Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).