Exogenous Application of Melatonin on the Preservation of Physicochemical and Enzymatic Qualities of Pepper Fruit from Chilling Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Treatments
2.3. Quality Analysis
2.3.1. Chilling Injury (CI) Index
2.3.2. Determination of Weight Loss (WL)
2.3.3. Determination of Respiration Rate
2.3.4. Determination of Firmness
2.3.5. Determination of Electrolyte Leakage (EL)
2.3.6. Determination of O2•− Radical
2.3.7. Determination of H2O2 Radical
2.3.8. Determination of Malonaldheide (MDA)
2.3.9. Determination of Glutathione (GSH), Glutathione Disulfide (GSSG), and GSH/GSSG Ratio
2.3.10. Determination of Ascorbic Acid (AsA), Dehydroascorbate (DHA), and AsA/DHA Ratio
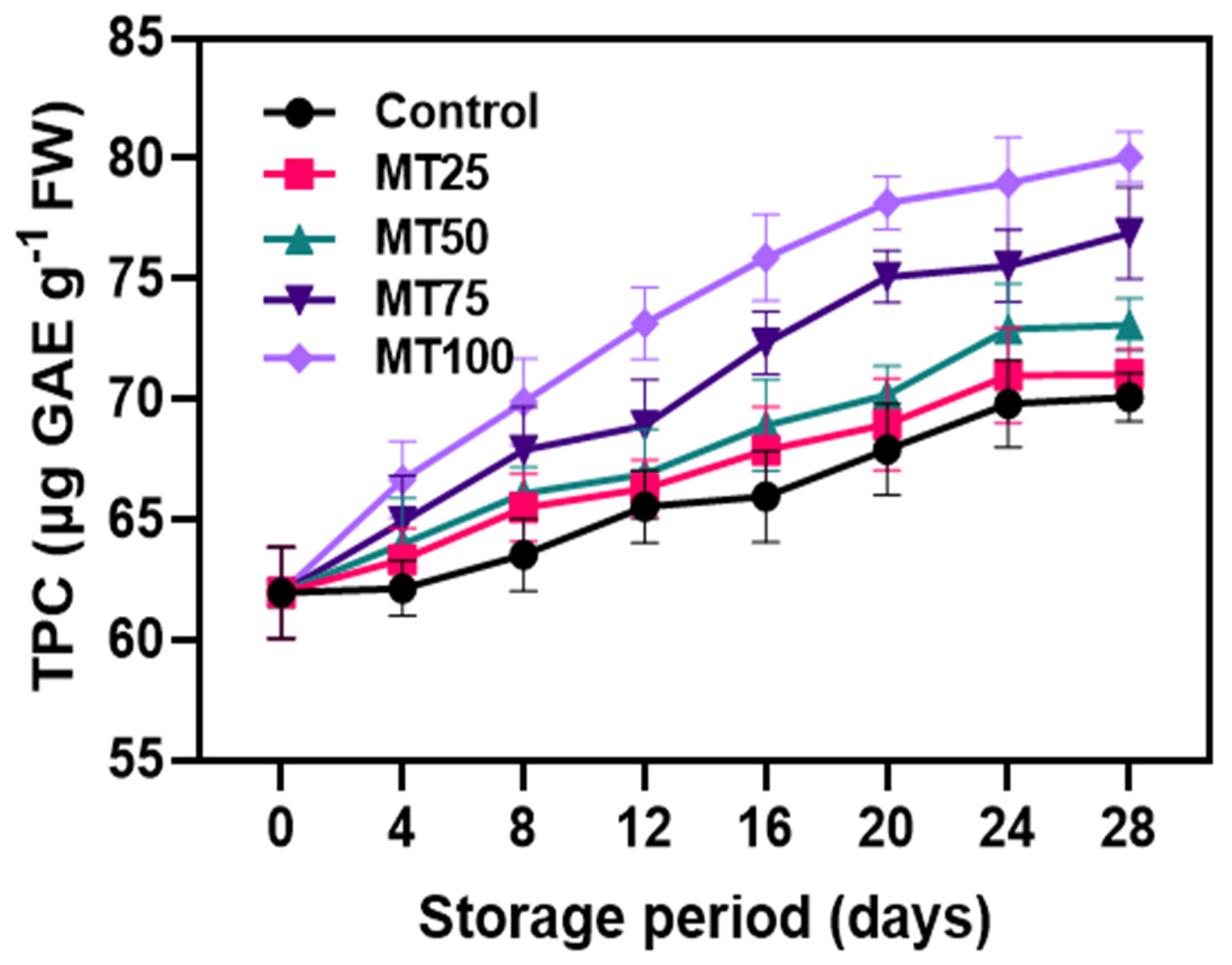
2.3.11. Determination of Total Phenolic Content (TPC)
2.3.12. Determination of Superoxide Dismutase (SOD)
2.3.13. Determination of Catalase (CAT)
2.3.14. Determination of Peroxidase (POD)
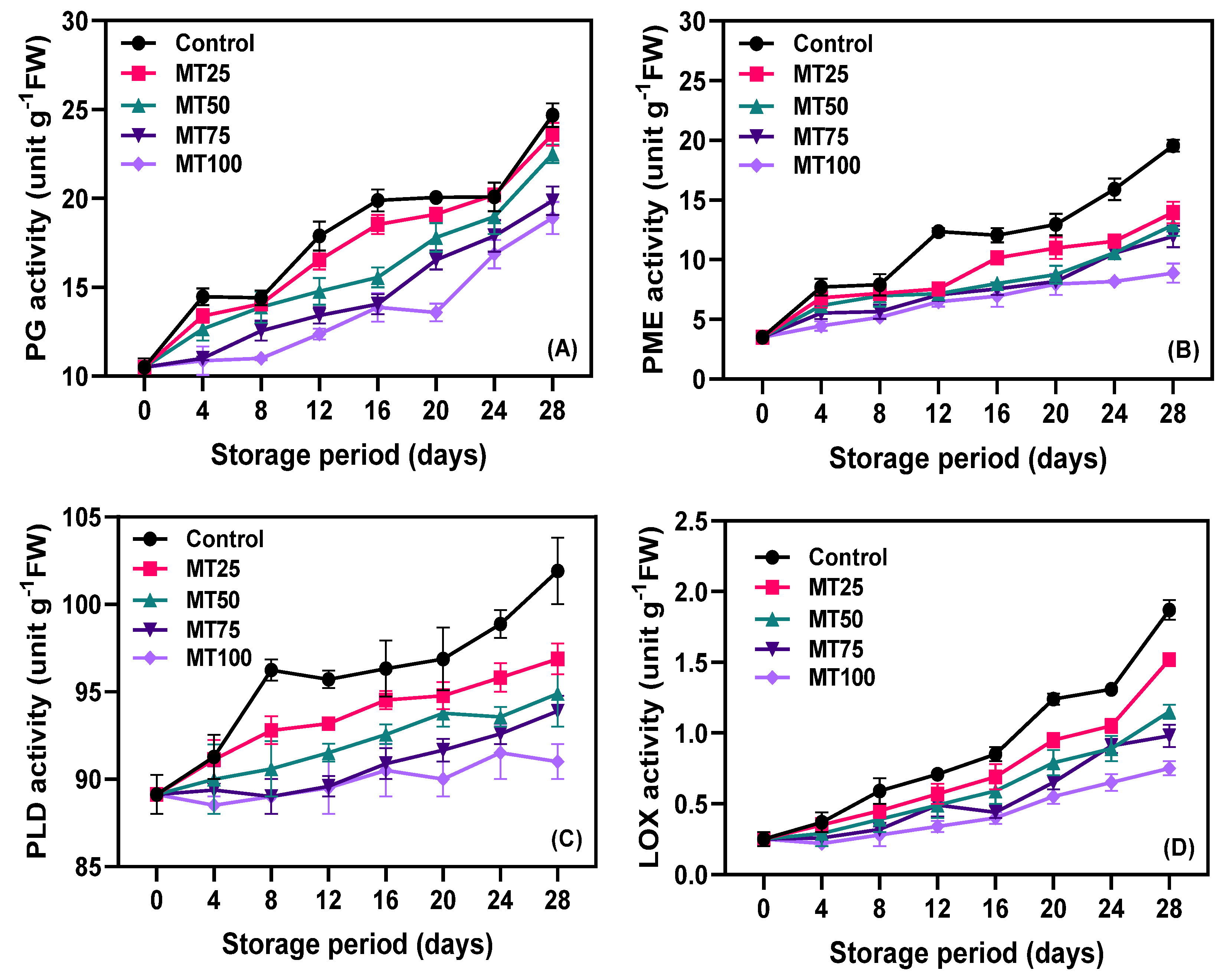
2.3.15. Determination of Polygalacturonase (PG) and Pectin Methylesterase (PME)
2.3.16. Determination of Phospholipase D (PLD)
2.3.17. Determination of Lipoxygenase (LOX)
2.4. Statistical Analysis
3. Results and Discussion
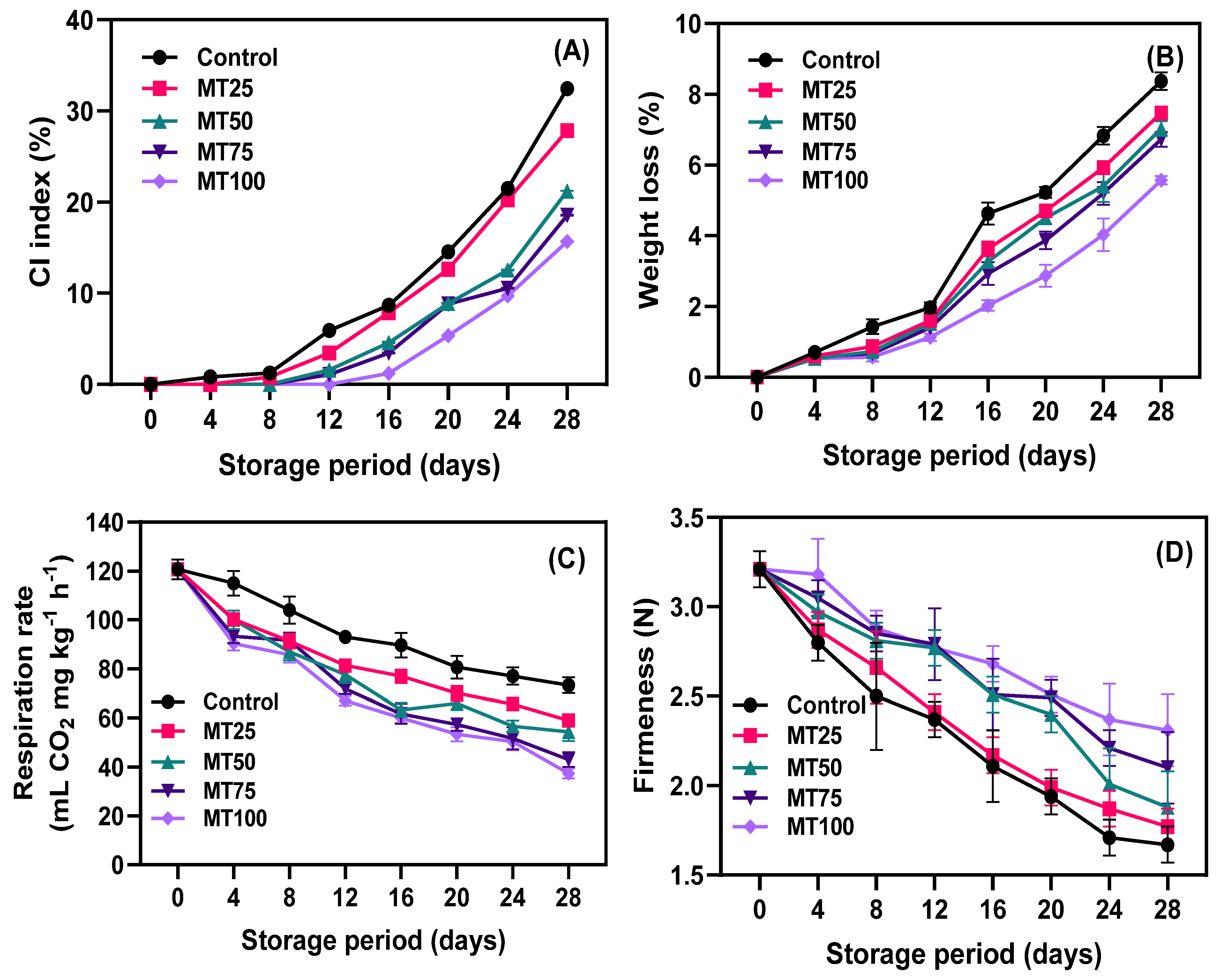
3.1. CI Index, WL, Respiration Rate, and Firmness
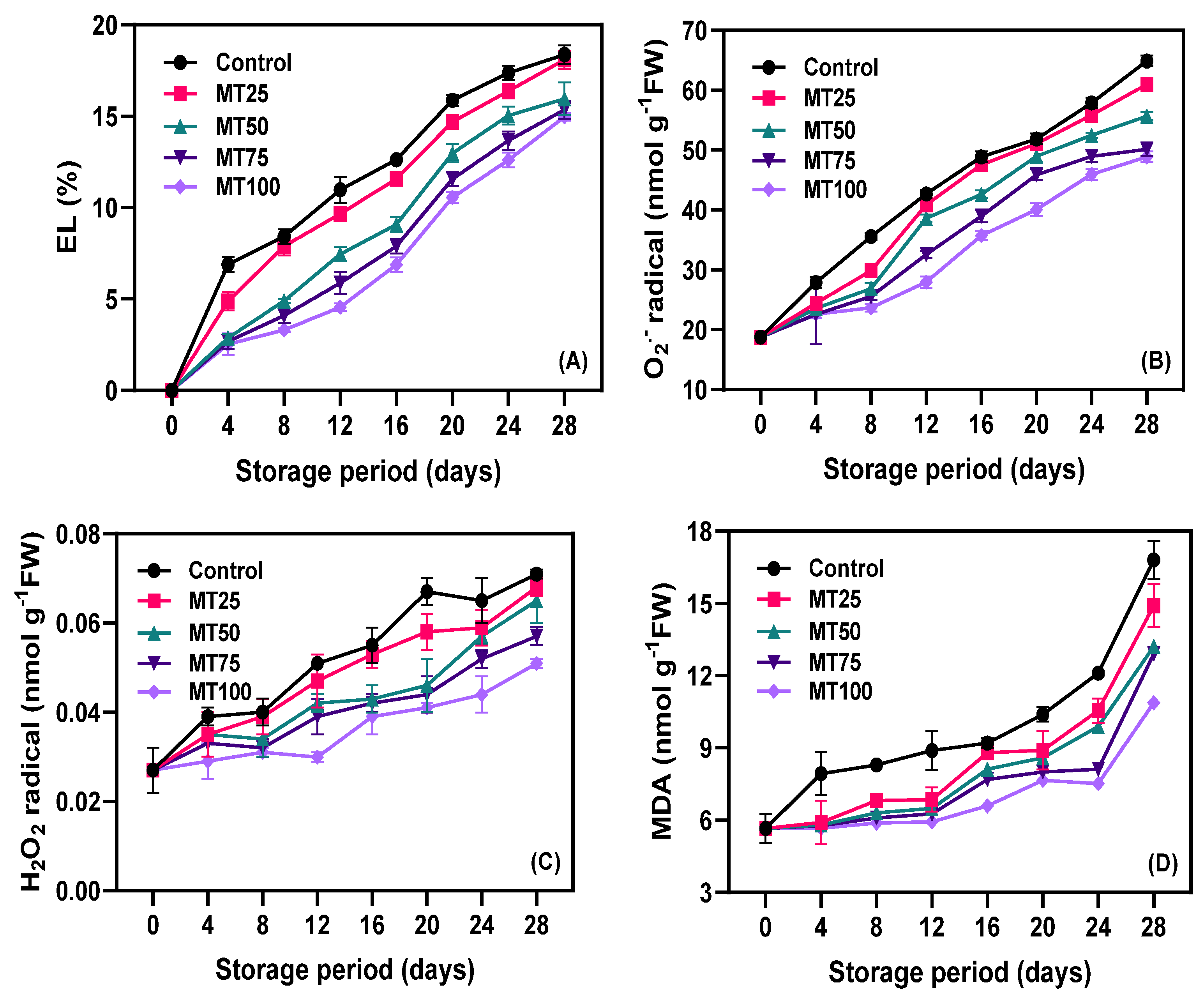
3.2. EL, ROS, and MDA
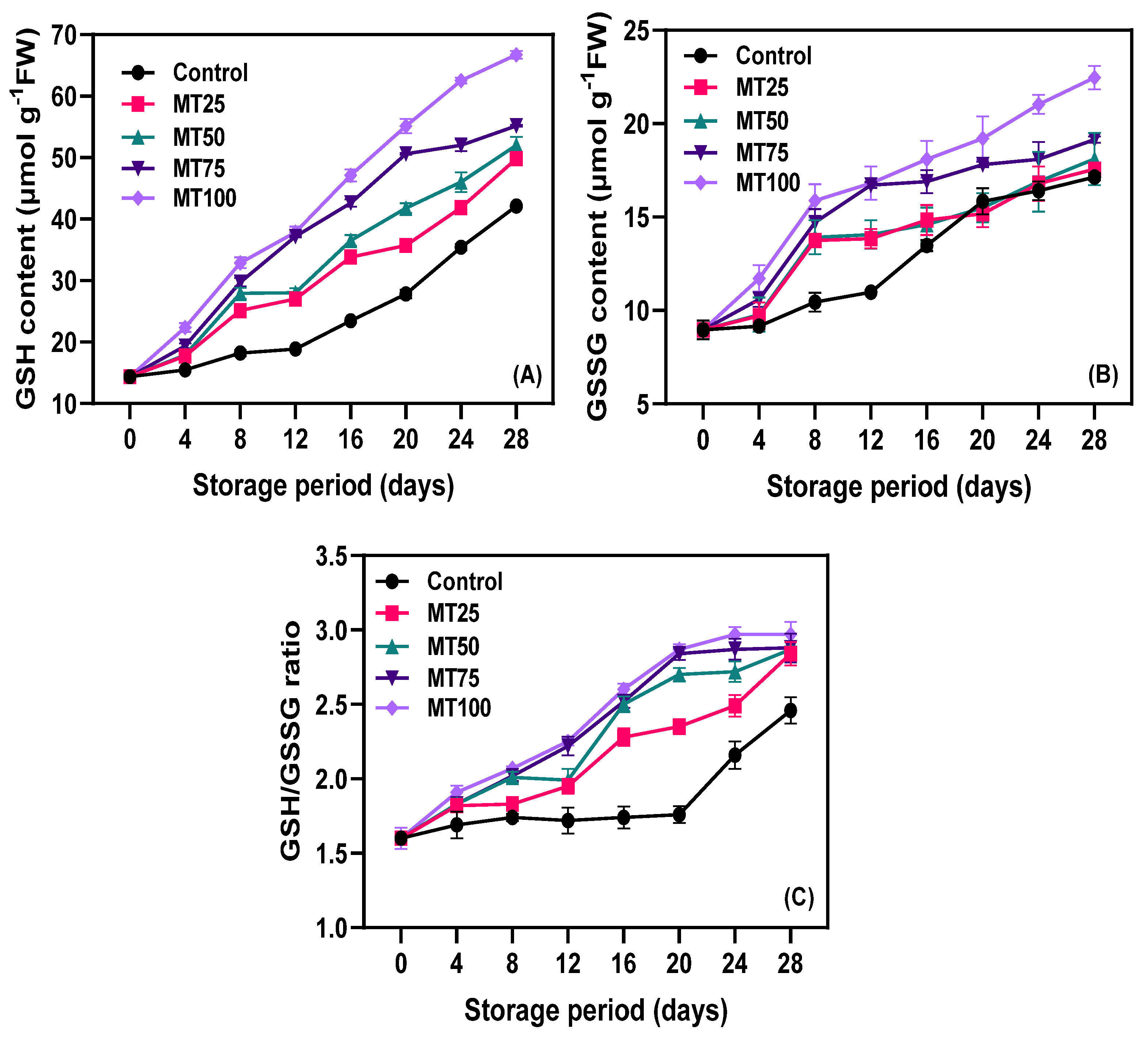
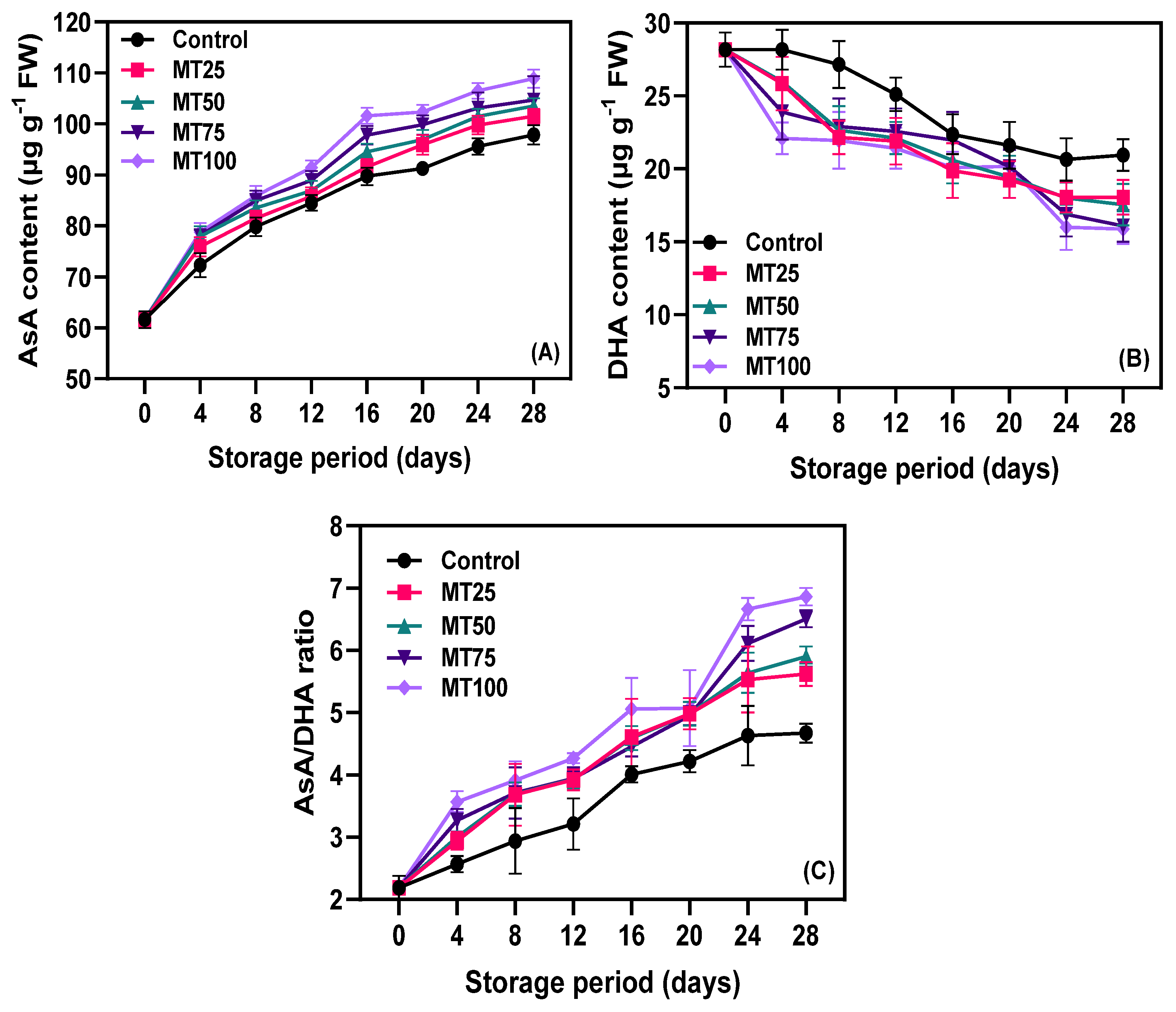
3.3. Non-Enzymatic Antioxidants (NEAs)
3.4. Enzyme Activities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobón-Suárez, A.; Giménez, M.J.; García-Pastor, M.E.; Zapata, P.J. Salicylic acid foliar application increases crop yield and quality parameters of green pepper fruit during postharvest storage. Agronomy 2021, 11, 2263. [Google Scholar] [CrossRef]
- Abdalla, M.; Taher, M.; Sanad, M.; Kamel, L. Chemical properties, phenolic profiles and antioxidant activities of pepper fruits. J. Agric. Chem. Biotechnol. 2019, 10, 133–140. [Google Scholar] [CrossRef]
- Wang, L.; Shen, X.; Chen, X.; Ouyang, Q.; Tan, X.; Tao, N. Exogenous application of melatonin to green horn pepper fruit reduces chilling injury during post-harvest cold storage by regulating enzymatic activities in the antioxidant system. Plants 2022, 11, 2367. [Google Scholar] [CrossRef] [PubMed]
- Konishi, A.; Terabayashi, S.; Itai, A. Relationship of cuticle development with water loss and texture of pepper fruit. Can. J. Plant. Sci. 2022, 102, 103–111. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.; Ramos, M.C.; Campos, M.J.; Díaz-Sánchez, I.; Cautain, B.; Mackenzie, T.A.; Vicente, F.; Corpas, F.J.; Palma, J.M. Pepper fruit extracts show anti-proliferative activity against tumor cells altering their NADPH-generating dehydrogenase and catalase profiles. Antioxidants 2023, 12, 1461. [Google Scholar] [CrossRef]
- Gil, M.I.; Tudela, J.A. Chapter 20.1-Fresh and fresh-cut fruit vegetables: Peppers. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Gil, M.I., Beaudry, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 521–525. [Google Scholar] [CrossRef]
- Li, P.; Zhang, R.; Zhou, H.; Mo, Y.; Wu, S.; Zhang, X.; Xie, Z.; Zhang, T.; Zhao, K.; Lv, J.; et al. Melatonin delays softening of post-harvest pepper fruits (Capsicum annuum L.) by regulating cell wall degradation, membrane stability and antioxidant systems. Postharvest Biol. Technol. 2024, 212, 112852. [Google Scholar] [CrossRef]
- Cao, S.; Song, C.; Shao, J.; Bian, K.; Chen, W.; Yang, Z. Exogenous melatonin treatment increases chilling tolerance and induces defense response in harvested peach fruit during cold storage. J. Agric. Food Chem. 2016, 64, 5215–5222. [Google Scholar] [CrossRef]
- Ahamad, S.; Asrey, R.; Singh, A.; Sethi, S.; Joshi, A.; Vinod, B.R.; Meena, N.; Menaka, M.; Choupdar, G. Melatonin treatment enhances bioactive compound retention, antioxidant activity and shelf-life of bell pepper (Capsicum annuum L.) during cold storage. Int. J. Food Sci. 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Ma, Q.; Zhang, X.; Luo, X.; Deng, Q. Exogenous melatonin treatment on post-harvest jujube fruits maintains physicochemical qualities during extended cold storage. PeerJ 2022, 10, e14155. [Google Scholar] [CrossRef]
- Kong, X.; Ge, W.; Wei, B.; Zhou, Q.; Zhou, X.; Zhao, Y.; Ji, S. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Shi, L.; Xu, L.; Shen, Z.; Chen, W.; Yang, Z. Melatonin increases chilling tolerance in post-harvest peach fruit by alleviating oxidative damage. Sci. Rep. 2018, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, S.; Bodaghi, H.; Ghasimi Hagh, Z. Alleviation of post-harvest chilling injury in sweet pepper using Salicylic acid foliar spraying incorporated with caraway oil coating under cold storage. Front. Plant Sci. 2022, 13, 999518. [Google Scholar] [CrossRef] [PubMed]
- Njie, A.; Zhang, W.; Dong, X.; Lu, C.; Pan, X.; Liu, Q. Effect of melatonin on fruit quality via decay inhibition and enhancement of antioxidative enzyme activities and genes expression of two mango cultivars during cold storage. Foods 2022, 11, 3209. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.L.; Kang, S.-M.; Jeoung, L.C.; Gross, K.; Woolf, A. Bell pepper (Capsicum annuum L.) fruits are susceptible to chilling injury at the breaker stage of ripeness. HortScience 2007, 42, 1659–1664. [Google Scholar] [CrossRef]
- Zhang, Y.; Huber, D.J.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Delay of post-harvest browning in litchi fruit by melatonin via the enhancing of antioxidative processes and oxidation repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Kantakhoo, J.; Imahori, Y. Antioxidative responses to pre-storage hot water treatment of red sweet pepper (Capsicum annuum L.) fruit during cold storage. Foods 2021, 10, 3031. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ding, F. Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Pareek, S.; Mani, S.; Domínguez-Avila, J.A.; González-Aguilar, G.A. A Melatonin Treatment Delays Postharvest Senescence, Maintains Quality, Reduces Chilling Injury, and Regulates Antioxidant Metabolism in Mango Fruit. J. Food Qual. 2022, 2022, e2379556. [Google Scholar] [CrossRef]
- Tan, C.K.; Ali, Z.M.; Ismail, I.; Zainal, Z. Effects of 1-methylcyclopropene and modified atmosphere packaging on the antioxidant capacity in pepper “Kulai” during low-temperature storage. Sci. World J. 2012, 2012, 474801. [Google Scholar] [CrossRef]
- Ngoc, T.T.B.; Thu, V.M.; Hien, N.T.P.; Tra, H.T.T.; Anh, H.D.; Hue, T.T. Effect of exogenous melatonin on antioxidant enzyme activities and membrane lipid peroxidation in avocado fruit during ripening. Vietnam. J. Biotechnol. 2022, 20, 495–504. [Google Scholar] [CrossRef]
- Priya Sethu, K.M.; Prabha, T.N.; Tharanathan, R.N. Post-harvest biochemical changes associated with the softening phenomenon in Capsicum annuum fruits. Phytochemistry 1996, 42, 961–966. [Google Scholar] [CrossRef]
- Yi, C.; Qu, H.X.; Jiang, Y.M.; Shi, J.; Duan, X.W.; Joyce, D.C.; Li, Y.B. ATP-induced changes in energy status and membrane integrity of harvested litchi fruit and its relation to pathogen resistance. J. Phytopathol. 2008, 156, 365–371. [Google Scholar] [CrossRef]
- Kissinger, M.; Tuvia-Alkalai, S.; Shalom, Y.; Fallik, E.; Elkind, Y.; Jenks, M.A.; Goodwin, M.S. Characterization of physiological and biochemical factors associated with post-harvest water loss in ripe pepper fruit during storage. J. Am. Soc. Hortic. Sci. 2005, 130, 735–741. [Google Scholar] [CrossRef]
- Ge, W.; Luo, M.; Sun, H.; Wei, B.; Zhou, X.; Zhou, Q.; Ji, S. The CaMYB340 transcription factor induces chilling injury in post-harvest bell pepper by inhibiting fatty acid desaturation. Plant J. 2022, 111, 800–818. [Google Scholar] [CrossRef] [PubMed]
- Medina-Santamarina, J.; Guillén, F.; Mihaela Iasmina Madalina, I.; Ruiz-Aracil, M.; Valero, D.; Castillo, S.; Serrano, M. Melatonin Treatments Reduce Chilling Injury and Delay Ripening, Leading to Maintenance of Quality in Cherimoya Fruit. Int. J. Mol. Sci. 2023, 24, 3787. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative stress associated with chilling injury in immature fruit: Post-harvest technological and biotechnological solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bel, P.; Egea, I.; Sánchez-Ballesta, M.T.; Martinez-Madrid, C.; Fernandez-Garcia, N.; Romojaro, F.; Olmos, E.; Estrella, E.; Bolarín, M.C.; Flores, F.B. Understanding the mechanisms of chilling injury in bell pepper fruits using the proteomic approach. J. Proteom. 2012, 75, 5463–5478. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, G.; Tengku, M.; Mohamed, M.; Ding, P.; Mohd Ghazali, H. Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. 2015, 190, 187–194. [Google Scholar] [CrossRef]
- Ullah, A.; Abbasi, N.A.; Shafique, M.; Qureshi, A.A. Influence of edible coatings on biochemical fruit quality and storage life of bell pepper cv. “Yolo Wonder”. J. Food Qual. 2017, 2017, e2142409. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef]
- Cherono, K.; Workneh, T.S. A review of the role of transportation on the quality changes of fresh tomatoes and their management in South Africa and other emerging markets. Int. Food Res. J. 2018, 25, 2211–2228. [Google Scholar]
- Sun, Y.; Li, M.; Ji, S.; Cheng, S.; Zhou, Q.; Zhou, X.; Li, M.; Wei, B. Effect of exogenous melatonin treatment on quality and softening of jujube fruit during storage. J. Food Process. Preserv. 2022, 46, e16662. [Google Scholar] [CrossRef]
- Draye, M.; Van Cutsem, P. Pectin methylesterases induce an abrupt increase of acidic pectin during strawberry fruit ripening. J. Plant Physiol. 2008, 165, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Shibata, Y.; Tateba, H.; Hatanaka, A.; Kajiwara, T. Changes of lipoxygenase and fatty acid hydroperoxide lyase activities in bell pepper fruits during maturation. Biosci. Biotechnol. Biochem. 1997, 61, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Imahori, Y.; Bai, J.; Ford, B.; Baldwin, E. Effect of storage temperature on chilling injury and activity of antioxidant enzymes in carambola ‘Arkin’ fruit. J. Food Process. Preserv. 2020, 45, e15178. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of pants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, R.; Yuan, L.; Yuan, G.; Zhao, M.; Zhu, S.; Hou, J.; Chen, G.; Wang, C. Response of photosynthetic capacity and antioxidative system of chloroplast in two wucai (Brassica campestris L.) genotypes against chilling stress. Physiol. Mol. Biol. Plants 2020, 26, 219–232. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Z.; Pan, J.; Li, J.; Li, X.; Khoo, H.E.; Dong, X. Melatonin treatment improves postharvest quality and regulates reactive oxygen species metabolism in “Feizixiao” litchi based on principal component analysis. Front. Plant Sci. 2022, 13, 965345. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.-Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Antoli, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell. Mol. Life Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.; Chai, H.; Cheng, N.; Yang, Y.; Wang, D.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, M.; Zhang, W.; Gao, Y.; Ma, X.; Cheng, S.; Chen, G. Exogenous melatonin activates the antioxidant system and maintains postharvest organoleptic quality in Hami melon (Cucumis. Melo Var. Inodorus Jacq.). Front. Plant Sci. 2023, 14, 1274939. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, C.; Bing, H.; Zhao, J.; Li, L.; Sun, P.; Li, T.; Du, D.; Zhao, J.; Wang, X.; et al. Integrated physiological, transcriptomic, and metabolomic analysis reveals the mechanism of guvermectin promoting seed germination in direct-seeded rice under chilling stress. J. Agric. Food Chem. 2023, 71, 7348–7358. [Google Scholar] [CrossRef]
- Esim, N.; ATICI, Ö. Relationships between Some endogenous signal compounds and the antioxidant system in response to chilling stress in maize (Zea mays L.) seedlings. Turk. J. Bot. 2016, 40, 37–44. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X. Melatonin-induced detoxification of organic pollutants and alleviation of phytotoxicity in selected horticultural crops. Horticulturae 2022, 8, 1142. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Zhu, L.; Ma, Z.; Wang, J.; Zhu, J. Exogenous melatonin improves plant iron deficiency tolerance via increased accumulation of polyamine-mediated nitric oxide. Int. J. Mol. Sci. 2016, 17, 1777. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-H.; Yu, C.-W.; Lin, C. Hydrogen peroxide functions as a stress signal in plants. Bot. Bull. Acad. Sin. 2005, 46, 1–10. [Google Scholar]
- Rahman, M.; Mian, M.; Ahmed, A.; Rohman, M. Roles of glutathione s-transferease in maize (Zea mays L.) under cold stress. Res. Agric. Livest. Fish. 2015, 2, 9–15. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Nuhu, F.; Gordon, A.; Sturmey, R.; Seymour, A.-M.; Bhandari, S. Measurement of glutathione as a tool for oxidative stress studies by high performance liquid chromatography. Molecules 2020, 25, 4196. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in Abiotic Stress Resistance and Tolerance. In Plant Growth Regulators: Signalling under Stress Conditions; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 239–273. [Google Scholar] [CrossRef]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin treatment improves post-harvest preservation and resistance of guava fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, C.; Wang, J.; Younas, S.; Zheng, H.; Zheng, L. Melatonin immersion affects the quality of fresh-cut broccoli (Brassica oleracea L.) during cold storage: Focus on the antioxidant system. J. Food Process. Preserv. 2020, 44, e14691. [Google Scholar] [CrossRef]
- Knight, J.; Madduma-Liyanage, K.; Mobley, J.A.; Assimos, D.G.; Holmes, R.P. Ascorbic acid intake and oxalate synthesis. Urolithiasis 2016, 44, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X. The physiological role of dehydroascorbic acid. FEBS Lett. 2002, 527, 5–9. [Google Scholar] [CrossRef]
- Sharova, E.I.; Medvedev, S.S.; Demidchik, V.V. Ascorbate in the apoplast: Metabolism and functions. Russ. J. Plant Physiol. 2020, 67, 207–220. [Google Scholar] [CrossRef]
- Hassan, A.M.A.; Zannou, O.; Pashazadeh, H.; Redha, A.A.; Koca, I. Drying date plum (Diospyros lotus L.) fruit: Assessing rehydration properties, antioxidant activity, and phenolic compounds. J. Food Sci. 2022, 87, 4394–4415. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, M.; Kabiri, R.; Hatami, A.; Oloumi, H.; Nasibi, F.; Tahmasei, Z. Exogenous application of melatonin mitigates the adverse effects of drought stress on morpho-physiological traits and secondary metabolites in Moldavian balm (Dracocephalum moldavica). Physiol. Mol. Biol. Plants 2019, 25, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhao, J.; Guo, L.; Kim, S.-C.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases: Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenphun, N.; Pham, N.H.; Rattanawut, J.; Venkatachalam, K. Exogenous Application of Melatonin on the Preservation of Physicochemical and Enzymatic Qualities of Pepper Fruit from Chilling Injury. Horticulturae 2024, 10, 550. https://doi.org/10.3390/horticulturae10060550
Charoenphun N, Pham NH, Rattanawut J, Venkatachalam K. Exogenous Application of Melatonin on the Preservation of Physicochemical and Enzymatic Qualities of Pepper Fruit from Chilling Injury. Horticulturae. 2024; 10(6):550. https://doi.org/10.3390/horticulturae10060550
Chicago/Turabian StyleCharoenphun, Narin, Nam Hoang Pham, Jessada Rattanawut, and Karthikeyan Venkatachalam. 2024. "Exogenous Application of Melatonin on the Preservation of Physicochemical and Enzymatic Qualities of Pepper Fruit from Chilling Injury" Horticulturae 10, no. 6: 550. https://doi.org/10.3390/horticulturae10060550
APA StyleCharoenphun, N., Pham, N. H., Rattanawut, J., & Venkatachalam, K. (2024). Exogenous Application of Melatonin on the Preservation of Physicochemical and Enzymatic Qualities of Pepper Fruit from Chilling Injury. Horticulturae, 10(6), 550. https://doi.org/10.3390/horticulturae10060550





