Can SPAD Values and CIE L*a*b* Scales Predict Chlorophyll and Carotenoid Concentrations in Leaves and Diagnose the Growth Potential of Trees? An Empirical Study of Four Tree Species
Abstract
1. Introduction
2. Materials and Methods
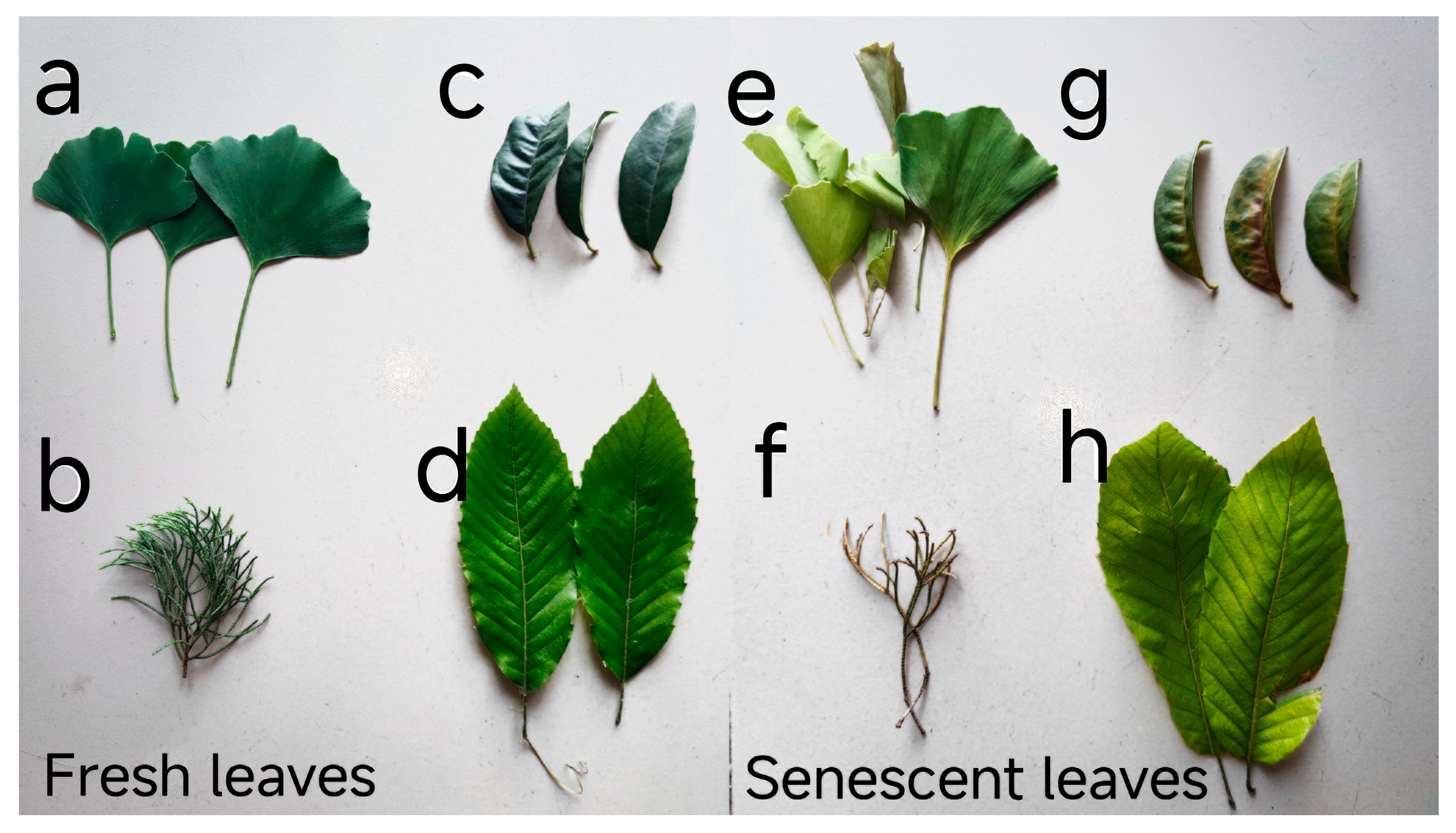
2.1. Experimental Site and Sampling
2.2. Plant Material
2.3. Measurements with SPAD-502 Chlorophyll Meter and CR-410 Automatic Color Difference Meter
2.4. Leaf Chlorophyll Concentrations
2.5. Data Analysis
3. Results
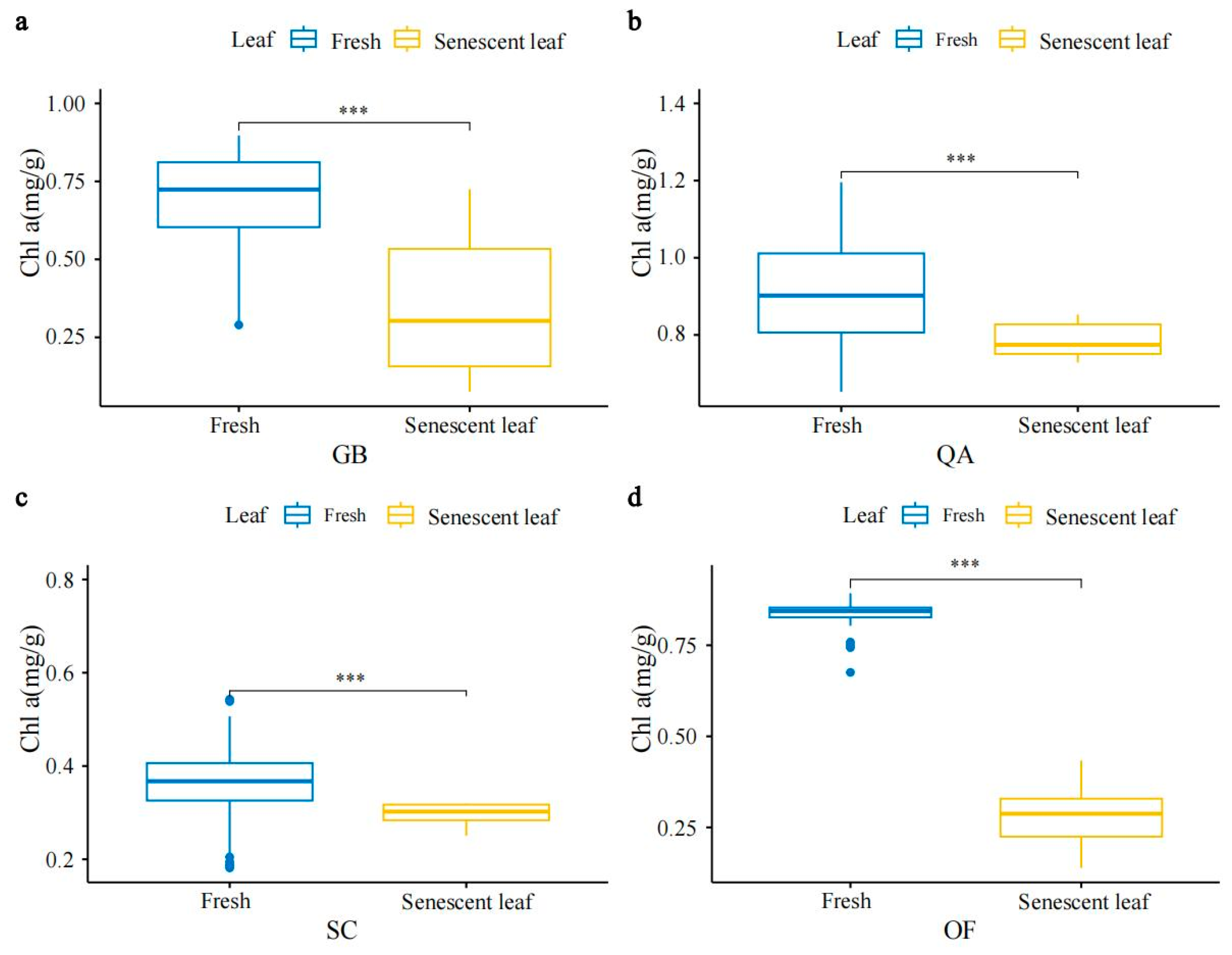
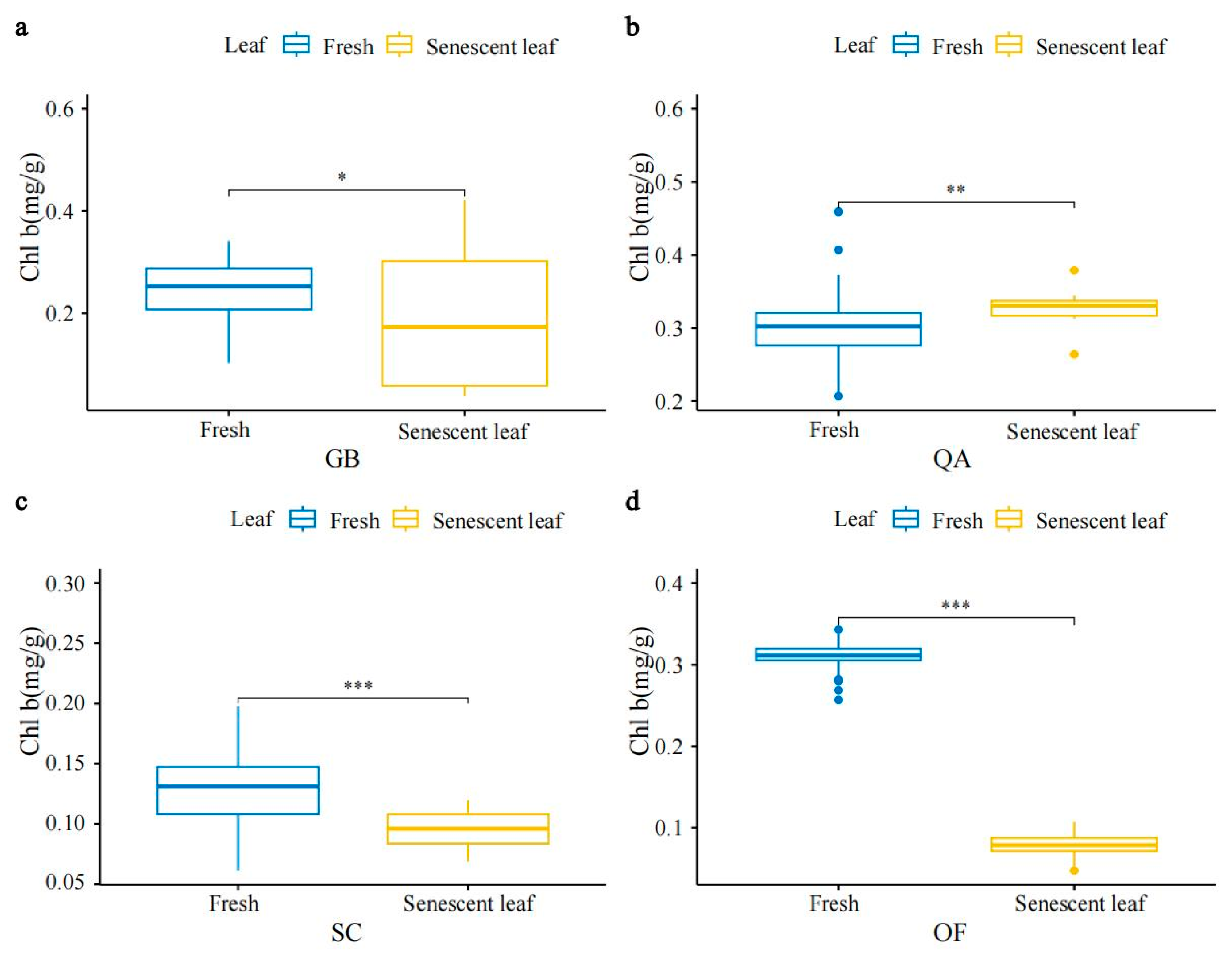
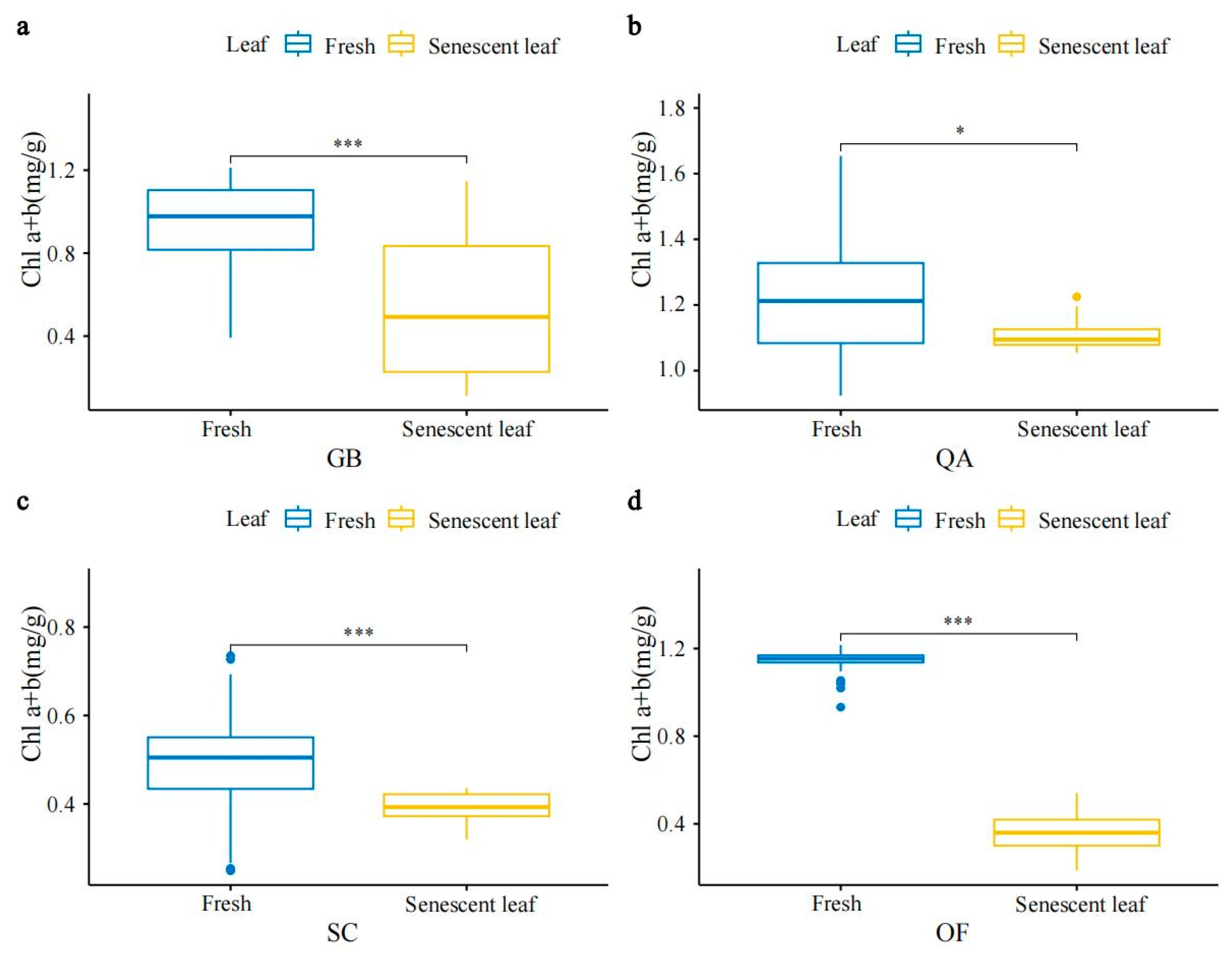
3.1. Characteristics of SPAD, Chlorophyll, and Carotenoid Concentrations in Four Ornamental Plants
3.2. The Relationship between SPAD, Chlorophyll, and Carotenoid Concentrations in Four Ornamental Plants
3.3. Correlations between Leaf Color Parameters, Chlorophylls, and Carotenoids
4. Discussion
4.1. SPAD Value as a Good Predictor for Photosynthetic Pigment Concentrations
4.2. Association Patterns of Leaf Color Parameters with Chlorophyll and Carotenoid Concentrations
4.3. Characteristics of SPAD, Chlorophyll, and Carotenoid Concentrations in Four Tree Species
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Sun, B.; Jiang, M.; Zheng, H.; Jian, Y.; Huang, W.; Yuan, Q.; Zheng, A.; Chen, Q.; Zhang, Y.; Lin, Y.; et al. Color-related chlorophyll and carotenoid concentrations of Chinese kale can be altered through CRISPR/Cas9 targeted editing of the carotenoid isomerase gene BoaCRTISO. Hortic. Res. 2020, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.B.; Boyd, R.A.; Ren, Y.D.; Lee, M.B.; Jones, S.I.; Ruiz-Vera, U.M.; Mcgrath, J.; Masters, M.D.; Ort, D.R. Reducing chlorophyll levels in seed-filling stages results in higher seed nitrogen without impacting canopy carbon assimilation. Plant Cell Environ. 2024, 47, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Demmig Adams, B.; Stewart, J.J.; LópezPozo, M.; Polutchko, S.K.; Adams, W.W. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.L.; Hao, D.L.; Huete, A.; Dechant, B.; Berry, J.; Chen, J.M.; Joiner, J.; Frankenberg, C.; Bond-Lamberty, B.; Ryu, Y.; et al. Optical vegetation indices for monitoring terrestrial ecosystems globally. Nat. Rev. Earth Environ. 2022, 3, 477–493. [Google Scholar] [CrossRef]
- Li, L.; Yuan, H. Chromoplast biogenesis and carotenoid accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Paciulli, M.; Palermo, M.; Chiavaro, E.; Pellegrini, N. Chlorophylls and colour changes in cooked vegetables. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 2, pp. 703–719. [Google Scholar]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Archetti, M.; Döring, T.F.; Hagen, S.B.; Hughes, N.M.; Leather, S.R.; Lee, D.W.; Lev-Yadun, S.; Manetas, Y.; Ougham, H.J.; Schaberg, P.G.; et al. Response to Sinkkonen: Ultraviolet reflectance in autumn leaves and the un-naming of colours. Trends Ecol. Evol. 2009, 24, 237–238. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Lee, D.W. Chlorophyll Catabolism and Leaf Coloration. Annu. Plant Rev. 2007, 26, 12–38. [Google Scholar]
- Wang, J.Q.; Gu, X.Y.; Dong, Y.L.; Wang, T.; Sun, Q.; Fu, S.; Yang, Y.; Huang, J.; Liang, C.; Xie, X.; et al. Advances in the endogenous and exogenous regulation of anthocyanins–the key to color change in eudicots. Crit. Rev. Plant Sci. 2023, 42, 217–238. [Google Scholar] [CrossRef]
- Wen, B.B.; Li, C.; Fu, X.L.; Li, D.; Li, L.; Chen, X.; Wu, H.; Cui, X.; Zhang, X.; Shen, H.; et al. Effects of nitrate deficiency on nitrate assimilation and chlorophyll synthesis of detached apple leaves. Plant Physiol. Biochem. 2019, 142, 363–371. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Guo, H. New advances in the regulation of leaf senescence by classical and peptide hormones. Front. Recent Dev. Plant Sci. 2022, 13, 923136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Han, Q.H.; Ding, C.B.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef] [PubMed]
- Mittelberger, C.; Yalcinkaya, H.; Pichler, C.; Gasser, J.; Scherzer, G.; Erhar, E.; Schumacher, S.; Holzner, B.; Janik, K.; Robatscher, R.; et al. Pathogen-induced leaf chlorosis: Products of chlorophyll breakdown found in degreened leaves of phytoplasma-infected apple (Malus × domestica Borkh.) and apricot (Prunus armeniaca L.) trees relate to the pheophorbide a oxygenase/phyllobilin pathway. J. Agric. Food Chem. 2017, 65, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Zhang, R.R.; Wang, Y.H.; Li, T.; Tan, G.F.; Tao, J.P.; Su, X.J.; Xu, Z.S.; Tian, Y.S.; Xiong, A.S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ji, J.; Wang, G.; Li, Z.; Wang, Y.; Fan, Y. Over-expression of LcPDS, LcZDS, and LcCRTISO, genes from wolfberry for carotenoid biosynthesis, enhanced carotenoid accumulation, and salt tolerance in tobacco. Front. Recent Dev. Plant Sci. 2020, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Velázquez, I.; Zavaleta-Mancera, H.A.; Arévalo-Galarza, M.L.; Suarez-Espinosa, J.; Garcia-Osorio, C.; Padilla-Chacon, D.; Galvan-Escobedo, I.G.; Jimenez-Bremont, J.F. Chlorophyll measurements in Alstroemeria sp. using SPAD-502 meter and the color space CIE L*a*b*, and its validation in foliar senescence. Photosynthetica 2022, 60, 230–239. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Liu, X.; Xue, J.; Ren, X.; Zhai, Y.; Zhang, X. The red/blue light ratios from light-emitting diodes affect growth and flower quality of Hippeastrum hybridum ‘Red Lion’. Front. Plant Sci. 2022, 13, 1048770. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.I.; Hong, G.; Albornoz, K.; Berlanga, M. Fresh grapevine (Vitis vinifera L.) leaves: Postharvest biology and handling recommendations. Sci. Hortic. 2022, 292, 110627. [Google Scholar] [CrossRef]
- Pinkard, E.A.; Patel, V.; Mohammed, C. Chlorophyll and nitrogen determination for plantation-grown Eucalyptus nitens and E. globulus using a non-destructive meter. For. Ecol. Manag. 2006, 223, 211–217. [Google Scholar] [CrossRef]
- Martín, I.; Alonso, N.; López, M.C.; Prieto, M.; Cadahia, C.; Eymar, E. Estimation of leaf, root, and sap nitrogen status using the SPAD-502 chlorophyll meter for ornamental shrubs. Commun. Soil. Sci. Plant Anal. 2007, 38, 1785–1803. [Google Scholar] [CrossRef]
- Li, H.Z.; Cui, L.J.; Dou, Z.G.; Wang, J.J.; Zhai, X.J.; Li, J.; Zhao, X.S.; Lei, Y.R.; Wang, J.Z.; Li, W. Hyperspectral analysis and regression modeling of SPAD measurements in leaves of three mangrove species. Forests 2023, 14, 1566. [Google Scholar] [CrossRef]
- Percival, G.C.; Keary, I.P.; Noviss, K. The potential of a chlorophyll content SPAD meter to quantify nutrient stress in foliar tissue of sycamore (Acer pseudoplatanus), English oak (Quercus robur), and European beech (Fagus sylvatica). Arboric. Urban. For. 2008, 34, 89–100. [Google Scholar] [CrossRef]
- Marenco, R.A.; Antezana-Vera, S.A.; Nascimento, H.C.S. Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Ferreira, I.Q.; Angelo Rodrigues, M. Assessing the potential use of two portable chlorophyll meters in diagnosing the nutritional status of plants. J. Plant Nutr. 2018, 41, 261–271. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.D. Assessment of spinach seedling health status and chlorophyll content by multivariate data analysis and multiple linear regression of leaf image features. Comput. Electron. Agric. 2018, 152, 281–289. [Google Scholar] [CrossRef]
- Jiang, X.P.; Zhen, J.N.; Miao, J.; Zhao, D.; Wang, J.; Jia, S. Assessing mangrove leaf traits under different pest and disease severity with hyperspectral imaging spectroscopy. Ecol. Indic. 2021, 129, 107901. [Google Scholar] [CrossRef]
- Li, G.Y.; Aubrey, D.P.; Sun, H.Z. Predictive capability of a leaf optical meter for determining leaf pigment status during senescence. Photosynthetica 2017, 55, 543–552. [Google Scholar] [CrossRef]
- Tan, X.; Wang, W.H.; Gao, L.; Wei, J.; Zhang, W.; Li, L.; Wu, J.J.; Wang, J.R.; Zhang, X.D.; Liao, X.; et al. The difference in leaf color quality of Cotinus coggygria during the coloration peak period affected by soil and topographic heterogeneity. Catena 2023, 228, 107140. [Google Scholar] [CrossRef]
- Hunt, E.R.; Daughtry, C.S.T. Chlorophyll meter calibrations for chlorophyll content using measured and simulated leaf transmittances. Agron. J. 2014, 106, 931–939. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, R.; McCabe, F. Response of chlorophyll, carotenoid and spad-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef]
- Borges, C.; Vega, R.R.A.; Chakraborty, S.; Weindorf, D.C.; Lopes, G.; Guilherme, L.R.G.; Curi, N.; Li, B.; Ribeiro, B.T. Pocket-sized sensor for controlled, quantitative and instantaneous color acquisition of plant leaves. J. Plant Physiol. 2022, 272, 153686. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, S.; Li, X.; Cunha, M.; Jayavelu, S.; Cammarano, D.; Fu, Y. Machine learning-based approaches for predicting SPAD values of maize using multi-spectral images. Remote Sens. 2022, 14, 1337. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Y.; Ren, Y.; Zhang, Z.; Geng, H. Prediction of chlorophyll content in multi-temporal winter wheat based on multispectral and machine learning. Front. Plant Sci. 2022, 13, 896408. [Google Scholar] [CrossRef]
- Pinzón-Sandoval, E.H.; Balaguera-López, H.E.; Almanza-Merchán, P.J. Evaluation of SPAD index for estimating nitrogen and magnesium contents in three blueberry varieties (Vaccinium corymbosum L.) on the Andean Tropics. Horticulturae 2023, 9, 269. [Google Scholar] [CrossRef]
- Loayza, H.; Calderón, R.A.; Gutiérrez, R.R.O.; Céspedes, F.E.; Quiroz, R. Estimation of relative chlorophyll concentrations in potato (Solanum tuberosum L.) leaflet using vegetation reflectance techniques. Ecol. Apl. 2022, 21, 91–101. [Google Scholar] [CrossRef]
- Coste, S.; Baraloto, C.; Leroy, C.; Marcon, E.; Renaud, A.; Richardson, A.D.; Roggy, J.C.; Schimann, H.; Uddling, J.; Herault, B. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: A calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 2010, 67, 607. [Google Scholar] [CrossRef]
- Bielinis, E.; Jozwiak, W.; Robakowski, P. Modelling of the relationship between the SPAD values and photosynthetic pigments content in Quercus petraea and Prunus serotina leaves. Dendrobiology 2015, 73, 125–134. [Google Scholar] [CrossRef]
- Atar, F.; Güney, D.; Bayraktar, A. Seasonal change of chlorophyll content (SPAD value) in some tree and shrub species. Turk. J. For. Sci.-Prague 2020, 4, 245–256. [Google Scholar] [CrossRef]
- Donnelly, A.; Yu, R.; Rehberg, C.; Meyer, G.; Young, E.B. Leaf chlorophyll estimates of temperate deciduous shrubs during autumn senescence using a SPAD-502 meter and calibration with extracted chlorophyll. Ann. For. Sci. 2020, 77, 30. [Google Scholar] [CrossRef]
- Wang, J.J.; Xu, Y.; Wu, G.F. The integration of species information and soil properties for hyperspectral estimation of leaf biochemical parameters in mangrove forest. Ecol. Indic. 2020, 115, 106467. [Google Scholar] [CrossRef]
- Liu, Y.; Hatou, K.; Aihara, T.; Kurose, S.; Akiyama, T.; Kohno, Y.; Lu, S.; Omasa, K. A robust vegetation index based on different UAV RGB images to estimate SPAD Values of naked barley leaves. Remote Sens. 2021, 13, 686. [Google Scholar] [CrossRef]
- Liu, J.J.; Lindenmayer, D.B.; Yang, W.J.; Ren, Y.; Campbell, M.J.; Wu, C.; Luo, Y.; Zhong, L.; Yu, M. Diversity and density patterns of large old trees in China. Sci. Total Environ. 2019, 655, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.P.; Li, M.; Jim, C.Y.; Liu, D. Environmental factors driving the spatial distribution pattern of venerable trees in Sichuan Province, China. Plants 2022, 11, 3581. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, C.; Pan, Y.J.; Zhou, L.H.; Hu, S.W.; Guo, Y.P.; Meng, Y.Y.; Song, K.; Pang, M.Y.; Li, H.; et al. Human activities and species biological traits drive the long-term persistence of old trees in human-dominated landscapes. Nat. Plants 2023, 9, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Jan, N.; Jitka, P.; Jiří, R.; Martina, S. SPAD chlorophyll meter reading can be pronouncedly affected by chloroplast movement. Photosynth. Res. 2010, 105, 265–271. [Google Scholar]
- Gao, J.F. Experimental Techniques of Plant Physiology; The World Book Publishing Company: Xi’an, China, 2000; pp. 99–103. [Google Scholar]
- Yuan, Y.; Wang, X.F.; Shi, M.M.; Wang, P. Performance comparison of RGB and multispectral vegetation indices based on machine learning for estimating Hopea hainanensis SPAD values under different shade conditions. Front. Plant Sci. 2022, 13, 928953. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, K.; Sun, X.C.; Wang, Y.H.; Zheng, P.F.; Shi, F.C. Characteristics of chlorophyll fuorescence in ten garden shrub species under fooding stress. Biologia 2022, 77, 339–350. [Google Scholar] [CrossRef]
- Hawkins, T.S.; Gardiner, E.S.; Comer, G.S. Modeling the relationship between extractable chlorophyll and SPAD-502 readings for endangered plant species research. J. Nat. Conserv. 2009, 17, 123–127. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ben, G.N.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant 2012, 146, 251–260. [Google Scholar]
- Shibaeva, T.G.; Mamaeva, A.V.; Sherudilo, E.G. Evaluation of a spad-502 plus chlorophyll meter to estimate chlorophyll content in leaves with interveinal chlorosis. Russ. J. Plant Physiol. 2020, 67, 690–696. [Google Scholar] [CrossRef]
- Silva, V.D.; Dos, A.L.; Brito-Rocha, E.; Dalmolin, A.C.; Mielke, M.S. Calibration of a multi-species model for chlorophyll estimation in seedlings of Neotropical tree species using hand-held leaf absorbance meters and spectral reflectance. iForest 2016, 9, 829–834. [Google Scholar] [CrossRef]
- Brown, L.A.; Williams, O.; Dash, J. Calibration and characterisation of four chlorophyll meters and transmittance spectroscopy for non-destructive estimation of forest leaf chlorophyll concentration. Agric. For. Meteorol. 2022, 323, 109059. [Google Scholar] [CrossRef]
- Itle, R.A.; Kabelka, E.A. Correlation Between L*a*b* Color Space Values and Carotenoid Content in Pumpkins and Squash (Cucurbita spp.). Hortscience 2009, 44, 633–637. [Google Scholar] [CrossRef]
- Shilpa, P.; Sadashiva, A.T.; Shivashankar, K.S. Carotenoid content in cherry tomatoes correlated to the colour space values L*, a*, b*: A non-destructive method of estimation. J. Hortic. Sci.-India 2020, 15, 27–34. [Google Scholar]
- Wang, Y.; Wang, D.Y.; Shi, P.H.; Omasa, K.J. Estimating rice chlorophyll content and leaf nitrogen concentration with a digital still color camera under natural light. Plant Methods 2014, 10, 36. [Google Scholar] [CrossRef]
- Valverde, J.C. Estimation of leaf nitrogen content from nondestructive methods in Eucalyptus tereticornis and Eucalyptus saligna plantations. Rev. Fac. Nac. Agron. Univ. Antioq. 2021, 74, 9655–9666. [Google Scholar] [CrossRef]
- Jagdeep-Singh; Varinderpal-Singh. Chlorophyll meter based precision nitrogen management in spring maize. J. Plant Nutr. 2023, 46, 17–27. [Google Scholar] [CrossRef]
- Ghosh, M.; Roychowdhury, A.; Dutta, S. SPAD Chlorophyll Meter-Based Real-Time Nitrogen Management in Manure-Amended Lowland Rice. J. Soil Sci. Plant Nutr. 2023, 23, 5993–6005. [Google Scholar] [CrossRef]
- Alridiwirsah; Tampubolon, K.; Basyuni, M.; Mustamu, N.E. SPAD Total Chlorophyll as an Initial Indicator of the Effect of 2,4-D Dimethyl Amine Herbicide for Lowland Rice and Barnyardgrass Weed. IOP 2023, 1241, 012007. [Google Scholar]
- Wu, G.Z.; Chen, X.Y.; Zang, Y.G.; Ye, Y.; Qiao, X.Q.; Zhang, W.Y.; Zhang, H.; Liu, L.J.; Zhang, Z.J.; Wang, Z.Q.; et al. An optimized strategy of nitrogen-split application based on the leaf positional differences in chlorophyll meter readings. J. Integr. Agric. 2023. [Google Scholar] [CrossRef]
- Maltese, N.E.; Maddonni, G.A.; Melchiori, R.J.M.; Caviglia, O.P. Plant nitrogen status at flowering and kernel set efficiency in early- and late-sown maize crops. Field Crops Res. 2021, 270, 108216. [Google Scholar] [CrossRef]
- Guizani, A.; Babay, E.; Askri, H.; Sialer, M.F.; Gharbi, F. Screening for drought tolerance and genetic diversity of wheat varieties using agronomic and molecular markers. Mol. Biol. Rep. 2024, 51, 432. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, Y.H.; Zheng, Z.Z.; Qiao, Y.C.; Hou, B.R.; Chen, J. A New Approach for Nitrogen Status Monitoring in Potato Plants by Combining RGB Images and SPAD Measurements. Remote Sens. 2022, 14, 4814. [Google Scholar] [CrossRef]
- Liu, N.; Liu, G.; Sun, H. Real-Time Detection on SPAD Value of Potato Plant Using an In-Field Spectral Imaging Sensor System. Sensors 2020, 20, 3430. [Google Scholar] [CrossRef]
- Byju, G.; Anand, M.H. Leaf color chart and chlorophyll-meter-based leaf nitrogen estimation and their threshold values for real-time nitrogen management in cassava. Commun. Soil. Sci. Plant Anal. 2009, 40, 2816–2832. [Google Scholar] [CrossRef]
- Swiader, J.M.; Moore, A. SPAD-chlorophyll response to nitrogen fertilization and evaluation of nitrogen status in dryland and irrigated pumpkins. J. Plant Nutr. 2002, 25, 1089–1100. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.Y.; Wang, J.; Guo, L.J.; Huang, J.S. Synergistic Effect between Trichoderma virens and Bacillus velezensis on the Control of Tomato Bacterial Wilt Disease. Horticulturae 2021, 7, 439. [Google Scholar] [CrossRef]
- Wu, G.S.; Jiang, Q.Y.; Bai, Y.H.; Tian, C.F.; Pan, W.; Jin, X.X.; Zhang, B.H. Nitrogen status assessment for multiple cultivars of strawberries using portable FT-NIR Spectrometers combined with cultivar recognition and multivariate analysis. IEEE ACCESS 2020, 8, 126039–126050. [Google Scholar] [CrossRef]
- Xiong, D.L.; Chen, J.; Yu, T.T.; Gao, W.L.; Ling, X.X.; Li, Y.; Peng, S.B.; Huang, J.L. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef]
- Lombard, K.; O’Neill, M.; Mexal, J.; Ulery, A.; Onken, B.; Bettmann, G.; Heyduck, R. Can soil plant analysis development values predict chlorophyll and total Fe in hybrid poplar? Agrofor. Syst. 2010, 78, 1–11. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, Z.Q.; Huang, H.J.; Yang, F.; Zhu, L.Y.; Han, D. Nitrogen fertilization in soil affects physiological characteristics and quality of green tea leaves. Hortscience 2018, 53, 715–722. [Google Scholar] [CrossRef]
- Bracke, J.; Elsen, A.; Adriaenssens, S.; Schoeters, L.; Vandendriessche, H.; Van, L.M.C. Application of proximal optical sensors to fine-tune nitrogen fertilization: Opportunities for woody ornamentals. Agronomy 2020, 9, 408. [Google Scholar] [CrossRef]
- Lafond, J. Rapid diagnosis of the nitrogen status of the wild lowbush blueberry. Int. J. Fruit. Sci. 2023, 23, 13–24. [Google Scholar] [CrossRef]
- LY/T 2737-2016; Regulation for Identification of Old and Notable Trees. The State Forestry Administration: Beijing, China, 2016.
- Shen, H.L.; Zhang, J.J.; Guo, C.S.; Gao, X.Y.; Chen, J.Y.; Chang, C.; Han, X.L.; Wang, L.J. Characterization and optimization of hydrothermal extraction of quercetin from Quercus leaves using response surface methodology. Can. Soc. Chem. Eng. 2021, 100, 598–606. [Google Scholar] [CrossRef]
- Wang, L.M.; Du, X.F.; Yue, D.M.; Chen, X.H. Catechin, rutin and quercetin in Quercus mongolica Fisch leaves exert inhibitory effects on multiple cancer cells. J. Food. Chem. 2022, 46, e14486. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Takashima, T.; Kabeya, D.; Hirose, T.; Kamata, N. Biomass Allocation and Leaf Chemical Defence in Defoliated Seedlings of Quercus serrata with Respect to Carbon–Nitrogen Balance. Ann. Bot. 2005, 95, 1025–1032. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.F.; Gao, W.Q. Defense pattern of Chinese cork oak across latitudinal gradients: Inffuences of ontogeny, herbivory, climate and soil nutrients. Sci. Rep. 2016, 6, 27269. [Google Scholar] [CrossRef]
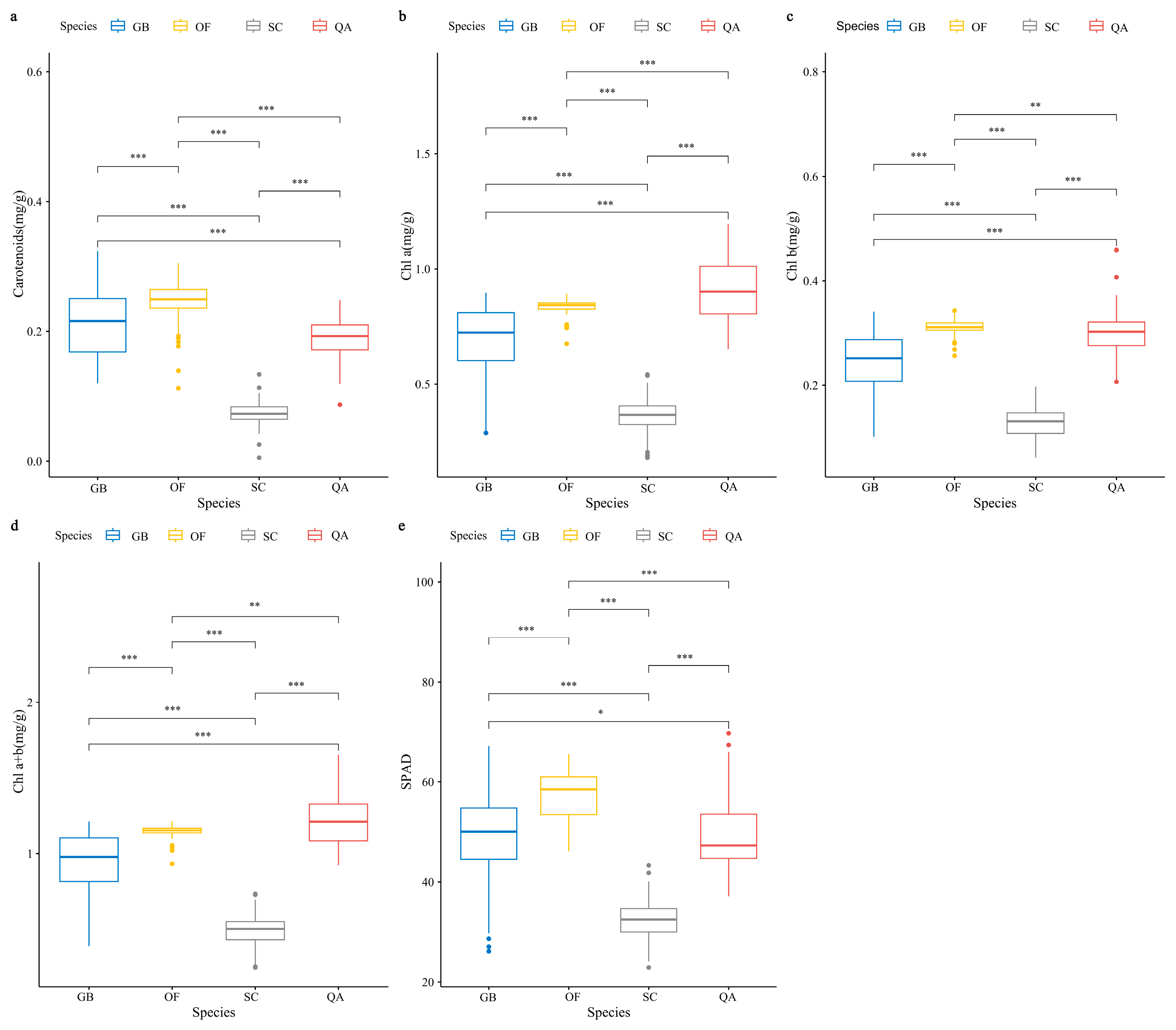
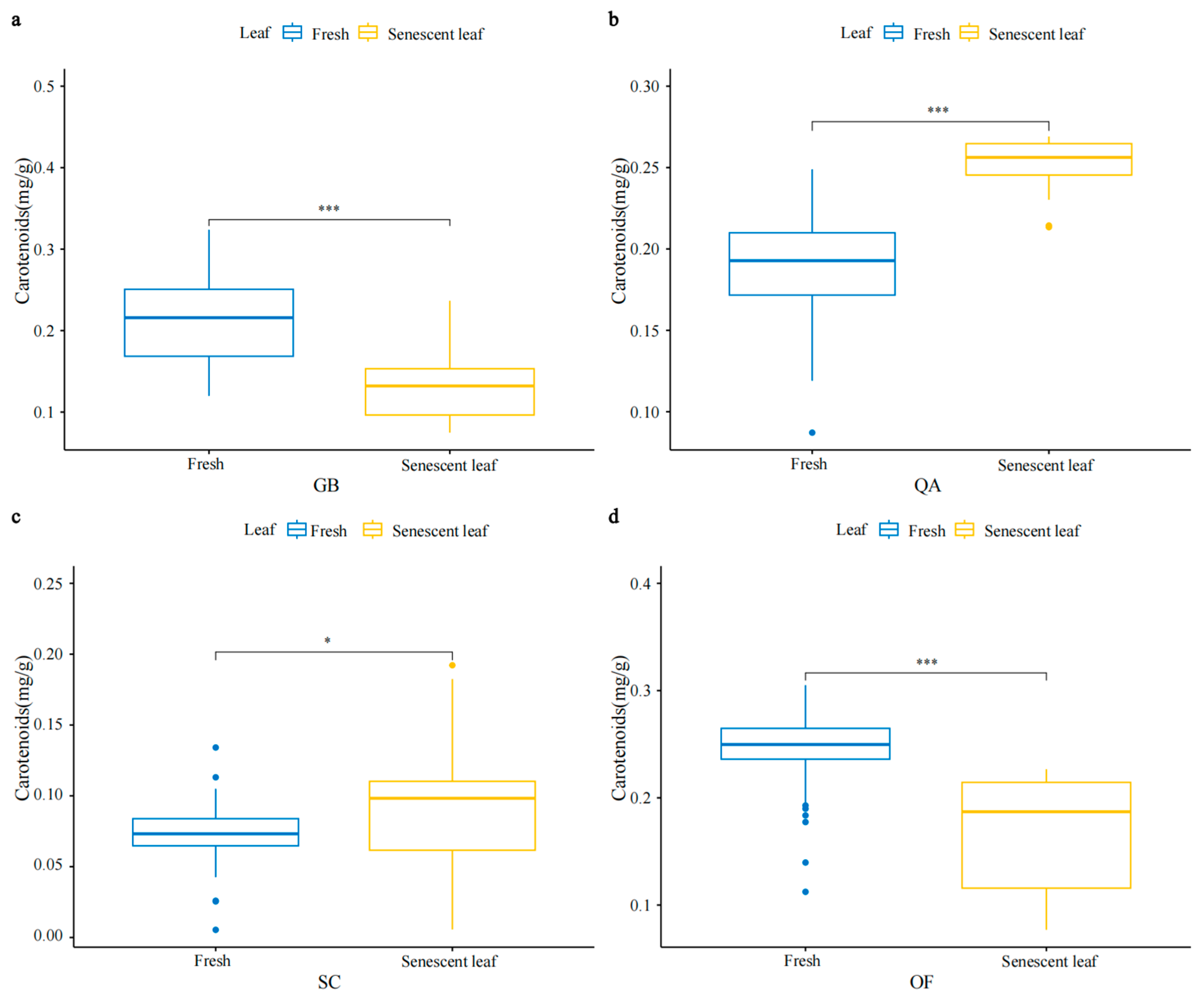

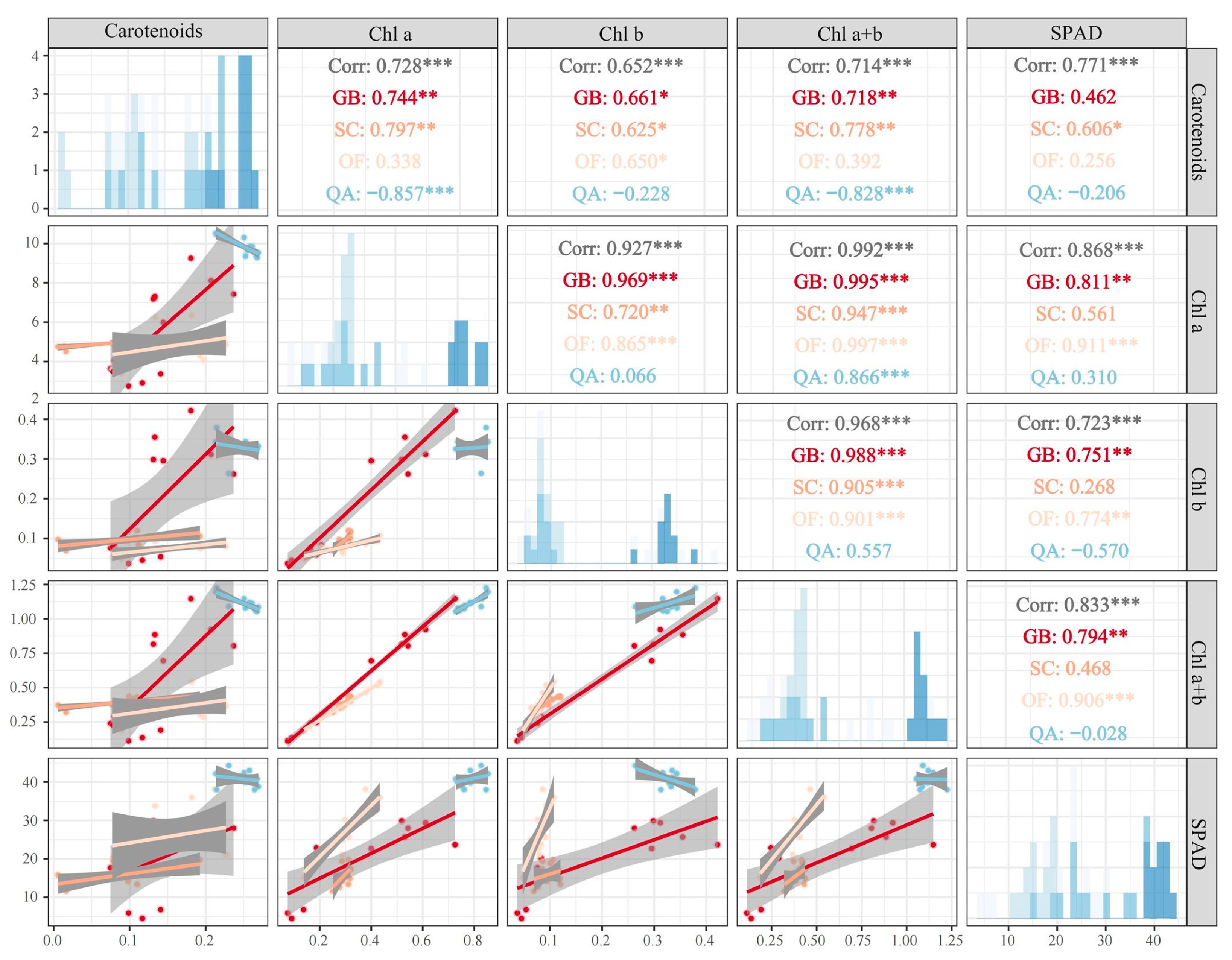
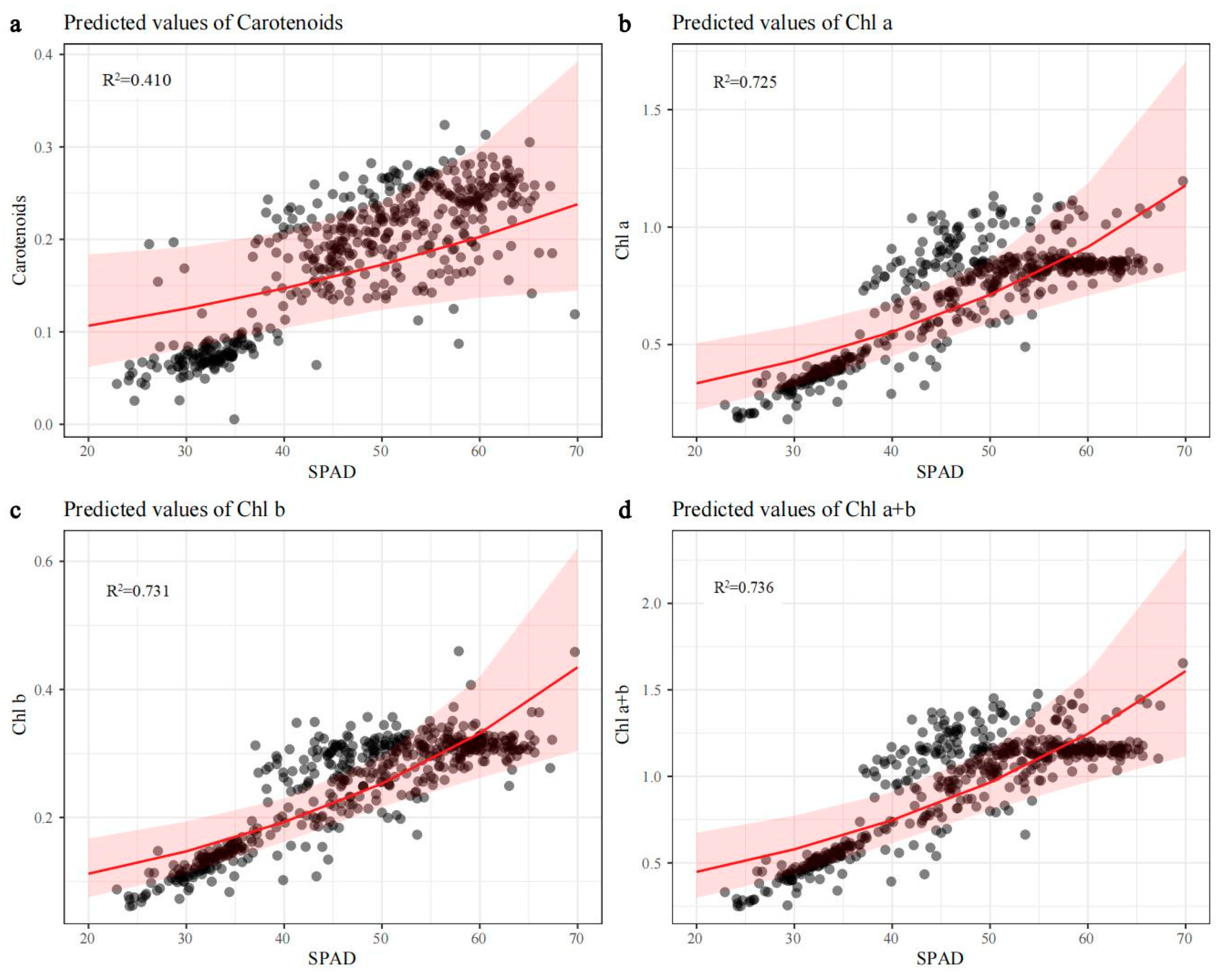
| Botanical Name | Life Form | Phylum | Family | Genus |
|---|---|---|---|---|
| Ginkgo biloba | deciduous tree | Gymnospermae | Ginkgoaceae | Ginkgo |
| Osmanthus fragrans | Evergreen tree or shrub | Angiospermae | Oleaceae | Osmanthus |
| Sabina chinensis ‘Kaizuca’ | Evergreen tree | Gymnospermae | Cupressaceae | Juniperus |
| Quercus acutissima | deciduous tree | Angiospermae | Fagaceae | Quercus |
| Species | CEI | NCCT |
|---|---|---|
| GB | 2.497219882 | 34.59333333 |
| OF | 2.188638527 | 41.86416667 |
| SC | 2.033736206 | 24.055 |
| QA | 1.198342869 | 44.827875 |
| Species | Leaf | Leaf Color Parameter | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| GB | Fresh | 99.8321 ± a | 0.9064 ± ab | 1.7913 ± ab |
| Senescent | 94.1925 ± c | 0.2575 ± d | 1.2183 ± d | |
| OF | Fresh | 99.8961 ± a | 0.9758 ± a | 1.6533 ± b |
| Senescent | 93.9725 ± cd | 0.2525 ± d | 1.22 ± d | |
| SC | Fresh | 99.8101 ± a | 0.8247 ± b | 1.944 ± a |
| Senescent | 93.9025 ± d | 0.4467 ± c | 1.2442 ± d | |
| QA | Fresh | 98.6988 ± b | 0.9412 ± a | 1.7459 ± b |
| Senescent | 93.8725 ± d | 0.2325 ± d | 1.4317 ± c | |
| Leaf | Leaf Color Parameter | Correlation | |||
|---|---|---|---|---|---|
| Carotenoids | Chl a | Chl b | Chl a+b | ||
| Fresh | L* | 0.120 ** | −0.324 ** | −0.182 ** | −0.298 ** |
| a* | 0.279 ** | 0.242 ** | 0.275 ** | 0.251 ** | |
| b* | −0.309 ** | −0.246 ** | −0.296 ** | −0.262 ** | |
| Senescent leaves | L* | 0.064 | −0.177 | −0.035 | −0.138 |
| a* | −0.108 | −0.324 * | −0.203 | −0.291 * | |
| b* | 0.334 * | 0.460 ** | 0.389 ** | 0.450 ** | |
| Total | L* | 0.123 ** | −0.085 | −0.045 | −0.078 |
| a* | 0.256 ** | 0.340 ** | 0.289 ** | 0.332 ** | |
| b* | −0.225 ** | −0.052 | −0.136 ** | −0.073 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Lu, L.; Shang, Y.; Ran, X.; Liu, Y.; Fang, Y. Can SPAD Values and CIE L*a*b* Scales Predict Chlorophyll and Carotenoid Concentrations in Leaves and Diagnose the Growth Potential of Trees? An Empirical Study of Four Tree Species. Horticulturae 2024, 10, 548. https://doi.org/10.3390/horticulturae10060548
Wei L, Lu L, Shang Y, Ran X, Liu Y, Fang Y. Can SPAD Values and CIE L*a*b* Scales Predict Chlorophyll and Carotenoid Concentrations in Leaves and Diagnose the Growth Potential of Trees? An Empirical Study of Four Tree Species. Horticulturae. 2024; 10(6):548. https://doi.org/10.3390/horticulturae10060548
Chicago/Turabian StyleWei, Lai, Liping Lu, Yuxin Shang, Xiaodie Ran, Yunpeng Liu, and Yanming Fang. 2024. "Can SPAD Values and CIE L*a*b* Scales Predict Chlorophyll and Carotenoid Concentrations in Leaves and Diagnose the Growth Potential of Trees? An Empirical Study of Four Tree Species" Horticulturae 10, no. 6: 548. https://doi.org/10.3390/horticulturae10060548
APA StyleWei, L., Lu, L., Shang, Y., Ran, X., Liu, Y., & Fang, Y. (2024). Can SPAD Values and CIE L*a*b* Scales Predict Chlorophyll and Carotenoid Concentrations in Leaves and Diagnose the Growth Potential of Trees? An Empirical Study of Four Tree Species. Horticulturae, 10(6), 548. https://doi.org/10.3390/horticulturae10060548






