Abstract
Essential oils (EOs) from Citrus sinensis (Rutaceae) possess diverse biological activities. However, a comprehensive comparison of their chemical composition and bioactivity across different plant parts has not been studied yet. The current research comparatively assesses the yield, chemical composition, chiral distribution, antioxidant properties, and larvicidal activity of EOs extracted from the peels, leaves, and flowers of C. sinensis. EOs extracted via hydro-distillation (HD) and steam distillation (SD) were analyzed by gas chromatography–mass spectrometry (GC-MS) and chiral GC-MS to explore their chemical composition and enantiomeric distribution. In addition, their larvicidal and antioxidant potentials were evaluated following standard protocols. Peels of C. sinensis exhibited significantly higher oil content (1.75–2.25%) compared to its leaves (0.75–0.78%) and flowers (0.20–0.25%). The GC-MS analysis identified around 60 compounds, including terpenoids, sesquiterpenoids, and oxygenated terpenoids in the HD and SD extractions. Higher concentrations of sabinene were found in flower extract (38.05–39.89%) and leaf extract (32.30–36.91%), while peel extract contained more than 90% limonene. The larvicidal activity of peel oil was primarily attributed to limonene, with an LC50 value of 0.0031 µL/mL. The current study reports the first chiral (GC-MS) analysis in the essential oil of the leaves and flowers of C. sinensis, paving the way for authenticity and purity. Furthermore, the chemical profiling of citrus EOs, particularly from the peel, demonstrates a safe and promising candidate for diverse biological applications.
1. Introduction
The genus Citrus, belonging to the Rutaceae family, is one of the most prominent fruits cultivated in tropical and subtropical regions worldwide [1]. Among these, sweet oranges (Citrus sinensis Osbeck) are thought to have originated in Southeast Asia, most likely in southern China, Vietnam, or the Assam region of India; however, the exact origins of citrus are still up for debate [2]. In addition to nutritional values, citrus fruits are enriched in various bioactive components such as flavonoids, phenols, limonoids, EOs, vitamin C, and carotenoids associated with health advantages [3]. Citrus is the principal source of EOs used from ancient times [4], and approximately 60% of citrus oils are produced from the peel of sweet oranges [5].
Typically, peels (rind), leaves, and flowers of the plant possess volatile constituents, versed with citrus essential oil (CEO) [6]. These constituents are biosynthesized by living organisms and are separated by chemical (solvent extraction) or physical methods (distillation) [7]. The two major methods for extracting CEOs are distillation (steam, dry, and hydro-distillation) and nonthermal extraction (cold pressing, supercritical CO2 fluid extraction, and solvent extraction). The extraction procedure has a significant impact on the volatile chemical profiles of CEOs [8]. EOs derived from citrus have intricate compositions, primarily consisting of sesquiterpenes, monoterpenes, and their oxygenated derivatives, which account for 85% to 99% of their volatile components [9]. The varying ecological and geographic conditions, maturity, and the timing of the harvest and the various extraction techniques are all factors that contribute to the chemotype in the EOs of Citrus species, which may alter the biological characteristics of these EOs [10].
CEOs are frequently used for flavoring, fragrances, cuisine, soft drinks, and liqueurs and are also present in a great variety of pharmaceutical preparations, as well as cosmetics, perfumes, detergents, and body products [11]. In addition, the antibacterial, insecticidal, and antioxidant efficacy of C. sinensis peel essential oil is widely recognized [12]. The pharmaceutical formulation produced by combining orange essential oil (Citrus sinensis L.) with β-cyclodextrin may reduce the risk of Alzheimer’s disease [13]. It has been reported that inhalation of CEOs influences the brain (hypothalamus, hippocampus, and piriform) activities and helps to restore the stress-induced cortex [14]. Similarly, preclinical and clinical studies carried out in rats reported immunosuppression and antidepressant activities of CEOs [15]. Additionally, they possess substantial fumigant properties against house flies, cockroaches, and mosquitoes, as well as larvicidal activity against the vector of malaria Anopheles labranchiae and the Aedes aegypti, yellow fever, and dengue fever vectors [16]. Limonene and myrcene, which give lemons their distinctive aroma, are also abundant in citrus peels mainly found in C. sinensis, as reported in the literature [17].
Even though numerous studies on citrus peel have been published, only a few studies are known about the chemical constituents, enantiomeric distribution, and biological activities of EOs from the leaves and flowers. Leaf extracts have potential use as a natural source of antioxidants and should be investigated further for their biological properties. In most of the research on Citrus spp., volatile and semi-volatile fraction composition uses data from the peel (rind) EOs [18]. To the best of our knowledge, no detailed investigation has been conducted to compare the EOs found in the peels, leaves, and flowers of C. sinensis. Despite reports on the larvicidal potential of C. sinensis oil, a knowledge gap exists regarding the activity of CEOs extracted from Nepalese origins. Hence, this study conducts a comparative analysis of the chemical profiles and enantiomeric ratios and larvicidal, along with antioxidant, activities of EOs extracted from peels, leaves, and flowers of C. sinensis collected from eastern parts of Nepal.
2. Materials and Methods
2.1. Sample Collection
C. sinensis, popularly known as sweet orange, samples were collected from the Sindhuli district with a latitude of 27°16′58″ N and a longitude of 85°58′47″ E in the eastern parts of Nepal. Fruit and leaves were taken during the initiation ripening period (November 2022), while flowers were collected at the start of the flowering season (May 2022) and identified by a horticulturist. The plant’s voucher specimen has been deposited in the National Herbarium and Plant Laboratories (KATH), Government of Nepal, Lalitpur, Nepal.
2.2. Extraction of Essential Oil
The extraction of essential oil from the peel, leaf, and flower of C. sinensis was carried out using two distinct distillation techniques: hydro- and steam distillation. Initially, fresh samples of peels, leaves, and flowers were washed with water and the glandular portion of the peel and leaves were then sliced and crushed, and the flowers were shed-dried. Then, the samples were subjected to both hydro- and steam distillation for 4–6 h using a modified Clevenger apparatus. The plant sample is mixed with water in the hydro-distillation technique, whereas the steam distillation apparatus is designed to pass steam generated in separate flasks through the plant sample [19]. The yield of extraction was expressed as a percentage (v/w) of the volume of the essential oil to the sample material (100 g) and stored at 4 °C in an ambered glass bottle for further analysis.
2.3. Chemical Profiling by Gas Chromatography–Mass Spectrometry
The Shimadzu GCMS-QP2010 Ultra operated in the electron impact (EI) mode (electron energy = 70 eV, scan range = 40–400 atomic mass units, scan rate = 3.0 scans/s) was used to investigate the chemical composition of the EOs of C. sinensis. A ZB-5 fused silica capillary column with a film thickness of 0.25 μm and a stationary phase of (5% phenyl)-polymethylsiloxane served as the GC column. Helium was used as the carrier gas; its column head pressure was 552 kPa, and its flow rate was 1.37 mL/min. In addition, 200 °C was the ion source temperature, and 250 °C was the injector temperature. The temperature was raised from an initial 50 °C to 260 °C in the GC oven using a gradual increase of 2 °C per minute. After preparing a 5% w/v solution of each sample in dichloromethane (CH2Cl2), 0.1 μL was injected using a 30:1 splitting ratio.
Based on their retention indices, which were calculated using a homologous series of n-alkanes as a guide, and on a comparison of their mass spectral fragmentation patterns with those found in published works and our internal MS library, the oil constituents were identified [20,21].
2.4. Enantiomeric Compound Analysis by Chiral GC/MS
The chiral analysis of the leaf, flower, and peel of C. sinensis EOs was carried out using a Shimadzu GCMS-QP2010S in EI mode (70 eV) and a B-Dex 325 with a chiral capillary GC column as described previously [22]. The peak area was used to calculate the ratios of enantiomers. The enantiomers were identified by comparing the retention times and mass spectrum fragmentation patterns using reference samples from Sigma-Aldrich (Milwaukee, WI, USA) [23].
2.5. Chemicals
DPPH (≤100%), ascorbic acid (≥98%), d-carvone (≥96%), β-pinene (≥99%), sabinene (≥75%), p-cymene (≥97%), linalool (≥95%), d-limonene (≥97%), and all other references were purchased from Sigma-Aldrich, Merck, Darmstadt, Germany. Solvents with high purity were purchased from Loba Chemie Pvt. Ltd., Mumbai, India.
2.6. Antioxidant Activity by DPPH Assay
The DPPH assay was performed to assess the antioxidant activities of EOs [24]. In short, 100 μL of the diluted samples of EOs ranging from 2 to 30 μL/mL in 10% DMSO (99% AR) was incubated in 96-well plates, and 100 μL 0.1 mM DPPH solution was added to the well plates; a 10% DMSO solution was utilized as a control along with ascorbic acid as standard purchased from Sigma-Aldrich, (Darmstadt, Germany). The well plate was incubated in the dark for 30 min at room temperature. Using a Gen5 well-plate reader (Epoch2, BioTek, Winooski, VT, USA), the absorbance of the sample and control were measured at 517 nm after 30 min. The experiment was performed in triplicate, and the percentage inhibition was evaluated according to the equation [25].
where Ao and As, represent the absorbance of the blank solution and sample, respectively.
2.7. Acute Toxicity Test
The OECD 425 guideline’s limit test dosage of 2000 mg/kg was used in an acute toxicity investigation [26,27]. After a 24-h fast, three female albino mice were given free access to water. A maximum dosage of 2000 mg/kg of OQ was given in stages, and each animal was observed separately for behavioral traits like restlessness, alertness, irritability, and fearfulness, for autonomic traits like defecation and urination, for neurologic traits like impulsive behavior, reaction, touch response, pain response, and gait, for physical traits like lacrimation, loss of hunger, tremors, hair erection, the amount of saliva, and diarrhea, and for morbidity or mortality following 24 h and then every day for a total of 7 days.
2.8. Larvicidal Assay
2.8.1. Sampling and Breeding of Aedes aegypti
A. aegypti larvae were obtained from Thapathali in Kathmandu, Nepal, and kept in a jar containing a 50:50 mixture of distilled water and water from the original breeding location. The larvae were fed crushed biscuits. The larvae were placed in 50 mL plastic cups with distilled water when they were at the pupal stage. The larvae were then placed in plastic cages and kept covered with nylon screens until the adults emerged. The mosquitoes were then provided with cotton swabs dipped in a 10% glucose solution. After hematophagy, the females were transferred to a 50 mL container made of plastic that included a piece of moistened filter paper for the oviposition stage. The larvae of the F1 generation were used in bioassays and kept in a heated chamber with an average temperature of 27 °C and a 12-h photoperiod.
2.8.2. Larvicidal Assay
The larvicidal activity was conducted according to WHO-2005, with slight modifications [28,29]. In brief, the 4th-instar larvae of A. aegypti were exposed to test EOs and standard compounds dissolved in alcohol. Different concentrations of these EOs were prepared and 10 larvae were placed in each polyethylene plastic container with test solutions (100 mL) at a temperature of 27 °C and a 12 h photoperiod. After 24 h, the mortality of larvae in both the treated and control groups was evaluated in terms of the 50% and 90% lethal concentrations (LC50 and LC90, respectively). Dead larvae were distinguished by their lack of movement upon touching with the tip of a thin brush. The experiment was conducted in triplicate.
2.9. Data Analysis
The IC50 value of an essential oil was determined using GraphPad Prism version 8. All the experiments were performed in triplicate, and the findings are presented as the mean ± standard deviation. R and R Studio (version 4.3.0) was used to generate Venn Diagrams and a heatmap to analyze common compounds and their correlation in EOs from peel, leaf, and flower via steam and hydro-distillation.
3. Results and Discussion
3.1. Isolation of EOs and Yields
The EOs of the peel, leaf, and flower of C. sinensis cultivated in the Himalayan country of Nepal were extracted from hydro-distillation and steam distillation. The oil contents in its different parts showed considerable variation, with yield percentages ranging from 0.20 to 2.15% for hydro-distillation extraction methods and from 0.22 to 1.75% for steam distillation, as shown in Table 1 along with the percentage yield from previous studies.

Table 1.
Yield percentage of essential oil from citrus peel, leaf, and flower.
The current study revealed that the peel of C. sinensis, which contains many oil glands made up of secretory cavities lined by multiple layers of specialized epithelial cells, had a higher yield percentage of oils than the leaf and flower. The results are consistent with earlier research, since the fruit’s peel has a higher concentration of terpenoids, such as limonene, and several other bioactive chemicals [34,35]. A greater proportion of EOs was obtained from peels through hydro-distillation compared to steam distillation. In contrast, steam distillation produced a higher percentage of volatile components in the case of the leaves and flowers of C. sinensis. Different extraction techniques and analyses lead to various percentage compositions of molecules that give off aromas in EOs [36].
3.2. Chemical Composition of Citrus EOs
GC-MS was used to identify the volatile compounds found in the CEOs. A total of 63, 60, and 60 compounds were identified from the peel, leaf, and flower, respectively, of C. sinensis by the hydro-distillation method, and 60, 59, and 62 compounds were identified respectively by the steam distillation technique. The ion chromatograms of GC-MS analysis are displayed in Figure S1A–F. Twenty compounds are common in the EOs of C. sinensis peels, leaves, and flowers obtained from steam distillation, whereas EOs obtained by the hydro-distillation method possess 16 common compounds (Figure 1). Twelve compounds are common in all six samples. The major compounds such as limonene, sabinene, linalool, myrcene, β-pinene, etc. have almost similar percentages in the EOs obtained from the two different techniques, but the percentage of minor constituents varies with the isolation techniques.
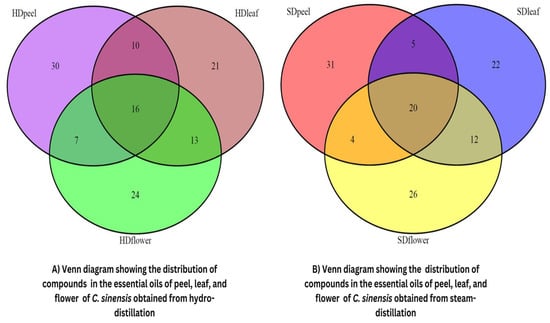
Figure 1.
Venn diagram showing the common number of majority components present in the essential oil of C. sinensis leaf, peel, and flower through hydro- and steam distillation techniques.
In the essential oil of C. sinensis peel, limonene is the most prominent compound, which comprises more than 90% of the total constituents (91.08% HD and 91.6% SD), followed by myrcene, linalool, sabinene, α-pinene, and terpinen-4-ol (Table 2). The findings validated Singh et al.’s exploration of 90.7% of the limonene in C. sinensis EOs [37]; 96.2% of limonene was likewise found in the EOs of C. sinensis, according to Michaelakis et al. [38]. Previous research has also reported similar findings [12]. Similarly, sabinene (36.91% HD and 32.89% SD) and limonene (21.35% HD and 19.71% SD) are the most dominant compounds in the EOs of C. sinensis leaf followed by terpinen-4-ol, myrcene, para-cymene, ꞵ-pinene, α-pinene, trans-ꞵ-ocimene, ẟ-3-carene, and the minor ones, almost comparable with the previously reported values [6,39,40]. The EOs of C. sinensis flower contain sabinene (39.46% HD and 38.05% SD), which is consistent with the previously reported value (31–48%) by Miguel et al. [32]. In addition, flower EOs contain linalool (8.27% HD and 7.12% SD) and ẟ-3-carene in significant amounts followed by myrcene, limonene, terpinen-4-ol, ꞵ-pinene, citronellal, α-pinene, and a minor amount of other terpenoids. The major constituents of citrus EOs from peels, leaves, and flowers are displayed in Table 2, and other constituents are displayed in Table S1.

Table 2.
Chemical composition of C. sinensis essential oil from the peel, leaf, and flower obtained by different extraction techniques.
Current research demonstrated that the percentage and quantity of constituents vary with the distillation methods. Although the major constituents and their percentages are almost similar in EOs obtained from both techniques, the numbers and percentages of minor constituents vary with the extraction technique. The obtained results are also supported by previous studies on other plants [41,42,43]. The findings of the current study demonstrate that EOs extracted from the peel, leaf, and flower contain 44, 34, and 43 common compounds, respectively, obtained from both the HD and SD extraction methods. The common constituents obtained from HD and SD are displayed by the Venn diagram in Figure 2.
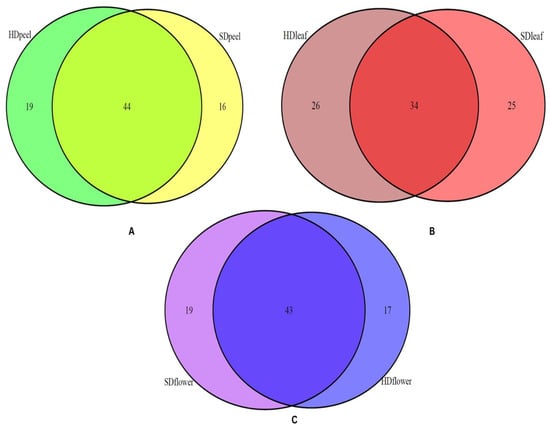
Figure 2.
Venn diagram displaying common constituents from two different distillation techniques in EOs of (A) peel (green and yellow color represent the total constituents obtained from HD and SD, respectively), (B) leaf (brown and red color represent the total constituents obtained from HD and SD, respectively), and (C) flower (blue and purple color represent the total constituents obtained from HD and SD, respectively).
The significant components found in different EOs from the peel, leaf, and flower are shown above in Figure 3. Active ingredients such as limonene, α-pinene, β-pinene, and β-myrcene are utilized as anesthetics and antiseptics in the perfume industry, flavorings, and medications [44]. Limonene is widely known for its anti-inflammatory and antimicrobial properties and pleasant fragrance [45]. Sabinene is a significant bicyclic monoterpene that occurs naturally and is employed in fine chemicals, advanced biofuels, flavorings, and perfume additives [46]. From the current study, sabinene was found to be dominant in the cases of leaf and flower, which was not reported earlier, so it can be a benchmark to study its pharmaco-activities and other biological activities. The chemical makeup of C. sinensis varies according to cultivars, harvest year, storage conditions, growing location, fruit variety, fruit part, climate, and degree of ripeness. Compounds, like β-sinensal and oxygenated sesquiterpenes, may be the maker compounds present in the EOs of the peel, leaf, and flower of C. sinensis, which have been reported from previous studies too [10,47]. The common compounds are also displayed in the heatmap diagram (Figure 4).
Figure 3.
Chemical structure of some significant compounds present in the peel, leaf, and flower of C. sinensis.
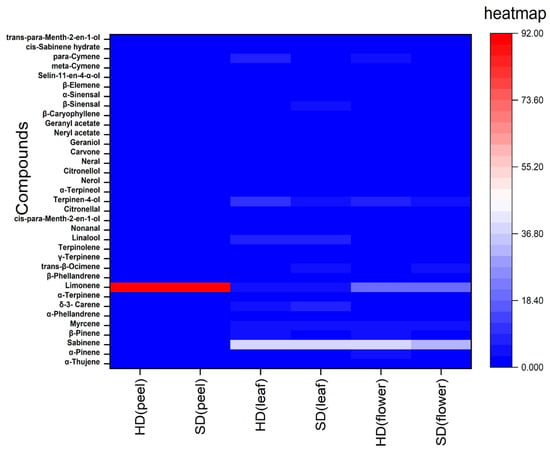
Figure 4.
Heatmap diagram showing the diversity and concentration of common chemical constituents in the leaf, flower, and peel of C. sinensis essential oil through hydro- and steam distillation, with different colors depending on the concentration and amount present.
3.3. Chiral Composition Analysis
The enantiomeric analysis depicts 8, 16, and 14 chiral terpenoids from the peel, leaf, and flower of C. sinensis, respectively. The relative percentage of the levorotatory and dextrorotatory forms of each chiral terpenoid are displayed in Table 3, and the ion chromatograms are displayed in Figure S2A–F.

Table 3.
Chiral distribution of essential oils of peel, leaf, and flower of C. sinensis.
Chiral GC-MS is one of the most widely used and effective techniques for authenticating and standardizing EOs [48]. During the biosynthesis of EOs in plant cells, high-purity enantiomeric compounds are formed due to the presence of stereoselective enzymes. So, it may be possible to distinguish between naturally occurring flavor molecules and nonstereoselective, synthesized racemic flavors. Chiral-GC may be used to evaluate the quality and distinguish between natural and artificial flavors [49]. Overall, 17 chiral compounds were discovered, with the most found in the essential oil leaf (16), flower (14), and peel (8). Aside from the leaf, both extraction techniques were found to contain identical types of enantiomeric chemicals in the flower and peel; nevertheless, the ratio of their composition varied somewhat in all types. Due to the inclusion of various carrier oils and other foreign substances, the EOs extracted from various plants may be contaminated. α-thujene, δ-3-carene, citronellal, and citronellol exist only in dextrorotatory form. Similarly, ꞵ-caryophyllene, ꞵ-elemene, and citronellyl acetate only exist in levorotatory form. Since terpenes make up a large portion of these oils, it is essential to determine how much of their overall activity is attributed to the parent EOs’ primary components. Previous research demonstrated that the active components in limonene are both its R-(+) and S-(−) enantiomers [38].
3.4. Antioxidant Activity
Antioxidant activity was undertaken by DPPH assay, and the IC50 value of each sample was evaluated. The IC50 value of peel, leaf, and flower essential oil through hydro- and steam distillation is displayed in Table 4. The antioxidant results revealed that EOs extracted by the steam-distillation technique exhibited significant antioxidant activities compared to EOs extracted from the hydro-distillation technique. The better antioxidant activity of EOs obtained from steam distillation may be due to the presence of additional ingredients and their synergistic or antagonistic effect on different compositional ratios. The result showed the most significant antioxidant activity in comparison to the previously reported value [50]. In general, EOs from peels, leaves, and flowers of citrus possess remarkable antioxidant activities.

Table 4.
IC50 value of samples from hydro- and steam distillation.
3.5. Acute Toxicity
Acute toxicity was employed to determine the minimum concentration of EOs responsible for serious toxicological effects or the deaths of animals. The result reveals that the essential oils from different parts do not exhibit noticeable toxicity even at high concentrations, i.e., 2000 mg/kg. The findings are consistent with previous studies [51]. Similarly, another study revealed that CEOs are nontoxic, noncarcinogenic, and nonmutagenic [52]. In agreement with these results, another study on acute toxicity assessment found limonene-rich essential oil to be safe and nonharmful [12,53] and suggested that it can be used safely in medical and biological applications. The oral acute toxicity of the oil extracted by both techniques is displayed in Table 5.

Table 5.
Acute toxicity (LD50) values of the peels, leaves, and flowers of C. sinensis.
3.6. Larvicidal Activity
Citrus essential oils from peels and leaves were tested against A. aegypti mosquito larvae (a vector of dengue fever) for its larvicidal activity. The peel’s EOs exhibited more effective larvicidal activities with low LC50 and LC90 values of 0.0183 and 0.0331 μL/mL, respectively, compared to the EOs of leaves having LC50 and LC90 values of 0.0479 and 0.0659 μL/mL, respectively (Table 6). The remarkable larvicidal activity of CEOs is also supported by a previous study of the larvicidal activity of CEOs against Anopheles labranchiae. The reported LC50 of CEOs against A. labranchiae was 0.07755 μL/mL [54]. Similarly, the larvicidal properties of β-cyclodextrin inclusion complexes of CEOs were also reported with an LC50 value of 0.02301 μL/mL [55].

Table 6.
Larvicidal activity of the CEOs.
Furthermore, the primary compounds responsible for the larvae’s deaths were identified by comparing the results with those of pure chemical compounds. The major constituent of citrus peel CEOs, d-limonene, exhibited the most significant larvicidal properties with an LC50 value of 0.0031 μL/mL, followed by β-pinene and p-cymene having LC50 0.0109 and 0.0148 μL/mL, respectively. On the other hand, d-carvone, sabinene, and linalool do not exhibit significant larvicidal properties. The results of larvicidal activity in terms of LC50 and LC90 along with mortality percentage at different concentrations are displayed in Table 6. The significant larvicidal activity of limonene was also supported by a previous study, in which limonene showed significant larvicidal activity against the larvae of Aedes albopictus with an LC50 of 39.7 µg/mL [56]. Additionally, (R)-(+)-limonene also showed remarkable larvicidal activity against larvae of A. albopictus and Culex pipiens with LC50 values of 9.7 ± 1.1 and 4.9 ± 0.5 mg/L, respectively [57]. Similarly, a comparable LC50 of β-pinene was reported (i.e., 12.1 ppm) against larvae of Aedes aegypti [58]. In addition, limonene, β-pinene, and p-cymene also exhibited larvicidal activity against larvae of Culex quinquefasciatus. But in contrast to the current study, sabinene, linalool, and d-carvone also exhibited noticeable larvicidal activity against larvae of C. quinquefasciatus [59]. The significant mortality rate found in citrus peels and leaves may therefore be attributable to the presence of the main chemical limonene, which also demonstrated strong larvicidal activity and can be taken into consideration for further research. Thus, the current study revealed that the EOs containing a high percentage of limonene show better larvicidal activities.
4. Conclusions
The results indicate that, apart from the fundamental chemical components, the yield, percentage composition, and properties of EOs vary with different extraction techniques. The EOs from peels demonstrated significantly better antioxidant and larvicidal properties compared to those from leaves. Similarly, toxicity analysis revealed that all three EOs are nontoxic and safe for use. On the other hand, the current study is the first to report the enantiomeric distribution and chiral composition of volatile substances in leaves and flowers. It can serve as the basis for future oil adulteration and authentication, as well as for the chemotaxonomy of C. sinensis. This study has paved the way for the potential commercial production of C. sinensis EOs in Nepal, as we observed similar volatile chemotypes and biological properties compared to those of other origins.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10060566/s1: Figure S1A–F: The ion chromatograms of compounds obtained from GC-MS analysis. Figure S2A–F: The ion chromatograms of enantiomeric compounds obtained from chiral GC-MS analysis. Table S1: Chemical Composition of Citrus sinensis essential oil by using GC/MS from peel, leaf, and flower.
Author Contributions
Conceptualization, N.P. and D.P.B.; methodology, D.P.B., R.R. and S.R.U.; validation, P.S. and A.A.; formal analysis, D.P.B., P.C., S.R.U. and R.R.; investigation, D.P.B., R.S., R.R. and S.R.U.; data curation, N.P., A.A. and P.S.; writing—original draft preparation, D.P.B., P.C. and S.R.U.; writing—review and editing, D.P.B., P.C., S.R.U., P.S., A.A. and N.P.; larvicidal activities, D.P.B. and R.R. supervision, N.P., A.A. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University Grants Commission, Nepal (PhD-77/78-S&T-05).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to express their gratitude toward the APRC, Lehi, USA, and the Natural Products Research Laboratory, Thapathali, Nepal, for laboratory support. The authors are thankful to Ambika Poudel, Aakash Ghimire, Anisha Pandey, Shrawan K. Regmi, Sanam Maharjan, and the support staff of NPRL for their technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the Origin and Evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, S.M.; Phi, N.T.L.; Sawamura, M. Chemical Composition of Peel Essential Oils of Sweet Oranges (Citrus sinensis) from Uganda and Rwanda. J. Essent. Oil Bear. Plants 2009, 12, 26–33. [Google Scholar] [CrossRef]
- Rodrigues Da Silva, L.; Silva, B. (Eds.) Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters Part I; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; ISBN 978-1-68108-239-4. [Google Scholar]
- Burt, S. Essential oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, V.; Paymal, N.; Quinton, C.; Tomi, F.; Luro, F. Investigations of the Chemical Composition and Aromatic Properties of Peel Essential oils throughout the Complete Phase of Fruit Development for Two Cultivars of Sweet Orange (Citrus sinensis (L.) Osb.). Plants 2022, 11, 2747. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, A.K.; Altyar, A.E.; Gad, H.A. Comparative Metabolic Study of Citrus sinensis Leaves Cultivars Based on GC–MS and Their Cytotoxic Activity. J. Pharm. Biomed. Anal. 2021, 198, 113991. [Google Scholar] [CrossRef]
- Li, G.; Xiang, S.; Pan, Y.; Long, X.; Cheng, Y.; Han, L.; Zhao, X. Effects of Cold-Pressing and Hydrodistillation on the Active Non-Volatile Components in Lemon Essential Oil and the Effects of the Resulting Oils on Aging-Related Oxidative Stress in Mice. Front. Nutr. 2021, 8, 689094. [Google Scholar] [CrossRef]
- Park, M.K.; Cha, J.Y.; Kang, M.; Jang, H.W.; Choi, Y. The Effects of Different Extraction Methods on Essential oils from Orange and Tangor: From the Peel to the Essential Oil. Food Sci. Nutr. 2024, 12, 804–814. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, K.; Deng, W.; Zhong, B.; Yang, W.; Chun, J. Chemical Composition and Antimicrobial Activity of Gannan Navel Orange (Citrus sinensis Osbeck Cv. Newhall) Peel Essential oils. Food Sci. Nutr. 2018, 6, 1431–1437. [Google Scholar] [CrossRef]
- Manzur, M.; Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Citrus sinensis Essential oils an Innovative Antioxidant and Antipathogenic Dual Strategy in Food Preservation against Spoliage Bacteria. Antioxidants 2023, 12, 246. [Google Scholar] [CrossRef]
- Bonaccorsi, I.; Sciarrone, D.; Cotroneo, A.; Mondello, L.; Dugo, P.; Dugo, G. Enantiomeric Distribution of Key Volatile Components in Citrus Essential oils. Rev. Bras. Farm. 2011, 21, 841–849. [Google Scholar] [CrossRef]
- Al Kamaly, O.; Numan, O.; Almrfadi, O.M.A.; Alanazi, A.S.; Conte, R. Separation and Evaluation of Potential Antioxidant, Analgesic, and Anti-Inflammatory Activities of Limonene-Rich Essential oils from Citrus sinensis (L.). Open Chem. 2022, 20, 1517–1530. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Tundis, R.; Loizzo, M.R.; Menichini, F. In Vitro Activities of Citrus medica L. Cv. Diamante (Diamante Citron) Relevant to Treatment of Diabetes and Alzheimer’s Disease. Phytother. Res. 2007, 21, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Fujiwara, R.; Tanida, M.; Nomura, J. Potential Antidepressant Effects of Lemon Odor in Rats. Eur. Neuropsychopharmacol. 1995, 5, 477–480. [Google Scholar] [CrossRef]
- Komori, T.; Fujiwara, R.; Tanida, M.; Nomura, J.; Yokoyama, M.M. Effects of Citrus Fragrance on Immune Function and Depressive States. Neuroimmunomodulation 1995, 2, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.; Setzer, W. Biological Activities and Safety of Citrus spp. Essential oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed]
- Toscano-Garibay, J.D.; Arriaga-Alba, M.; Sánchez-Navarrete, J.; Mendoza-García, M.; Flores-Estrada, J.J.; Moreno-Eutimio, M.A.; Espinosa-Aguirre, J.J.; González-Ávila, M.; Ruiz-Pérez, N.J. Antimutagenic and Antioxidant Activity of the Essential oils of Citrus sinensis and Citrus latifolia. Sci. Rep. 2017, 7, 11479. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Bouaziz, M.; Yangui, T.; Sayadi, S.; Dhouib, A. Disinfectant Properties of Essential oils from Salvia officinalis L. Cultivated in Tunisia. Food Chem. Toxicol. 2009, 47, 2755–2760. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 2007; Volume 4, p. 804. [Google Scholar]
- Satyal, P.; Jones, T.; Lopez, E.; McFeeters, R.; Ali, N.; Mansi, I.; Al-kaf, A.; Setzer, W. Chemotypic Characterization and Biological Activity of Rosmarinus Officinalis. Foods 2017, 6, 20. [Google Scholar] [CrossRef]
- Poudel, D.K.; Ojha, P.K.; Rokaya, A.; Satyal, R.; Satyal, P.; Setzer, W.N. Analysis of Volatile Constituents in Curcuma Species, Viz. C. Aeruginosa, C. Zedoaria, and C. Longa, from Nepal. Plants 2022, 11, 1932. [Google Scholar] [CrossRef]
- DeCarlo, A.; Johnson, S.; Okeke-Agulu, K.I.; Dosoky, N.S.; Wax, S.J.; Owolabi, M.S.; Setzer, W.N. Compositional Analysis of the Essential Oil of Boswellia Dalzielii Frankincense from West Africa Reveals Two Major Chemotypes. Phytochemistry 2019, 164, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Embarek, G.; Kokkalou, E.; Kefalas, P. Phenolic Profile and Antioxidant Activity of the Algerian Ripe Date Palm Fruit (Phoenix Dactylifera). Food Chem. 2005, 89, 411–420. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Gribaldo, L.; Gennari, A.; Blackburn, K.; Clemedson, C.; Deguercy, A.; Meneguz, A.; Pfaller, W.; Ruhdel, I. 3.1. Acute Toxicity. Altern. Lab. Anim. 2005, 33, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Saleem, U.; Rehman, A.-U.; Ahmad, B.; Froeyen, M.; Mirza, M.U.; Kee, L.Y.; Abdullah, I.; Ahmad, S. Toxicity Evaluation of the Naphthalen-2-Yl 3,5-Dinitrobenzoate: A Drug Candidate for Alzheimer Disease. Front. Pharmacol. 2021, 12, 607026. [Google Scholar] [CrossRef]
- WHO-2005. WHO. Instructions for Determining the Susceptibility or Resistance of Mosquito Larvae to Insecticides; WHO/VBC: Geneva, Switzerland, 1981; Volume 81, p. 807. [Google Scholar]
- Giatropoulos, A.; Papachristos, D.P.; Kimbaris, A.; Koliopoulos, G.; Polissiou, M.G.; Emmanouel, N.; Michaelakis, A. Evaluation of Bioefficacy of Three Citrus Essential oils against the Dengue Vector Aedes Albopictus (Diptera: Culicidae) in Correlation to Their Components Enantiomeric Distribution. Parasitol. Res. 2012, 111, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.P.; Poudel, D.K.; Satyal, P.; Khadayat, K.; Dhami, S.; Aryal, D.; Chaudhary, P.; Ghimire, A.; Parajuli, N. Volatile Compounds and Antioxidant and Antimicrobial Activities of Selected Citrus Essential oils Originated from Nepal. Molecules 2021, 26, 6683. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Vashist, H. Hydrodistillation and Comparative Report of Percentage Yield on Leaves and Fruit Peels from Different Citrus Plants of Rutaceae Family. J. Plant Sci. 2015, 10, 75–78. [Google Scholar] [CrossRef]
- Miguel, M.G.; Dandlen, S.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Duarte, A.; Faisca, J. Essential Oils of Flowers of Citrus sinensis and Citrus clementina Cultivated in Algarve, Portugal. Acta Hortic. 2008, 89–94. [Google Scholar] [CrossRef]
- Dewi, I.A.; Prastyo, A.M.; Wijana, S. Extraction of Essential Oil from Baby Java Orange (Citrus sinensis) Solid Waste Using Water and Steam Distillation. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012054. [Google Scholar] [CrossRef]
- Voo, S.S.; Grimes, H.D.; Lange, B.M. Assessing the Biosynthetic Capabilities of Secretory Glands in Citrus Peel. Plant Physiol. 2012, 159, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Brezo-Borjan, T.; Švarc-Gajić, J.; Morais, S.; Delerue-Matos, C.; Rodrigues, F.; Lončarević, I.; Pajin, B. Chemical and Biological Characterisation of Orange (Citrus sinensis) Peel Extracts Obtained by Subcritical Water. Processes 2023, 11, 1766. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus Essential oils: Extraction, Authentication and Application in Food Preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Mishra, P.K.; Dubey, N.K. Chemical Profile, Antifungal, Antiaflatoxigenic and Antioxidant Activity of Citrus Maxima Burm. and Citrus sinensis (L.) Osbeck Essential oils and Their Cyclic Monoterpene, Dl-Limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Michaelakis, A.; Papachristos, D.; Kimbaris, A.; Koliopoulos, G.; Giatropoulos, A.; Polissiou, M.G. Citrus Essential oils and Four Enantiomeric Pinenes against Culex pipiens (Diptera: Culicidae). Parasitol. Res. 2009, 105, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Eldahshan, O.A.; Halim, A.F. Comparison of the Composition and Antimicrobial Activities of the Essential oils of Green Branches and Leaves of Egyptian Navel Orange (Citrus sinensis (L.) Osbeck Var. Malesy). Chem. Biodivers. 2016, 13, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Kasali, A.A.; Lawal, O.A.; Eshilokun, A.O.; Olaniyan, A.A.; Opoku, A.R.; Setzer, W.N. Citrus Essential Oil of Nigeria. Part V: Volatile Constituents of Sweet Orange Leaf Oil (Citrus sinensis). Nat. Prod. Commun. 2011, 6, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Grzeszczuk, M.; Jadczak, D. Comparison of the Chemical Composition of Essential oils Isolated by Water-Steam Distillation and Hydrodistillation from Garden Thyme (Thymus vulgaris L.). J. Essent. Oil Bear. Plants 2016, 19, 832–842. [Google Scholar] [CrossRef]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Comparison of Chemical Composition and Biological Properties of Essential oils Obtained by Hydrodistillation and Steam Distillation of Laurus nobilis L. Plant Foods Hum. Nutr. 2020, 75, 495–504. [Google Scholar] [CrossRef]
- Bhalla, P.; Varshney, V.K. Comparative Study of Hydro- and Steam-Water Distillation for Isolation of Essential Oils from Needles of Cupressus Torulosa D. Don. J. Essent. Oil Bear. Plants 2023, 26, 1161–1171. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus Lemon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect against Listeria Monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Meryem, S.; Mohamed, D.; Nour-eddine, C.; Faouzi, E. Chemical Composition, Antibacterial and Antioxidant Properties of Three Moroccan Citrus Peel Essential oils. Sci. Afr. 2023, 20, e01592. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Liu, H.; Liu, W.; Zhang, R.; Xian, M.; Liu, H. Biosynthesis and Production of Sabinene: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1535–1544. [Google Scholar] [CrossRef]
- Dongre, P.; Doifode, C.; Choudhary, S.; Sharma, N. Botanical Description, Chemical Composition, Traditional Uses and Pharmacology of Citrus sinensis: An Updated Review. Pharmacol. Res.-Mod. Chin. Med. 2023, 8, 100272. [Google Scholar] [CrossRef]
- Paudel, P.N.; Satyal, P.; Setzer, W.N.; Awale, S.; Watanabe, S.; Maneenet, J.; Satyal, R.; Acharya, A.; Phuyal, M.; Gyawali, R. Chemical-Enantiomeric Characterization and In-Vitro Biological Evaluation of the Essential oils from Elsholtzia Strobilifera (Benth.) Benth. and E. blanda (Benth.) Benth. from Nepal. Nat. Prod. Commun. 2023, 18, 1934578X231189325. [Google Scholar] [CrossRef]
- Hong, J.H.; Khan, N.; Jamila, N.; Hong, Y.S.; Nho, E.Y.; Choi, J.Y.; Lee, C.M.; Kim, K.S. Determination of Volatile Flavour Profiles of Citrus Spp. Fruits by SDE-GC–MS and Enantiomeric Composition of Chiral Compounds by MDGC–MS. Phytochem. Anal. 2017, 28, 392–403. [Google Scholar] [CrossRef]
- Torres-Alvarez, C.; Núñez González, A.; Rodríguez, J.; Castillo, S.; LEssential oils-Rivas, C.; Báez-González, J.G. Chemical Composition, Antimicrobial, and Antioxidant Activities of Orange Essential Oil and Its Concentrated Oils. CyTA-J. Food 2016, 15, 129–135. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In Vitro Evaluation of Acute Toxicity of Five Citrus Spp. Essential oils towards the Parasitic Mite Varroa Destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R.; Young, R. Essential Oil Profiles. In Essential Oil Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 187–482. ISBN 978-0-443-06241-4. [Google Scholar]
- Miya, G.; Nyalambisa, M.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical Profiling, Toxicity and Anti-Inflammatory Activities of Essential oils from Three Grapefruit Cultivars from KwaZulu-Natal in South Africa. Molecules 2021, 26, 3387. [Google Scholar] [CrossRef]
- El-Akhal, F.; Lalami, A.E.O.; Guemmouh, R. Larvicidal Activity of Essential oils of Citrus sinensis and Citrus aurantium (Rutaceae) Cultivated in Morocco against the Malaria Vector Anopheles labranchiae (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 2015, 5, 458–462. [Google Scholar] [CrossRef]
- Galvão, J.G.; Silva, V.F.; Ferreira, S.G.; França, F.R.M.; Santos, D.A.; Freitas, L.S.; Alves, P.B.; Araújo, A.A.S.; Cavalcanti, S.C.H.; Nunes, R.S. β-Cyclodextrin Inclusion Complexes Containing Citrus sinensis (L.) Osbeck Essential Oil: An Alternative to Control Aedes aegypti Larvae. Thermochim. Acta 2015, 608, 14–19. [Google Scholar] [CrossRef]
- Jian, R.; Lin, Y.; Li, Y.; Wu, W.; Ren, X.; Liang, Z.; Kong, L.; Cai, J.; Lao, C.; Wu, M.; et al. Larvicidal Activity of Two Rutaceae Plant Essential oils and Their Constituents Against Aedes albopictus (Diptera: Culicidae) in Multiple Formulations. J. Med. Entomol. 2022, 59, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Theochari, I.; Giatropoulos, A.; Papadimitriou, V.; Karras, V.; Balatsos, G.; Papachristos, D.; Michaelakis, A. Physicochemical Characteristics of Four Limonene-Based Nanoemulsions and Their Larvicidal Properties against Two Mosquito Species, Aedes albopictus and Culex pipiens molestus. Insects 2020, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Gonzalez Audino, P.; Seccacini, E.; Licastro, S.; Zerba, E.; Masuh, H. Larvicidal effect of Eucalyptus grandis essential oil and turpentine and their major components on Aedes aegypti larvae. J. Am. Mosq. Control Assoc. 2007, 23, 299–303. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Correa-Basurto, J.; Rodríguez-Valdez, L.M.; Sánchez-Torres, L.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. In Vitro and in Silico Studies of Terpenes, Terpenoids and Related Compounds with Larvicidal and Pupaecidal Activity against Culex quinquefasciatus Say (Diptera: Culicidae). Chem. Cent. J. 2018, 12, 53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).