Enhancing Secondary Metabolite Production in Pelargonium graveolens Hort. Cell Cultures: Eliciting Effects of Chitosan and Jasmonic Acid on Bioactive Compound Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Germination
2.2. Callus Establishment
2.3. Inducing the Formation of Callus with Elicitors
2.3.1. Preparation of Elicitors
2.3.2. Callus Inoculation
2.4. Sample Extraction
2.4.1. Total Phenolic Contents
2.4.2. Total Flavonoid Contents
2.5. Quantification of Phenolic Compounds Using HPLC-DAD
2.6. Extraction of Samples, and Enzymes Activities
2.6.1. Peroxidase (POD) Activity
2.6.2. Superoxide Dismutase (SOD) Activity
2.7. DPPH Activity
2.8. Anti-Tyrosinase Activity
2.9. Statistical Analysis
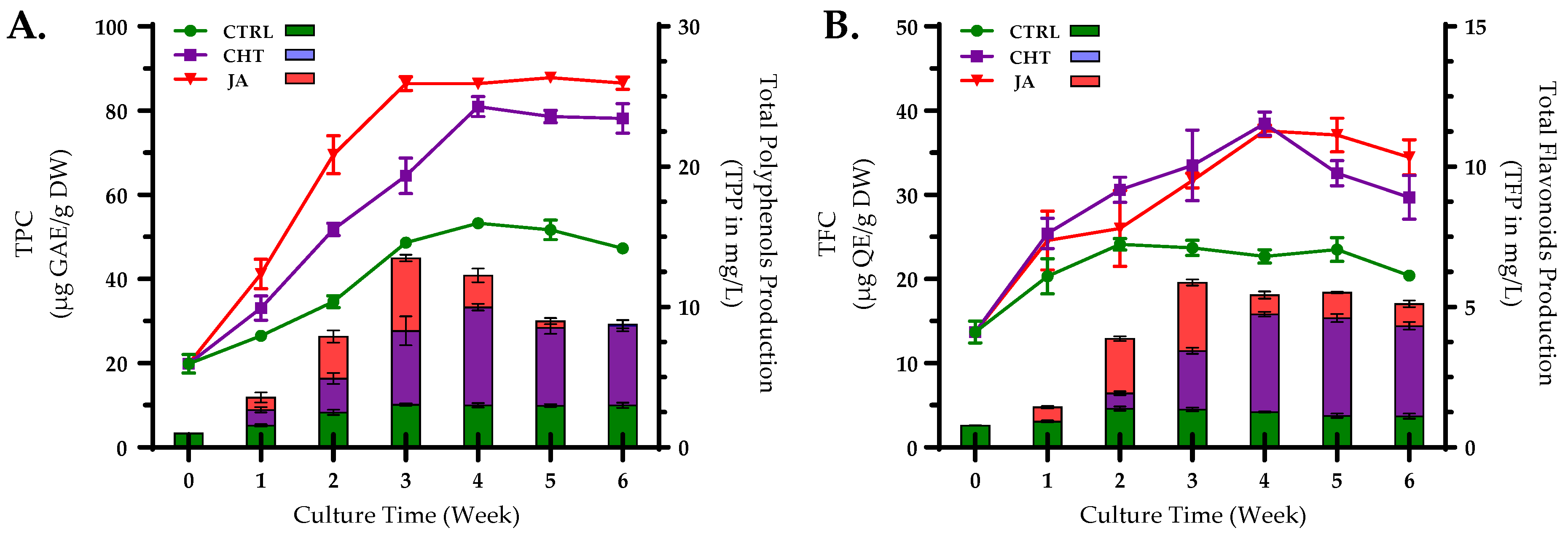
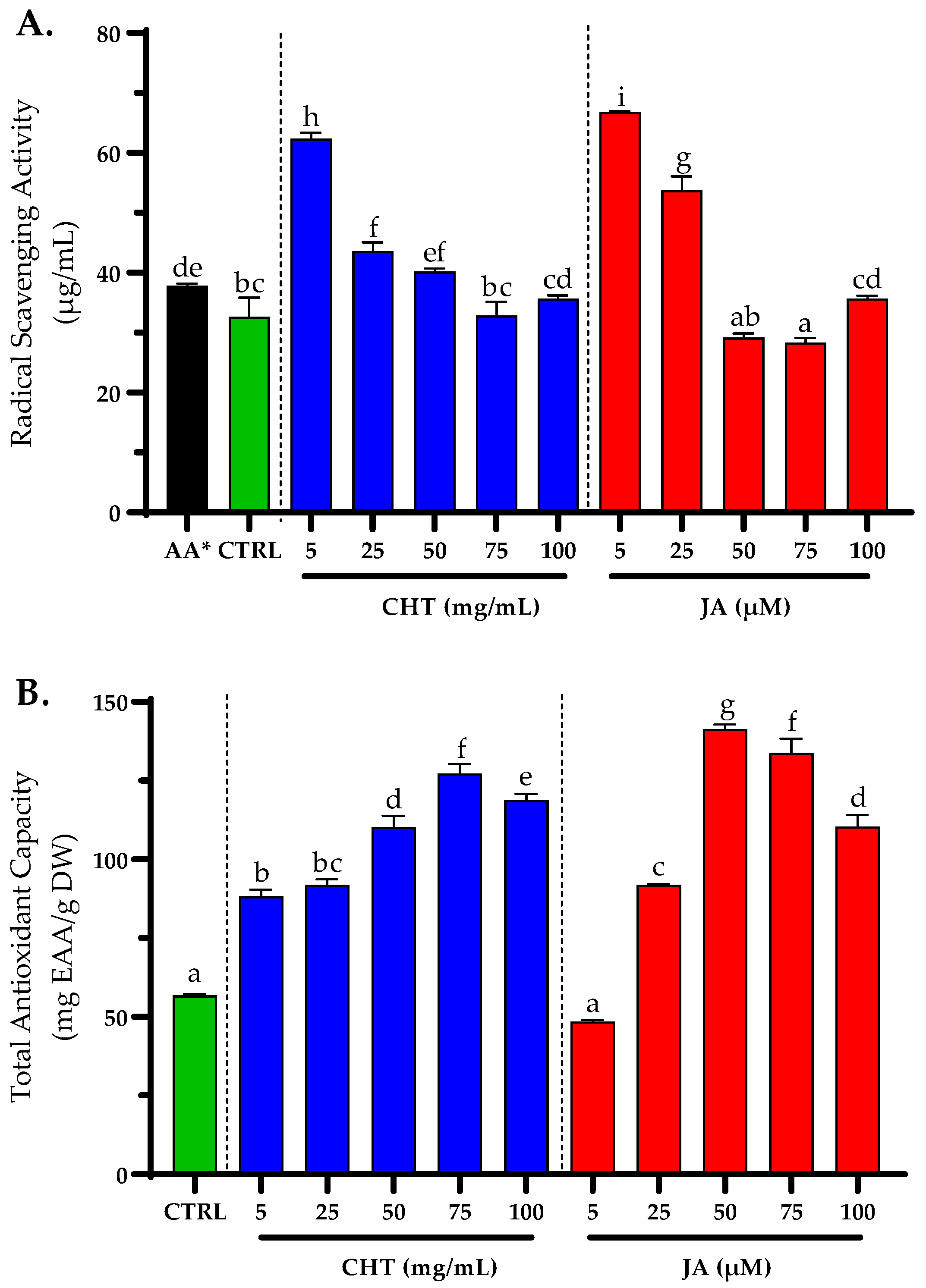
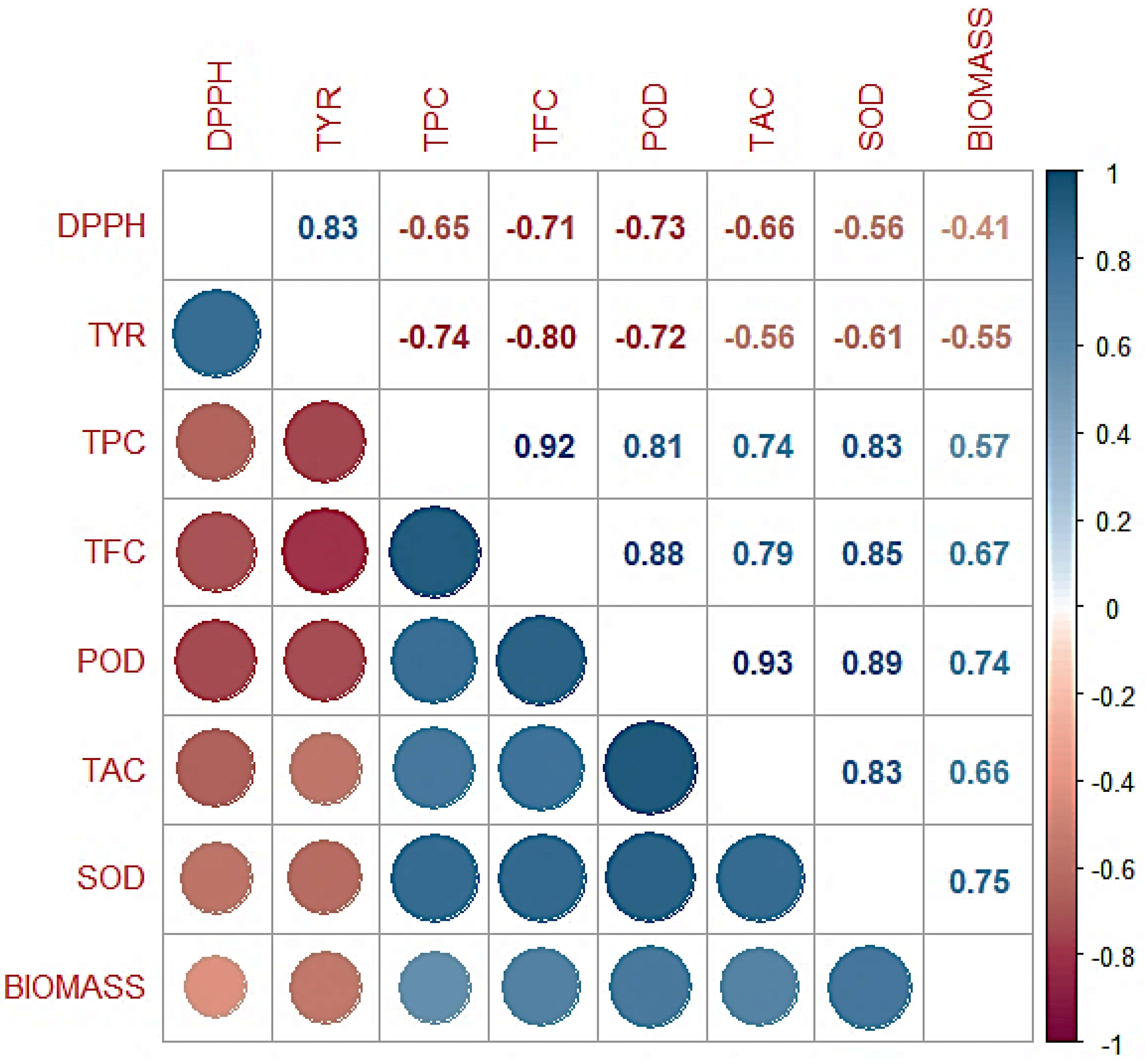
3. Results and Discussion
3.1. Preliminary Tests with Different Concentrations of Elicitors
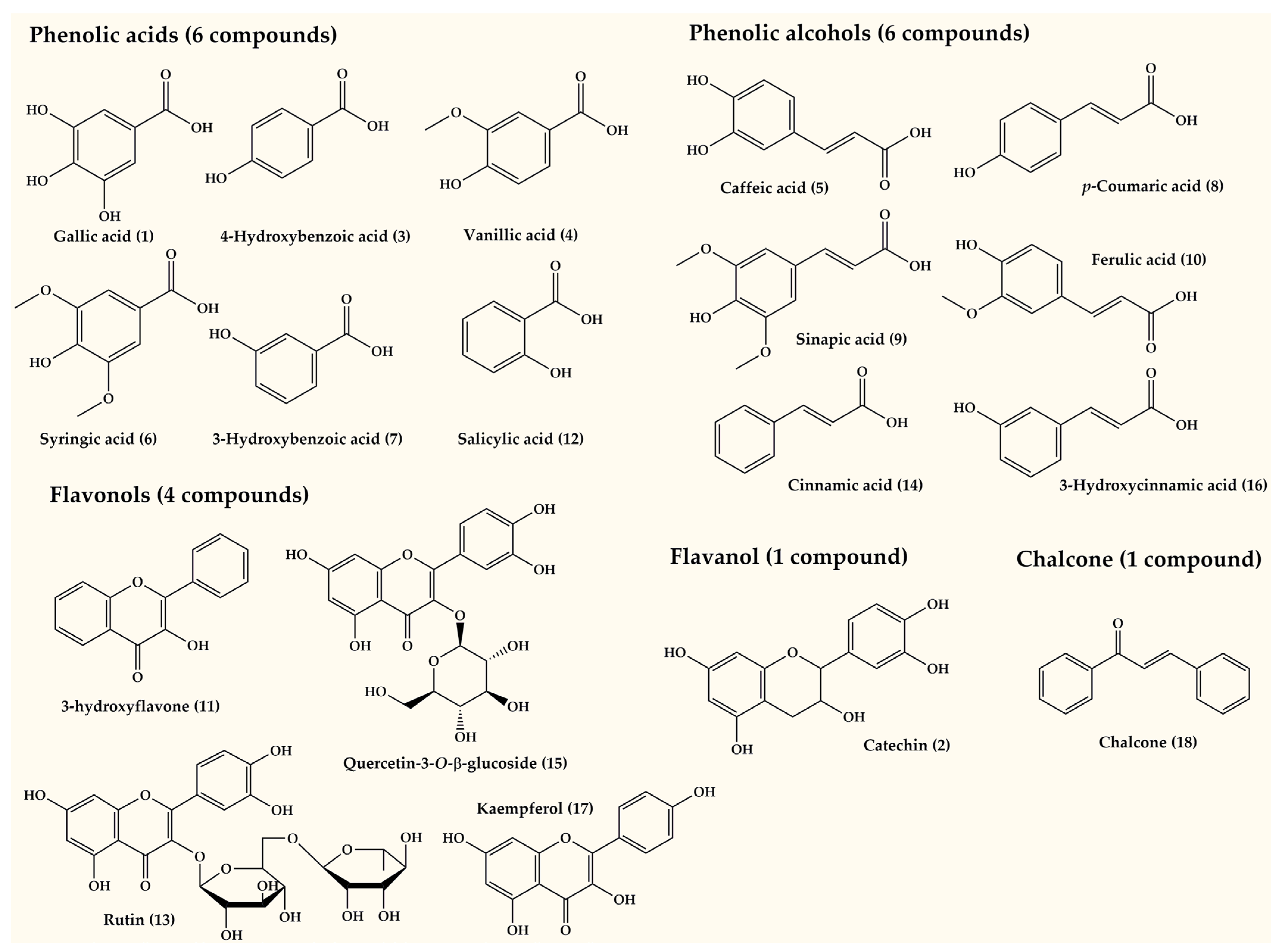
3.2. Phenolic Compounds Quantification Using HPLC-DAD
3.3. Antioxidant Enzymes Quantification
3.4. Antioxidant Activity
3.5. Tyrosinase Inhibition
3.6. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Kalske, A. Plant Secondary Metabolite Diversity and Species Interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (Cell) Factories for Advanced Production of Plant Secondary Metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef]
- Gupta, S.; Chauhan, D.; Mehla, K.; Sood, P.; Nair, A. An Overview of Nutraceuticals: Current Scenario. J. Basic Clin. Pharm. 2010, 1, 55. [Google Scholar] [PubMed]
- Wawrosch, C.; Zotchev, S.B. Production of Bioactive Plant Secondary Metabolites through in Vitro Technologies—Status and Outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Verpoorte, R. Manipulating Indole Alkaloid Production by Catharanthus roseus Cell Cultures in Bioreactors: From Biochemical Processing to Metabolic Engineering. Phytochem. Rev. 2007, 6, 435–457. [Google Scholar] [CrossRef]
- Zárate, R.; Verpoorte, R. Strategies for the Genetic Modification of the Medicinal Plant Catharanthus roseus (L.) G. Don. Phytochem. Rev. 2007, 6, 475–491. [Google Scholar] [CrossRef]
- Humbal, A.; Pathak, B. Influence of Exogenous Elicitors on the Production of Secondary Metabolite in Plants: A Review (‘“VSI: Secondary Metabolites”’). Plant Stress 2023, 100166. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nadira, U.A.; Bibi, N.; Cao, F.; He, X.; Zhang, G.; Wu, F. Secondary Metabolism and Antioxidants Are Involved in the Tolerance to Drought and Salinity, Separately and Combined, in Tibetan Wild Barley. Environ. Exp. Bot. 2015, 111, 1–12. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of Plant Secondary Metabolites: A Historical Perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Anil, K.; Das, S.N.; Podile, A.R. Induced Defense in Plants: A Short Overview. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 669–679. [Google Scholar] [CrossRef]
- Hammond-Kosack, K. Responses to Plant Pathogens. Biochem. Mol. Biol. Plants 2000, 1102–1109. [Google Scholar]
- Eder, J.; Cosio, E.G. Elicitors of Plant Defense Responses. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 148, pp. 1–36. ISBN 0074-7696. [Google Scholar]
- Ramachandra Rao, S.; Tripathi, U.; Ravishankar, G.A. Biotransformation of Digitoxin in Cell Cultures of Capsicum Frutescens in the Presence of β-Cyclodextrin. Biocatal. Biotransform. 2002, 20, 137–143. [Google Scholar] [CrossRef]
- Boller, T. Chemoperception of Microbial Signals in Plant Cells. Annu. Rev. Plant Biol. 1995, 46, 189–214. [Google Scholar] [CrossRef]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen Derived Elicitors: Searching for Receptors in Plants. Mol. Plant Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical Composition and Antioxidant and Antimicrobial Activity of Essential Oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of Portuguese-Grown Geranium Oil (Pelargonium Sp.). J. Essent. Oil Res. 2004, 16, 490–495. [Google Scholar] [CrossRef]
- Lohani, A.; Mishra, A.K.; Verma, A. Cosmeceutical Potential of Geranium and Calendula Essential Oil: Determination of Antioxidant Activity and in Vitro Sun Protection Factor. J. Cosmet. Dermatol. 2019, 18, 550–557. [Google Scholar] [CrossRef] [PubMed]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic Contents and in Vitro Investigation of the Antioxidant, Enzyme Inhibitory, Photoprotective, and Antimicrobial Effects of the Organic Extracts of Pelargonium graveolens Growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Lalli, J.Y.Y.; Van Zyl, R.L.; Van Vuuren, S.F.; Viljoen, A.M. In Vitro Biological Activities of South African Pelargonium (Geraniaceae) Species. S. Afr. J. Bot. 2008, 74, 153–157. [Google Scholar] [CrossRef]
- Cock, I.E.; Selesho, M.I.; Van Vuuren, S.F. A Review of the Traditional Use of Southern African Medicinal Plants for the Treatment of Selected Parasite Infections Affecting Humans. J. Ethnopharmacol. 2018, 220, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Kardan-Yamchi, J.; Mahboubi, M.; Kazemian, H.; Hamzelou, G.; Feizabadi, M.M. The Chemical Composition and Anti-Mycobacterial Activities of Trachyspermum copticum and Pelargonium graveolens Essential Oils. Recent Pat. Anti-Infect. Drug Discov. 2020, 15, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.; Singh, P.; Pant, S.; Chauhan, N.; Lohani, H. Potentiation of Antimicrobial Activity of Ciprofloxacin by Pelargonium graveolens Essential Oil against Selected Uropathogens. Phytother. Res. 2011, 25, 1225–1228. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Hamdi, N. Phytochemical Composition and Antimicrobial Activities of the Essential Oils and Organic Extracts from Pelargonium graveolens Growing in Tunisia. Lipids Health Dis. 2012, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, M.N.; Kameli, A.; Saidi, F. Essential Oil of Algerian Rose-Scented Geranium (Pelargonium graveolens): Chemical Composition and Antimicrobial Activity against Food Spoilage Pathogens. Food Control 2013, 34, 208–213. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, B.; Kumar, A.; Kumar, R.; Dubey, N.K.; Gupta, R. Assessment of Pelargonium graveolens Oil as Plant-based Antimicrobial and Aflatoxin Suppressor in Food Preservation. J. Sci. Food Agric. 2008, 88, 2421–2425. [Google Scholar] [CrossRef]
- Fan, G.; Li, X.; Wang, X.; Zhai, Q.; Zhan, Y. Chitosan Activates Defense Responses and Triterpenoid Production in Cell Suspension Cultures of Betula platyphylla Suk. Afr. J. Biotechnol. 2010, 9, 2816. [Google Scholar]
- Elateeq, A.A.; Saad, Z.; Eissa, M.; Ullah, S. Effect of Chitosan and Light Conditions on the Production of Callus Biomass, Total Flavonoids and Total Phenolics in Ginkgo biloba L. Al-Azhar J. Agric. Res. 2021, 46, 28–42. [Google Scholar] [CrossRef]
- Lucho-Constantino, G.G.; Zaragoza-Martínez, F.; Ponce-Noyola, T.; Cerda-García-Rojas, C.M.; Trejo-Tapia, G.; Esparza-García, F.; Ramos-Valdivia, A.C. Antioxidant Responses under Jasmonic Acid Elicitation Comprise Enhanced Production of Flavonoids and Anthocyanins in Jatropha curcas Leaves. Acta Physiol. Plant. 2017, 39, 165. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Khan, M.A.; Mahmood, T.; Ahmad, M.; Chaudhary, M.F.; Khan, M.A. Shoot Regeneration and Free-Radical Scavenging Activity in Silybum marianum L. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 101, 371–376. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ahmad, I.; Mehmood, Z.; Mohammad, F. Screening of Some Indian Medicinal Plants for Their Antimicrobial Properties. J. Ethnopharmacol. 1998, 62, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Loukili, E.L.H.; Abrigach, F.; Bouhrim, M.; Bnouham, M.; Fauconnier, M.; Ramdani, M. Chemical Composition and Physicochemical Analysis of Opuntia Dillenii Extracts Grown in Morocco. J. Chem. 2021, 2021, 8858929. [Google Scholar] [CrossRef]
- Khan, T.; Khan, T.; Hano, C.; Abbasi, B.H. Effects of Chitosan and Salicylic Acid on the Production of Pharmacologically Attractive Secondary Metabolites in Callus Cultures of Fagonia indica. Ind. Crops Prod. 2019, 129, 525–535. [Google Scholar] [CrossRef]
- Lagrimini, L. Plant Peroxidases: Under-and over-Expression in Transgenic Plants and Physiological Consequences. Plant Peroxidases 1980, 1990, 59–69. [Google Scholar]
- Taibi, M.; Elbouzidi, A.; Ou-Yahia, D.; Dalli, M.; Bellaouchi, R.; Tikent, A.; Roubi, M.; Gseyra, N.; Asehraou, A.; Hano, C.; et al. Assessment of the Antioxidant and Antimicrobial Potential of Ptychotis verticillata Duby Essential Oil from Eastern Morocco: An In Vitro and In Silico Analysis. Antibiotics 2023, 12, 655. [Google Scholar] [CrossRef]
- Elbouzidi, A.; Taibi, M.; Laarej, S.; El Hassania, L.; Haddou, M.; El Hachlafi, N.; Naceiri Mrabti, H.; Baraich, A.; Bellaouchi, R.; Asehraou, A. Chemical Profiling of Volatile Compounds of the Essential Oil of Grey-Leaved Rockrose (Cistus albidus L.) and Its Antioxidant, Anti-Inflammatory, Antibacterial, Antifungal, and Anticancer Activity In Vitro and In Silico. Front. Chem. 2024, 12, 1334028. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N. Essential Oils of Mentha viridis Rich Phenolic Compounds Show Important Antioxidant, Antidiabetic, Dermatoprotective, Antidermatophyte and Antibacterial Properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- El Aanachi, S.; Gali, L.; Rammali, S.; Bensouici, C.; Aassila, H.; Dari, K. In Vitro Study of the Antioxidant, Photoprotective, Anti-Tyrosinase, and Anti-Urease Effects of Methanolic Extracts from Leaves of Six Moroccan Lamiaceae. J. Food Meas. Charact. 2021, 15, 1785–1795. [Google Scholar] [CrossRef]
- Namdeo, A.G.; Ingawale, D.K. Ashwagandha: Advances in Plant Biotechnological Approaches for Propagation and Production of Bioactive Compounds. J. Ethnopharmacol. 2021, 271, 113709. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of Secondary Metabolites from Cell and Organ Cultures: Strategies and Approaches for Biomass Improvement and Metabolite Accumulation. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total Phenolic and Flavonoid Contents and Antioxidant Activity of Ginger (Zingiber officinale Rosc.) Rhizome, Callus and Callus Treated with Some Elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Gadzovska Simic, S.; Tusevski, O.; Maury, S.; Delaunay, A.; Joseph, C.; Hagège, D. Effects of Polysaccharide Elicitors on Secondary Metabolite Production and Antioxidant Response in Hypericum perforatum L. Shoot Cultures. Sci. World J. 2014, 2014, 609649. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and Biotic Elicitors-Role in Secondary Metabolites Production through in Vitro Culture of Medicinal Plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; InTech: Rijeka, Croatia, 2016; Volume 17, pp. 247–277. [Google Scholar]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Chun, S.W.; Chung, Y.S.; Lee, S.Y.; Park, S.U. Influence of Chitosan, Salicylic Acid and Jasmonic Acid on Phenylpropanoid Accumulation in Germinated Buckwheat (Fagopyrum esculentum Moench). Foods 2019, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kastell, A.; Smetanska, I. Chitosan or Yeast Extract Enhance the Accumulation of Eight Phenolic Acids in Cell Suspension Cultures of Malus × domestica Borkh. J. Hortic. Sci. Biotechnol. 2014, 89, 93–99. [Google Scholar] [CrossRef]
- Sayed, M.; Khodary, S.E.A.; Ahmed, E.S.; Hammouda, O.; Hassan, H.M.; El-Shafey, N.M. Elicitation of Flavonoids by Chitosan and Salicylic Acid in Callus of Rumex vesicarius L. In Proceedings of the IX International Symposium on In Vitro Culture and Horticultural Breeding 1187, Giza, Egypt, 13 March 2016; pp. 165–176. [Google Scholar]
- Shah, M.; Jan, H.; Drouet, S.; Tungmunnithum, D.; Shirazi, J.H.; Hano, C.; Abbasi, B.H. Chitosan Elicitation Impacts Flavonolignan Biosynthesis in Silybum marianum (L.) Gaertn Cell Suspension and Enhances Antioxidant and Anti-Inflammatory Activities of Cell Extracts. Molecules 2021, 26, 791. [Google Scholar] [CrossRef] [PubMed]
- Orlita, A.; Sidwa-Gorycka, M.; Paszkiewicz, M.; Malinski, E.; Kumirska, J.; Siedlecka, E.M.; Łojkowska, E.; Stepnowski, P. Application of Chitin and Chitosan as Elicitors of Coumarins and Furoquinolone Alkaloids in Ruta graveolens L. (Common Rue). Biotechnol. Appl. Biochem. 2008, 51, 91–96. [Google Scholar] [CrossRef]
- Taurino, M.; Ingrosso, I.; D’amico, L.; De Domenico, S.; Nicoletti, I.; Corradini, D.; Santino, A.; Giovinazzo, G. Jasmonates Elicit Different Sets of Stilbenes in Vitis vinifera Cv. Negramaro Cell Cultures. SpringerPlus 2015, 4, 49. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The Effects of Jasmonic Acid and Methyl Jasmonate on Rosmarinic Acid Production in Mentha × piperita Cell Suspension Cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Joseph, C.; Hagege, D. Jasmonic Acid Elicitation of Hypericum perforatum L. Cell Suspensions and Effects on the Production of Phenylpropanoids and Naphtodianthrones. Plant Cell Tissue Organ Cult. (PCTOC) 2007, 89, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, R.; Tian, Y.; Wang, H.; Ma, F.; Liu, C.; Liang, W.; Li, C. Exogenous Chitosan Enhances the Resistance of Apple to Glomerella Leaf Spot. Sci. Hortic. 2023, 309, 111611. [Google Scholar] [CrossRef]
- Ali, M.; Ayyub, C.M.; Silverman, E.; Hussain, Z.; Iqbal, S.; Ayyub, S.; Akram, B. Antioxidant, Lipid Peroxidation and Cell Membrane Stability Influence Yield in Cucumis sativus L. by Chitosan Application under Different Sowing Times. JPAA 2020, 5, 59–69. [Google Scholar]
- Al-Dhabaan, F.A.; Mostafa, M.; Almoammar, H.; Abd-Elsalam, K.A. Chitosan-Based Nanostructures in Plant Protection Applications. Nanobiotechnology Appl. Plant Prot. 2018, 2018, 351–384. [Google Scholar]
- Zaragoza-Martínez, F.; Lucho-Constantino, G.G.; Ponce-Noyola, T.; Esparza-García, F.; Poggi-Varaldo, H.; Cerda-García-Rojas, C.M.; Trejo-Tapia, G.; Ramos-Valdivia, A.C. Jasmonic Acid Stimulates the Oxidative Responses and Triterpene Production in Jatropha curcas Cell Suspension Cultures through Mevalonate as Biosynthetic Precursor. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 47–56. [Google Scholar] [CrossRef]
- Woch, N.; Laha, S.; Gudipalli, P. Salicylic Acid and Jasmonic Acid Induced Enhanced Production of Total Phenolics, Flavonoids, and Antioxidant Metabolism in Callus Cultures of Givotia moluccana (L.) Sreem. Vitr. Cell. Dev. Biol. -Plant 2023, 59, 227–248. [Google Scholar] [CrossRef]
- Samadi, S.; Saharkhiz, M.J.; Azizi, M.; Samiei, L.; Ghorbanpour, M. Exploring Potential of Multi-Walled Carbon Nanotubes to Establish Efficient Callogenesis, Elicitation of Phenolic Compounds and Antioxidative Activities in Thyme Plants (Thymus daenensis): An in Vitro Assay. S. Afr. J. Bot. 2023, 157, 602–613. [Google Scholar] [CrossRef]
- Khan, H.; Khan, T.; Ahmad, N.; Zaman, G.; Khan, T.; Ahmad, W.; Batool, S.; Hussain, Z.; Drouet, S.; Hano, C. Chemical Elicitors-Induced Variation in Cellular Biomass, Biosynthesis of Secondary Cell Products, and Antioxidant System in Callus Cultures of Fagonia indica. Molecules 2021, 26, 6340. [Google Scholar] [CrossRef]
- Benhamou, N. Elicitor-Induced Plant Defence Pathways. Trends Plant Sci. 1996, 1, 233–240. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor Signal Transduction Leading to Production of Plant Secondary Metabolites. Biotechnology Advances 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Zhu, J.-F. Study on Antimicrobial Activity of Chitosan with Different Molecular Weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Wasternack, C.; Parthier, B. Jasmonate-Signalled Plant Gene Expression. Trends Plant Sci. 1997, 2, 302–307. [Google Scholar] [CrossRef]
- Elbouzidi, A.; Taibi, M.; Ouassou, H.; Ouahhoud, S.; Ou-Yahia, D.; Loukili, E.H.; Aherkou, M.; Mansouri, F.; Bencheikh, N.; Laaraj, S.; et al. Exploring the Multi-Faceted Potential of Carob (Ceratonia siliqua Var. Rahma) Leaves from Morocco: A Comprehensive Analysis of Polyphenols Profile, Antimicrobial Activity, Cytotoxicity against Breast Cancer Cell Lines, and Genotoxicity. Pharmaceuticals 2023, 16, 840. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Li, J.-K.; Liu, X.-D.; Shen, L.; Zeng, W.-M.; Qiu, G.-Z. Natural Plant Polyphenols for Alleviating Oxidative Damage in Man: Current Status and Future Perspectives. Trop. J. Pharm. Res. 2016, 15, 1089–1098. [Google Scholar] [CrossRef]
- Girgih, A.; Ichoron, N.; Akinsola, A.; Igoli, J. Free Radicals and Antioxidant Quenching Properties of Plant Phytochemicals in the Management of Oxidative Stress. In Plant Food Phytochemicals and Bioactive Compounds in Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2024; pp. 202–240. [Google Scholar]
- Engwa, G.A. Free Radicals and the Role of Plant Phytochemicals as Antioxidants against Oxidative Stress-Related Diseases. In Phytochemicals: Source of Antioxidants and Role in Disease Prevention; IntechOpen: London, UK, 2018; Volume 7, pp. 49–74. [Google Scholar]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Ali, M.B.; Yu, K.-W.; Hahn, E.-J.; Paek, K.-Y. Methyl Jasmonate and Salicylic Acid Elicitation Induces Ginsenosides Accumulation, Enzymatic and Non-Enzymatic Antioxidant in Suspension Culture Panax ginseng Roots in Bioreactors. Plant Cell Rep. 2006, 25, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Chong, T.M.; Abdullah, M.A.; Fadzillah, N.M.; Lai, O.M.; Lajis, N.H. Jasmonic Acid Elicitation of Anthraquinones with Some Associated Enzymic and Non-Enzymic Antioxidant Responses in Morinda Elliptica. Enzym. Microb. Technol. 2005, 36, 469–477. [Google Scholar] [CrossRef]
- Piotrowska, A.; Bajguz, A.; Czerpak, R.; Kot, K. Changes in the Growth, Chemical Composition, and Antioxidant Activity in the Aquatic Plant Wolffia arrhiza (L.) Wimm. (Lemnaceae) Exposed to Jasmonic Acid. J. Plant Growth Regul. 2010, 29, 53–62. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Mapunya, B.M.; Houghton, P.J.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase Inhibition by Extracts and Constituents of Sideroxylon inerme L. Stem Bark, Used in South Africa for Skin Lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Semmarath, W.; Arjsri, P.; Umsumarng, S.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Limtrakul (Dejkriengkraikul), P. Determination of Phenolic Content, Antioxidant Activity, and Tyrosinase Inhibitory Effects of Functional Cosmetic Creams Available on the Thailand Market. Plants 2021, 10, 1383. [Google Scholar] [CrossRef]
- Ali, L.; Khan, S.; Nazir, M.; Raiz, N.; Naz, S.; Zengin, G.; Mukhtar, M.; Parveen, S.; Shazmeen, N.; Saleem, M. Chemical Profiling, in Vitro Biological Activities and Pearson Correlation between Phenolic Contents and Antioxidant Activities of Caragana Brachyantha Rech. f. S. Afr. J. Bot. 2021, 140, 189–193. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef]
- Dalvand, H.; Hamdi, S.M.M.; Ahmadvand, H. Phytochemicals Analysis and Antioxidant Potential of Hydroalcoholic Extracts of Fresh Fruits of Pistacia Atlantica and Pistacia Khinjuk. Plant Sci. Today 2024, 11, 513–520. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]




| Elicitors | Concentrations | Tags |
|---|---|---|
| No-elicitor (control) | - | CTRL |
| Chitosan (mg/mL) | 5 | CHT1 |
| 25 | CHT2 | |
| 50 | CHT3 | |
| 75 | CHT4 | |
| 100 | CHT5 | |
| Jasmonic acid (µM) | 5 | JA1 |
| 25 | JA2 | |
| 50 | JA3 | |
| 75 | JA4 | |
| 100 | JA5 |
| Elicitor | Concentrations | Initiation Day | Callus Characteristics | Maximum Biomass DW (g/100 mL) | Optimum Values | ||
|---|---|---|---|---|---|---|---|
| Color | Texture | TPC (µg GAE/g DW) | TFC (µg QE/g DW) | ||||
| CTRL | - | 3rd | LG | F | 5.73 ± 0.23 a | 53.24 ± 0.52 b | 24.10 ± 0.70 cd |
| CHT (mg/mL) | 5 | 7th | LB | C | 6.80 ± 0.19 a | 51.72 ± 1.23 b | 20.33 ± 1.06 b |
| 25 | 7th | LB | C | 8.67 ± 0.65 b | 42.11 ± 1.40 a | 16.56 ± 2.45 a | |
| 50 | 3rd | DG | F | 13.32 ± 0.38 e | 62.41 ± 0.33 d | 28.59 ± 0.51 e | |
| 75 | 4th | GB | C | 12.34 ± 0.73 de | 80.94 ± 2.38 f | 38.45 ± 0.62 h | |
| 100 | 3rd | DG | C | 10.04 ± 0.64 bc | 82.67 ± 1.02 f | 32.23 ± 1.21 fg | |
| JA (µM) | 5 | 4th | LG | C | 12.22 ± 0.32 de | 54.03 ± 0.46 bc | 21.61 ± 0.23 bc |
| 25 | 5th | LB | C | 11.09 ± 0.41 cd | 52.19 ± 0.83 b | 26.88 ± 0.74 de | |
| 50 | 3rd | DG | C | 15.62 ± 0.98 f | 86.41 ± 1.62 g | 37.61 ± 0.53 h | |
| 75 | 3rd | DG | F | 16.65 ± 0.70 f | 73.62 ± 1.04 e | 33.54 ± 0.48 g | |
| 100 | 3rd | DG | C | 13.43 ± 0.94 e | 57.13 ± 0.95 c | 29.32 ± 0.81 ef | |
| No. | Compounds | Quantity (mg/100 g DW of Sample) | ||
|---|---|---|---|---|
| CTRL | CHT (75 mg/mL) | JA (50 µM) | ||
| 1 | Gallic acid | - | 0.91 ± 0.28 | - |
| 2 | Catechin | 79.27 ± 0.68 | 1279.84 ± 0.07 | 911.83 ± 0.05 |
| 3 | 4-Hydroxybenzoic acid | 208.92 ± 0.01 | 410.41 ± 0.01 | 292.75 ± 0.02 |
| 4 | Vanillic acid | - | - | - |
| 5 | Caffeic acid | 299.32 ± 0.48 | 140.25 ± 0.12 | 278.16 ± 0.96 |
| 6 | Syringic acid | - | - | 60.87 ± 0.03 |
| 7 | 3-hydroxybenzoic acid | - | - | 921.37 ± 0.00 |
| 8 | p-Coumaric acid | 286.93 ± 0.41 | - | 2248.33 ± 0.53 |
| 9 | Sinapic acid | 669.40 ± 1.24 | - | - |
| 10 | Ferulic acid | - | 350.75 ± 0.18 | 1562.87 ± 1.75 |
| 11 | 3-hydroxyflavone | - | - | 1012.41 ± 0.00 |
| 12 | Salicylic acid | 18.28 ± 0.07 | 57.99 ± 0.03 | 692.10 ± 0.21 |
| 13 | Rutin | 0.53 ± 0.07 | 0.59 ± 0.64 | - |
| 14 | Cinnamic acid | - | 54.67 ± 0.29 | 395.11 ± 1.45 |
| 15 | Quercetin 3-O-β-D-glucoside | 2760.51 ± 6.22 | 3328.10 ± 2.07 | 8177.46 ± 2.07 |
| 16 | 3-hydroxycinnamic acid | 2799.25 ± 4.83 | 6834.61 ± 6.03 | 6377.09 ± 2.41 |
| 17 | Kaempferol | 223.37 ± 0.83 | 886.99 ± 0.41 | 1031.81 ± 1.24 |
| 18 | Chalcone | 0.79 ± 0.03 | 557.91 ± 0.02 | 927.35 ± 0.03 |
| Total Phenolic acids (mg/100 g) | 8216.37 ± 8.0661 | 13,602.01 ± 6.447 | 24,891.40 ± 4.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbouzidi, A.; Taibi, M.; Baraich, A.; Haddou, M.; Loukili, E.H.; Asehraou, A.; Mesnard, F.; Addi, M. Enhancing Secondary Metabolite Production in Pelargonium graveolens Hort. Cell Cultures: Eliciting Effects of Chitosan and Jasmonic Acid on Bioactive Compound Production. Horticulturae 2024, 10, 521. https://doi.org/10.3390/horticulturae10050521
Elbouzidi A, Taibi M, Baraich A, Haddou M, Loukili EH, Asehraou A, Mesnard F, Addi M. Enhancing Secondary Metabolite Production in Pelargonium graveolens Hort. Cell Cultures: Eliciting Effects of Chitosan and Jasmonic Acid on Bioactive Compound Production. Horticulturae. 2024; 10(5):521. https://doi.org/10.3390/horticulturae10050521
Chicago/Turabian StyleElbouzidi, Amine, Mohamed Taibi, Abdellah Baraich, Mounir Haddou, El Hassania Loukili, Abdeslam Asehraou, François Mesnard, and Mohamed Addi. 2024. "Enhancing Secondary Metabolite Production in Pelargonium graveolens Hort. Cell Cultures: Eliciting Effects of Chitosan and Jasmonic Acid on Bioactive Compound Production" Horticulturae 10, no. 5: 521. https://doi.org/10.3390/horticulturae10050521
APA StyleElbouzidi, A., Taibi, M., Baraich, A., Haddou, M., Loukili, E. H., Asehraou, A., Mesnard, F., & Addi, M. (2024). Enhancing Secondary Metabolite Production in Pelargonium graveolens Hort. Cell Cultures: Eliciting Effects of Chitosan and Jasmonic Acid on Bioactive Compound Production. Horticulturae, 10(5), 521. https://doi.org/10.3390/horticulturae10050521







