Influence of pH on the Growth of Verticillium longisporum and Verticillium Stripe Severity in Canola (Brassica napus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Effects of pH on In Vitro Fungal Growth
2.2. Effects of pH on Verticillium Stripe Severity under Semi-Hydroponic Conditions
2.3. Effects of pH on Verticillium Stripe Severity and Yields at Maturity
2.4. Statistical Analysis
3. Results
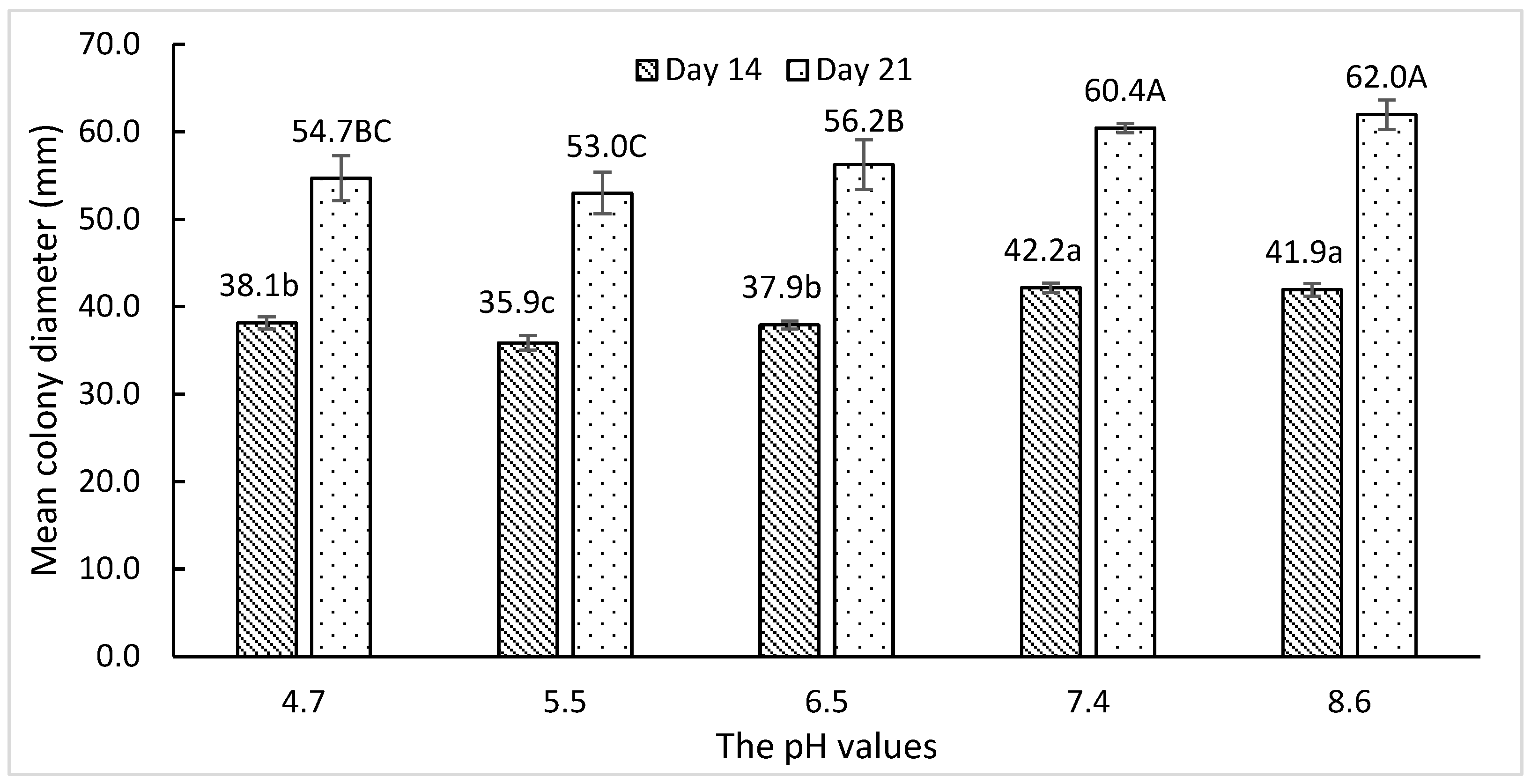
3.1. Effects of pH on In Vitro Fungal Growth
3.2. Effects of pH on Verticillium Stripe Severity under Semi-Hydroponic Conditions
3.3. Effects of pH on Verticillium Stripe Severity and Yields at Maturity
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Canadian Food Inspection Agency. Verticillium Stripe—Verticillium Longisporum. 2018. Available online: https://inspection.canada.ca/plant-health/invasive-species/plant-diseases/verticillium-stripe/eng/1420746212959/1420746213803 (accessed on 23 September 2023).
- Heale, J.B.; Karapapa, V.K. The Verticillium Threat to Canada’s Major Oilseed Crop: Canola. Can. J. Plant Pathol. 1999, 21, 1–7. [Google Scholar] [CrossRef]
- Dunker, S.; Keunecke, H.; Steinbach, P.; Von Tiedemann, A. Impact of Verticillium longisporum on Yield and Morphology of Winter Oilseed Rape (Brassica napus) in Relation to Systemic Spread in the Plant. J. Phytol. 2008, 156, 698–707. [Google Scholar] [CrossRef]
- Kamchen, R. The Rise of Verticillium Stripe. Canola Digest. 2023. Available online: https://canoladigest.ca/january-2023/the-rise-of-verticillium-stripe/ (accessed on 23 September 2023).
- Cruz, D.R.; Leandro, L.F.S.; Munkvold, G.P. Effects of Temperature and pH on Fusarium oxysporum and Soybean Seedling Disease. Plant Dis. 2019, 103, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Rollins, J.A.; Dickman, M.B. pH Signaling in Sclerotinia sclerotiorum: Identification of a pacC/RIM1 Homolog. Appl. Environ. Microbiol. 2001, 67, 75–81. [Google Scholar] [CrossRef]
- Goswami, B.; Rahaman, M.; Hoque, A.; Bhuiyan, K.; Mian, I. Variations in Different Isolates of Rhizoctonia solani Based on Temperature and pH. Bangladesh J. Agric. Res. 2011, 36, 389–396. [Google Scholar] [CrossRef]
- Gossen, B.D.; Kasinathan, H.; Cao, T.; Manolii, V.P.; Strelkov, S.E.; Hwang, S.-F.; McDonald, M.R. Interaction of pH and Temperature Affect Infection and Symptom Development of Plasmodiophora brassicae in Canola. Can. J. Plant Pathol. 2013, 35, 294–303. [Google Scholar] [CrossRef]
- Sbeiti, A.A.L.; Mazurier, M.; Ben, C.; Rickauer, M.; Gentzbittel, L. Temperature Increase Modifies Susceptibility to Verticillium Wilt in Medicago Spp. and May Contribute to the Emergence of More Aggressive Pathogenic Strains. Front. Plant Sci. 2023, 14, 1109154. [Google Scholar] [CrossRef] [PubMed]
- Baard, S.W.; Pauer, G.D.C. Effect of Alternate Drying and Wetting of the Soil, Fertilizer Amendment, and pH on the Survival of Microsclerotia of Verticillium dahliae. Phytophylactica 1981, 13, 165–168. [Google Scholar]
- Fayzalla, E.-S.A.; Shabana, Y.M.; Mahmoud, N.S. Effect of Environmental Conditions on Wilting and Root Rot Fungi Pathogenic to Solanaceous Plants. Plant Pathol. J. 2008, 7, 27–33. [Google Scholar] [CrossRef]
- Rampersad, S.N. A Study of Environmental Factors That Affect Survival of Pumpkin Isolates of Verticillium dahliae. HortScience 2010, 45, 1211–1217. [Google Scholar] [CrossRef]
- Hu, X.; Bai, Y.; Chen, T.; Hu, D.; Yang, J.; Xu, X. An Optimized Method for in Vitro Production of Verticillium dahliae Microsclerotia. Eur. J. Plant Pathol. 2013, 136, 225–229. [Google Scholar] [CrossRef]
- Li, F.; Matloob, M.; Nzabanita, C.; Li, Y. Growth, Sporulation and Germination of Verticillium alfalfae on Media. Eur. J. Plant Pathol. 2021, 161, 383–395. [Google Scholar] [CrossRef]
- Cui, J.; Strelkov, S.E.; Fredua-Agyeman, R.; Hwang, S.F. Development of Optimized Verticillium longisporum Inoculation Techniques for Canola (Brassica napus). Can. J. Plant Pathol. 2022, 45, 92–102. [Google Scholar] [CrossRef]
- Yang, C.; Fredua-Agyeman, R.; Hwang, S.-F.; Gorim, L.Y.; Strelkov, S.E. Optimizing the Evaluation of Root System Architectural Traits in Brassica napus. Can. J. Plant Sci. 2024, cjps-2023-0169. [Google Scholar] [CrossRef]
- Wang, Y.; Strelkov, S.E.; Hwang, S.-F. Blackleg Yield Losses and Interactions with Verticillium Stripe in Canola (Brassica napus) in Canada. Plants 2023, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Dix, N.J.; Webster, J. Fungal Ecology; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Deacon, J.W. Introduction to Modern Mycology; Blackwell Science Inc.: Chichester, UK, 1985; ISBN 978-0-632-01156-8. [Google Scholar]
- Chaudhary, S.; Kumar, M.; Sengar, R.S.; Chand, P.; Mishra, P.; Tomar, A. Effect of Nutrient Status, Temperature and pH on Mycelial Growth, Sclerotial Production and Germination of Rhizoctonia solani Isolated from Paddy Fields. Prog. Agric. 2018, 18, 82. [Google Scholar] [CrossRef]
- Dutta, B.K. Effect of the Chemical and Physical Condition of the Soil on Verticillium Wilt of Antirrhinum. Plant Soil 1981, 63, 217–225. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.-D.; Zhang, D.-D.; Zhang, Y.-Y.; Wang, D.; Song, J.; Zhang, J.; Li, R.; Kong, Z.-Q.; Klosterman, S.J.; et al. Biological Characteristics of Verticillium dahliae MAT1-1 and MAT1-2 Strains. Int. J. Mol. Sci. 2021, 22, 7148. [Google Scholar] [CrossRef]
- Kabir, Z.; Bhat, R.G.; Subbarao, K.V. Comparison of Media for Recovery of Verticillium dahliae from Soil. Plant Dis. 2004, 88, 49–55. [Google Scholar] [CrossRef]
- Bautista-Jalón, L.S.; Frenkel, O.; Tsror (Lahkim), L.; Malcolm, G.M.; Gugino, B.K.; Lebiush, S.; Hazanovsky, M.; Milgroom, M.G.; Del Mar Jiménez-Gasco, M. Genetic Differentiation of Verticillium dahliae Populations Recovered from Symptomatic and Asymptomatic Hosts. Phytopathology 2021, 111, 149–159. [Google Scholar] [CrossRef]
- Depotter, J.R.L.; Deketelaere, S.; Inderbitzin, P.; Tiedemann, A.V.; Höfte, M.; Subbarao, K.V.; Wood, T.A.; Thomma, B.P.H.J. Verticillium longisporum, the Invisible Threat to Oilseed Rape and Other Brassicaceous Plant Hosts. Mol. Plant Pathol. 2016, 17, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Malca, L.; Erwin, D.C.; Moje, W. Effect of pH and Carbon and Nitrogen Sources on the Growth of Verticillium albo-Atrum. Phytopathology 1966, 55, 401–406. [Google Scholar]
- Amalraj, A.; Taylor, J.; Sutton, T. A Hydroponics Based High Throughput Screening System for Phytophthora Root Rot Resistance in Chickpea (Cicer arietinum L.). Plant Methods 2019, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Baquy, M.A.-A.; Li, J.-Y.; Xu, C.-Y.; Mehmood, K.; Xu, R.-K. Determination of Critical pH and Al Concentration of Acidic Ultisols for Wheat and Canola Crops. Solid Earth 2017, 8, 149–159. [Google Scholar] [CrossRef]
- Van Wyk, P.S.; Baard, S.W. Germination of Conidia of Verticillium dahliae in Soil. Plant Soil 1971, 35, 601–611. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kaminski, D.; Graham, J.; Pradhan, M.; Froese, R.D.; Bargen, E.; Buss, T.; Clouson, N.; Farooq, A.; Heard, J.; et al. Survey of Canola Diseases in Manitoba in 2022. Can. J. Plant Pathol. 2023, 103, 126–129. [Google Scholar]
- Akhavan, A.; Peru, C.; Avila, R.; Fernando, D.; Gilroyed, J.; Esau, B.; Huffman, T.; Jacob, C.; Bond, J.; Montreuil, N.; et al. Survey of Canola Diseases in Saskatchewan, 2022. Can. J. Plant Pathol. 2023, 103, 121–125. [Google Scholar]
- Harding, M.W.; Daniels, G.C.; Hill, T.B.; Xue, S.; Sarkes, A.; Yang, Y.; Feng, J. Canola Disease Survey in Alberta, 2022. Can. J. Plant Pathol. 2023, 103, 110–112. [Google Scholar]
- Manitoba Agriculture Soil Fertility Guide. 2017. Available online: https://www.gov.mb.ca/agriculture/crops/soil-fertility/soil-fertility-guide/pubs/soil_fertility_guide.pdf (accessed on 23 September 2023).
- Canola Council of Canada Effects of Soil Characteristics. Canola Encyclopedia. 2023. Available online: https://www.canolacouncil.org/canola-encyclopedia/field-characteristics/effects-of-soil-characteristics/#:~:text=Most%20cultivated%20soils%20in%20western,northeast%20British%20Columbia%2C%20and%20Ontario (accessed on 23 September 2023).
- Les, H. Grainews. 5 August 2020. Available online: https://www.grainews.ca/columns/les-henry-geography-of-acid-soils-in-the-prairie-provinces/ (accessed on 23 September 2023).
- Donald, C.; Porter, I. Integrated Control of Clubroot. J. Plant Growth Regul. 2009, 28, 289–303. [Google Scholar] [CrossRef]
- Gladders, P.; Smith, J.A.; Kirkpatrick, L.; Clewes, E.; Grant, C.; Barbara, D.; Barnes, A.V.; Lane, C.R. First Record of Verticillium Wilt (Verticillium longisporum) in Winter Oilseed Rape in the UK. New Dis. Rep. 2011, 23, 8. [Google Scholar] [CrossRef]
- Tzelepis, G.; Bejai, S.; Sattar, M.N.; Schwelm, A.; Ilbäck, J.; Fogelqvist, J.; Dixelius, C. Detection of Verticillium Species in Swedish Soils Using Real-Time PCR. Arch. Microbiol. 2017, 199, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Chapara, V.; Al Salman, D.; Azizi, A.; Del Río Mendoza, L.E. First Report of Verticillium Stripe of Canola Caused by Verticillium longisporum in North Dakota. Plant Dis. 2023, 107, 4026. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Wang, R.; Wu, M.; Yang, L.; Li, G.; Zhang, J. Identification and Virulence Test of a New Pathogen That Causes Verticillium Striping on Rapeseed in Northwestern China. Oil Crop Sci. 2024, 9, 8–19. [Google Scholar] [CrossRef]
- Struck, C.; Rüsch, S.; Strehlow, B. Control Strategies of Clubroot Disease Caused by Plasmodiophora brassicae. Microorganisms 2022, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Faggian, R.; Hanson, M.; Kennedy, R.; Petch, G.; Wakeham, A. Assessment of the Response of Plasmodiophora brassicae in Contaminated Horticultural Land, Using Lime-based Fertilizer Concentrations. Food Energy Secur. 2017, 6, e00122. [Google Scholar] [CrossRef]
- Niwa, R.; Kumei, T.; Nomura, Y.; Yoshida, S.; Osaki, M.; Ezawa, T. Increase in Soil pH Due to Ca-Rich Organic Matter Application Causes Suppression of the Clubroot Disease of Crucifers. Soil Biol. Biochem. 2007, 39, 778–785. [Google Scholar] [CrossRef]
- Chai, A.L.; Xie, X.W.; Shi, Y.X.; Li, B.J. Research Status of Clubroot (Plasmodiophora brassicae) on Cruciferous Crops in China. Can. J. Plant Pathol. 2014, 36, 142–153. [Google Scholar] [CrossRef]

| Plant Height (mm) *** | Disease Severity (0–4 Scale) *** | Biomass (g 10−1 Plants) *** | ||||
|---|---|---|---|---|---|---|
| pH | Control | Inoculated | Control | Inoculated | Control | Inoculated |
| 4.4 ± 0.01 | 81 ± 3.8 A | 59 ± 4.3 ab | 0 A | 1.19 ab | 0.27 ± 0.03 A | 0.11 ± 0.01 ab |
| 5.4 ± 0.10 | 84 ± 4.4 A | 66 ± 2.5 a | 0 A | 0.72 a | 0.27 ± 0.01 AB | 0.15 ± 0.02 a |
| 6.3 ± 0.02 | 82 ± 3.7 A | 56 ± 1.3 b | 0 A | 1.59 bc | 0.26 ± 0.01 AB | 0.12 ± 0.01 ab |
| 7.5 ± 0.01 | 79 ± 5.1 A | 56 ± 8.5 b | 0 A | 2.10 d | 0.24 ± 0.02 AB | 0.10 ± 0.03 b |
| 8.4 ± 0.03 | 77 ± 2.2 A | 59 ± 1.9 ab | 0 A | 1.91 cd | 0.22 ± 0.01 B | 0.11 ± 0.01 ab |
| Plant Height (cm) | Disease Severity (0–4 Scale) *** | Seed Yield (g 10−1 Plants) | ||||
|---|---|---|---|---|---|---|
| pH | Control | Inoculated | Control | Inoculated | Control | Inoculated |
| 5.6 ± 0.27 | 58.4 ± 3.9 C | 54.2 ± 1.7 b (n.s.) | 0 A | 0.33 c | 0.88 ± 0.13 C | 0. 79 ± 0.14 a (n.s.) |
| 6.5 ± 0.24 | 87.5 ± 5.8 B | 85.4 ± 5.8 a (n.s.) | 0 A | 1.24 b | 1.35 ± 0.07 B | 0.76 ± 0.02 a (***) |
| 7.2 ± 0.21 | 79.8 ± 9.4 B | 93.7 ± 6.8 a (*) | 0 A | 1.24 b | 1.50 ± 0.11 AB | 0.71 ± 0.03 a (***) |
| 7.8 ± 0.16 | 113 ± 5.5 A | 91.4 ± 3.5 a (***) | 0 A | 1.58 a | 1.66 ± 0.05 A | 0.61 ± 0.03 a (***) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Strelkov, S.E.; Hwang, S.-F. Influence of pH on the Growth of Verticillium longisporum and Verticillium Stripe Severity in Canola (Brassica napus). Horticulturae 2024, 10, 554. https://doi.org/10.3390/horticulturae10060554
Wang Y, Strelkov SE, Hwang S-F. Influence of pH on the Growth of Verticillium longisporum and Verticillium Stripe Severity in Canola (Brassica napus). Horticulturae. 2024; 10(6):554. https://doi.org/10.3390/horticulturae10060554
Chicago/Turabian StyleWang, Yixiao, Stephen E. Strelkov, and Sheau-Fang Hwang. 2024. "Influence of pH on the Growth of Verticillium longisporum and Verticillium Stripe Severity in Canola (Brassica napus)" Horticulturae 10, no. 6: 554. https://doi.org/10.3390/horticulturae10060554
APA StyleWang, Y., Strelkov, S. E., & Hwang, S.-F. (2024). Influence of pH on the Growth of Verticillium longisporum and Verticillium Stripe Severity in Canola (Brassica napus). Horticulturae, 10(6), 554. https://doi.org/10.3390/horticulturae10060554






