A Review of Traditional Applications, Geographic Distribution, Botanical Characterization, Phytochemistry, and Pharmacology of Hypericum ascyron L.
Abstract
1. Introduction
2. Materials and Methods
3. Traditional Applications
4. Geographical Distribution
5. Botanical Description
6. Phytochemistry
6.1. Phloroglucinols
6.2. Xanthones and Dibenzo-1,4-dioxane Derivatives
6.3. Flavonoids
6.4. Phenolics
6.5. Steroids and Triterpenoids
6.6. Volatile Components
6.7. Other Components
7. Pharmacology
7.1. Antioxidant Activity
7.2. Antidiabetic Activity
7.3. Anti-Inflammatory and Analgesic Effects
7.4. Antidepressant Activity
7.5. Cytotoxicity
7.6. Antimicrobial Activity
7.7. Hepatoprotective Effect
7.8. Other Pharmacological Effects
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hashida, W.; Tanaka, N.; Kashiwada, Y.; Sekiya, M.; Ikeshiro, Y.; Takaishi, Y. Tomoeones A-H, cytotoxic phloroglucinol derivatives from Hypericum ascyron. Phytochemistry 2008, 69, 2225–2230. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Liu, J.; Sun, B.; Wei, G.; Li, Y.; Zhang, J.; Yao, G.; Luo, Z.; Xue, Y.; et al. Hyperascyrones A-H, polyprenylated spirocyclic acylphloroglucinol derivatives from Hypericum ascyron Linn. Phytochemistry 2015, 115, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.M.; Long, X.W.; Yang, X.W.; Xia, F.; Khan, A.; Yan, H.; Deng, J.; Li, X.; Xu, G. seco-Polycyclic polyprenylated acylphloroglucinols with unusual carbon skeletons from Hypericum ascyron. Tetrahedron Lett. 2017, 58, 2113–2117. [Google Scholar] [CrossRef]
- Hu, J.W.; Shi, M.J.; Wang, J.J.; Li, L.; Jiang, J.D.; Ji, T.F. Methylated polycyclic polyprenylated acylphloroglucinol derivatives from Hypericum ascyron. J. Nat. Prod. 2018, 81, 2348–2356. [Google Scholar] [CrossRef]
- Zhen, B.; Hu, J.W.; Wang, J.J.; Shi, M.J.; Li, L.; Ci, R.; Jiang, J.D.; Ji, T.F. Hyperascyrins L-N, rare methylated polycyclic polyprenylated acylphloroglucinol derivatives from Hypericum ascyron. J. Asian Nat. Prod. Res. 2019, 21, 409–418. [Google Scholar] [CrossRef]
- Li, Z.P.; Kim, J.Y.; Ban, Y.J.; Park, K.H. Human neutrophil elastase (HNE) inhibitory polyprenylated acylphloroglucinols from the flowers of Hypericum ascyron. Bioorg. Chem. 2019, 90, 103075. [Google Scholar] [CrossRef]
- Niwa, K.; Tanaka, N.; Tatano, Y.; Yagi, H.; Kashiwada, Y. Hypascyrins A-E, prenylated acylphloroglucinols from Hypericum ascyron. J. Nat. Prod. 2019, 82, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xia, J.; Qian, M.Y.; Wang, X.R.; Hu, B.; Liu, X.S.; Wu, L. Ascyrones A-E, type B bicyclic ployprenylated acylphloroglucinol derivatives from Hypericum ascyron. Chin. J. Nat. Med. 2022, 20, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Yue, G.G.; Li, X.R.; Xu, G.; Lau, C.B. Structurally diverse spirocyclic polycyclic polyprenylated acylphloroglucinols from Hypericum ascyron Linn. and their anti-tumor activity. Phytochemistry 2023, 212, 113727. [Google Scholar] [CrossRef]
- Zhang, E.H.; Chen, Y.; Zhang, L. Antidepressant polyprenylated acylphloroglucinols from Hypericum ascyron. J. Asian Nat. Prod. Res. 2023, 23, 474–481. [Google Scholar] [CrossRef]
- Tian, L.R. Chemical and Bioactive Studies on Hypericum ascyron L. and Morus alba Linn. Master’s Thesis, Shandong University, Jinan, China, 2023. [Google Scholar]
- Hu, L.H.; Yip, S.C.; Sim, K.Y. Xanthones from Hypericum ascyron. Phytochemistry 1999, 52, 1371–1373. [Google Scholar] [CrossRef]
- Chae, S.; Lee, S.Y.; Kim, J.S.; Bae, K.H.; Kim, S.K.; Kang, S.S. Constituents from Hypericum ascyron. Kor. J. Pharmacogn. 2006, 37, 162–168. [Google Scholar]
- Hashida, W.; Tanaka, N.; Takaishi, Y. Prenylated xanthones from Hypericum ascyron. J. Nat. Med. 2007, 61, 371–374. [Google Scholar] [CrossRef]
- Li, X.M.; Luo, X.G.; Si, C.L.; Wang, N.; Zhou, H.; He, J.F.; Zhang, T.C. Antibacterial active compounds from Hypericum ascyron L. induce bacterial cell death through apoptosis pathway. Eur. J. Med. Chem. 2015, 96, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Luo, X.G.; Li, K.; Wang, N.; Hua, E.B.; Zhang, Y.; Zhang, T.C. Difference in protective effects of three structurally similar flavonoid glycosides from Hypericum ascyron against H2O2-induced injury in H9c2 cardiomyoblasts. Mol. Med. Rep. 2015, 12, 5423–5428. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.J.; Oh, K.Y.; Kim, D.; Song, H.; Kim, J.Y.; Oh, S.; Ryu, H.W. Metabolomic profiling, antioxidant and anti-inflammatory activities of Hypericum species growing in South Korea. Nat. Prod. Commun. 2017, 12, 1041–1044. [Google Scholar] [CrossRef]
- Zhang, X.F. Polyphenol Chemical Constituents from Two Hypericum. Master’s Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2017. [Google Scholar]
- Yan, M.; Xiao, S.; Chen, F.; Zhou, Y. Chemical constituents of Hypericum ascyron L. Nat. Prod. Res. Dev. 2014, 26, 1785–1788. [Google Scholar] [CrossRef]
- Park, H.J.; Kwon, S.H.; Yun, S.Y.; Lee, K.T. Isolation of steroids and flavonoids from the herbs of Hypericum ascyron L. Kor. J. Pharmacogn. 2000, 31, 39–44. [Google Scholar]
- Gao, Y.; Han, L.; Sun, L.; Zheng, D.; Huang, X.S.; Yu, S.S. Chemical constituents from Hypericum ascyron. Chin. J. Nat. Med. 2007, 5, 413–416. [Google Scholar]
- Zhao, J.L. Study on Chemical Constituents of Hypericum ascyron. Master’s Thesis, Anhui University, Hefei, China, 2020. [Google Scholar] [CrossRef]
- Yang, P. Study of Hypericum ascyron L. Antidepressant Effect and Volatile Oil Component Analysis. Master’s Thesis, Heilongjiang University of Chinese Medicine, Harbin, China, 2008. [Google Scholar]
- Xu, F.; Gao, W.; Xing, J.; Zhao, J.; Xu, F.; Ji, T.; Gu, Z. Study on the chemical constituents of Hypericum ascyron L. Northwest Pharm. J. 2016, 31, 331–333. [Google Scholar]
- Wang, J.; Wang, N.; Yao, X.; Ishii, R.; Kitanaka, S. Inhibitory activity of Chinese herbal medicines toward histamine release from mast cells and nitric oxide production by macrophage-like cell line, RAW 264.7. J. Nat. Med. 2006, 60, 73–77. [Google Scholar] [CrossRef]
- Lv, J.M.; Jia, W.; Li, C.Y. The anti-inflammatory and analgesic effects of Hypericum ascyron L. extract. Pract. Clin. J. Integr. Tradit. Chin. West. Med. 2008, 8, 87–89. [Google Scholar]
- Lee, Y.Y.; Yang, W.K.; Han, J.E.; Kwak, D.; Kim, T.H.; Saba, E.; Kim, S.D.; Lee, Y.C.; Kim, J.S.; Kim, S.H.; et al. Hypericum ascyron L. extract reduces particulate matter-induced airway inflammation in mice. Phytother. Res. 2021, 35, 1621–1633. [Google Scholar] [CrossRef]
- Chon, S.U.; Heo, B.G.; Park, Y.S.; Kim, D.K.; Gorinstein, S. Total phenolics level, antioxidant activities and cytotoxicity of young sprouts of some traditional Korean salad plants. Plant Foods Hum. Nutr. 2009, 64, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Song, Y.; Zhang, L. α-Glucosidase inhibitory and antioxidant properties and antidiabetic activity of Hypericum ascyron L. Med. Chem. Res. 2011, 20, 809–816. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, G.W.; Pan, J.H.; Yan, J.K. Studies on the α-glucosidase inhibitory effect and antioxidant activity of total flavonoids from Hypericum ascyron L. Chin. J. Anal. Lab. 2013, 32, 11–15. [Google Scholar] [CrossRef]
- Li, X.M.; Luo, X.G.; Wang, N.; Zhou, H.; Si, C.L.; Li, K.; Ma, N.; Zhang, T.C. The extract of Hypericum ascyron L. induces bacterial cell death through apoptosis pathway. J. Ethnopharmacol. 2015, 166, 205–210. [Google Scholar] [CrossRef]
- Niwa, K.; Tanaka, N.; Shimomoto, Y.; Tsuji, D.; Kim, S.Y.; Kojoma, M.; Itoh, K.; Chen, C.H.; Lee, K.H.; Kashiwada, Y. Hyperdioxanes, dibenzo-1,4-dioxane derivatives from the roots of Hypericum ascyron. J. Nat. Med. 2021, 75, 907–914. [Google Scholar] [CrossRef]
- Li, X.M.; Luo, X.G.; Ma, N.; Li, K.; Li, W.; Ma, D.Y.; Zhang, T.C. Quality and antitumour activity evaluation of extract of Hypericum ascyron. Biomed. Chromatogr. 2015, 29, 47–52. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Gao, Q.; Cao, X.; Li, Y.; Li, X.; Min, Z.; Yu, Y.; Guo, Y.; Shuai, L. Extractive from Hypericum ascyron L. promotes serotonergic neuronal differentiation in vitro. Stem Cell Res. 2018, 31, 42–50. [Google Scholar] [CrossRef]
- Zhang, G.W.; Zhou, J.; Liu, Y.H. Chromatographic analysis of total flavonoids from Hypericum ascyron L.and its tyrosinase inhibitory effect. J. Nanchang Univ. Nat. Sci. 2013, 37, 448–451. [Google Scholar]
- Zhou, J.; Zhang, G.W.; Hu, M.M. Inhibitory effect on xanthine oxidase and antioxidant activity of total flavonoids from Hypericum ascyron L. J. Food Sci. Biotechnol. 2013, 32, 353–357. [Google Scholar]
- Li, Y.X.; Meng, Q.M. Herbalogical study on Forsythiae fructus. J. Chin. Med. Mater. 2002, 25, 435–437. [Google Scholar]
- Jiangsu New Medical College. Dictionary of Traditional Chinese Medicine, 1st ed.; Shanghai People’s Publishing House: Shanghai, China, 1977; pp. 1002–1003. [Google Scholar]
- Flora of China Editorial Committee of Chinese Academy of Sciences. The Flora of China; Science Press: Beijing, China, 1990; Volume 50, pp. 43–45. [Google Scholar]
- Fu, L.G.; Chen, T.Q.; Lang, K.Y.; Hong, T.; Lin, Q. Higher Plants of China; Qingdao Publishing House: Qingdao, China, 2000; Volume 4. [Google Scholar]
- Celaj, O.; Durán, A.G.; Cennamo, P.; Scognamiglio, M.; Fiorentino, A.; Esposito, A.; D’Abrosca, B. Phloroglucinols from Myrtaceae: Attractive targets for structural characterization, biological properties and synthetic procedures. Phytochem. Rev. 2021, 20, 259–299. [Google Scholar] [CrossRef]
- Xu, F.H.; Ding, Y.; Wang, Q. Chemical constituents of ethyl acetate fraction from Hypericum ascyron. J. Chin. Med. Mater. 2016, 39, 322–325. [Google Scholar] [CrossRef]
- Hu, Y.L.; Hu, K.; Kong, L.M.; Xia, F.; Yang, X.W.; Xu, G. Norascyronones A and B, 2,3,4-nor-polycyclic polyprenylated acylphloroglucinols from Hypericum ascyron. Org. Lett. 2019, 21, 1007–1010. [Google Scholar] [CrossRef]
- Zheng, Q.M.; Qin, L.P.; Zheng, H.C.; Guo, C.; Chen, Y.; Zhang, C.; Zhang, Q.Y.; Han, T. Quantitative phytochemical analysis of eleven Hypericum species growing in China. Acad. J. Second Mil. Med. Univ. 2003, 24, 457–459. [Google Scholar]
- Xiao, C.Y.; Mu, Q.; Gibbons, S. The phytochemistry and pharmacology of Hypericum. Prog. Chem. Org. Nat. Prod. 2020, 112, 85–182. [Google Scholar] [CrossRef]
- Panda, S.S.; Chand, M.; Sakhuja, R.; Jain, S.C. Xanthones as potential antioxidants. Curr. Med. Chem. 2013, 20, 4481–4507. [Google Scholar] [CrossRef]
- Niwa, K.; Tanaka, N.; Kim, S.Y.; Kojoma, M.; Kashiwada, Y. Hyperdioxane A, a conjugate of dibenzo-1,4-dioxane and sesquiterpene from Hypericum ascyron. Org. Lett. 2018, 20, 5977–5980. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, S.I.; You, Y.O.; Yu, H.H.; Han, S.H.; Kim, K.J. Xanthone with antibacterial activity against methicillin-resistant Staphylococcus aureus from Hypericum ascyron L. J. Dent. Res. 2003, 82, B81. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Komissarenko, N.F.; Levashova, I.G.; Zhdanova, V.P. Flavonoids of Hypericum ascyron. Chem. Nat. Compd. 1992, 28, 508. [Google Scholar] [CrossRef]
- Li, G.X. Studies on the Bioactive Constituents of Hypericum ascyron L. Master’s Thesis, Yanbian University, Yanji, China, 2011. [Google Scholar]
- Wang, Z.; Wang, X. Studies on the constituents of honghanlian (Hypericum ascyron L.). Acta Pharm. Sin. 1980, 15, 365–367. [Google Scholar]
- Song, Y.L. Studies on Active Constituents of Hypericum ascyron, Haloxylon ammodendron, Sophora japonica L. and Quercus aliena Blume. Master’s Thesis, Henan University, Zhengzhou, China, 2010. [Google Scholar]
- Huang, X.L. Preliminary studies on the constituents of flavonoids in Hypericum ascyron L. Acta Sci. Nat. Univ. Sunyatseni 1980, 19, 101–104. [Google Scholar]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar] [CrossRef]
- Zang, L.; Xu, H.; Huang, C.; Wang, C.; Wang, R.; Chen, Y.; Wang, L.; Wang, H. A link between chemical structure and biological activity in triterpenoids. Recent Pat. Anticancer Drug Discov. 2022, 17, 145–161. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Biological activity and structural diversity of steroids containing aromatic rings, phosphate groups, or halogen atoms. Molecules 2023, 28, 5549. [Google Scholar] [CrossRef]
- Chen, C.; Wei, G.; Zhu, H.; Guo, Y.; Li, X.N.; Zhang, J.; Liu, Y.; Yao, G.; Luo, Z.; Xue, Y.; et al. A new 3,4-seco-oleanane-type triterpenoid with an unusual enedione moiety from Hypericum ascyron. Fitoterapia 2015, 103, 227–230. [Google Scholar] [CrossRef]
- Zhu, H.C. Studies on the Chemical Constituents and Bioactivities of Hypericum sampsonii and Hypericum ascyron. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2014. [Google Scholar]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential oils and health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar]
- Hu, Y.L.; Li, X.R.; Xu, G. Carascynol A, a hybrid of caryophyllane-type terpenoid and a C6 unit degraded by polyprenylated acylphloroglucinols from Hypericum ascyron. Nat. Prod. Bioprospect. 2022, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. Study on the Extraction, Purification and Biological Activity of Flavonoids from Hypericum ascyron L. Master’s Thesis, Nanchang University, Nanchang, China, 2013. [Google Scholar]
- Jung, H.; Moon, T.C.; Lee, E.; Son, K.H.; Kim, H.P.; Kang, S.S.; Bae, K.H.; An, R.B.; Kwon, D.Y.; Chang, H.W. Screening of arachidonic acid cascade related enzymes inhibitors from Korean indigenous plants (2). J. Pharm. Soc. Korea. 2003, 47, 69–77. [Google Scholar]
- Hong, E.J.; Park, H.J.; Kim, N.H.; Jo, J.B.; Lee, J.E.; Su-Bin; Lim, S.B.; Ahn, D.H.; Jung, H.Y.; Cho, Y.J. Inhibitory effect of Hypericum ascyron on pro-inflammatory responses in lipopolysaccharide-induced RAW 264.7 cells. J. Appl. Biol. Chem. 2017, 60, 363–372. [Google Scholar] [CrossRef]
- Meng, L.K.; Li, J.; Zhang, C.L.; Gao, C.J.; Wu, Z.H. Study on the selection of active sites in the effect of Hypericum ascyron L. on adjuvant arthritis in rats. Chin. Med. Mod. Distance Educ. China 2020, 18, 127–129. [Google Scholar]
- Li, X.M.; Luo, X.G.; He, J.F.; Wang, N.; Zhou, H.; Yang, P.L.; Zhang, T.C. Induction of apoptosis in human cervical carcinoma HeLa cells by active compounds from Hypericum ascyron L. Oncol. Lett. 2018, 15, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Gibbons, S.; Stavri, M.; Smith, E.; Zhou, F.S.; Hu, C.Q. Antibacterial effects of Hypericum ascyron. and Hypericum japonicum. against multidrug-resistant Staphylococcus aureus. Pharm. Biol. 2006, 44, 157–159. [Google Scholar] [CrossRef]
- Diao, L.; Chen, H.J. A study on the protective effect of extracts from Hypericum ascyron L. on carbon tetrachloride and alcohol induced liver injury in mice. Heilongjiang Anim. Sci. Vet. Med. 2015, 39, 151–153. [Google Scholar]
- Jiang, C.T.; Xu, Z.Y.; Ni, G.Y.; Wang, R.R.; Chen, X.K. Clinical observation on the treatment of 100 cases of asthmatic chronic bronchitis with Hypericum ascyron L. Anhui Med. J. 1980, 3, 38–40. [Google Scholar]
- Zhou, Z.D.; Han, C.H.; Chen, S.H.; Wang, P.; Wang, J. Pharmacological research on Hypericum ascyron L. Anhui Med. J. 1980, 3, 48–51. [Google Scholar]
- Lee, E.H.; Kim, M.U.; Kang, I.K.; Park, K.I.; Cho, Y.J. Inhibitory activities of extracts from Hypericum ascyron L. on biological enzymes. J. Korean Soc. Food Sci. Nutr. 2018, 47, 7–14. [Google Scholar] [CrossRef]
- Li, Y.B.; Ge, Z.H.; Jia, Y.L.; Ren, Y.T. Experimental study on the in vitro elimination of cerebral cysticercosis by Hypericum ascyron L. Inf. Tradit. Chin. Med. 1995, 12, 47. [Google Scholar]



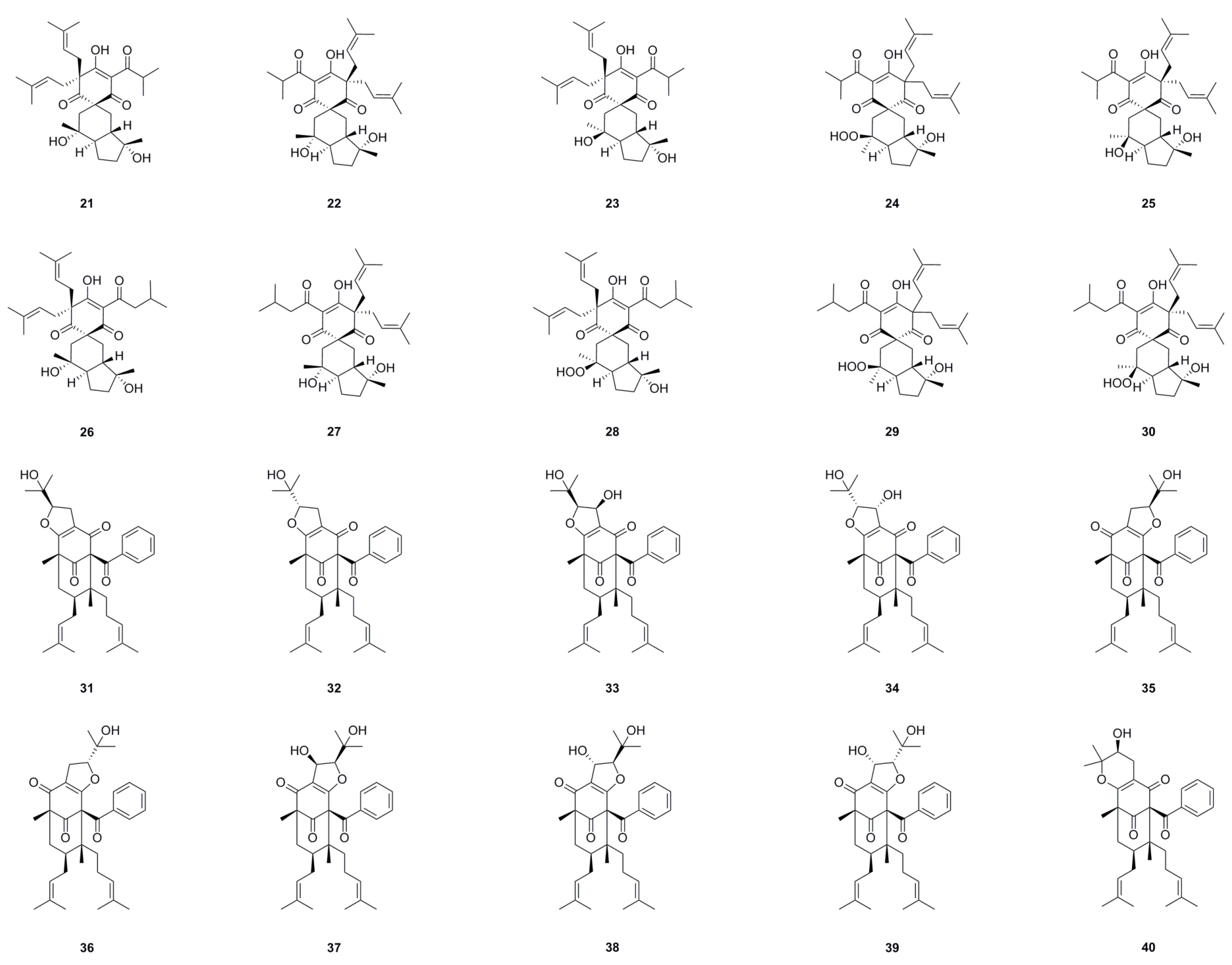
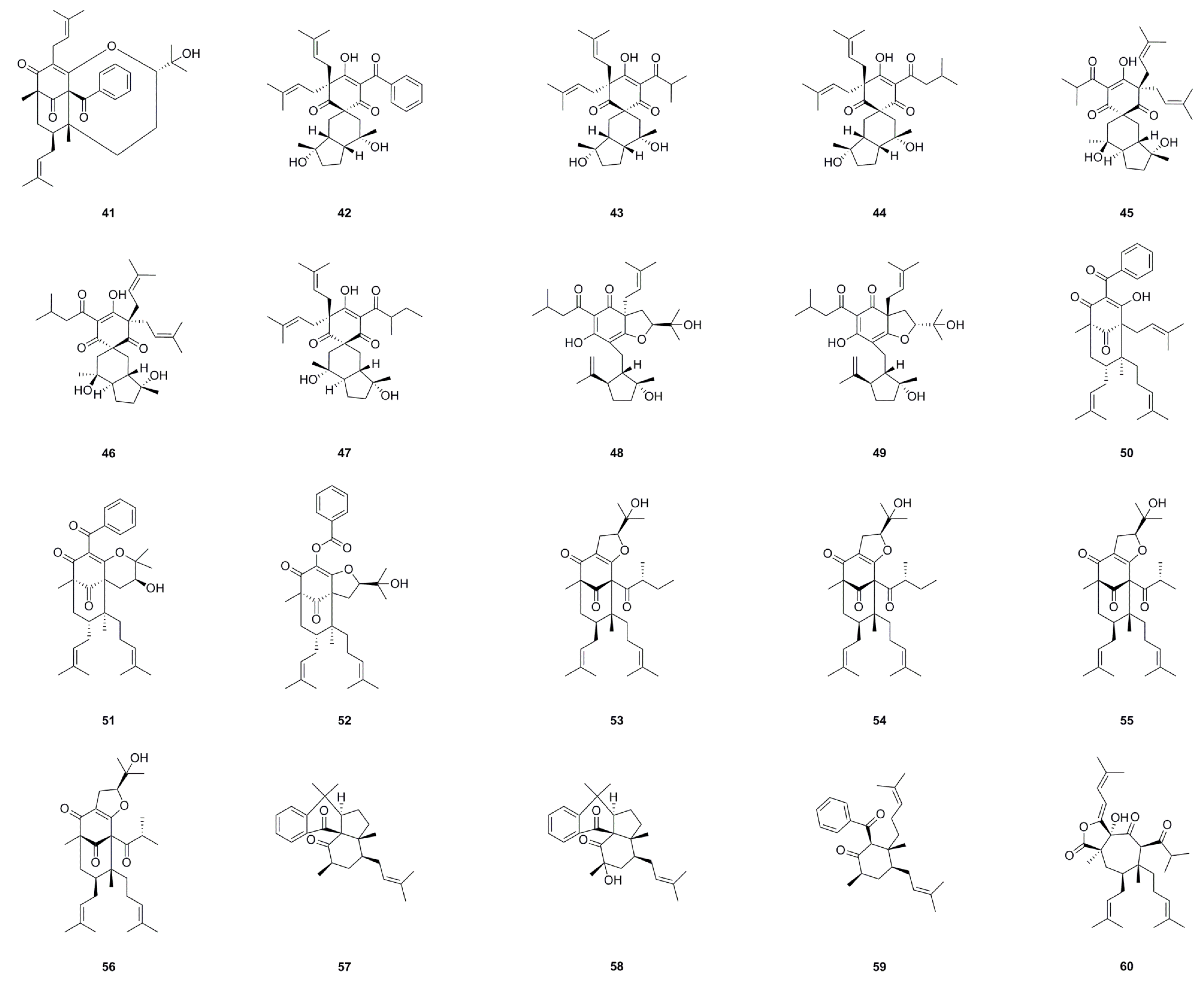
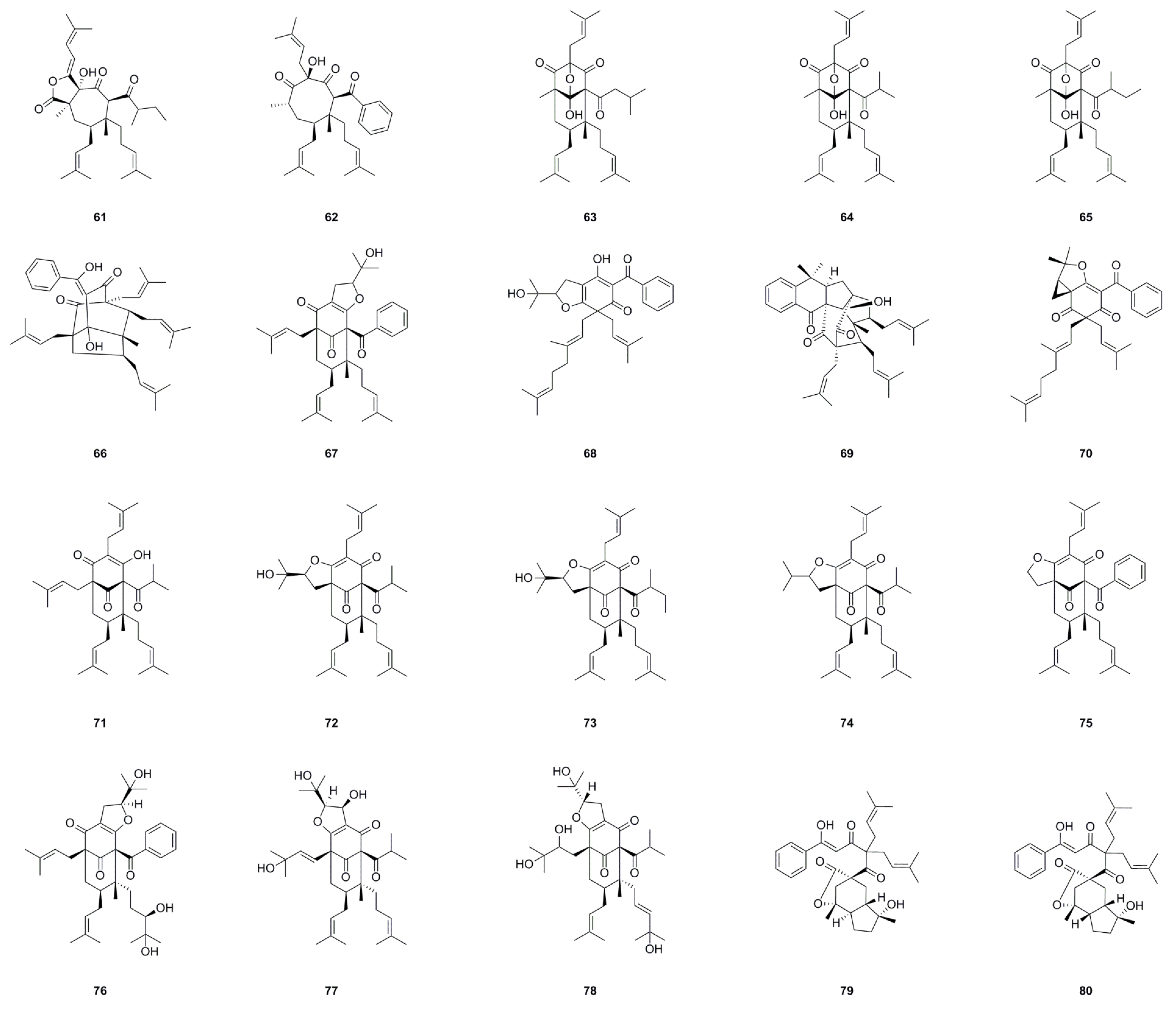
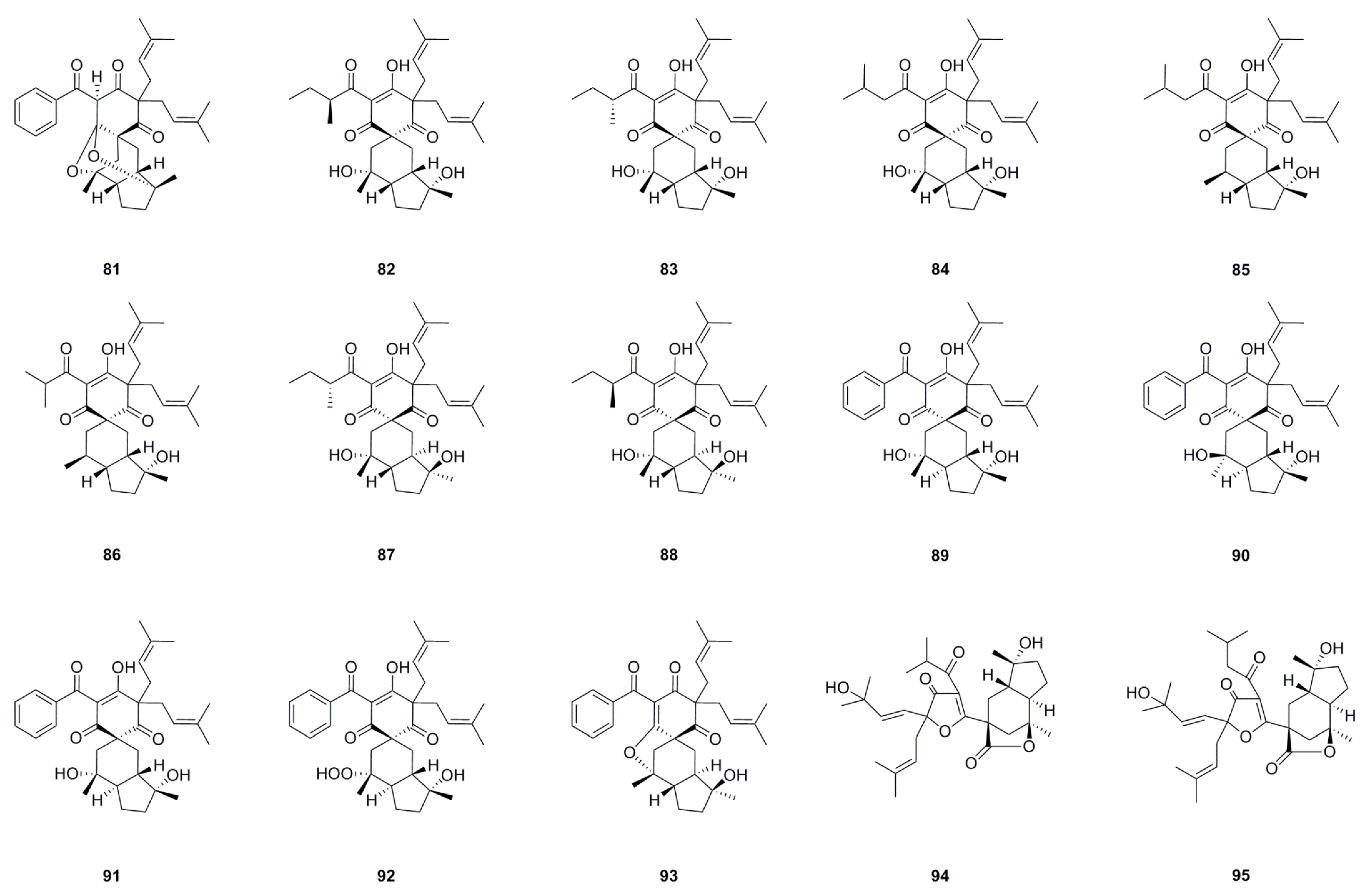
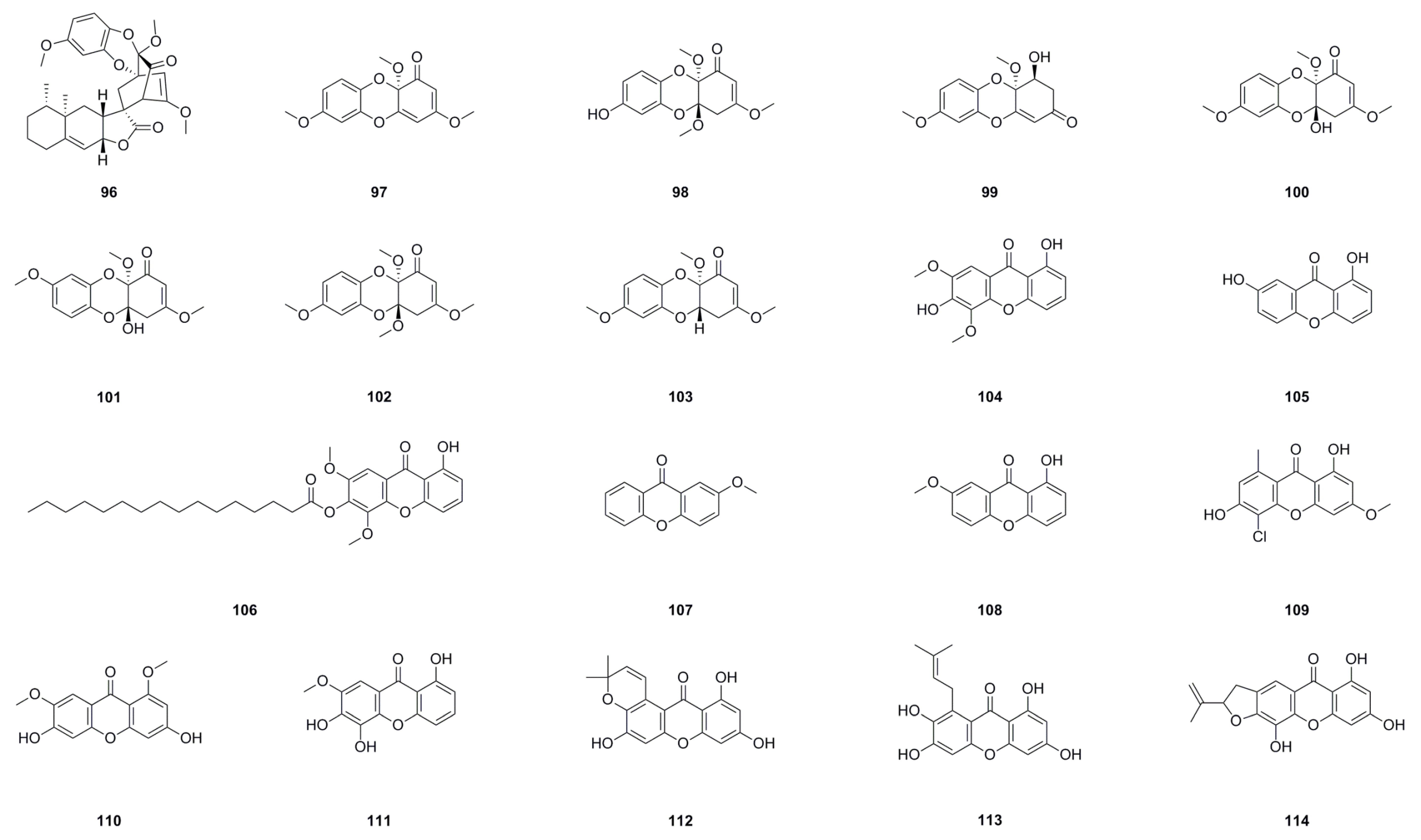

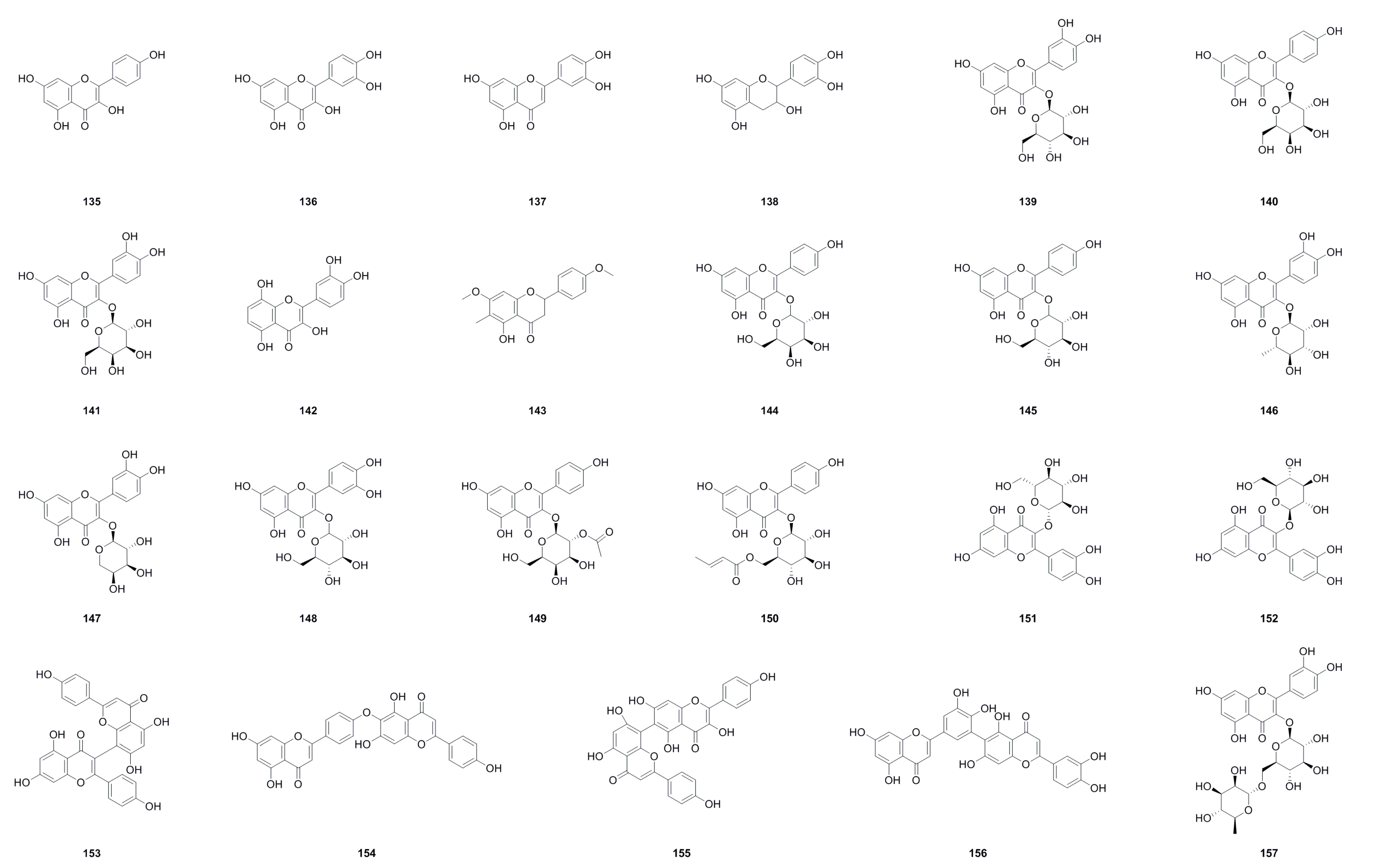


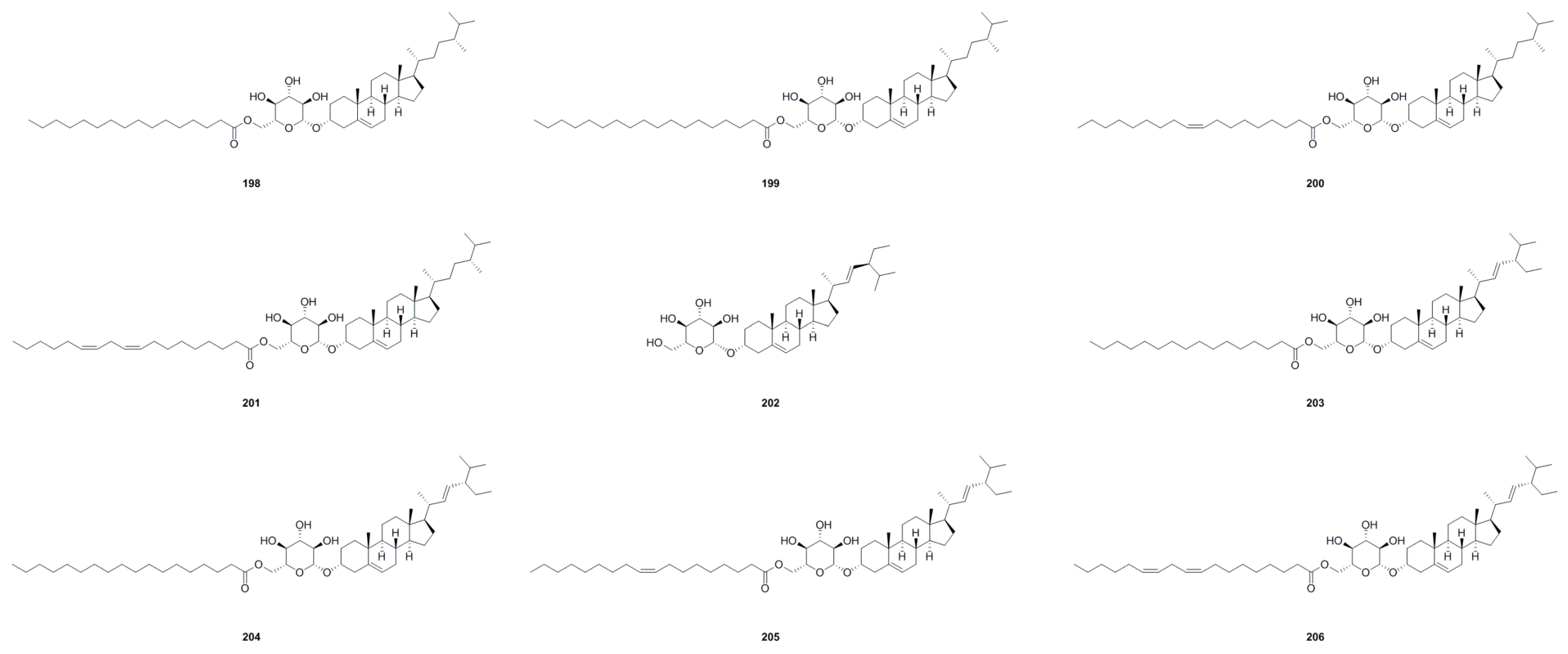
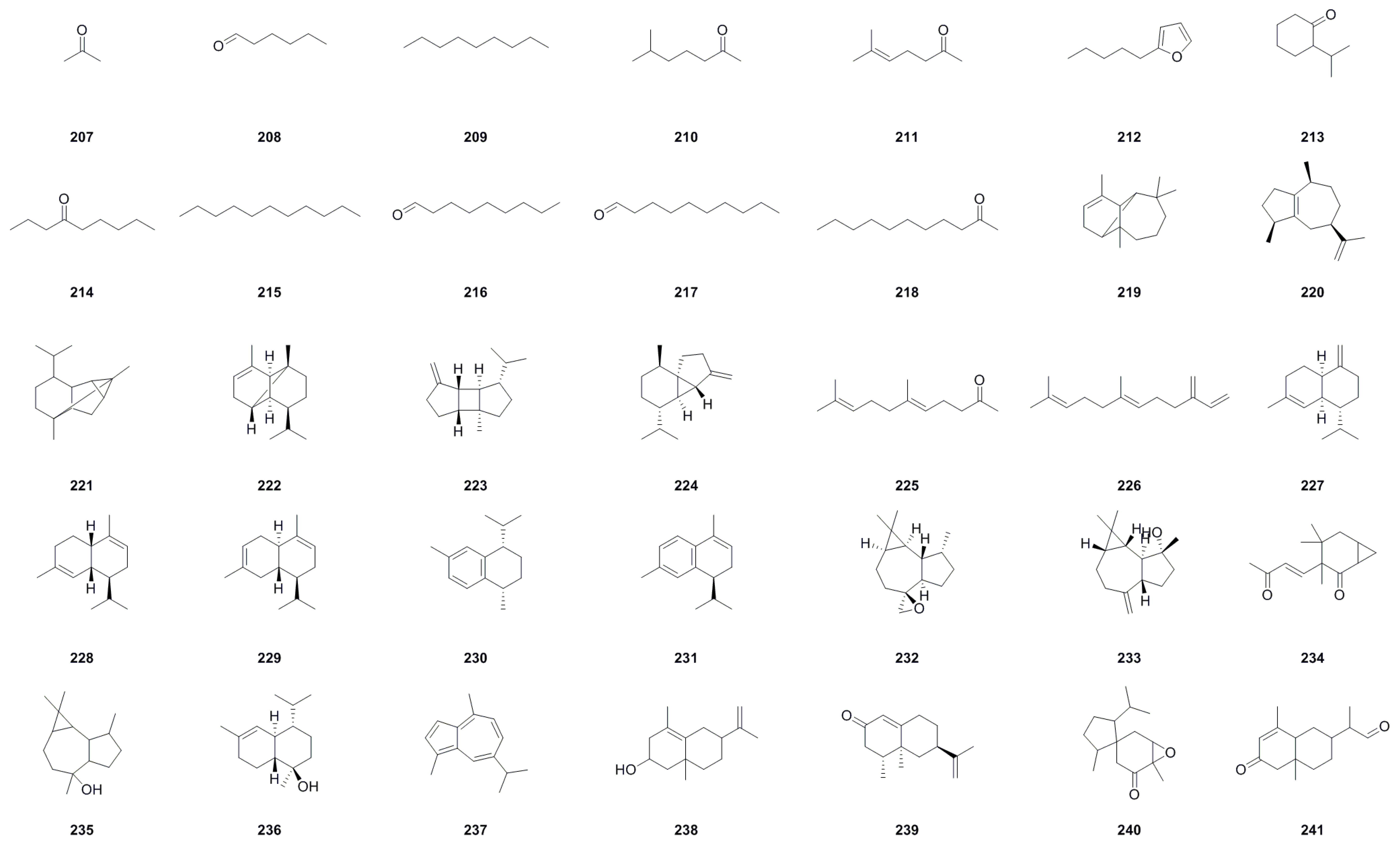

| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Ref. |
|---|---|---|---|---|---|---|
| 1. | Ascyrone A | C34H44O6 | 548.3138 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, ROESY, HMBC, CD | [8] |
| 2. | Ascyrone B | C34H44O7 | 564.3087 | aerial parts | , UV, HRESIMS, 1H NMR, 13C NMR, ROESY, HSQC, HMBC, CD | [8] |
| 3. | Ascyrone C | C34H44O6 | 548.3138 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, HMBC, ROESY, CD | [8] |
| 4. | Ascyrone D | C34H44O6 | 548.3138 | aerial parts | , UV, HRESIMS, 1H NMR, 13C NMR, ROESY, CD | [8] |
| 5. | Ascyrone E | C34H42O5 | 530.3032 | aerial parts | , UV, HRESIMS, 1H NMR, 13C NMR, HMBC, ROESY, CD | [8] |
| 6. | Longistylione A | C34H44O5 | 532.3189 | roots | HRESIMS, 1H NMR, 13C NMR | [4] |
| aerial parts | 1D NMR, 2D NMR, CD | [34] | ||||
| 7. | Longistylione B | C34H44O5 | 532.3189 | aerial parts | 1H NMR, 13C NMR, CD | [8] |
| roots | HRESIMS, 1H NMR, 13C NMR | [4] | ||||
| aerial parts | 1D NMR, 2D NMR, CD | [34] | ||||
| 8. | Longistylione C | C34H44O5 | 532.3189 | aerial parts | 1H NMR, 13C NMR, CD | [8] |
| roots | HRESIMS, 1H NMR, 13C NMR | [4] | ||||
| aerial parts | 1D NMR, 2D NMR, CD | [34] | ||||
| 9. | Longistylione D | C34H44O5 | 532.3189 | aerial parts | 1H NMR, 13C NMR, CD | [8] |
| roots | HRESIMS, 1H NMR, 13C NMR | [4] | ||||
| aerial parts | 1D NMR, 2D NMR, CD | [34] | ||||
| 10. | Hyperascyrin N | C34H44O6 | 548.3138 | aerial parts | 1H NMR, 13C NMR, CD | [8] |
| 11. | Hypascyrin A | C31H46O5 | 498.3345 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HMBC, NOESY, CD | [7] |
| 12. | Hypascyrin B | C30H44O5 | 484.3189 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, CD | [7] |
| 13. | Hypascyrin C | C31H46O5 | 498.3345 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, CD | [7] |
| 14. | Hypascyrin D | C31H48O6 | 516.3451 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HMBC, NOESY, CD | [7] |
| 15. | Hypascyrin E | C31H46O5 | 498.3345 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HMBC, NOESY, CD | [7] |
| 16. | ent-Hyphenrone J | C30H44O5 | 484.3189 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, CD | [7] |
| 17. | Hyphenrone K | C31H46O5 | 498.3345 | roots | , 1H NMR, 13C NMR, CD | [7] |
| 18. | Hypercalin B | C33H42O5 | 518.3032 | aerial parts | 1H NMR, 13C NMR | [1] |
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] | ||||
| 19. | Hypercalin C | C30H44O5 | 484.3189 | roots | 1H NMR, 13C NMR | [7] |
| aerial parts | 1H NMR, 13C NMR | [1] | ||||
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] | ||||
| 20. | 3,5-Dihydroxy-4-{[(1R,2S,5S)-2-hydroxy-2-methyl-5-(1-methylethenyl)cyclopentyl]methyl}-2-(3-methylbutanoyl)-6,6-bis(3-methylbut-2-enyl)cyclohexa-2,4-dien-1-one | C31H46O5 | 498.3345 | aerial parts | 1H NMR, 13C NMR | [1] |
| 21. | Tomoeone A | C30H44O6 | 500.3138 | roots | 1H NMR, 13C NMR | [7] |
| aerial parts | 1H NMR, 13C NMR | [2] | ||||
| aerial parts | , IR, HRESIMS, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY | [1] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] | ||||
| aerial parts | UV, HRESIMS, 1H NMR, 13C NMR, HMBC, NOESY | [11] | ||||
| 22. | Tomoeone B | C30H44O6 | 500.3138 | aerial parts | 1H NMR, 13C NMR, CD | [2] |
| aerial parts | , IR, HRESIMS, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY | [1] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] | ||||
| 23. | Tomoeone C | C30H44O6 | 500.3138 | aerial parts | , IR, HRESIMS, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY | [1] |
| 24. | Revised tomoeone C | C30H44O7 | 516.3087 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] |
| 25. | Tomoeone D | C30H44O6 | 500.3138 | aerial parts | , IR, HRESIMS, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY | [1] |
| 26. | Tomoeone E | C31H46O6 | 514.3294 | aerial parts | 1H NMR, 13C NMR, CD | [2] |
| aerial parts | , IR, HRESIMS, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY | [1] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] | ||||
| 27. | Tomoeone F | C31H46O6 | 514.3294 | aerial parts | 1H NMR, 13C NMR, CD | [2] |
| aerial parts | , IR, HRESIMS, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY | [1] | ||||
| 28. | Tomoeone G | C31H46O6 | 514.3294 | aerial parts | , IR, HRESIMS, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY | [1] |
| 29. | Revised tomoeone G | C31H46O7 | 530.3244 | aerial parts | 1H NMR, 13C NMR, CD | [2] |
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] | ||||
| 30. | Tomoeone H | C31H46O7 | 530.3244 | roots | 1H NMR, 13C NMR | [7] |
| aerial parts | , IR, HRESIMS, 1H NMR, 13C NMR, COSY, HSQC, HMBC, NOESY | [1] | ||||
| aerial parts | 1H NMR, 13C NMR, CD | [2] | ||||
| 31. | Hyperascyrin A | C34H44O5 | 532.3189 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, HSQC, HMBC, NOESY, CD | [4] |
| 32. | Hyperascyrin B | C34H44O5 | 532.3189 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, NOESY, CD | [4] |
| 33. | Hyperascyrin C | C34H44O6 | 548.3138 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, HMBC, NOESY, CD | [4] |
| 34. | Hyperascyrin D | C34H44O6 | 548.3138 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, NOESY, CD | [4] |
| 35. | Hyperascyrin E | C34H44O5 | 532.3189 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, HMBC, NOESY, CD | [4] |
| 36. | Hyperascyrin F | C34H44O5 | 532.3189 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, HMBC, NOESY, CD | [4] |
| 37. | Hyperascyrin G | C34H44O6 | 548.3138 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, NOESY, CD | [4] |
| 38. | Hyperascyrin H | C34H44O6 | 548.3138 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, NOESY, CD | [4] |
| 39. | Hyperascyrin I | C34H44O6 | 548.3138 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, NOESY, CD | [4] |
| 40. | Hyperascyrin J | C34H44O5 | 532.3189 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, HMBC, NOESY, CD | [4] |
| 41. | Hyperascyrin K | C34H44O5 | 532.3189 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, HMBC, NOESY, CD | [4] |
| 42. | Hyperascyrone A | C33H42O6 | 534.2981 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, DEPT, 1H-1H COSY, NOESY, HMBC, CD | [2] |
| 43. | Hyperascyrone B | C30H44O6 | 500.3138 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [2] |
| 44. | Hyperascyrone C | C31H46O6 | 514.3294 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [2] |
| 45. | Hyperascyrone D | C30H44O6 | 500.3138 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [2] |
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] | ||||
| 46. | Hyperascyrone E | C31H46O6 | 514.3294 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [2] |
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] | ||||
| 47. | Hyperascyrone F | C31H46O6 | 514.3294 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [2] |
| 48. | Hyperascyrone G | C31H46O6 | 514.3294 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [2] |
| 49. | Hyperascyrone H | C31H46O6 | 514.3294 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [2] |
| 50. | Hyperascyrin L | C34H44O4 | 516.3240 | aerial parts | UV, IR, HRESIMS, 1H NMR, 13C NMR, HSQC, HMBC, NOESY, CD | [5] |
| 51. | Hyperascyrin M | C34H44O5 | 532.3189 | aerial parts | UV, IR, HRESIMS, 1H NMR, 13C NMR, HSQC, HMBC, NOESY, CD | [5] |
| 52. | Hyperascyrin N | C34H44O6 | 548.3138 | aerial parts | UV, IR, HRESIMS, 1H NMR, 13C NMR, HSQC, HMBC, NOESY, CD | [5] |
| 53. | Hypermongone A | C32H48O5 | 512.3502 | aerial parts | HRESIMS, 1H NMR, 13C NMR | [5] |
| 54. | Hypermongone B | C32H48O5 | 512.3502 | aerial parts | HRESIMS, 1H NMR, 13C NMR | [5] |
| whole plant | MS, 1H NMR, 13C NMR | [3] | ||||
| 55. | Hypermongone C | C31H46O5 | 498.3345 | aerial parts | HRESIMS, 1H NMR, 13C NMR | [5] |
| 56. | Hypermongone D | C31H46O5 | 498.3345 | aerial parts | HRESIMS, 1H NMR, 13C NMR | [5] |
| 57. | Norascyronone A | C26H34O2 | 378.2559 | aerial parts | , mp, UV, IR, ESIMS, HRESIMS, 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HSQC, NOESY, HMBC, CD | [43] |
| 58. | Norascyronone B | C26H34O3 | 394.2508 | aerial parts | , UV, IR, ESIMS, HRESIMS, 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HSQC, NOESY, HMBC, CD | [43] |
| 59. | Norascyronone C | C26H36O2 | 380.2715 | aerial parts | , UV, IR, ESIMS, HRESIMS, 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HSQC, NOESY, HMBC, CD | [43] |
| 60. | Ascyronone A | C31H46O5 | 498.3345 | whole plant | UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY | [3] |
| 61. | Ascyronone B | C32H48O5 | 512.3502 | whole plant | UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY | [3] |
| 62. | Ascyronone C | C33H46O4 | 506.3396 | whole plant | UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY | [3] |
| 63. | Ascyronone D | C32H48O5 | 512.3502 | whole plant | UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY | [3] |
| 64. | Hyperibone J | C31H46O5 | 498.3345 | whole plant | MS, 1H NMR, 13C NMR | [3] |
| 65. | Hyperscabrone G | C32H48O5 | 512.3502 | whole plant | MS, 1H NMR, 13C NMR | [3] |
| 66. | Ascyronone E | C38H50O4 | 570.3709 | flowers | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [6] |
| 67. | Ascyronone F | C38H50O5 | 586.3658 | flowers | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [6] |
| 68. | Ascyronone G | C33H42O5 | 518.3032 | flowers | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, NOESY, HMBC, CD | [6] |
| 69. | Hypelodin B | C38H48O4 | 568.3553 | flowers | MS, 1H NMR, 13C NMR | [6] |
| 70. | Hypercohin K | C33H40O4 | 500.2927 | flowers | MS, 1H NMR, 13C NMR | [6] |
| 71. | Hyperforin | C35H52O4 | 536.3866 | aerial parts | HPLC | [44] |
| 72. | Furohyperforin | C35H52O5 | 552.3815 | flowers | MS, 1H NMR, 13C NMR | [6] |
| whole plant | EI-MS, 1H NMR, 13C NMR | [24] | ||||
| 73. | Furoadhyperforin | C36H54O5 | 566.3971 | whole plant | EI-MS, 1H NMR, 13C NMR | [24] |
| 74. | Hypercohin G | C35H52O4 | 536.3866 | flowers | MS, 1H NMR, 13C NMR | [6] |
| 75. | Hyphenrone X | C35H44O4 | 528.3240 | flowers | MS, 1H NMR, 13C NMR | [6] |
| 76. | Ascyronine A | C38H52O7 | 620.3713 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HMQC, HMBC, ROESY, CD | [10] |
| 77. | Ascyronine B | C35H52O7 | 584.3713 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HMQC, HMBC, ROESY, CD | [10] |
| 78. | Ascyronine C | C35H54O8 | 602.3819 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HMQC, HMBC, ROESY, CD | [10] |
| 79. | Hunascynol A | C33H42O6 | 534.2981 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 80. | Hunascynol B | C33H42O6 | 534.2981 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 81. | Hunascynol C | C33H40O5 | 516.2876 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 82. | Hunascynol D | C31H46O6 | 514.3294 | aerial parts | , mp, UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 83. | Hunascynol E | C31H46O6 | 514.3294 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 84. | Hunascynol F | C31H46O6 | 514.3294 | aerial parts | , mp, UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 85. | Hunascynol G | C31H46O5 | 498.3345 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 86. | Hunascynol H | C30H44O5 | 484.3189 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 87. | Hunascynol I | C31H46O6 | 514.3294 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 88. | Hunascynol J | C31H46O6 | 514.3294 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, CD | [9] |
| 89. | Hyperbeanol A | C33H42O6 | 534.2981 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] |
| 90. | Hyperbeanol B | C33H42O6 | 534.2981 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] |
| 91. | Hyperbeanol C | C33H42O6 | 534.2981 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] |
| 92. | Hyperbeanol D | C33H42O7 | 550.2931 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] |
| 93. | Hypercohone G | C33H40O5 | 516.2876 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [9] |
| 94. | Hyperascone A | C30H42O7 | 514.2931 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, NOESY, HMBC, CD | [11] |
| 95. | Hyperascone B | C31H44O7 | 528.3087 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMQC, NOESY, HMBC, CD | [11] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Ref. |
|---|---|---|---|---|---|---|
| 96. | Hyperdioxane A | C30H34O8 | 522.2254 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, CD | [47] |
| 97. | Hyperdioxane B | C15H14O6 | 290.0790 | roots | , mp, UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, CD | [47] |
| 98. | Hyperdioxane C | C15H16O7 | 308.0896 | roots | UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, ROESY, CD | [32] |
| 99. | Hyperdioxane D | C14H14O6 | 278.0790 | roots | UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, ROESY, CD | [32] |
| 100. | Hyperdioxane E | C15H16O7 | 308.0896 | roots | UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, ROESY, CD | [32] |
| 101. | Hyperdioxane F | C15H16O7 | 308.0896 | roots | UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, ROESY, CD | [32] |
| 102. | Sampsone B | C16H18O7 | 322.1053 | roots | , UV, IR, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, CD | [32] |
| 103. | 3,7,10a-Trimethoxy-1,4,4a,10a-tetrahydrodibenzo-p-dioxin-1-one | C15H16O6 | 292.0947 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, ROESY, CD | [32] |
| 104. | 1,6-Dihydroxy-5,7-dimethoxyxanthone | C15H12O6 | 288.0634 | aerial parts | EI-MS, 1H NMR, 13C NMR | [13] |
| 105. | Euxanthone | C13H8O4 | 228.0423 | aerial parts | 1H NMR, 13C NMR | [13] |
| aerial parts | EI-MS, 1H NMR, 13C NMR | [12] | ||||
| 106. | 6-O-palmitolyl-1,6-dihydroxy-5,7-dimethoxyxanthone | C31H42O7 | 526.2931 | aerial parts | UV, EI-MS, 1H NMR, 13C NMR | [13] |
| 107. | 2-Methoxyxanthone | C14H10O3 | 226.0630 | aerial parts | EI-MS, 1H NMR, 13C NMR | [12] |
| 108. | 1-Hydroxy-7-methoxyxanthone | C14H10O4 | 242.0579 | aerial parts | EI-MS, 1H NMR, 13C NMR | [12] |
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] | ||||
| 109. | 5-Chloro-1,6-dihydroxy-3-methoxy-8-methylxanthone | C15H11ClO5 | 306.0295 | aerial parts | UV, IR, HRESIMS, 1H NMR, 13C NMR, DEPT, HMBC, NOESY | [12] |
| 110. | 3,6-Dihydroxy-1,7-dimethoxyxanthone | C15H12O6 | 288.0634 | aerial parts | UV, IR, HRESIMS, 1H NMR, 13C NMR, DEPT, HMQC, HMBC, NOESY | [12] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 111. | 7-Methoxy-1,5,6-trihydroxyxanthone | C14H10O6 | 274.0477 | aerial parts | EI-MS, 1H NMR, 13C NMR | [12] |
| 112. | Toxyloxanthone B | C18H14O6 | 326.0790 | aerial parts | EI-MS, 1H NMR, 13C NMR | [12] |
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] | ||||
| 113. | 1,3,6,7-Tetrahydroxy-8-(3-methylbut-2-enyl)xanthone | C18H16O6 | 328.0947 | aerial parts | EI-MS, 1H NMR, 13C NMR | [12] |
| whole plant | MS, 1H NMR, 13C NMR | [48] | ||||
| 114. | 1,3,5-Trihydroxy-6,7-[2′-(1-methylethenyl)-dihydrofurano]-xanthone | C18H14O6 | 326.0790 | aerial parts | IR, HRESIMS, 1H NMR, 13C NMR, HMBC | [14] |
| 115. | 1,3,5-Trihydroxy-6,7-[2′-(1-hydroxy-1-methylethyl)-dihydrofurano]-xanthone | C18H16O7 | 344.0896 | aerial parts | IR, HRESIMS, 1H NMR, 13C NMR, HMBC | [14] |
| 116. | 1,3,5-Trihydroxy-6-O-prenyl-xanthone | C18H16O6 | 328.0947 | aerial parts | IR, HRESIMS, 1H NMR, 13C NMR, HMBC | [14] |
| 117. | 1,3,5-Trihydroxy-3′,3′-dimethyl-2H-pyran[6,7]xanthen-9-one | C18H14O6 | 326.0790 | aerial parts | MS, 1H NMR, 13C NMR | [14] |
| 118. | 1,3-Dihydroxy-5-methoxyxanthone | C14H10O5 | 258.0528 | aerial parts | MS, 1H NMR, 13C NMR | [14] |
| 119. | 1,3,5,6-Tetrahydroxyxanthone | C13H8O6 | 260.0321 | aerial parts | MS, 1H NMR, 13C NMR | [14] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 120. | 1,7-Dihydroxyxanthone | C13H8O4 | 228.0423 | aerial parts | ESI-MS, 1H NMR | [19] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] | ||||
| 121. | 2,3-Dimethoxyxanthone | C15H12O4 | 256.0736 | whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] |
| 122. | 1,3,6,7-Tetrahydroxyxanthone | C13H8O6 | 260.0321 | aerial parts | ESI-MS, 1H NMR | [19] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 123. | 6-Deoxyisojacareubin | C18H14O5 | 310.0841 | whole plant | 1H NMR, 13C NMR | [22] |
| 124. | Paxanthone | C19H16O6 | 340.0947 | whole plant | 1H NMR, 13C NMR | [22] |
| 125. | 1,3,5-Trihydroxy-6-O-(3-methylbut-2-enyl)-xanthone | C18H16O6 | 328.0947 | aerial parts | UV, HRESIMS, 1H NMR, 13C NMR | [11] |
| 126. | 3,7-Dihydroxy-1,6-dimethoxy-xanthone | C15H12O6 | 288.0634 | aerial parts | UV, HRESIMS, 1H NMR, 13C NMR, HMBC | [11] |
| 127. | Subalatin | C24H20O9 | 452.1107 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 128. | Sampsone C | C18H16O8 | 360.0845 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 129. | Deprenylated rheediaxanthone | C18H16O6 | 328.0947 | aerial parts | UV, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HMQC, HMBC | [11] |
| 130. | 1,3,7-Trihydroxy-xanthone | C13H8O5 | 244.0372 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 131. | 1,5-Dihydroxy-8-methoxy-xanthone | C14H10O5 | 258.0528 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 132. | 1,4,8-Trihydroxy-xanthone | C13H8O5 | 244.0372 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 133. | 3,4-Dihydroxy-2-methoxy-xanthone | C14H10O5 | 258.0528 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 134. | 2-Hydroxy-xanthone | C13H8O3 | 212.0473 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Ref. |
|---|---|---|---|---|---|---|
| 135. | Kaempferol | C15H10O6 | 286.0477 | aerial parts | mp, UV | [50] |
| aerial parts | 1H NMR, EI-MS | [13] | ||||
| aerial parts | mp, ESI-MS, 1H NMR, 13C NMR | [21] | ||||
| aerial parts | MS, 1H NMR, 13C NMR | [14] | ||||
| aerial parts | 1H NMR, 13C NMR | [51] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [19] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] | ||||
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| whole plant | mp, 1H NMR, 13C NMR | [29] | ||||
| whole plant | Chemical reactions, mp, UV, IR, 13C NMR | [20] | ||||
| whole plant | Chemical reactions, mp, UV, IR | [52] | ||||
| whole plant | ESI-MS, 1H NMR, 13C NMR | [53] | ||||
| whole plant | HPLC | [35] | ||||
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] | ||||
| whole plant | UPLC-Q-TOF-MS | [17] | ||||
| 136. | Quercetin | C15H10O7 | 302.0427 | aerial parts | mp, UV | [50] |
| aerial parts | HPLC | [44] | ||||
| aerial parts | 1H NMR, EI-MS | [13] | ||||
| aerial parts | MS, 1H NMR, 13C NMR | [14] | ||||
| aerial parts | mp, ESI-MS, 1H NMR, 13C NMR | [21] | ||||
| aerial parts | 1H NMR, 13C NMR | [51] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [19] | ||||
| aerial parts | MS, 1H NMR, 13C NMR, DEPT | [15] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] | ||||
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| whole plant | Chemical reactions, mp, UV, IR, 13C NMR | [20] | ||||
| whole plant | mp, ESI-MS, 1H NMR, 13C NMR | [53] | ||||
| whole plant | HPLC | [35] | ||||
| whole plant | UPLC-Q-TOF-MS | [17] | ||||
| whole plant | Chemical reactions, mp, IR | [54] | ||||
| whole plant | Chemical reactions, elemental analysis, mp, UV, IR, 1H NMR | [52] | ||||
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] | ||||
| 137. | Luteolin | C15H10O6 | 286.0477 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 138. | Catechin | C15H14O6 | 290.0790 | whole plant | UPLC-Q-TOF-MS | [17] |
| 139. | Isoquercitrin | C21H20O12 | 464.0955 | whole plant | Chemical reactions, elemental analysis, mp, UV, IR | [52] |
| whole plant | Chemical reactions, mp, UV, IR, 1H NMR, 13C NMR | [20] | ||||
| whole plant | HPLC | [35] | ||||
| 140. | Trifolin | C21H20O11 | 448.1006 | aerial parts | , mp, UV, acid and enzymatic hydrolysis | [50] |
| 141. | Hyperoside | C21H20O12 | 464.0955 | aerial parts | , mp, UV, acid and enzymatic hydrolysis | [50] |
| aerial parts | HPLC | [44] | ||||
| aerial parts | mp, ESI-MS, 1H NMR, 13C NMR | [21] | ||||
| aerial parts | 1H NMR, 13C NMR | [51] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [19] | ||||
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| whole plant | Chemical reactions, mp, UV, IR, MS, 1H NMR | [54] | ||||
| whole plant | Chemical reactions, elemental analysis, mp, UV, IR, 1H NMR | [52] | ||||
| whole plant | mp, 1H NMR, 13C NMR | [53] | ||||
| whole plant | mp, 1H NMR, 13C NMR | [29] | ||||
| whole plant | HPLC | [35] | ||||
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] | ||||
| whole plant | UPLC-Q-TOF-MS | [17] | ||||
| 142. | 3,5,8,3′,4′-Pentahydroxyflavone | C15H10O7 | 302.0427 | whole plant | ESI-MS, 1H NMR, 13C NMR | [53] |
| whole plant | mp, 1H NMR, 13C NMR | [29] | ||||
| 143. | 6-Methyl-5-hydroxy-7,4′-dimethoxyflavanone | C18H18O5 | 314.1154 | whole plant | 1H NMR, 13C NMR | [22] |
| 144. | Kaempferol-3-O-β-D-galactoside | C21H20O11 | 448.1006 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [19] |
| whole plant | UPLC-Q-TOF-MS | [17] | ||||
| 145. | Kaempferol 3-O-β-D-glucoside | C21H20O11 | 448.1006 | aerial parts | 1H NMR, 13C NMR | [51] |
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [19] | ||||
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| whole plant | 1H NMR, 13C NMR | [53] | ||||
| whole plant | mp, 1H NMR, 13C NMR | [29] | ||||
| whole plant | HPLC | [35] | ||||
| whole plant | UPLC-Q-TOF-MS | [17] | ||||
| 146. | Quercitrin | C21H20O11 | 448.1006 | whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] |
| 147. | Quercetin 3-O-α-L-arabinofuranoside | C20H18O11 | 434.0849 | aerial parts | mp, ESI-MS, 1H NMR, 13C NMR | [21] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 148. | Quercetin-3-O-β-D-glucoside | C21H20O12 | 464.0955 | aerial parts | 1H NMR, 13C NMR | [51] |
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [19] | ||||
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| whole plant | mp, 1H NMR, 13C NMR | [53] | ||||
| whole plant | mp, 1H NMR, 13C NMR | [29] | ||||
| whole plant | UPLC-Q-TOF-MS | [17] | ||||
| 149. | Kaempferol 3-O-β-(2′′-acetyl) galactopyranoside | C23H22O12 | 490.1111 | aerial parts | MS, 1H NMR, 13C NMR, DEPT | [15] |
| 150. | kaempferol-3-O-(6′′-O-crotonoyl)-β-D-glucopyranoside | C25H24O12 | 516.1268 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [19] |
| 151. | Isoquercetin | C21H20O12 | 464.0955 | aerial parts | UV, ESI-MS, 1H NMR, 13C NMR | [16] |
| 152. | Isohyperoside | C21H20O12 | 464.0955 | aerial parts | UV, ESI-MS, 1H NMR, 13C NMR | [16] |
| 153. | 3,8”-Biapigenin | C30H18O10 | 538.0900 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 154. | Hinokiflavone | C30H18O10 | 538.0900 | aerial parts | UV, 1H NMR | [11] |
| 155. | Kaempferol (6–8′′) apigenin | C30H18O11 | 554.0849 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| 156. | 5’,3′′′-Dihydroxyrobustaflavone | C30H18O12 | 570.0798 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| 157. | Rutin | C27H30O16 | 610.1534 | whole plant | Chemical reactions, elemental analysis, mp, UV, IR | [52] |
| aerial parts | HPLC | [44] | ||||
| whole plant | HPLC | [35] | ||||
| whole plant | mp, EI-MS, 1H NMR, 13C NMR | [42] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Ref. |
|---|---|---|---|---|---|---|
| 158. | p-Hydroxybenzoic acid | C7H6O3 | 138.0317 | aerial parts | 1H NMR, 13C NMR | [51] |
| aerial parts | ESI-MS, 1H NMR | [19] | ||||
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 159. | 4-Hydroxybenzoic acid methyl ester | C8H8O3 | 152.0473 | aerial parts | EI-MS, 1H NMR, 13C NMR | [13] |
| 160. | Protocatechuic acid | C7H6O4 | 154.0266 | aerial parts | 1H NMR, 13C NMR | [51] |
| aerial parts | ESI-MS, 1H NMR | [19] | ||||
| aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] | ||||
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 161. | Protocatechuic acid methyl ester | C8H8O4 | 168.0423 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| 162. | Vanillic acid | C8H8O4 | 168.0423 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 163. | p-Coumaric acid | C9H8O3 | 164.0473 | aerial parts | ESI-MS, 1H NMR | [19] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 164. | Gallic acid | C7H6O5 | 170.0215 | aerial parts | TLC, 1H NMR | [19] |
| 165. | Ethyl gallate | C9H10O5 | 198.0528 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 166. | 3-O-Caffeoylquinic acid methyl ester | C17H20O9 | 368.1107 | aerial parts | MS, 1H NMR, 13C NMR | [14] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| 167. | Methyl caffeate | C10H10O4 | 194.0579 | aerial parts | MS, 1H NMR, 13C NMR | [14] |
| 168. | 5,7-Dihydroxy-2-isopropylchromone | C12H12O4 | 220.0736 | aerial parts | MS, 1H NMR, 13C NMR | [14] |
| aerial parts | UV, 1H NMR | [11] | ||||
| 169. | Hyperfaberol F | C13H14O4 | 234.0892 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 170. | 2,2’,5,6’-Tetrahydroxybenzophenone | C13H10O5 | 246.0528 | whole plant | 1H NMR, 13C NMR, DEPT | [22] |
| 171. | 2,4-Dihydroxy-6-(4-hydroxybenzoyloxyl-)-benzoyl acid | C14H10O7 | 290.0427 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| 172. | 2,4-Dihydroxy-6-(3,4-dihydroxybenzoyloxyl-)-benzoyl acid | C14H10O8 | 306.0376 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| 173. | 2-(3,4-Dihydroxybenzoyl)-2,4,6-trihydroxy-3(2H)-benzofuranone | C15H10O8 | 318.0376 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| 174. | Chlorogenic acid | C16H18O9 | 354.0951 | whole plant | UPLC-Q-TOF-MS | [17] |
| whole plant | UV | [54] | ||||
| 175. | 4-O-Caffeoylquinic acid | C16H18O9 | 354.0951 | whole plant | UPLC-Q-TOF-MS | [17] |
| 176. | 5-O-Caffeoylquinic acid | C16H18O9 | 354.0951 | whole plant | UPLC-Q-TOF-MS | [17] |
| 177. | 9,9’-O-Di-(E)-feruloyl-(-)-secoisolariciresinol | C40H42O12 | 714.2676 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [19] |
| 178. | Cinchonain Ia | C24H20O9 | 452.1107 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Ref. |
|---|---|---|---|---|---|---|
| 179. | Friedelin | C30H50O | 426.3862 | aerial parts | EI-MS, 1H NMR, 13C NMR | [12] |
| aerial parts | , 1H NMR, 13C NMR | [58] | ||||
| aerial parts | 1H NMR, 13C NMR | [59] | ||||
| whole plant | EI-MS, 1H NMR, 13C NMR | [24] | ||||
| 180. | Glutinol | C30H50O | 426.3862 | whole plant | 1H NMR, 13C NMR, DEPT | [22] |
| 181. | Betulinic acid | C30H48O3 | 456.3603 | aerial parts | 1H NMR, 13C NMR | [13] |
| aerial parts | UV, 1H NMR, 13C NMR | [11] | ||||
| whole plant | ESI-MS, 1H NMR, 13C NMR | [53] | ||||
| whole plant | mp, 1H NMR, 13C NMR | [29] | ||||
| whole plant | 1H NMR, 13C NMR, DEPT | [22] | ||||
| 182. | 3,4-seco-Olean-13(18)-ene-12,19-dione-3-oic acid | C30H46O4 | 470.3396 | aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HMBC, X-ray | [59] |
| aerial parts | , UV, IR, HRESIMS, 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HMBC, CD, X-ray | [58] | ||||
| 183. | Betulinic acid methyl ester | C31H50O3 | 470.3760 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 184. | Ursolic acid | C30H48O3 | 456.3603 | whole plant | ESI-MS, 1H NMR, 13C NMR | [53] |
| whole plant | mp, 1H NMR, 13C NMR | [29] | ||||
| 185. | 19α-Hydroxyursolic acid | C30H48O4 | 472.3553 | aerial parts | mp, ESI-MS, 1H NMR, 13C NMR | [21] |
| 186. | 6β,19α-Dihydroxy ursolic acid | C30H48O5 | 488.3502 | aerial parts | mp, ESI-MS, 1H NMR, 13C NMR | [21] |
| 187. | Myriaboric acid | C30H46O6 | 502.3294 | aerial parts | mp, ESI-MS, 1H NMR, 13C NMR | [21] |
| 188. | Ergosterol peroxide | C28H44O3 | 428.3290 | aerial parts | 1H NMR, 13C NMR | [59] |
| 189. | β-Sitosterol | C29H50O | 414.3862 | aerial parts | 1H NMR | [13] |
| aerial parts | TLC | [19] | ||||
| aerial parts | TLC, ESI-MS, 1H NMR | [21] | ||||
| aerial parts | 1H NMR, 13C NMR | [51] | ||||
| whole plant | GC-MS | [20] | ||||
| whole plant | Chemical reaction, mp, TLC | [42] | ||||
| whole plant | 1H NMR, 13C NMR, DEPT | [22] | ||||
| 190. | Campesterol | C28H48O | 400.3705 | whole plant | GC-MS | [20] |
| aerial parts | 1H NMR, 13C NMR | [51] | ||||
| 191. | Stigmasterol | C29H48O | 412.3705 | aerial parts | TLC, ESI-MS, 1H NMR | [21] |
| aerial parts | 1H NMR, 13C NMR | [51] | ||||
| whole plant | GC-MS | [20] | ||||
| whole plant | 1H NMR, 13C NMR | [22] | ||||
| 192. | β-Sitosterol-β-D-glucoside | C35H60O6 | 576.4390 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| whole plant | ESI-MS, 1H NMR, 13C NMR | [53] | ||||
| whole plant | mp, 1H NMR, 13C NMR | [29] | ||||
| whole plant | Chemical reaction, mp, TLC | [42] | ||||
| 193. | β-Sitosterol-β-D-glucoside palmitoyl ester | C51H90O7 | 814.6687 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 194. | β-Sitosterol-β-D-glucoside stearoyl ester | C53H94O7 | 842.7000 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 195. | β-Sitosterol-β-D-glucoside oleoyl ester | C53H92O7 | 840.6843 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 196. | β-Sitosterol-β-D-glucoside linoleoyl ester | C53H90O7 | 838.6687 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 197. | Campesterol-β-D-glucoside | C34H58O6 | 562.4233 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 198. | Campesterol-β-D-glucoside palmitoyl ester | C50H88O7 | 800.6530 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 199. | Campesterol-β-D-glucoside stearoyl ester | C52H92O7 | 828.6843 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 200. | Campesterol-β-D-glucoside oleoyl ester | C52H90O7 | 826.6687 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 201. | Campesterol-β-D-glucoside linoleoyl ester | C52H88O7 | 824.6530 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 202. | Stigmasterol-β-D-glucoside | C35H58O6 | 574.4233 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 203. | Stigmasterol-β-D-glucoside palmitoyl ester | C51H88O7 | 812.6530 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 204. | Stigmasterol-β-D-glucoside stearoyl ester | C53H92O7 | 840.6843 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 205. | Stigmasterol-β-D-glucoside oleoyl ester | C53H90O7 | 838.6687 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| 206. | Stigmasterol-β-D-glucoside linoleoyl ester | C53H88O7 | 836.6530 | whole plant | IR, 1H NMR, 13C NMR | [20] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Ref. |
|---|---|---|---|---|---|---|
| 207. | Acetone | C3H6O | 58.0419 | whole plant | GC-MS | [23] |
| 208. | Hexanal | C6H12O | 100.0888 | whole plant | GC-MS | [23] |
| 209. | Nonane | C9H20 | 128.1565 | whole plant | GC-MS | [23] |
| 210. | 2-Heptanone, 6-methyl- | C8H16O | 128.1201 | whole plant | GC-MS | [23] |
| 211. | 5-Hepten-2-one, 6-methyl- | C8H14O | 126.1045 | whole plant | GC-MS | [23] |
| 212. | Furan, 2-pentyl- | C9H14O | 138.1045 | whole plant | GC-MS | [23] |
| 213. | Cyclohexanone,2-(1-methylethyl)- | C9H16O | 140.1201 | whole plant | GC-MS | [23] |
| 214. | 4-Nonanone | C9H18O | 142.1358 | whole plant | GC-MS | [23] |
| 215. | Undecane | C11H24 | 156.1878 | whole plant | GC-MS | [23] |
| 216. | Nonanal | C9H18O | 142.1358 | whole plant | GC-MS | [23] |
| 217. | Decanal | C10H20O | 156.1514 | whole plant | GC-MS | [23] |
| 218. | 2-Undecanone | C11H22O | 170.1671 | whole plant | GC-MS | [23] |
| 219. | Tricyclo[5.4.0.0(2,8)]undec-9-ene,2,6,6,9-tetramethyl- | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 220. | Azulene,1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethenyl)-,[1s-(1α,4α,7α)]- | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 221. | 1,2,4-Metheno-1H-indene, octahydro-1,7a-dimethyl-5-(1-methylethyl)- | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 222. | Copaene | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 223. | Cyclobuta[1,2-a:3,4-a′]dicyclopentene, decahydro-3a-methyl-6-methylene-1-(1-methylethyl)-, (1S,3aS,3bR,6aS,6bR)- | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 224. | β-Cubebene | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 225. | 6,10-Dimethyl-5,9-undecadien-2-one | C13H22O | 194.1671 | whole plant | GC-MS | [23] |
| 226. | 1,6,10-Dodecatriene,7,11-dimethyl-3-methylene-,(E)- | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 227. | γ-Muurolene | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 228. | α-muurolene | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 229. | β-Cadinene | C15H24 | 204.1878 | whole plant | GC-MS | [23] |
| 230. | Calamenene | C15H22 | 202.1722 | whole plant | GC-MS | [23] |
| 231. | α-calacorene | C15H20 | 200.1565 | whole plant | GC-MS | [23] |
| 232. | Aromadendrene oxide 2 | C15H24O | 220.1827 | whole plant | GC-MS | [23] |
| 233. | (-)-Spathulenol | C15H24O | 220.1827 | whole plant | GC-MS | [23] |
| 234. | 3,4,4-Trimethyl-3-(3-oxo-but-1-enyl)-bicyclo[4.1.0]heptan-2-one | C14H20O2 | 220.1463 | whole plant | GC-MS | [23] |
| 235. | 1H-Cycloprop[e]azulen-4-ol, decahydro-1,1,4,7-tetramethyl- | C15H26O | 222.1984 | whole plant | GC-MS | [23] |
| 236. | α-Cadinol | C15H26O | 222.1984 | whole plant | GC-MS | [23] |
| 237. | Azulene,1,4-dimethyl-7-(1-methylethyl)- | C15H18 | 198.1409 | whole plant | GC-MS | [23] |
| 238. | 6-Isopropenyl-4,8a-dimethyl-,1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | C15H24O | 220.1827 | whole plant | GC-MS | [23] |
| 239. | (+)-Nootkatone | C15H22O | 218.1671 | whole plant | GC-MS | [23] |
| 240. | spiro[4.5]decan-7-one,1,8-dimethyl-8,9-epoxy-4-isopropyl- | C15H24O2 | 236.1776 | whole plant | GC-MS | [23] |
| 241. | 2-(4a,8-Dimethyl-6-oxo-1,2,3,4,4a,5,6,8a-octahydro-naphthalen-2-yl)-propionaldehyde | C15H22O2 | 234.1620 | whole plant | GC-MS | [23] |
| 242. | 2-Pentadecanone, 6,10,14-trimethyl- | C18H36O | 268.2766 | whole plant | GC-MS | [23] |
| 243. | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 278.1518 | whole plant | GC-MS | [23] |
| 244. | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296.3079 | whole plant | GC-MS | [23] |
| 245. | Nonadecane | C19H40 | 268.3130 | whole plant | GC-MS | [23] |
| 246. | 5,9,13-Pentadecatrien-2-one, 6,10,14-trimethyl-,(E,E)- | C18H30O | 262.2297 | whole plant | GC-MS | [23] |
| 247. | Hexadecanoic acid, methyl ester | C17H34O2 | 270.2559 | whole plant | GC-MS | [23] |
| 248. | Dibutyl phthalate | C16H22O4 | 278.1518 | whole plant | GC-MS | [23] |
| 249. | n-Hexadecanoic acid | C16H32O2 | 256.2402 | whole plant | GC-MS | [23] |
| 250. | Hexadecanoic acid, ethyl ester | C18H36O2 | 284.2715 | whole plant | GC-MS | [23] |
| 251. | Eicosane | C20H42 | 282.3287 | whole plant | GC-MS | [23] |
| 252. | Heneicosane | C21H44 | 296.3443 | whole plant | GC-MS | [23] |
| 253. | Octadecane | C18H38 | 254.2974 | whole plant | EI-MS, 1H NMR | [24] |
| 254. | Octacosane | C28H58 | 394.4539 | whole plant | EI-MS, 1H NMR | [24] |
| 255. | n-Hexacosanol | C26H54O | 382.4175 | aerial parts | GC-MS | [13] |
| 256. | n-Octacosanol | C28H58O | 410.4488 | aerial parts | GC-MS | [13] |
| whole plant | 1H NMR, 13C NMR | [24] | ||||
| 257. | n-Nonacosanol | C29H60O | 424.4644 | aerial parts | mp, EI-MS, 1H NMR | [21] |
| 258. | n-Triacontanol | C30H62O | 438.4801 | aerial parts | GC-MS | [13] |
| 259. | Hendecanoic acid | C11H22O2 | 186.1620 | whole plant | 1H NMR, 13C NMR | [24] |
| 260. | Octadecanoic acid | C18H36O2 | 284.2715 | whole plant | EI-MS, 1H NMR, 13C NMR | [24] |
| 261. | Arachidic acid | C20H40O2 | 312.3028 | whole plant | EI-MS, 1H NMR | [24] |
| 262. | Lignoceric acid | C24H48O2 | 368.3654 | aerial parts | GC-MS | [13] |
| 263. | Hexacosanoic acid | C26H52O2 | 396.3967 | aerial parts | GC-MS | [13] |
| whole plant | EI-MS, 1H NMR | [24] | ||||
| 264. | Octacosanoic acid | C28H56O2 | 424.4280 | aerial parts | GC-MS | [13] |
| 265. | Triacontanoic acid | C30H60O2 | 452.4593 | aerial parts | GC-MS | [13] |
| whole plant | EI-MS, 1H NMR | [24] |
| No. | Name | Formula | Exact Theoretical Molecular Weight | Source | Characterization Method | Ref. |
|---|---|---|---|---|---|---|
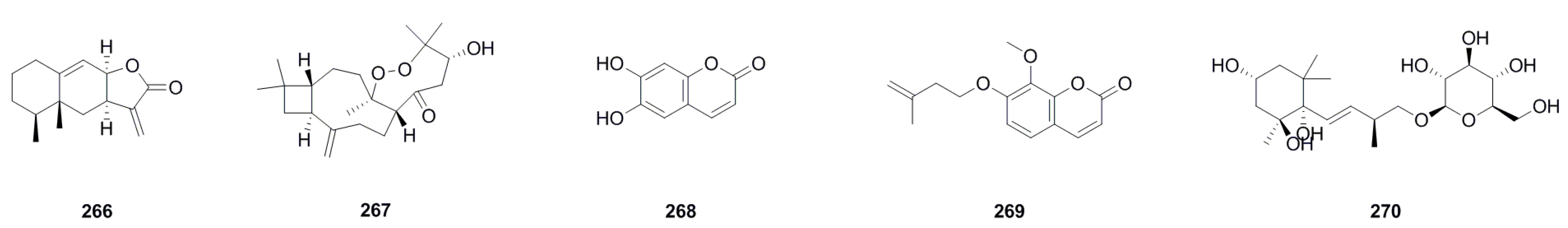
| 266. | Eremophil-9,11(13)-dien-8β,12-olide | C15H20O2 | 232.1463 | roots | , UV, IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, HMBC, NOESY, CD | [47] |
| 267. | Carascynol A | C21H34O4 | 350.2457 | aerial parts | IR, HRESIMS, 1H NMR, 13C NMR, 1H-1H COSY, DEPT, HMBC, ROESY, X-ray | [61] |
| 268. | Esculetin | C9H6O4 | 178.0266 | aerial parts | ESI-MS, 1H NMR, 13C NMR | [18] |
| 269. | 7-Isopentenyloxy-8-methoxycoumarin | C15H16O4 | 260.1049 | aerial parts | UV, 1H NMR, 13C NMR | [11] |
| 270. | Bridelionoside C | C20H36O9 | 420.2359 | whole plant | 1H NMR, 13C NMR | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhou, Y.; Rui, X.; Ye, Z.; Zheng, L.; Zang, H.; Zhong, Y. A Review of Traditional Applications, Geographic Distribution, Botanical Characterization, Phytochemistry, and Pharmacology of Hypericum ascyron L. Horticulturae 2024, 10, 555. https://doi.org/10.3390/horticulturae10060555
Liu M, Zhou Y, Rui X, Ye Z, Zheng L, Zang H, Zhong Y. A Review of Traditional Applications, Geographic Distribution, Botanical Characterization, Phytochemistry, and Pharmacology of Hypericum ascyron L. Horticulturae. 2024; 10(6):555. https://doi.org/10.3390/horticulturae10060555
Chicago/Turabian StyleLiu, Meihui, Yongmei Zhou, Xiaoxiao Rui, Zi Ye, Linyu Zheng, Hao Zang, and Yuan Zhong. 2024. "A Review of Traditional Applications, Geographic Distribution, Botanical Characterization, Phytochemistry, and Pharmacology of Hypericum ascyron L." Horticulturae 10, no. 6: 555. https://doi.org/10.3390/horticulturae10060555
APA StyleLiu, M., Zhou, Y., Rui, X., Ye, Z., Zheng, L., Zang, H., & Zhong, Y. (2024). A Review of Traditional Applications, Geographic Distribution, Botanical Characterization, Phytochemistry, and Pharmacology of Hypericum ascyron L. Horticulturae, 10(6), 555. https://doi.org/10.3390/horticulturae10060555








