Practical Guidelines for Farm Waste Utilization in Sustainable Kale Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Propagation under Different Growing Media
2.1.1. Growing Media Preparation and Experimental Setup
2.1.2. Data Collection
- Physicochemical Properties of Growing Media
- Seed Germination Test
- n = the number of seeds that were germinated on day D;
- D = the number of days from the beginning of germination.
- Seedling Growth Characteristics
2.1.3. Statistical Analysis
2.2. Efficacy of Selected Growing Media as the Soil Amendment in Different Rates for Kale Production
2.2.1. Soil Amendment Preparation and Experimental Site Setup
2.2.2. Data Collection and Analysis
- Chemical Properties of Soil Before and After Treatment
- Growth and Yield Assessment
- Yield Quality Determination
- Ac = control reaction absorbance; As = sample reaction absorbance.
- Yield Estimation and Economic Benefit Analysis
- 305 = 365 (number of days in a year) − 60 (days for a growing period).
2.2.3. Statistical Analysis
3. Results
3.1. Seedling Propagation under Different Growing Media
3.1.1. Physicochemical Properties of Growing Media
| Treatment | pH (1:10 H2O) | EC (dS m−1) (1:10 H2O) | Total N (%) | Total P (%) | Total K (%) |
|---|---|---|---|---|---|
| T1 | 6.53 ± 0.16 f | 0.037 ± 0.000 fg | 5.69 ± 0.02 a | 0.13 ± 0.04 de | 0.30 ± 0.00 e |
| T2 | 5.64 ± 0.20 g | 0.030 ± 0.000 g | 5.20 ± 0.03 b | 0.01 ± 0.00 f | 0.27 ± 0.02 e |
| T3 | 9.38 ± 0.09 a | 0.040 ± 0.012 f | 3.74 ± 0.15 d | 0.06 ± 0.01 ef | 0.70 ± 0.00 a |
| T4 | 7.31 ± 0.10 e | 0.120 ± 0.000 a | 4.33 ± 0.03 c | 0.47 ± 0.03 a | 0.59 ± 0.03 b |
| T5 | 7.65 ± 0.01 d | 0.050 ± 0.000 e | 5.17 ± 0.06 b | 0.06 ± 0.03 ef | 0.52 ± 0.04 c |
| T6 | 7.54 ± 0.29 de | 0.090 ± 0.000 c | 5.32 ± 0.21 b | 0.42 ± 0.05 a | 0.47 ± 0.03 cd |
| T7 | 8.39 ± 0.24 b | 0.103 ± 0.006 b | 4.25 ± 0.03 c | 0.30 ± 0.06 b | 0.62 ± 0.04 b |
| T8 | 8.00 ± 0.04 c | 0.070 ± 0.000 d | 3.78 ± 0.03 d | 0.25 ± 0.07 bc | 0.52 ± 0.02 c |
| T9 | 8.12 ± 0.03 bc | 0.070 ± 0.000 d | 3.52 ± 0.08 e | 0.19 ± 0.06 cd | 0.45 ± 0.01 d |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Treatment | Organic Matter (%) | C/N Ratio | Bulk Density (g m−3) | Total Pore Space (%) | Water-Holding Capacity (%) |
|---|---|---|---|---|---|
| T1 | 79.13 ± 0.74 e | 8.07 ± 0.05 g | 0.16 ± 0.00 f | 45.27 ± 0.75 b | 41.46 ± 0.86 c |
| T2 | 77.46 ± 0.17 f | 8.63 ± 0.04 f | 0.16 ± 0.01 f | 12.35 ± 1.06 f | 6.79 ± 0.84 h |
| T3 | 130.17 ± 0.42 a | 20.20 ± 0.77 a | 0.35 ± 0.01 b | 51.22 ± 1.00 a | 45.19 ± 0.99 b |
| T4 | 46.59 ± 0.42 h | 6.24 ± 0.04 h | 0.37 ± 0.00 a | 28.62 ± 1.17 c | 25.26 ± 1.14 d |
| T5 | 107.26 ± 0.75 b | 12.04 ± 0.15 d | 0.24 ± 0.00 e | 15.89 ± 0.92 e | 12.27 ± 0.82 f |
| T6 | 59.50 ± 0.89 g | 6.50 ± 0.34 h | 0.27 ± 0.00 d | 23.64 ± 1.18 d | 18.48 ± 1.01 e |
| T7 | 82.05 ± 1.36 d | 11.20 ± 0.23 e | 0.31 ± 0.01 c | 12.85 ± 0.89 f | 8.49 ± 1.24 g |
| T8 | 83.40 ± 0.99 d | 12.80 ± 0.07 c | 0.27 ± 0.00 d | 12.55 ± 0.93 f | 7.88 ± 0.41 gh |
| T9 | 91.28 ± 0.84 c | 15.05 ± 0.40 b | 0.31 ± 0.00 c | 51.63 ± 0.75 a | 47.10 ± 0.92 a |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
3.1.2. Germination Index
3.1.3. Growth Characteristics of Kale Seedling
3.2. Efficacy of Selected Growing Media as the Soil Amendment in Different Rates for Kale Production
3.2.1. Selected Chemical Properties of Soil before and after Treatment for Kale Production
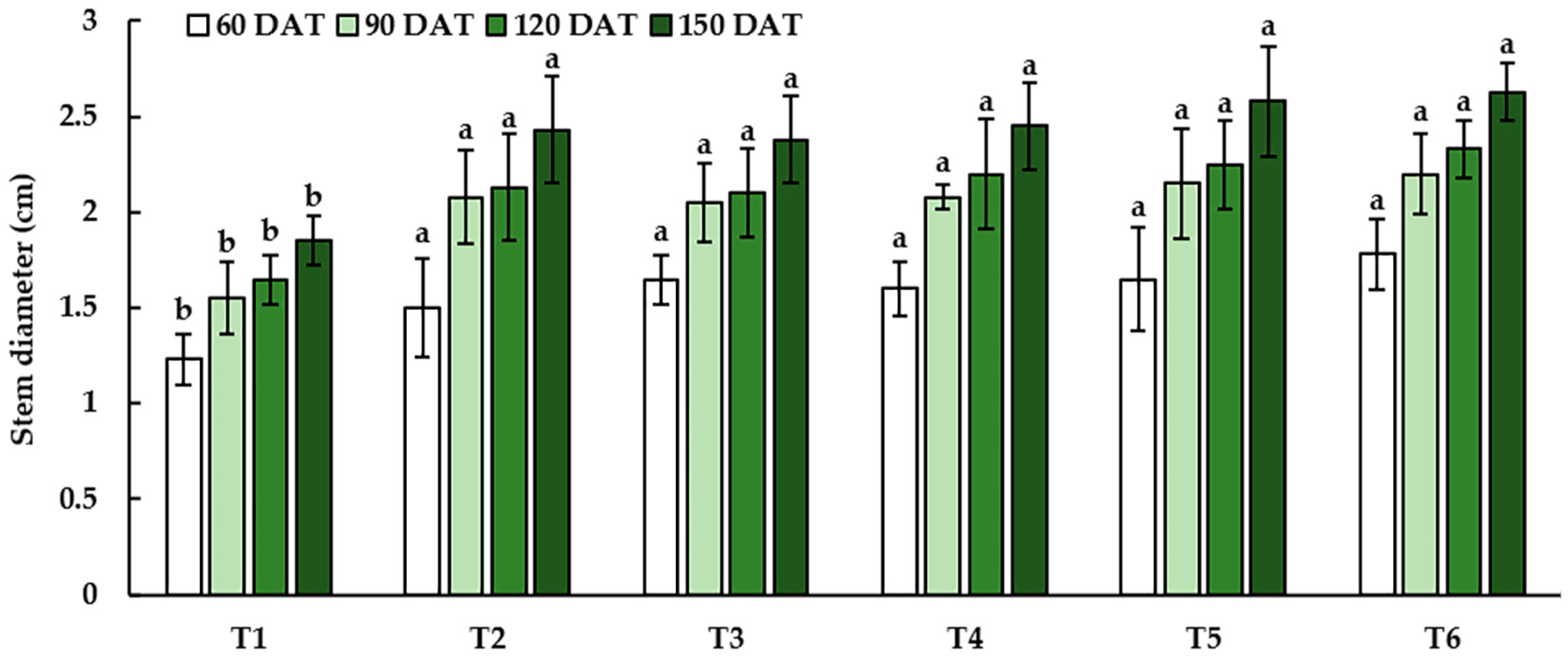
3.2.2. Growth and Yield Characteristics
3.2.3. Yield Quality
3.2.4. Economic Profitability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groenbaek, M.; Jensen, S.; Neugart, S.; Schreiner, M.; Kidmose, U.; Kristensen, H.L. Nitrogen split dose fertilization, plant age and frost effects on phytochemical content and sensory properties of curly kale (Brassica oleracea L. var. sabellica). Food Chem. 2016, 197, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, N.; Fanta, S.W. Kale: Review on nutritional composition, bio- active compounds, anti-nutritional factors, health beneficial properties, and value-added products. Cogent Food Agric. 2020, 6, 1811048. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Alfawaz, H.A.; Wani, K.; Alrakayan, H.; Alnaami, A.M.; Al-Daghri, N.M. Awareness, knowledge and attitude towards ‘superfood’ kale and its health benefits among Arab adults. Nutrients 2022, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Fadigas, J.C.; Santos, A.M.P.; Jesus, R.M.; Lima, D.C.; Fragoso, W.D.; David, J.M.; Ferreira, S.L.C. Use of multivariate analysis techniques for the characterization of analytical results for the determination of the mineral composition of kale. Microchem. J. 2010, 96, 352–356. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, I.; Petřík, I.; Tarkowska, D.; Lepeduš, H.; Vujčić, V.; Radić Brkanac, S.; Novák, O.; Salopek-Sondi, B. Correlations between phytohormones and drought tolerance in selected Brassica crops: Chinese cabbage, white cabbage and kale. Int. J. Mol. Sci. 2018, 19, 2866. [Google Scholar] [CrossRef]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestková, J.; Novák, O.; Lepeduš, H.; Vujčić Bok, V.; Radić Brkanac, S.; Strnad, M.; Salopek-Sondi, B. Early Brassica crops responses to salinity stress: A comparative analysis between Chinese cabbage, white cabbage and kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef]
- Bauer, N.; Tkalec, M.; Major, N.; Talanga Vasari, A.; Tokić, M.; Vitko, S.; Ban, D.; Ban, S.G.; Salopek-Sondi, B. Mechanisms of kale (Brassica oleracea var. acephala) tolerance to individual and combined stresses of drought and elevated temperature. Int. J. Mol. Sci. 2022, 23, 11494. [Google Scholar] [CrossRef]
- Arenas, M.; Vavrina, C.S.; Cornell, J.A. Coir as an alternative to peat in media for tomato transplant production. Hortic. Sci. 2002, 37, 309–312. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, T.; Gulfam, A.; Saleem, A. Growth and flowering of gerbera as influenced by various horticultural substrates. Pak. J. Bot. 2012, 44, 291–299. [Google Scholar]
- Popradit, A.; Wiangnon, J.; Jitrabiab, P.; Pakvilai, N. Organic fertilizer application using leaf waste according to Maejo engineering method 1. Thai Environ. Eng. J. 2022, 36, 47–54. [Google Scholar]
- Ahn, J.; Oh, S.; Kang, Y.J.; Kim, K.; Moon, S.-K.; Moon, B.; Myung, S.; Kim, M.-S.; Lee, Y.K.; Ko, K. Effect of oak tree sawdust fermentation period on peanut seed germination, seedling biomass, and morphology. Horticulturae 2021, 7, 182. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal: A review. Biol. Fertil. Soils. 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Velde, M.V.D.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type, ratio, and nutrient levels in growing media affects seedling production and plant performance. Agronomy 2020, 10, 1421. [Google Scholar] [CrossRef]
- Suryani, S.; Rahmawati, R.; Raimon; Akbar, Y.; Sabri, Y.; Lubis, R.; Nalarsih, R. Biochar and local microorganism from VCO liquid waste improves the quality of soil chemical properties and kale production. Int. J. Dev. Econ. Sustain. 2022, 7, 79–85. [Google Scholar]
- Tsai, C.C.; Chang, Y.F. Kinetics of C mineralization of biochars in three excessive compost-fertilized soils: Effects of feedstocks and soil properties. Agronomy 2020, 10, 1749. [Google Scholar] [CrossRef]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E.; Gascó, G. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci. Hortic. Amst. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- Steiner, C.; Harttung, T. Biochar as a growing media additive and peat substitute. Solid Earth 2014, 5, 995–999. [Google Scholar] [CrossRef]
- Pakvilai, N.; Tuamkartok, N. Sustainable Use of compost from grease trap waste and water hyacinth on the growth rate of Chinese kale. In Proceeding of the 7th GoGreen Summit 2021, Manila, Philippines, 14–15 October 2021. [Google Scholar]
- Waqas, M.; Hashim, S.; Humphries, U.W.; Ahmad, S.; Noor, R.; Shoaib, M.; Naseem, A.; Hlaing, P.T.; Lin, H.A. Composting processes for agricultural waste management: A comprehensive review. Processes 2023, 11, 731. [Google Scholar] [CrossRef]
- National Bureau of Agricultural Commodity and Food Standards. Thai Agricultural Standard TAS 9503: Compost; Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2005.
- Palakit, K.; Duangsathaporn, K.; Lumyai, P.; Sangram, N.; Sikareepaisarn, P.; Khantawan, C. Efficiency of Biochar and bio-fertilizers derived from maize debris as soil amendments. Environ. Nat. Resour. J. 2018, 16, 79–90. [Google Scholar]
- Zhang, L.; Sun, X. Using cow dung and spent coffee grounds to enhance the two-stage co-composting of green waste. Bioresour. Technol. 2017, 245, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining organic carbon in soils: Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1934, 63, 251–263. [Google Scholar] [CrossRef]
- Di Gioia, F.; Bellis, P.D.; Mininni, C.; Santamaria, P.; Serio, F. Physicochemical, agronomical and microbiological evaluation of alternative growing media for the production of rapini (Brassica rapa L.) microgreens. J. Sci. Food Agric. 2017, 97, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; He, Q.; Mousavi, S.M.N.; Abbey, L. Evaluation of aging methods on the surface characteristics of hydrochar and germination indices for kale seeds. Horticulturae 2023, 9, 545. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Abas, N.A.; Zahra, S.M. Impact of salinity stress on germination of water spinach (Ipomoea aquatica). Annu. Res. Rev. Biol. 2019, 31, 1–12. [Google Scholar] [CrossRef]
- Office of Soil Survey and Land Use Planning. Miracle of Soil: Soil Series for Economic Plants Cultivation in Thailand; Land Development Department, Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2005. [Google Scholar]
- Burt, R. Soil Survey Laboratory Methods Manual; Soil Survey Investigations Report, No. 42, Version 4.0; Natural Resources Conservation Service Soils: Washington, DC, USA, 2004.
- Mackinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Yu, X.L.; Hu, Q.L.; Huang, Y. Study on extracting methods and characteristics of chlorophyll in peony. J. Luoyang Norm. Univ. 2005, 5, 113–115. [Google Scholar]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Clive, D.; Kriengkrai, M. The influence of different light spectra on physiological responses, antioxidant capacity and chemical compositions in two holy basil cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef]
- Limbu, S.M.; Shoko, A.P.; Lamtane, H.A.; Kishe-Machumu, M.A.; Joram, M.C.; Mbonde, A.S.; Mgana, H.F.; Mgaya, Y.D. Fish polyculture system integrated with vegetable farming improves yield and economic benefits of small-scale farmers. Aquac. Res. 2017, 48, 3613–3644. [Google Scholar] [CrossRef]
- Soil Survey Staff. National Soil Survey Handbook: Soil Properties and Qualities; Natural Resources Conservation Service, United States Department of Agriculture (USDA): Washington, DC, USA, 2017.
- Rosen, C.J.; Eliason, R. Nutrient Management for Commercial Fruit and Vegetable Crops in Minnesota; University of Minnesota Extension Service: Retrieved from the University of Minnesota Digital Conservancy: Paul, MN, USA, 2005; p. 15. [Google Scholar]
- Ur Rahman, S.; Xuebin, Q.; Riaz, L.; Yasin, G.; Noor Shah, A.; Shahzad, U.; Du, Z. The interactive effect of pH variation and cadmium stress on wheat (Triticum aestivum L.) growth, physiological and biochemical parameters. PLoS ONE 2021, 16, e0253798. [Google Scholar] [CrossRef] [PubMed]
- Laghmouchi, Y.; Belmehdi, O.; Bouyahya, A.; Senhaji, N.S.; Abrini, J. Effect of temperature, salt stress and pH on seed germination of medicinal plant Origanum compactum. Biocatal. Agric. Biotechnol. 2017, 10, 156–160. [Google Scholar] [CrossRef]
- Gillespie, D.P.; Kubota, C.; Miller, S.A. Effects of low pH of hydroponic nutrient solution on plant growth, nutrient uptake, and root rot disease incidence of basil (Ocimum basilicum L.). HortScience 2020, 55, 1251–1258. [Google Scholar] [CrossRef]
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ carbohydrate polymers as plant growth promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Jo, J.S.; Ha, S.Y.; Jung, J.Y.; Kim, J.S.; Nam, J.B.; Yang, J.K. Effects of lignocellulosic growing media to the prevention of forest soil erosion. J. Korean Wood Sci. Technol. 2017, 45, 419–431. [Google Scholar]
- Rosen, C.J.; Halbach, T.R.; Swanson, B.T. Horticultural uses of municipal solid waste composts. HortTechnol. 1993, 3, 167–173. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Saez-Tovar, J.; Martinez-Sabater, E.; Gruda, N.S.; Egea-Gilabert, C. Promising composts as growing media for the production of baby leaf lettuce in a floating system. Agronomy 2020, 10, 1540. [Google Scholar] [CrossRef]
- Antonious, G.F. Soil amendments for agricultural production. In Organic Fertilizers—From Basic Concepts to Applied Outcomes; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 157–187. [Google Scholar]
- Limwikran, T.; Kheoruenromne, I.; Suddhiprakarn, A.; Prakongkep, N.; Gilkes, R.J. Most plant nutrient elements are retained by biochar in soil. Soil Syst. 2019, 3, 75. [Google Scholar] [CrossRef]
- Lima, J.R.d.S.; Goes, M.d.C.C.d.; Hammecker, C.; Antonino, A.C.D.; Medeiros, É.V.d.; Sampaio, E.V.d.S.B.; Leite, M.C.d.B.S.; Silva, V.P.d.; de Souza, E.S.; Souza, R. Effects of poultry manure and biochar on acrisol soil properties and yield of common bean. A short-term field experiment. Agriculture 2021, 11, 290. [Google Scholar] [CrossRef]
- Yang, T.; Xing, X.; Gao, Y.; Ma, X. An environmentally friendly soil amendment for enhancing soil water availability in drought-prone soils. Agronomy 2022, 12, 133. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Hill, R.R.; Snyder, J.C. Chicken manure enhanced yield and quality of field-grown kale and collard greens. J. Environ. Sci. Health B 2014, 49, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Dalorima, T.; Khandaker, M.M.; Zakaria, A.J.; Hasbullah, M. Impact of organic fertilizations in improving bris soil conditions and growth of watermelon (Citrullus lanatus). Bulg. J. Agric. Sci. 2018, 24, 112–118. [Google Scholar]
- Naguib, A.E.M.M.; El-Baz, F.K.; Salama, Z.A.; Hanaa, H.A.E.B.; Ali, H.F.; Gaafar, A.A. Enhancement of phenolics, flavonoids and glucosinolates of broccoli (Brassica olaracea, var. Italica) as antioxidants in response to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2012, 11, 135–142. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P.; Heeb, A. Influence of different types of fertilizers on the major antioxidant components of tomatoes. J. Food Compos. Anal. 2006, 19, 20–27. [Google Scholar] [CrossRef]
- Sałata, A.; Nurzynska-Wierdak, R.; Kalisz, A.; Kunicki, E.; Ibáñez-Asensio, S.; Moreno-Ramón, H. Effects of organic cropping on phenolic compounds and antioxidant capacity of globe artichoke herbs. Agronomy 2022, 12, 192. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. Effects of nitrogen fertilization on synthesis of primary and secondary metabolites in three varieties of Kacip Fatimah (Labisia pumila Blume). Int. J. Mol. Sci. 2011, 12, 5238–5254. [Google Scholar] [CrossRef]
- Majid, W.H.D.W.; Yusoff, M.M.; Misran, A.; Arisah, F.M. Effects of fertilizer rate on growth performance and phytochemical compounds of Gynura procumbens. Asian J. Plant Sci. 2024, 23, 132–145. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef]
- El-shony, M.A.M.; Farid, M.I.; El-Kamar, F.A.; Abbas, M.H.H.; Abbas, H.H. Ameliorating a sandy soil using biochar and compost amendments and their implications as slow-release fertilizers on plant growth. Egypt. J. Soil Sci. 2019, 59, 305–322. [Google Scholar]



| Treatment | Formulation | ||||
|---|---|---|---|---|---|
| Peat Moss (%) | Decomposed Sawdust (%) | Biochar (%) | Farm Waste Compost (%) | Total (%) | |
| T1 | 100 | 0 | 0 | 0 | 100 |
| T2 | 0 | 100 | 0 | 0 | 100 |
| T3 | 0 | 0 | 100 | 0 | 100 |
| T4 | 0 | 0 | 0 | 100 | 100 |
| T5 | 0 | 50 | 50 | 0 | 100 |
| T6 | 0 | 50 | 0 | 50 | 100 |
| T7 | 0 | 20 | 40 | 40 | 100 |
| T8 | 0 | 33.34 | 33.33 | 33.33 | 100 |
| T9 | 0 | 25 | 50 | 25 | 100 |
| Treatment | Germination Rate (%) | Germination Index | Mean Germination Time (Day) |
|---|---|---|---|
| T1 | 63.33 ± 5.77 bc | 20.79 ± 2.52 de | 3.68 ± 0.74 b |
| T2 | 65.00 ± 5.00 bc | 24.17 ± 1.36 bcd | 3.47 ± 0.29 bc |
| T3 | 28.33 ± 2.89 e | 7.71 ± 1.63 f | 4.22 ± 0.20 a |
| T4 | 46.67 ± 2.89 d | 16.19 ± 2.16 e | 3.27 ± 0.49 bc |
| T5 | 65.00 ± 5.00 bc | 26.03 ± 3.77 bc | 2.77 ± 0.22 c |
| T6 | 75.00 ± 5.00 a | 27.49 ± 3.39 ab | 3.13 ± 0.51 bc |
| T7 | 78.33 ± 5.77 a | 31.44 ± 4.49 a | 2.72 ± 0.12 c |
| T8 | 71.67 ± 2.89 ab | 26.28 ± 0.70 bc | 2.81 ± 0.09 c |
| T9 | 56.67 ± 5.77 c | 21.17 ± 3.10 cde | 3.21 ± 0.51 bc |
| p-value | 0.000 | 0.000 | 0.005 |
| Treatment | Plant Height (cm) | Leaf Number | Root Length (cm) | Shoot Fresh Weight (mg plant−1) | Root Fresh Weight (mg plant−1) |
|---|---|---|---|---|---|
| T1 | 5.00 ± 0.50 b | 3.83 ± 0.29 a | 5.60 ± 0.26 de | 7.60 ± 0.30 a | 1.40 ± 0.10 de |
| T2 | 3.83 ± 0.29 c | 2.53 ± 0.15 c | 6.03 ± 0.15 bc | 2.93 ± 0.12 e | 2.63 ± 0.15 b |
| T3 | 1.77 ± 0.25 d | 2.20 ± 0.20 c | 0.70 ± 0.26 h | 1.77 ± 0.15 f | 0.23 ± 0.15 f |
| T4 | 5.03 ± 0.25 b | 3.77 ± 0.15 a | 5.17 ± 0.15 fg | 7.00 ± 0.20 b | 1.53 ± 0.15 d |
| T5 | 3.73 ± 0.25 c | 2.97 ± 0.15 b | 5.33 ± 0.15 ef | 3.50 ± 0.20 d | 1.27 ± 0.06 e |
| T6 | 4.90 ± 0.17 b | 3.80 ± 0.26 a | 6.40 ± 0.26 a | 6.83 ± 0.15 b | 3.17 ± 0.15 a |
| T7 | 5.70 ± 0.36 a | 3.87 ± 0.23 a | 5.73 ± 0.15 cd | 7.37 ± 0.12 a | 2.67 ± 0.15 b |
| T8 | 4.60 ± 0.36 b | 3.57 ± 0.12 a | 6.23 ± 0.06 ab | 6.87 ± 0.12 b | 3.07 ± 0.12 a |
| T9 | 3.83 ± 0.35 c | 3.50 ± 0.30 a | 4.97 ± 0.15 g | 5.60 ± 0.20 c | 1.93 ± 0.15 c |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Treatment | pH (1:1 H2O) | EC (dS m−1) (1:5 H2O) | Organic Matter (g kg−1) | Total N (g kg−1) | Available P (mg kg−1) | Available K (mg kg−1) |
|---|---|---|---|---|---|---|
| Soil before treatment | ||||||
| 6.29 ± 0.04 | 0.14 ± 0.02 | 37.00 ± 1.01 | 1.90 ± 0.08 | 32.08 ± 0.49 | 418.81 ± 7.42 | |
| Soil after treatment | ||||||
| T1 | 5.97 ± 0.05 a | 0.20 ± 0.01 d | 34.97 ± 1.17 d | 1.01 ± 0.03 d | 12.10 ± 0.28 c | 339.02 ± 8.13 c |
| T2 | 5.65 ± 0.07 d | 0.23 ± 0.01 c | 37.15 ± 1.56 bc | 1.08 ± 0.05 bc | 20.45 ± 4.77 b | 332.39 ± 20.46 c |
| T3 | 5.74 ± 0.03 bcd | 0.25 ± 0.02 b | 37.84 ± 0.56 b | 1.10 ± 0.02 b | 24.61 ± 2.15 a | 352.30 ± 9.39 c |
| T4 | 5.69 ± 0.08 cd | 0.26 ± 0.01 b | 36.12 ± 0.74 cd | 1.05 ± 0.02 cd | 20.33 ± 1.28 b | 408.72 ± 21.50 b |
| T5 | 5.77 ± 0.08 bc | 0.29 ± 0.00 a | 40.36 ± 0.71 a | 1.17 ± 0.02 a | 14.66 ± 1.64 c | 421.99 ± 4.70 ab |
| T6 | 5.84 ± 0.05 b | 0.30 ± 0.01 a | 40.70 ± 1.44 a | 1.18 ± 0.04 a | 15.12 ± 2.41 c | 435.27 ± 4.69 a |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Treatment | Yield (t ha−1) | |||
|---|---|---|---|---|
| 60 DAT | 90 DAT | 120 DAT | 150 DAT | |
| T1 | 1.59 ± 0.15 c | 3.02 ± 0.46 c | 6.74 ± 0.30 c | 4.06 ± 0.75 d |
| T2 | 3.78 ± 0.95 b | 6.61 ± 1.10 b | 6.95 ± 0.38 c | 6.06 ± 0.96 c |
| T3 | 4.79 ± 0.67 ab | 9.04 ± 0.95 a | 10.31 ± 0.96 b | 6.52 ± 1.07 c |
| T4 | 3.96 ± 0.61 b | 9.73 ± 0.59 a | 11.13 ± 0.87 b | 6.86 ± 1.05 bc |
| T5 | 3.94 ± 0.60 b | 9.36 ± 0.44 a | 11.61 ± 0.82 b | 8.09 ± 0.56 b |
| T6 | 5.40 ± 0.56 a | 9.89 ± 0.47 a | 15.24 ± 1.33 a | 10.53 ± 0.82 a |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 |
| Treatment | Chlorophyll A (mg g−1 FW) | Chlorophyll B (mg g−1 FW) | Total Chlorophyll (mg g−1 FW) | Carotenoid (mg g−1 FW) | Total Phenolic (mg GAE g−1 DW) | Flavonoid (mg g−1 DW) | DPPH Radical Scavenging (%) |
|---|---|---|---|---|---|---|---|
| T1 | 0.92 ± 0.01 e | 0.40 ± 0.04 b | 1.31 ± 0.05 d | 1.25 ± 0.07 d | 22.08 ± 0.88 a | 29.02 ± 0.36 a | 47.05 ± 2.49 a |
| T2 | 0.91 ± 0.01 e | 0.35 ± 0.00 c | 1.26 ± 0.01 e | 1.53 ± 0.01 b | 19.84 ± 1.06 b | 28.08 ± 0.34 b | 46.71 ± 0.81 ab |
| T3 | 0.94 ± 0.01 d | 0.37 ± 0.01 bc | 1.31 ± 0.01 d | 1.44 ± 0.01 c | 16.59 ± 0.51 c | 27.12 ± 0.31 c | 44.86 ± 0.78 bc |
| T4 | 1.04 ± 0.01 c | 0.39 ± 0.01 b | 1.43 ± 0.02 c | 1.56 ± 0.01 b | 17.76 ± 0.24 c | 27.94 ± 0.17 b | 45.04 ± 1.03 abc |
| T5 | 1.13 ± 0.02 b | 0.40 ± 0.01 b | 1.53 ± 0.02 b | 1.93 ± 0.02 a | 17.29 ± 1.59 c | 27.30 ± 0.75 c | 43.57 ± 0.30 c |
| T6 | 1.15 ± 0.00 a | 0.44 ± 0.01 a | 1.59 ± 0.01 a | 1.97 ± 0.01 a | 17.02 ± 2.22 c | 28.07 ± 0.40 b | 44.44 ± 0.65 c |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thepsilvisut, O.; Srikan, N.; Chutimanukul, P.; Athinuwat, D.; Chuaboon, W.; Marubodee, R.; Ehara, H. Practical Guidelines for Farm Waste Utilization in Sustainable Kale Production. Horticulturae 2024, 10, 525. https://doi.org/10.3390/horticulturae10050525
Thepsilvisut O, Srikan N, Chutimanukul P, Athinuwat D, Chuaboon W, Marubodee R, Ehara H. Practical Guidelines for Farm Waste Utilization in Sustainable Kale Production. Horticulturae. 2024; 10(5):525. https://doi.org/10.3390/horticulturae10050525
Chicago/Turabian StyleThepsilvisut, Ornprapa, Nuengruethai Srikan, Preuk Chutimanukul, Dusit Athinuwat, Wilawan Chuaboon, Rusama Marubodee, and Hiroshi Ehara. 2024. "Practical Guidelines for Farm Waste Utilization in Sustainable Kale Production" Horticulturae 10, no. 5: 525. https://doi.org/10.3390/horticulturae10050525
APA StyleThepsilvisut, O., Srikan, N., Chutimanukul, P., Athinuwat, D., Chuaboon, W., Marubodee, R., & Ehara, H. (2024). Practical Guidelines for Farm Waste Utilization in Sustainable Kale Production. Horticulturae, 10(5), 525. https://doi.org/10.3390/horticulturae10050525






