Potassium Nutrition Induced Salinity Mitigation in Mungbean [Vigna radiata (L.) Wilczek] by Altering Biomass and Physio-Biochemical Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Parameters
2.2. Biochemical Analyses
2.2.1. Nitrate Reductase Activity
2.2.2. Nitrite Reductase Activity
2.2.3. Total Free Amino Acids
2.2.4. Total Soluble Proteins
2.2.5. Chlorophyll and Carotenoids Content
2.3. Mineral Analyses
2.4. Statistical Analyses
3. Results
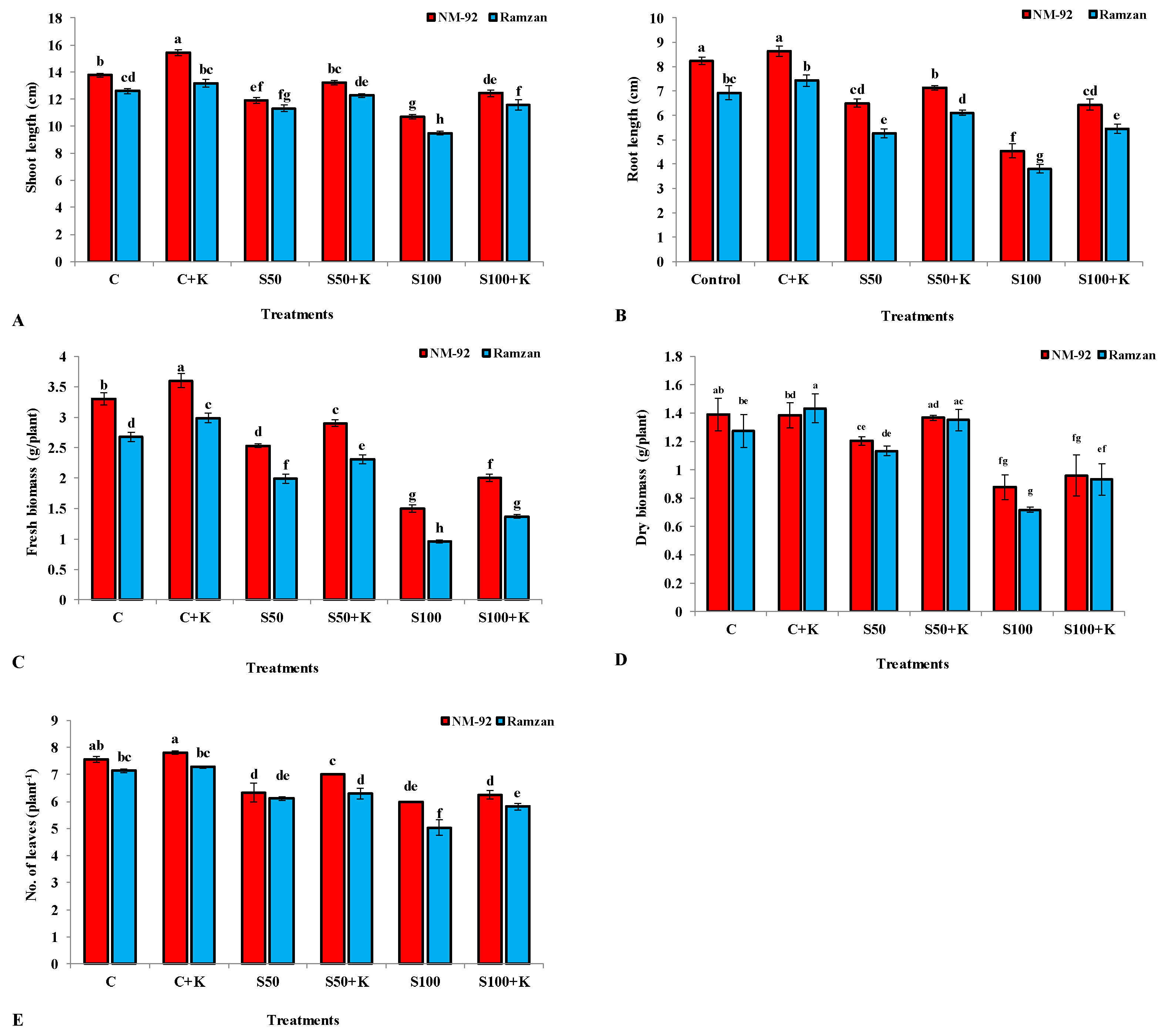
3.1. Growth Parameters
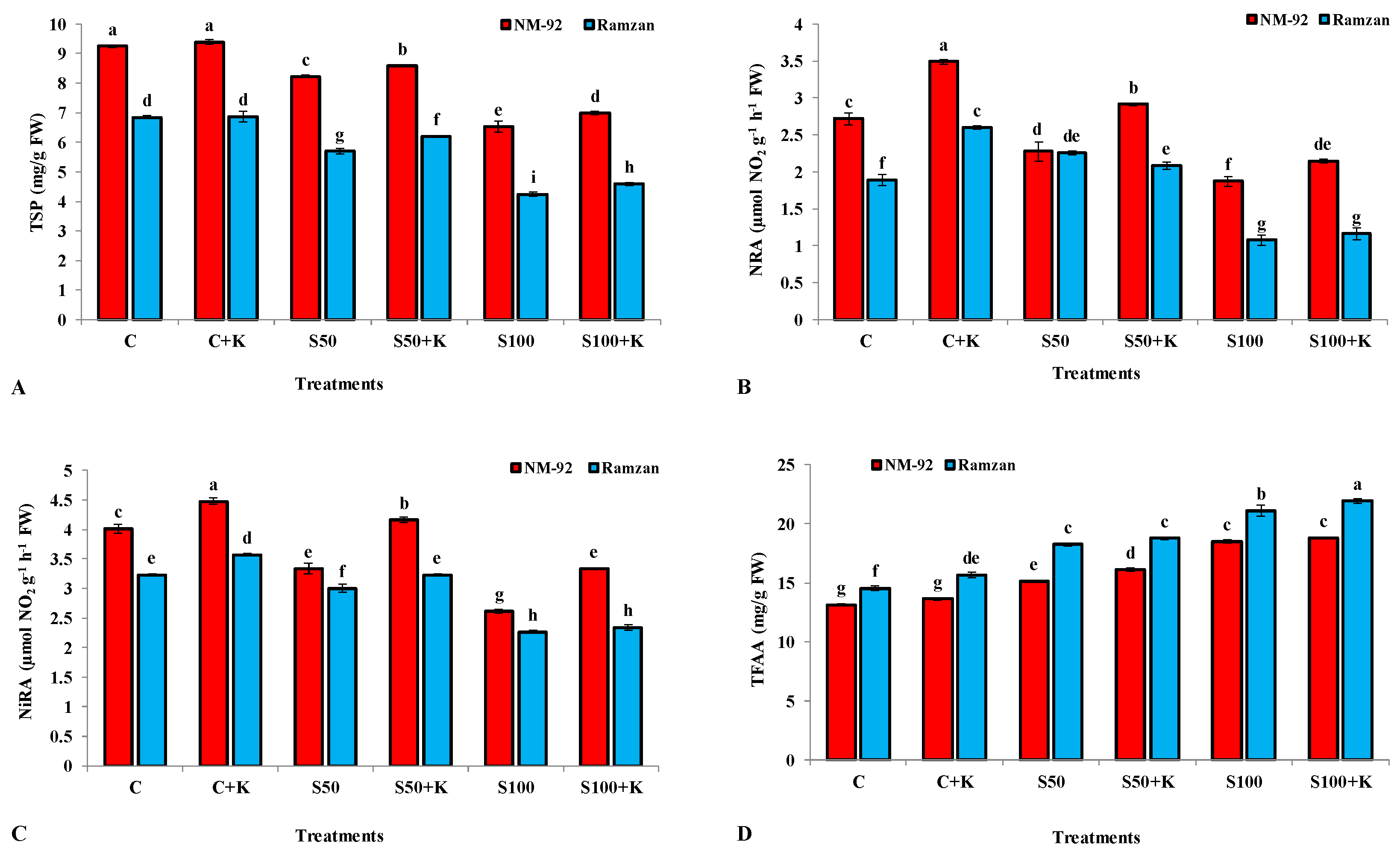
3.2. Biochemical Parameters
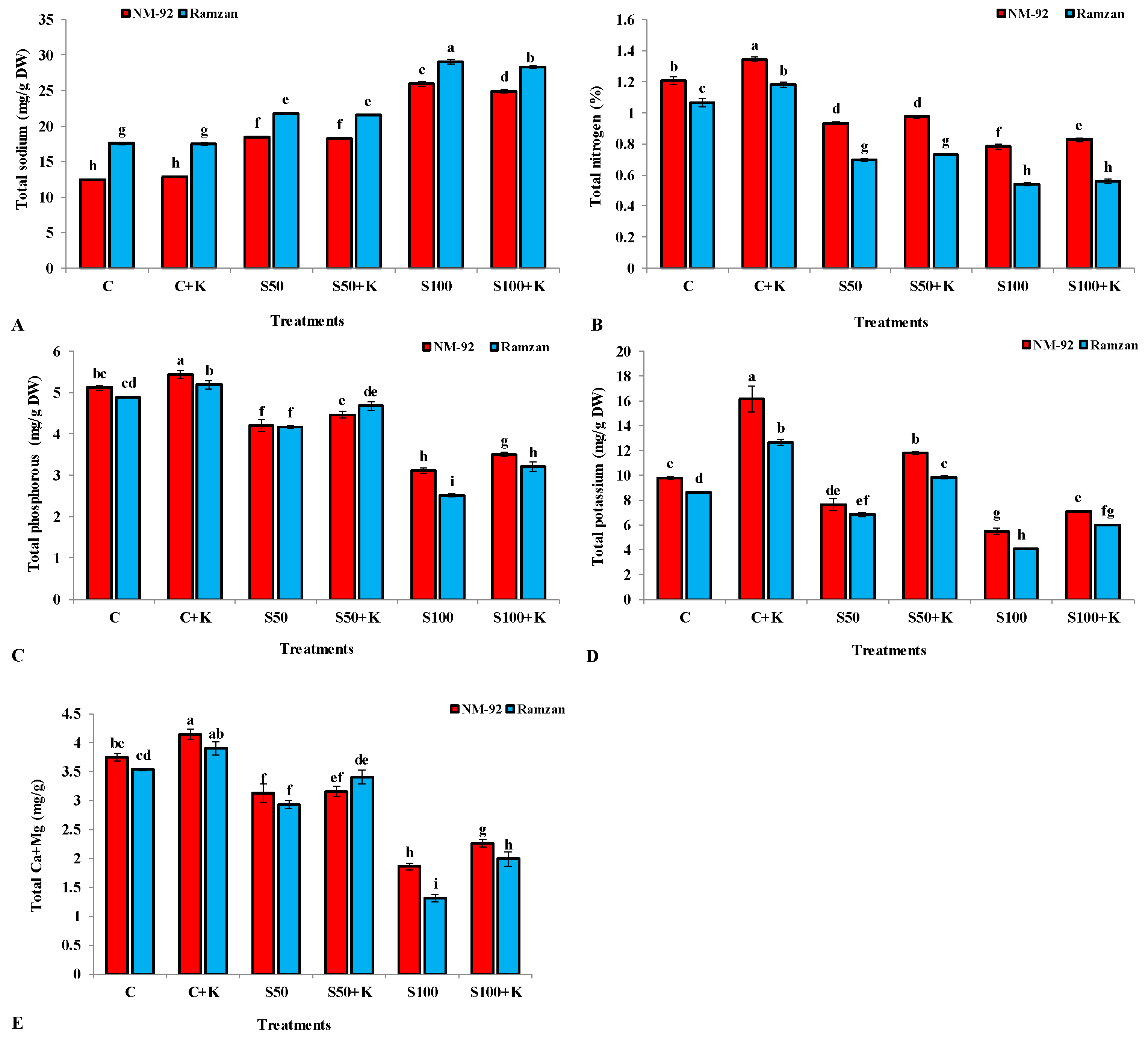
3.3. Nutrient Uptake
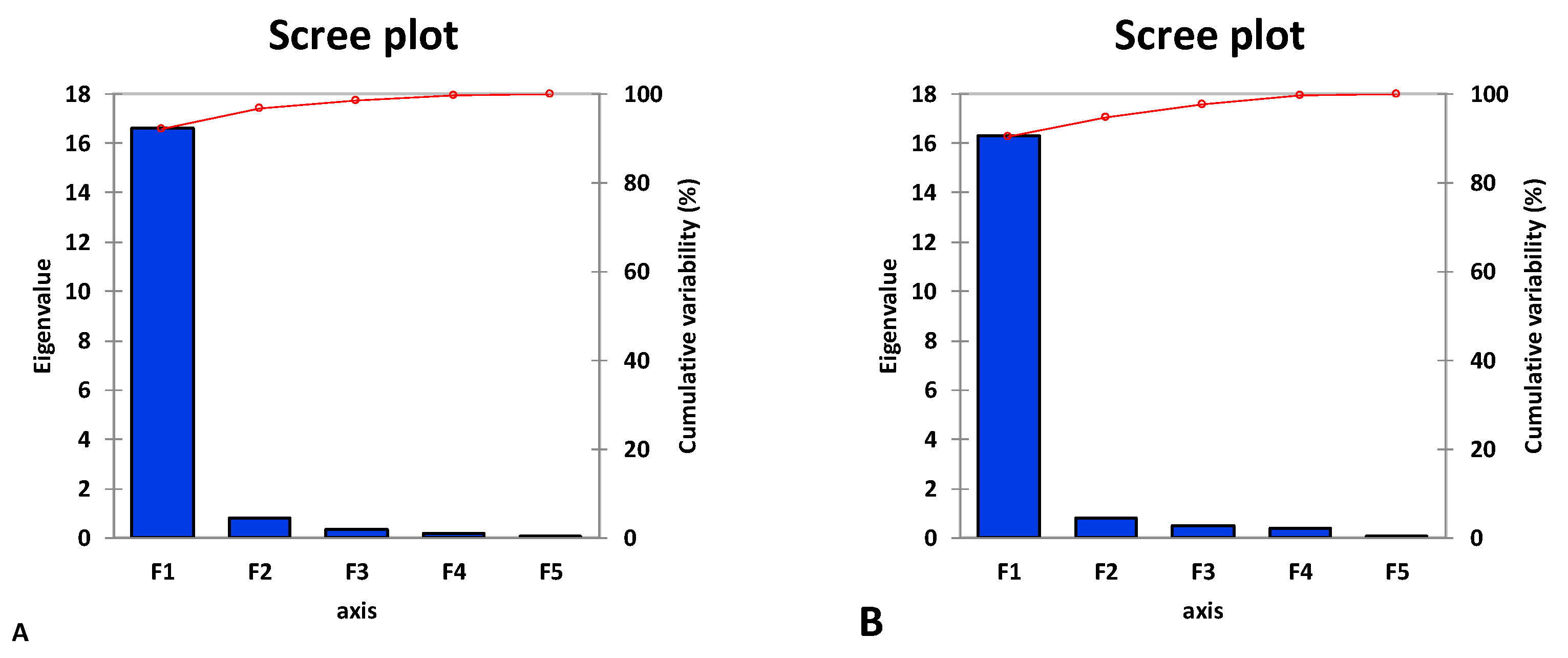
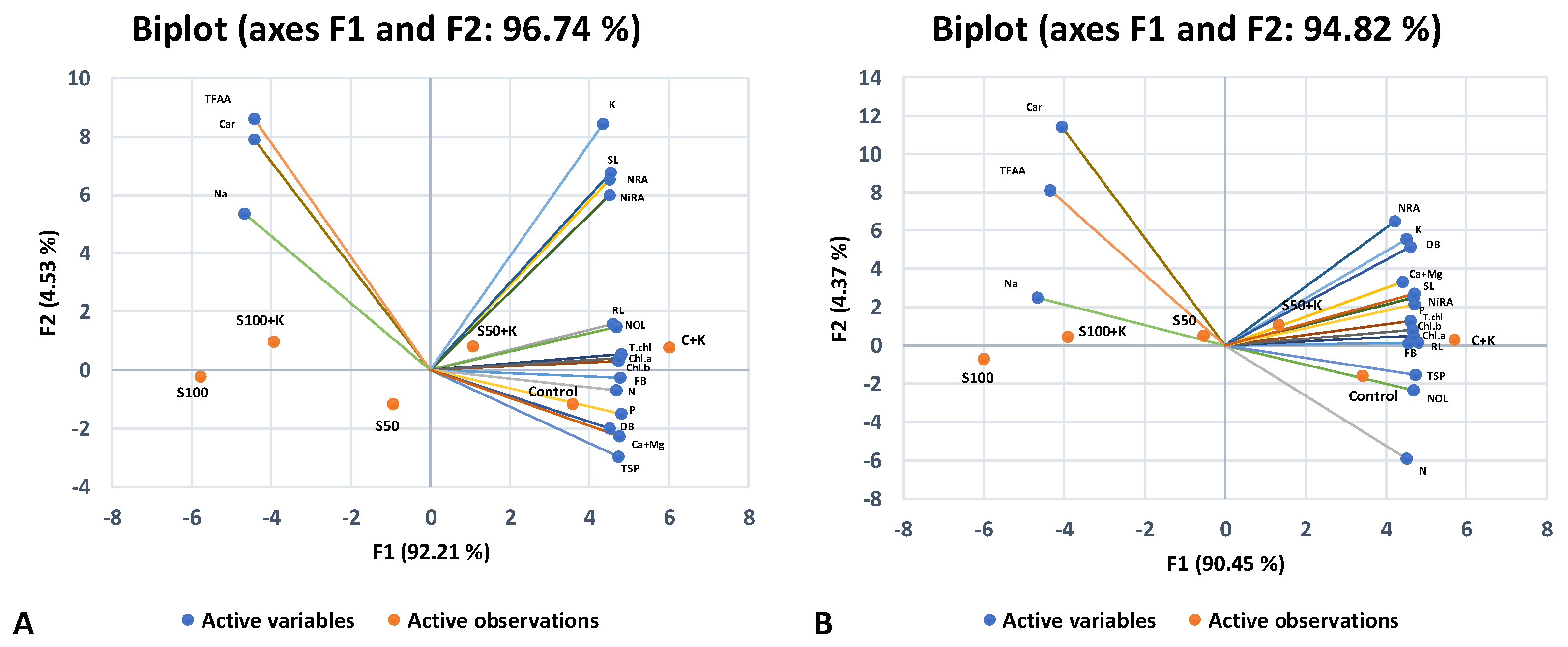
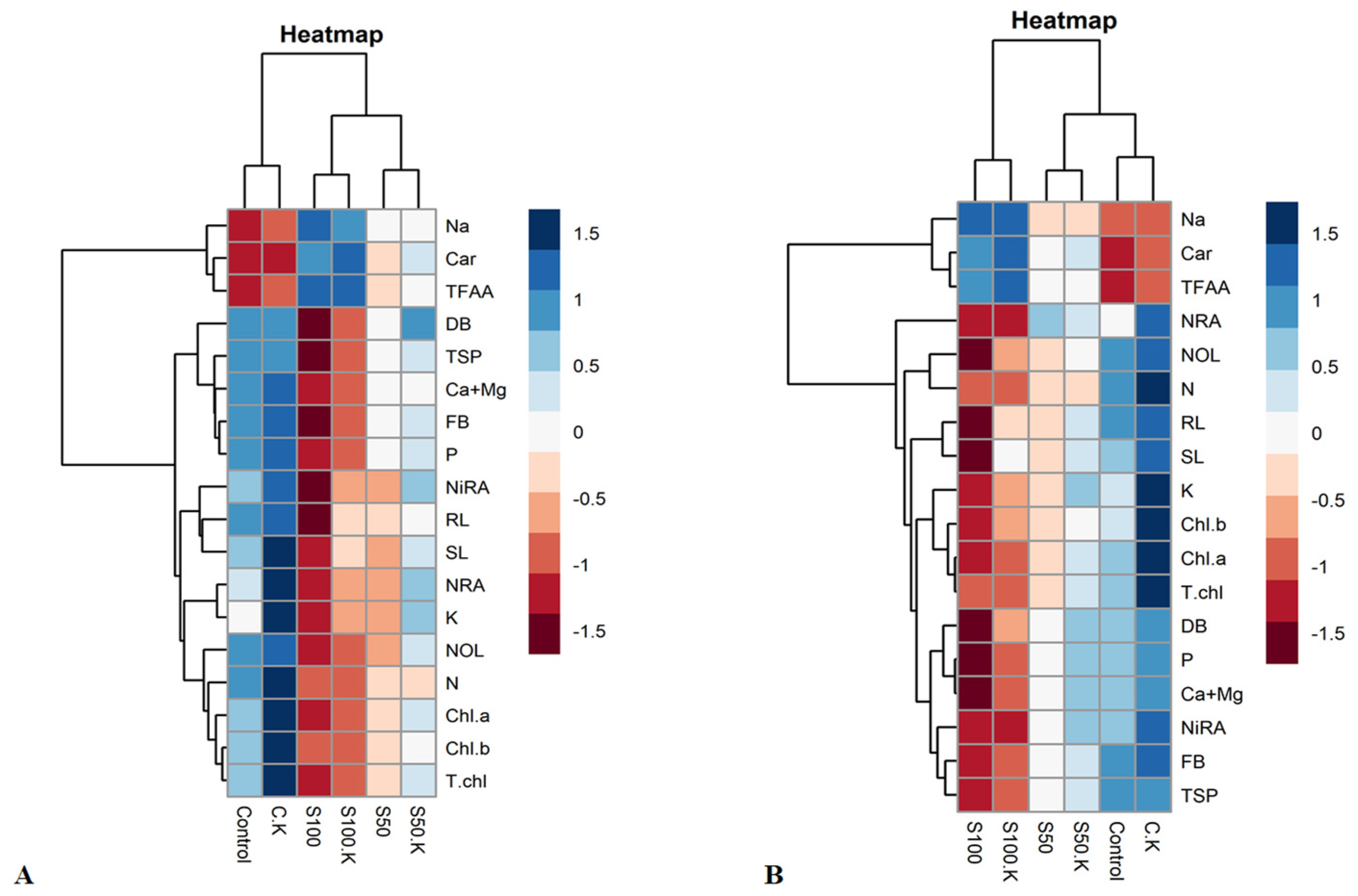
3.4. Principal Component and Heatmap Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mustafa, G.; Akhtar, M.S.; Abdullah, R. Global concern for salinity on various agro-ecosystems. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Akhtar, M., Ed.; Springer: Singapore, 2019; pp. 1–19. [Google Scholar]
- Montanarella, L.; Badraoui, M.; Chude, V.; Costa, I.; Mamo, T.; Yemefack, M.; Aulang, M.; Yagi, K.; Hong, S.Y.; Vijarnsorn, P. Status of the World’s Soil Resources: Main Report; Food and Agriculture Organization of the United Nation: Rome, Italy, 2015. [Google Scholar]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J.J.C. Salinity stress in arid and semi-arid climates: Effects and management in field crops. Clim. Change Agric. 2019, 13, 201–226. [Google Scholar]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, P.; Harisha, R.; Kohli, M.; Naik, P.K.; Sagar, S.; Shashidhar, B.; Patel, M.K.; Ganesh, B.T.; Madhusudhan, B.; Chethan Kumar, K.J. Exploring the World of Mungbean: Uncovering its Origins, Taxonomy, Genetic Resources and Research Approaches. Int. J. Plant Soil Sci. 2023, 35, 614–635. [Google Scholar] [CrossRef]
- Matloob, A.; Jabran, K.; Farooq, M.; Khaliq, A.; Aslam, F.; Abbas, T.; Ehsanullah; Zaman, U.; Irshad, S.; Chauhan, B.S. Water-Wise Cultivation of Basmati Rice in Pakistan. In Modern Techniques of Rice Crop Production; Springer: Singapore, 2022; pp. 187–229. [Google Scholar]
- Ashraf, M.Y.; Zaib-UN-Nisa, N.; Shani, M.Y.; Naz, A.; Azmat, M.; Ashraf, I. Salicylic acid seed priming improved dry biomass and ionic efficiency of mungbean [Vigna radiata (L) wilczek] under salt stress conditions. Pak. J. Bot. 2023, 55, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Wang, H.-W.; Sun, D.-W.J. Phytohormones in postharvest storage of fruit and vegetables: Mechanisms and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 2969–2983. [Google Scholar] [CrossRef]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants—Natural biological resources for sustainable plant production. Microorganisms 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Rakhmankulova, Z.; Shuyskaya, E.; Toderich, K.; Voronin, P. Elevated atmospheric CO2 concentration improved C4 xero-halophyte Kochia prostrata physiological performance under saline conditions. Plants 2021, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Fariduddin, Q.; Khan, T.A.; Hayat, S. Epibrassinolide reverses the stress generated by combination of excess aluminum and salt in two wheat cultivars through altered proline metabolism and antioxidants. S. Afr. J. Bot. 2017, 112, 391–398. [Google Scholar] [CrossRef]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef]
- Nasrallah, A.K.; Kheder, A.A.; Kord, M.A.; Fouad, A.S.; El-Mogy, M.M.; Atia, M.A. Mitigation of salinity stress effects on broad bean productivity using calcium phosphate nanoparticles application. Horticulturae 2022, 8, 75. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Halotolerant plant growth-promoting fungi and bacteria as an alternative strategy for improving nutrient availability to salinity-stressed crop plants. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Singapore, 2019; pp. 103–146. [Google Scholar]
- Ketehouli, T.; Idrice Carther, K.F.; Noman, M.; Wang, F.-W.; Li, X.-W.; Li, H.Y. Adaptation of plants to salt stress: Characterization of Na+ and K+ transporters and role of CBL gene family in regulating salt stress response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 450967. [Google Scholar] [CrossRef] [PubMed]
- Sym, G.J. Optimisation of the in-vivo assay conditions for nitrate reductase in barley (Hordeum vulgare L. cv. Igri). J. Sci. Food Agric. 1984, 35, 725–730. [Google Scholar] [CrossRef]
- Ramarao, C.; Patil, V.; Dhak, B.; Kadrekar, S.B. A simple in vivo method for the determination of nitrite reductase activity in rice roots. Z. Pflanzenphysiol. 1983, 109, 81–85. [Google Scholar] [CrossRef]
- Hamilton, P.; Van Slyke, D.J. Amino acid determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Davies, B. Carotenoids in higher plants. In Lipids and Lipid Polymers in Higher Plants; Springer: Berlin/Heidelberg, Germany, 1977; pp. 199–217. [Google Scholar]
- Lowther, J.R. Use of a single sulphuric acid-hydrogen peroxide digest for the analysis of Pinus radiata needles. Commun. Soil Sci. Plant Anal. 1980, 11, 175–188. [Google Scholar] [CrossRef]
- Hide, J.J.S. Diagnosis and Improvement of Saline and Alkali Soils; US Salinity Laboratory Staff; Richards, L.A., Ed.; US Department of Agriculture: Washington, DC, USA, 1954; 160p.
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Chang, S.; Jackson, M.L. Soil Phosphorus Fractions in some representative soils 1. J. Soil Sci. 1958, 9, 109–119. [Google Scholar] [CrossRef]
- Ramzan, F.; Younis, A. Use of biostimulants in tolerance of drought stress in agricultural crops. In Emerging Plant Growth Regulators in Agriculture; Academic Press: Cambridge, MA, USA, 2022; pp. 429–446. [Google Scholar]
- Dutta, P.; Bera, A.K. Effect of NaCl salinity on seed germination and seedling growth of mungbean cultivars. Legume Res.-Int. J. 2014, 37, 161–164. [Google Scholar] [CrossRef]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef]
- Alharbi, K.; Al-Osaimi, A.A.; Alghamdi, B.A. Sodium chloride (NaCl)-induced physiological alteration and oxidative stress generation in Pisum sativum (L.): A toxicity assessment. ACS Omega 2022, 7, 20819–20832. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.Y.; Rafique, N.; Ashraf, M.; Azhar, N.; Marchand, M. Effect of supplemental potassium (K+) on growth, physiological and biochemical attributes of wheat grown under saline conditions. J. Plant Nutr. 2013, 36, 443–458. [Google Scholar] [CrossRef]
- Alharbi, B.M.; Elhakem, A.H.; Alnusairi, G.S.; Soliman, M.H.; Hakeem, K.R.; Hasan, M.M.; Abdelhamid, M.T. Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ. 2021, 67, 208–220. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Ayub, Q.; Khan, S.M.; Hussain, I.; Gurmani, A.R.; Naveed, K.; Mehmood, A.; Ali, S.; Ahmad, T.; Haq, N.; Hussain, A. Mitigating the adverse effects of NaCl salinity on pod yield and ionic attributes of okra plants by silicon and gibberellic acid application. Italus Hortus 2021, 28, 1–59. [Google Scholar] [CrossRef]
- Abbas, S.; Javed, M.T.; Ali, Q.; Azeem, M.; Ali, S. Nutrient deficiency stress and relation with plant growth and development. In Engineering Tolerance in Crop Plants against Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2021; pp. 239–262. [Google Scholar]
- Hyder, S.; Gondal, A.S.; Rizvi, Z.F.; Iqbal, R.; Hannan, A.; Sahi, S.T. Antagonism of selected fungal species against Macrophomina phaseolina (Tassi) goid, causing charcoal rot of Mungbean. Pak. J. Bot. 2022, 54, 1129–1138. [Google Scholar] [CrossRef]
- Liu, B.; Soundararajan, P.; Manivannan, A. Mechanisms of silicon-mediated amelioration of salt stress in plants. Plants 2019, 8, 307. [Google Scholar] [CrossRef]
- Singh, R.B.; Rao, V.P.; Sengar, R.S. Study of salinity induced oxidative stress and antioxidant responses in callus cultures of sugarcane. Ecol. Genet. Genom. 2023, 26, 100164. [Google Scholar]
- Hesari, N.; Szegő, A.; Mirmazloum, I.; Pónya, Z.; Kiss-Bába, E.; Kolozs, H.; Gyöngyik, M.; Vasas, D.; Papp, I. High-nitrate-supply-induced transcriptional upregulation of ascorbic acid biosynthetic and recycling pathways in cucumber. Plants 2023, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory, Transport and Signaling Mechanisms; Springer: Cham, Switzerland, 2018; pp. 85–136. [Google Scholar]
- Dubey, R.S.; Srivastava, R.K.; Pessarakli, M. Physiological mechanisms of nitrogen absorption and assimilation in plants under stressful conditions. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2021; pp. 579–616. [Google Scholar]
- Anwar, T.; Qureshi, H.; Fatimah, H.; Siddiqi, E.H.; Anwaar, S.; Moussa, I.M.; Adil, M.F. Elucidating effect of ZnO-Nanoparticles and melatonin on physiological adjustments and growth of Solanum melongena under salinity stress. Horticulturae 2023, 322, 112455. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, S.H.; Namich, A.A.M.; Abdel-Sattar, R.R. Effect of salicylic acid and potassium citrate on cotton plant under salt stress. Fresenius Environ. Bull. 2017, 26, 1091–1100. [Google Scholar]
- Lysenko, V.; Rajput, V.D.; Kumar Singh, R.; Guo, Y.; Kosolapov, A.; Usova, E.; Varduny, T.; Chalenko, E.; Yadronova, O.; Dmitriev, P.; et al. Chlorophyll fluorometry in evaluating photosynthetic performance: Key limitations, possibilities, perspectives and alternatives. Physiol. Mol. Biol. Plants 2022, 28, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Ashraf, M.Y.; Saleem, M. Shift in physiological and biochemical processes in wheat supplied with zinc and potassium under saline condition. J. Plant Nutr. 2018, 41, 19–28. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Tariq, S.; Saleem, M.; Khan, M.A.; Hassan, S.W.U.; Sadef, Y. Calcium and zinc mediated growth and physio-biochemical changes in mungbean grown under saline conditions. J. Plant Nutr. 2020, 43, 512–525. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar]
- Mostofa, M.G.; Saegusa, D.; Fujita, M.; Tran, L.S.P. Hydrogen sulfide regulates salt tolerance in rice by maintaining Na+/K+ balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front. Plant Sci. 2015, 6, 171620. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Khattak, R.; Irshad, M.; Mahmood, Q.; An, P. Effect of saline irrigation water on the leachability of salts, growth and chemical composition of wheat (Triticum aestivum L.) in saline-sodic soil supplemented with phosphorus and potassium. J. Soil Sci. Plant Nutr. 2016, 16, 604–620. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Rehman, M.; Yang, M.; Fahad, S.; Saleem, M.H.; Liu, L.; Liu, F.; Deng, G. Morpho-physiological traits, antioxidant capacity, and nitrogen metabolism in ramie under nitrogen fertilizer. Agron. J. 2020, 112, 2988–2997. [Google Scholar] [CrossRef]
- Okon, O.G. Effect of salinity on physiological processes in plants. In Microorganisms in Saline Environments: Strategies and Functions; Springer: Cham, Switzerland, 2019; pp. 237–262. [Google Scholar]
- Rehman, S.; Abbas, G.; Shahid, M.; Saqib, M.; Farooq, A.B.U.; Hussain, M.; Murtaza, B.; Amjad, M.; Naeem, M.A.; Farooq, A. Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 171, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Shagol, C.C.; Kim, K.; Han, S.; Sa, T. Spore associated bacteria regulates maize root K+/Na+ ion homeostasis to promote salinity tolerance during arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2018, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Mekdad, A.A.; Shaaban, A.; Rady, M.M.; Ali, E.F.; Hassan, F.A. Integrated application of K and Zn as an avenue to promote sugar beet yield, industrial sugar quality, and K-use efficiency in a salty semi-arid agro-ecosystem. Agronomy 2021, 11, 780. [Google Scholar] [CrossRef]
- Podder, S.; Ray, J.; Das, D.; Sarker, B.C. Effect of salinity (NaCl) on germination and seedling growth of mungbean (Vigna radiata L.). J. Biosci. Agric. Res. 2020, 24, 2012–2019. [Google Scholar] [CrossRef]
- Fiaz, M.; Ahmed, I.; Hassan, S.M.U.; Niazi, A.K.; Khokhar, M.F.; Farooq, M.A.; Arshad, M. Antibiotics induced changes in nitrogen metabolism and antioxidative enzymes in mung bean (Vigna radiata). Sci. Total Environ. 2023, 873, 162449. [Google Scholar] [CrossRef]

| (a) | |||
| Sr. no# | Treatment Description | Designation | Concentration of NaCl/K2SO4 |
| 1. | Control | C | 0 mM NaCl + 0 mM K2SO4 |
| 2. | Control + Potassium sulfate | C + K | 0 mM NaCl + 50 mM K2SO4 |
| 3. | Salinity (50 mM NaCl) | S50 | 50 mM NaCl + 0 mM K2SO4 |
| 4. | Salinity (50 mM NaCl) + Potassium sulfate | S50 + K | 50 mM NaCl + 50 mM K2SO4 |
| 5. | Salinity (100 mM NaCl) | S100 | 100 mM NaCl + 0 mM K2SO4 |
| 6. | Salinity (100 mM NaCl) + Potassium sulfate | S100 + K | 100 mM NaCl + 50 mM K2SO4 |
| (b) | |||
| Contents | Composition of Stock Solution | Concentration per Liter | |
| MgSO4·7H2O | 24.6 g/100 mL | 2 mL/L | |
| Ca(NO3)2·4H2O | 23.6 g/100 mL | 5 mL/L | |
| KH2PO4 | 13.6 g/100 mL | 1 mL/L | |
| KNO3 | 10.1 g/100 mL | 5 mL/L | |
| H3BO3 | 2.86 g/L | 1 mL/L | |
| MnCl2·4H2O | 1.82 g/L | 1 mL/L | |
| ZnSO4·7H2O | 0.22 g/L | 1 mL/L | |
| CuSO4·5H2O | 0.09 g/L | 1 mL/L | |
| MoO3 | 0.01 g/L | 1 mL/L | |
| Fe-DTPA | 50.0 mg/L | ||
| (a) | ||||||||||
| Source | DF | SL | RL | NOL | FB | DB | NiRA | NRA | TFAA | TSP |
| Variety | 1 | 12.48 *** | 10.53 *** | 2.66 ** | 3.14 *** | 0.012 NS | 4.73 *** | 4.58 *** | 55.70 *** | 52.76 *** |
| Treatment | 5 | 12.48 *** | 11.49 *** | 3.68 *** | 3.68 *** | 0.368 *** | 1.98 *** | 2.103 *** | 41.74 *** | 7.904 *** |
| Variety × Treatment | 5 | 0.50 * | 0.07 NS | 0.10 NS | 0.002 NS | 0.039 NS | 0.19 *** | 0.13 *** | 0.67 ** | 0.011 NS |
| Error | 24 | 0.15 | 0.12 | 0.06 | 0.014 | 0.018 | 0.012 | 0.007 | 0.12 | 0.024 |
| (b) | ||||||||||
| Source | DF | Chl. a | Chl. b | T.Chl | Car | N | P | K | Na | (Ca + Mg) |
| Variety | 1 | 0.26 *** | 0.205 *** | 0.929 *** | 12.26 *** | 0.419 *** | 0.358 *** | 25.00 *** | 131.45 *** | 0.366 *** |
| Treatment | 5 | 0.067 *** | 0.052 *** | 0.236 *** | 4.15 *** | 0.355 *** | 5.589 *** | 71.23 *** | 174.70 *** | 5.126 *** |
| Variety × Treatment | 5 | 0.0001 NS | 0.002 ** | 0.0025 NS | 0.03 NS | 0.004 ** | 0.112 ** | 1.46 * | 1.048 *** | 0.1005 ** |
| Error | 24 | 0.0004 | 0.0003 | 0.001 | 0.014 | 0.0007 | 0.022 | 0.38 | 0.108 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shani, M.Y.; Ashraf, M.Y.; Butt, A.K.; Abbas, S.; Nasif, M.; Khan, Z.; Mauro, R.P.; Cannata, C.; Gul, N.; Ghaffar, M.; et al. Potassium Nutrition Induced Salinity Mitigation in Mungbean [Vigna radiata (L.) Wilczek] by Altering Biomass and Physio-Biochemical Processes. Horticulturae 2024, 10, 549. https://doi.org/10.3390/horticulturae10060549
Shani MY, Ashraf MY, Butt AK, Abbas S, Nasif M, Khan Z, Mauro RP, Cannata C, Gul N, Ghaffar M, et al. Potassium Nutrition Induced Salinity Mitigation in Mungbean [Vigna radiata (L.) Wilczek] by Altering Biomass and Physio-Biochemical Processes. Horticulturae. 2024; 10(6):549. https://doi.org/10.3390/horticulturae10060549
Chicago/Turabian StyleShani, Muhammad Yousaf, M. Yasin Ashraf, Ammara Khalid Butt, Shahid Abbas, Muhammad Nasif, Zafran Khan, Rosario Paolo Mauro, Claudio Cannata, Nimra Gul, Maria Ghaffar, and et al. 2024. "Potassium Nutrition Induced Salinity Mitigation in Mungbean [Vigna radiata (L.) Wilczek] by Altering Biomass and Physio-Biochemical Processes" Horticulturae 10, no. 6: 549. https://doi.org/10.3390/horticulturae10060549
APA StyleShani, M. Y., Ashraf, M. Y., Butt, A. K., Abbas, S., Nasif, M., Khan, Z., Mauro, R. P., Cannata, C., Gul, N., Ghaffar, M., & Amin, F. (2024). Potassium Nutrition Induced Salinity Mitigation in Mungbean [Vigna radiata (L.) Wilczek] by Altering Biomass and Physio-Biochemical Processes. Horticulturae, 10(6), 549. https://doi.org/10.3390/horticulturae10060549








