Abstract
Zero-discharge and low-input aquaponics systems are a promising alternative to the intensive agricultural and aquacultural production systems currently used, ensuring high environmental sustainability. However, new approaches and management practices are needed to increase their productivity to reach the yields of classic production systems. In this context, the present study investigated for the first time the potential of two biostimulants to improve lettuce performance in aquaponics, whether coupled or decoupled, with hydroponics serving as a control. A comprehensive evaluation was conducted to assess the plant functional (focusing on the photosynthetic process evaluation) and growth responses at the whole-plant level. In addition, the nutritional state of the leaves was determined and metabolomic analysis was performed at the cellular level, the latter also for the first time in aquaponics research. The results demonstrated the limitations that coupled aquaponics poses in relation to lettuce growth, function and metabolism, which were already obvious from the 12th day of the experiment. Indicatively, the plants grown under coupled aquaponics exhibited a notable decrease in the leaf fresh weight, potassium content and nitrogen content, with reductions of 80%, 60%, and 30%, respectively, in comparison to the hydroponics control. However, the combined physiological and metabolomic data indicate that these plants down-regulate processes and metabolism to acclimate to low nutrient levels in lettuce leaves rather than experiencing damage. The application of biostimulants did not significantly optimize the plants’ performance, though one of them appeared to be effective in improving some aspects of the photochemical efficiency. The decoupled and hydroponics systems resulted in similarly high yields and efficiency in terms of plant function, without any marked contribution from the biostimulants. We conclude that the decoupled aquaponics system has been successful in achieving yields comparable to those of hydroponics, with lower chemical inputs. Future studies should focus on examining other biostimulants in this system to further improve its performance while maintaining its environmental benefits within a circular economy framework.
1. Introduction
Aquaponics is an innovative and highly sustainable cultivation system, operating in a circular economy framework. Crop production in a soilless setup is combined with aquaculture in a closed-loop system [1]. Water is the common element of these two sub-systems, constantly recirculating between them. Fish metabolism and uneaten feed enrich the water with nutrients, which are subsequently absorbed by plants, thereby cleaning the water for re-use in the aquaculture unit. Plant nutrition is succeeded by diluted nutrients in the recirculating water, thus replacing chemical fertilizers. The main advantage of aquaponics is the low environmental footprint for food production, based on saving water resources and minimizing inputs [2]. Aquaponics has been developed during the last 20 years as a zero-discharge production system. Both commercial aquaculture and classic hydroponics dispose of large quantities of wastewater [3]. In the first case, a daily discharge of 10–40% wastewater is typically practiced to maintain the water quality, a percentage that is reduced to 20% when recirculating aquaculture systems (RASs) are employed [4]. Additionally, the versatility of aquaponics installation in terms of the space, sub-system size and crop–fish pairing makes it appropriate for a large range of operating scales, from domestic and urban agriculture—reducing the food miles—to commercial-scale production [5,6]. Consequently, well-developed aquaponics may contribute to the sustainable intensification of agricultural production required to meet the food demands of the expanding human population, which is predicted to increase by 50–70% until 2050 [7].
The closed-loop, coupled aquaponics system described above has certain limitations that prevent it from being equally or more productive compared to conventional cultivation systems such as hydroponics [8,9,10]. The limitations concern the sub-optimal levels of some nutrients in the fish-derived water, resulting mainly in K and Fe deficiencies in crops [11,12]. A modification that has been introduced to address this issue is decoupled aquaponics; the fish tanks and the crop sub-system are separated and independently controlled in open loops [9,13]. This separation allows, in addition to pH regulation at optimum levels for fish and crops, the supplementation of deficient nutrients to adequately meet crop requirements. In this case, the water is not returned to the fish tanks, ensuring their welfare in the absence of chemicals. However, the inputs of the aquaponics system increase, compromising its overall sustainability and low-input character.
Plant biostimulants (BSs) have been reported to enhance plant performance by triggering physiological mechanisms and processes, thereby leading to increased nutrient use efficiency and tolerance to abiotic stress [7,14]. By definition, BSs are not fertilizers or soil improvers. Instead they modify a plant’s primary and/or secondary metabolism, resulting in improved nutrient acquisition and/or product quality traits, with the latter being connected to stress-combating substances [15,16]. The modes of action of BSs are highly diversified and depend on various factors, such as their nature, dosage, application technique, crop species, growth stage, and environmental conditions [14]. In his pivotal review, Du Jardin [17] conclusively examines three levels of BS action. At the whole plant level, increased root-to-shoot micronutrients transport and the protection of photosynthetic apparatus have been reported, among many other physiological processes affected. The next level is the agricultural function in the context of crop performance, which includes improved mineral composition and nutrient-foraging capacity. Finally, there is the level of economy and environmental services, where the use of BSs results in a higher yield, improved product quality and lower inputs. However, as research continues to identify, formulate, and produce new BS products with improved performance, it is necessary to investigate their effects and, more importantly, to focus on the mechanisms by which the plant responds. Focusing solely on crop yield does not provide sufficient information on the underlying mechanisms and modes of action. Therefore, it is crucial to delve deeper into the subject by also studying the plant’s functional traits and utilizing metabolomics, which focuses on specific targets of metabolic pathways and substances. Metabolomics analysis can provide valuable insights into plant function when considered in association with plant physiology and, at the macroscale, growth performance.
The combination of the benefits of aquaponics and BSs was the core idea of the research presented in this paper. Our objective was to assess whether this integrated cultivation system could utilize the aforementioned triggering of plant metabolism by BSs to overcome the limitations of nutrient acquisition by aquaponics crops while maintaining its sustainable and low-input nature. Toward this direction, the effects of two distinct formulations of BS on lettuce performance and the possible mechanisms involved were investigated in coupled and decoupled aquaponics systems, with hydroponics as a control. A comprehensive evaluation of the plant functional and growth responses was conducted at the whole-plant level. Additionally, the nutritional state of the leaves was determined, and metabolomics analysis was performed at the cellular level.
2. Materials and Methods
2.1. Experimental Setup
The experiment was carried out in the pilot-scale aquaponics greenhouse of the University of Thessaly in Velestino (39°44′ N, 22°79′ E), Central Greece. The gothic arch greenhouse is 440 m2 in area and 5 m high. The largest area is covered by the hydroponics unit (360 m2) where the crops are cultivated, while the RAS is installed in a closed chamber (80 m2) under controlled environmental conditions. A full description of both the hydroponics and the RAS sub-systems, as well as the environmental control within the RAS chamber and the whole greenhouse, can be found in our previous articles [8,9]. Briefly, the RAS sub-system comprises three fish tanks, each with a volume of 1.3 m3, connected with the mechanical filter (0.5 m3, Combi Bio 15, ProfiDrum, Retford, UK) and the bio-filter. The latter is filled with ceramic rings (15 mm) and K1 (1 mm, Kaldness media), colonized by the nitrifying bacteria that perform the conversion of the ammonia exerted by fish to nitrates to be absorbed by plants.
The crop cultivation unit consists of 18 hydroponic channels (8.5 × 0.22 m), positioned 50 cm above the ground. The pilot aquaponics greenhouse setup allows the concurrent implementation of three cultivation systems, i.e., coupled (CAP) aquaponics, decoupled (DCAP) aquaponics and hydroponics (HP), in separate channels. In the CAP channels, the crops are irrigated with water directly derived by the RAS, after its pH value is adjusted to increase the nutrients availability. This water is returned, after sterilization, to the fish tanks, completing the cycle. The DCAP channels also use RAS water, but this is enriched with fertilizers to meet the nutrient concentration targets used for HP. The latter is the typical HP nutrient solution for each crop species tested, which exclusively employs chemical fertilizers.
Two factors were tested in the current experiment, i.e., the cultivation system and BS, each with three levels. For the former, HP, DCAP and CAP were studied. In each of these groups, three BS management schemes were carried out; no BS, BS1 and BS2, with the latter two referring to different liquid formulations, the compositions of which are described in Table 1. Subsequently, the experimental design included the following 9 treatments:
- (1)
- HP (Hydroponics—no biostimulant)
- (2)
- HP-BS1 (Hydroponics—1st biostimulant formulation)
- (3)
- HP-BS2 (Hydroponics—2nd biostimulant formulation)
- (4)
- DCAP (Decoupled aquaponics—no biostimulant)
- (5)
- DCAP-BS1 (Decoupled aquaponics—1st biostimulant formulation)
- (6)
- DCAP-BS2 (Decoupled aquaponics—2nd biostimulant formulation)
- (7)
- CAP (Coupled aquaponics—no biostimulant)
- (8)
- CAP-BS1 (Coupled aquaponics—1st biostimulant formulation)
- (9)
- CAP-BS2 (Coupled aquaponics—2nd biostimulant formulation)

Table 1.
The composition of each BS formulation, as well as its pH and the amino acids present. The concentrations are given in percentage (%) weight per volume (w/v).
Table 1.
The composition of each BS formulation, as well as its pH and the amino acids present. The concentrations are given in percentage (%) weight per volume (w/v).
| BS1 | BS2 | |
|---|---|---|
| Free amino acids | 12.5 | 14.4 |
| Nitrogen (N) total | 3 | 3 |
| Organic nitrogen (N) | 3 | 3 |
| Potassium oxide (K2O) | 1.2 | 1.2 |
| Total amino acids | 14.5 | 16.8 |
| Low-molecular-weight peptides | 45 | 49.2 |
| Organic material | - | 28.8 |
| Azotobacter chroococcum | No | Yes |
| pH | 5.8 | 5.5 |
| Amino acids | Ala, Arg, Asp, Cys, Gly, Glu, Hyp, His, Iso, Leu, Lys, Met, Phe, Pro, Ser, Thr, Tyr, Va | |
Lettuce plants (Lactuca sativa cv. Station) were grown on perlite (Hydroperl 33L, Nordiaagro, Athens, Greece) slabs, 4 plants per slab, irrigated with drippers. A total of 540 plants were randomly allocated to the 9 treatments, resulting in 60 plants/treatment. The HP plants were fertigated using a standard nutrient solution for lettuce crops, specifically formulated for the Mediterranean climatic conditions [18] (16.4 mM N-NO3, 1.3 mM N-NH4, 1.4 mM P, 8.0 mM K+, 4.8 mM Ca2+, 1.1 mM Mg2+, 1.4 mM S-SO4, 35.0 μM Fe, 30.0 μΜ B, 0.8 μΜ Cu, 5.0 μΜ Zn, 5.0 μΜ Mn, 0.5 μΜ Mo).
In order to achieve the target concentration in the DCAP treatment, the RAS solution was analyzed weekly. Based on the results of the analyses, the amount of each fertilizer needed to achieve the target was determined and the input was adjusted accordingly. A customized controller (Argos Electronics, Evia, Greece) continually and automatically controlled the irrigation and fertilization of all the treatments.
The mean pH value of the irrigation solution in all the treatments was set at 5.6, according to Aslanidou et al. (2022) [8]. The electrical conductivity (EC) of the solution was measured weekly with a portable instrument (Combo pH-EC-TDS-Temp, 98130 Hanna Instruments, Woonsocket, RI, USA) and varied among the treatments. In particular, the mean EC in the nutrient solution of the HP and DCAP treatments was 2.5 dS m−1 throughout the experiment, whilst the mean EC of the CAP treatment was 0.84 dS m−1. Both BSs were applied by foliar spraying three times during the experiment (on Day (D)7, D27 and D40), at a rate of 1.5 L/ha.
Red tilapia fish (Oreochromis spp.) were reared in the RAS system, with a total biomass of 32.7 kg, thus a stocking of 8.4 kg m−3. The fish were fed ad libitum three times a day with Prodac Pond Sticks Color (crude protein 29.0%, crude ash 5.7%, crude fibers 3.3%, crude fat 2.9%, moisture 4.8%, omega 6 42.2%, omega 3 5.7%) [8].
The duration of the experiment was 56 days, from November 2021 to January 2022.
2.2. Measurements
2.2.1. Plant Growth Assessment
Three harvests were performed on D26, D38 and D56 to assess both the fresh weight of the aerial plant part and the number of leaves developed up to each date (six plants per treatment).
2.2.2. Plant Physiology Parameters
The concentration of total chlorophylls (chl), i.e., chl a plus b, was measured through the SPAD index (SPAD 502Plus, Konica Minolta, Osaka, Japan). Two measurements per plant were taken, and twenty plants per treatment were measured on five dates throughout the growing period (D12, D19, D27, D34, D54). All the measurements were performed between 9:00 and 10:00 am to ensure similar light conditions.
The photochemical reflectance index (PRI) was measured in the lettuce leaves with a portable instrument, PlantPen PRI 210 (Photon Systems Instruments, Drásov, Czech Republic), during clear days between 10:00 and 11:30 am. The measurements were performed on the same dates and plants, and following the same replication as per the SPAD determination.
The in vivo chl a fluorescence was assessed in mature, unshaded leaves (20 replicates/treatment) on the same dates as above with the Handy PEA+ fluorimeter (Hansatech Instruments Ltd., King’s Lynn, UK). Prior to each measurement, the leaves were dark-adapted for 30 min with the use of a leaf clip. After this period, the leaves were illuminated with 3000 µmol photons m−2 s−1 of a red LED array at 650 nm for 3 s and the transients of chl a fluorescence were recorded (at 50 µsecs, 100 µsecs, 300 µsecs, 2 msecs and 30 msecs). The data were analyzed with PeaPlus Software v.1-13 (Hansatech Instruments Ltd., King’s Lynn, UK). Table S1 summarizes the main parameters derived from the JIP test, according to Strasser et al. [19].
The total phenolics content was measured 3 times during the experimental period (10 replicates/treatment, on D26, D38, D56), following the Folin–Ciocalteu method [20]. Dry samples of lettuce leaves (0.27 g each, 10 replicates/treatment) were extracted in methanol 50% and maintained at 40 °C for 1 h in a water bath. The extract (0.05 mL) mixed with 0.25 mL Folin–Ciocalteu reagent, 0.75 mL of Na2CO3 (20% w/v) and 3.95 mL of water was left for 2 h at room temperature and subsequently the absorbance was read at 760 nm (double beam spectrophotometer, Shimadzu UV-1900). The concentration of the total leaf phenolics was expressed as gallic acid equivalents (GAE, g g−1 leaf dry weight), following a standard curve with known gallic acid concentrations.
The leaf nutritional state (N, K+, Ca+2, Na+) was determined twice during the experiment (6 replications per treatment, on D38 and D56). The total nitrogen content was measured using the Kjeldahl nitrogen method. The K+, Ca+2, and Na+ concentrations of the leaves were analyzed with a Flame Photometer (JENWAY, PFP7 Flame Photometer) after the extraction of 0.5 g of each sample with a 20 mL hydrochloric solution (6%), followed by dilution up to 50 mL with deionized water.
2.2.3. Metabolomics Analysis
Sampling for the metabolomics analysis was conducted on D54, just before the final harvest. Four mature leaves per treatment were cut and immediately stored in liquid nitrogen. The plant samples (0.5 g) were pulverized under liquid nitrogen and extracted with 1.4 mL of 100% methanol (pre-cooled 4 °C) according to [21]. Adonitol (100 μL of 0.2 mg mL−1 aqueous solution) was added to the mixtures as a quantitative standard. The mixtures were then centrifuged (11,000× g, 10 min, 4 °C), the supernatants were transferred to glass vials and 0.75 mL of chloroform (−20 °C) and 1.5 mL of dd water (pre-cooled at 4 °C) were added. The mixtures were centrifuged (2200× g, 15 min, 4 °C) and 0.15 mL of the polar phase was transferred to glass vials. The samples were left to dry in a vacuum dryer. The dried residues were then re-dissolved in 40 μL of 20 mg ml−1 methoxyamine hydrochloride in pyridine. The mixtures were shaken in a water bath (2 h, 37 °C) and then 0.7 mL of N-Methyl-N-(trimethtlsilyl)-trifluoroacetamide (MSTFA) was added, vortexed and incubated in a water bath (30 min, 37 °C). The aliquots were transferred into 1.5 mL autosampler vials with glass inserts and stored at −20 °C.
Gas chromatography–mass spectrometry (GC–MS) analysis was performed in a Thermo Trace Ultra GC equipped with an ISQ MS and TriPlus RSH autosampler (Switzerland). A 1 μL sample volume was injected with a split ratio of 70:1. The separations were carried out on a TR-5MS capillary column (30 m × 0.25 mm× 0.25 μm). The injector temperature was set at 220 °C, the ion source at 230 °C, and the interface at 250 °C, while a constant flow rate of the carrier gas (He) was set at 1 mL min−1. The GC temperature program was set at 70 °C for 5 min, then increased to 240 °C at a rate of 8 C min−1 and held at 240 °C for 15 min. After a 5 min solvent delay, the mass range of m/z 50–600 was recorded. The mass spectra were acquired in electron impact ionization (EI) mode. The peak area integration and chromatogram visualization were performed using the Xcalibur program. For the peak identification, mass spectra tick evaluation was performed and the NIST11 database was used. Quantification of the detected metabolites was assessed based on the relative response compared to the internal standard adonitol and expressed as the relative abundance [21,22].
2.3. Statistical Analysis
Data were subjected to a two-way ANOVA where the cultivation system (CAP, DCAP and HP) and the presence of a BS (BS1, BS 2, no BS) were the two main factors, followed by Tukey’s post hoc test in cases where the prerequisites of the ANOVA (Levene’s test for the homogeneity of variances and the Shapiro–Wilk test for the normal distribution) were met. Otherwise, the non-parametric Kruskal–Wallis test was performed, followed by Dunn’s post hoc test. The level of significance was set at 95% (p ≤ 0.05) in all the tests. The analyses were conducted using the open-access JASP v.0.18.1 software (JASP Team 2022 Computer Software). The generation of the heatmap was based on the relative abundance of the detected metabolites, which was determined using the Log2(fc) metric, and was carried out using GraphPad Prism 9.0 [21].
3. Results
Figure 1 illustrates the growth response of lettuce to both the cultivation system and the presence of a BS, as measured at three harvests during the growing period. The fresh weight of the aerial plant part (Figure 1a) was significantly lower in the CAP treatments than in the HP and DCAP, regardless of the presence of a BS. From the first harvest on D26, the CAP plants exhibited a 72.6% reduction in fresh weight compared to the other two treatments. This difference was maintained at the intermediate harvest on D38 and reached 80% at the final one on D54. In all cases, BS application did not affect the results, while no interactions between the cultivation system and BS were recorded. A comparable pattern of effects was observed in the number of leaves (Figure 1b). On D26 and D54, the CAP plants produced 21.7% to 28.7% fewer leaves respectively, compared to the HP and DCAP plants in all the BS sub-treatments. As shown above, lettuce growth was found to be influenced solely by the cultivation system used, demonstrating the inferiority of the CAP plants, and not by the presence of a BS.
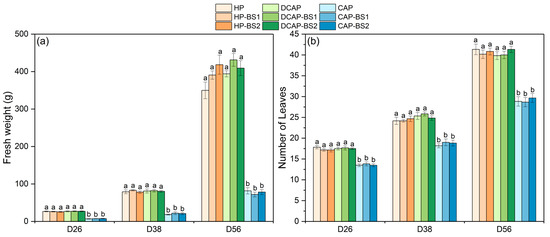
Figure 1.
Growth parameters of lettuce measured at the three harvests (Mean ± Stdev, n = 6): (a) aerial plant part fresh weight and (b) the number of leaves per plant. Different letters denote statistically significant differences between treatments on each measurement (p ≤ 0.05).
The total chl content of the lettuce leaves, as depicted by the SPAD values (Figure 2a), showed limited variation during the growing period. The peak values were observed during the initial two measurements on D12 and D19 for all the treatments. Thereafter, the values gradually decreased until the final harvest. The CAP plants showed a significantly lower chl content than the HP and DCAP, with the differences being significant at the first two measurements and D34. The PRI index (Figure 2b), which is directly related to the light use efficiency (LUE) of the photosynthetic apparatus, was highest at the beginning of the experiment (D12), but the values of all the CAP treatments were half of those for the HP and DCAP treatments. On D19, the CAP treatments experienced a considerable decline, reaching negative values, which only partially recovered by the end of the experiment. At D54, just before the final harvest, the differences among the treatments were levelled out and the PRI reached its minimum levels. The cultivation system was the only factor that significantly influenced the PRI profile, except for the last measurement. The effects of both BSs were not statistically significant in any case. An interaction between the two factors was recorded on D27.
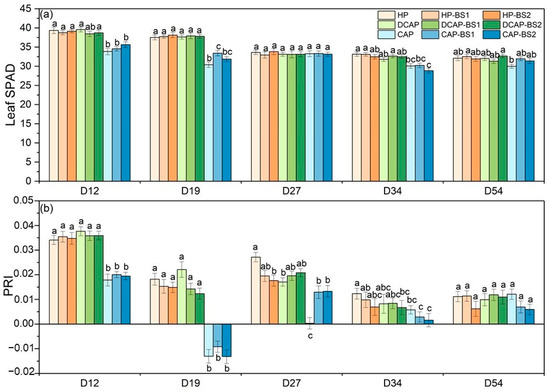
Figure 2.
The SPAD index of lettuce leaves (a) and the PRI index of leaf reflectance (b) measured at five time points during the experimental period (Mean ± Stdev, n = 20). Different letters denote statistically significant differences between treatments on each day (p ≤ 0.05).
The in vivo fluorescence measurements are presented in the radar plots in Figure 3. This provides information on the potential effects of the treatments on all the events related to the functioning of photosystem II (PSII) and certain processes of photosystem I (PSI). We have chosen to display only the three most representative days out of the five sampling days. Already from D12, the CAP plants showed the first signs of stress, as depicted by the lower values of PItotal, which is a photosynthetic performance index. In parallel, the reduced Sm implies changes in the acceptor site and a possibly reduced pool size of e-primary acceptors. Additionally, decreases in φΕο and φRo were evident, which relate to the quantum yield of electron transport through the PSII to intermediate and final acceptors, respectively. The 1-Vi and 1/Vi parameters concern events around the PSI, the function of which was reduced in the CAP lettuce to the lowest level compared to all the other treatments, but the differences were not statistically significant. The energy fluxes per active reaction center (RC), like the absorption flux through the PSII antenna chl (ABS/RC), trapped (TRo/RC) and dissipated (DIo/RC) energy fluxes were considerably higher in the CAP plants compared to HP and DCAP. The application of BS1 and BS2 partially alleviated the stress situation in the CAP treatment and BS1 improved the HP performance, although its effect was not statistically significant, except for PItotal. In the second measurement on D19, the situation was comparable to that on D12, with two changes: a deterioration of CAP in terms of the PItotal and DIo/RC, and the greater positive contribution of the BS to the outcome. Notably, the PItotal in CAP significantly decreased by 60% compared to HP, while the DIo/RC increased by the same percentage. This indicates that enhanced dissipated energy was the major contributor to the decline in the PItotal. Post hoc tests revealed significant pairwise differences between both the CAP-BS1/BS2 and CAP plants in most of the fluorescence parameters on D20, suggesting an ameliorating effect of BS application. This was also the case for both the DCAP and the HP treatments. At the final measurement on D54, the differences in the CAP treatments compared with HP and DCAP were minimized and mainly concerned the energy fluxes, which were slightly reduced in CAP, CAP-BS1, and CAP-BS2. The statistical analysis showed that the cultivation system was the only factor significantly affecting the results. No significant differences were found between the HP and DCAP treatments in all the fluorescence parameters and measurement time points, regardless of the presence of a BS.
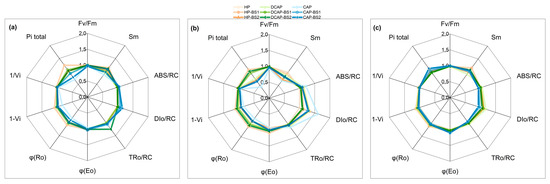
Figure 3.
Spider plots of JIP parameters derived from chl a fluorescence OJIP transient curves in lettuce (mean values, n = 20) for 6 experimental days: (a) Day 12; (b) Days 19 and 20; and (c) Day 54. Values are normalized to the values of the HP treatment.
The concentration of phenolics remained relatively stable throughout the experimental period (Figure 4). The CAP treatments generally exhibited higher values than the other treatments, except for the first measurement on D26. On this date, the only statistically significant differences concerned HP and DCAP-BS2, which were lower than DCAP and HP-BS1. On D38, HP exhibited the lowest value (13 mg GAE/100 g DW) and CAP-BS2 the highest (21 mg GAE/100 g DW). On this date, all the CAP-related treatments were significantly higher than all the HP-related ones and DCAP-BS2. Noteworthy is the low value in DCAP-BS1, which was similar with the HP treatment, although DCAP and DCAP-BS2 were 30% higher, close to the CAP treatment levels. In the last measurement, the difference within the DCAP treatments was minimized, while the three CAP treatments exhibited higher levels in comparison to all the others. Indicatively, CAP reached a 47% increase over HP.
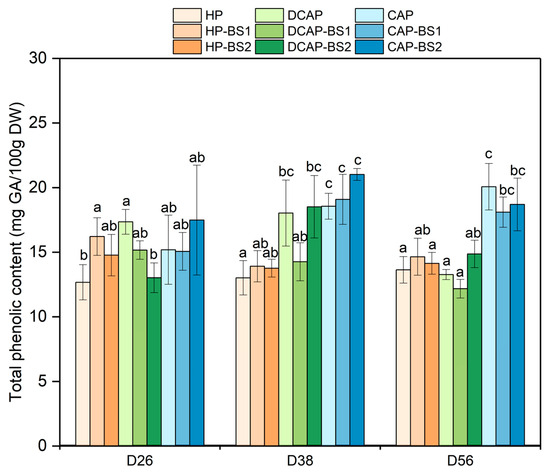
Figure 4.
The total phenolics content of lettuce leaves measured at three time points during the experimental period (Mean ± Stdev, n = 10). Different letters denote statistically significant differences between treatments (p ≤ 0.05) on each day.
The nutritional state of the lettuce leaves in respect to the nitrogen, potassium, sodium, and calcium content is presented in Table 2. On D38, the total N concentration was similar among the treatments, with only CAP-BS2 showing significantly lower values by 22% than HP. However, all the CAP treatments exhibited a reduction in the K+ content, reaching a 30% decrease compared to the others, a result that was also statistically significant. In the opposite direction were the Na+ and Ca2+ levels of the CAP treatments, showing an increase of 172–193% for Na+ and 90–107% for Ca2+ in comparison to HP. No differences were found between any of the HP and DCAP treatments in the results on D38. In the final harvest, D54, the nutritional status of all the CAP plants, regardless of the presence of a BS, had deteriorated for all the measured nutrients. The N content was lower by almost 30% and the K+ content by 60%. Na+ was 4–5.7 times higher in CAP than in HP, reaching 12.01 mg g DW−1, with all the above differences being statistically significant. The K+ levels of DCAP-BS2 were noteworthy as they were significantly lower than all the DCAP and HP treatments yet double those of the CAP treatments. In contrast, the differences in the Ca2+ concentration were diminished on D56, with only the CAP treatment exhibiting a significant decrease of 23% compared to all three HP treatments.

Table 2.
Lettuce leaf concentrations of total N, K+, Na+, and Ca2+, expressed as mg g DW−1 (Avd ± Sd, n = 6) determined on two sampling dates, D38 and D56. Different letters within the columns indicate significant differences (p ≤ 0.05) among treatments. At the bottom of the table, the significant impact of the cultivation system (CS) on each element and date is marked with an asterisk (p ≤ 0.05), while the dash in BS denotes no statistically significant effect.
A heatmap was created to depict the profile of the lettuce polar metabolites and the variation in their levels, showing the abundance of each metabolite as its deviation from the corresponding control (HP) for this metabolite (Figure 5). According to the analysis, 49 metabolites were identified and grouped into the following categories: organic acids (17 metabolites), amino acids (9 metabolites), water-soluble sugars (15 metabolites), sugar alcohols (5 metabolites), and others (3 metabolites). In the DCAP, DCAP-BS2, and HP-BS2 treatments, a large number of metabolites, i.e., 27, 26, and 23 compounds, respectively, exhibited a significantly increased concentration compared to HP. Contrarily, in both the CAP and CAP-BS1 treatments, 10 metabolites with significantly decreased abundance compared to the control were detected. The common response in all the treatments was a downward trend in threonine, xylose and butyric acid, and increased abundance of aspartic acid and glycine, oxalic and succinic acids, as well as meso-erythrol.
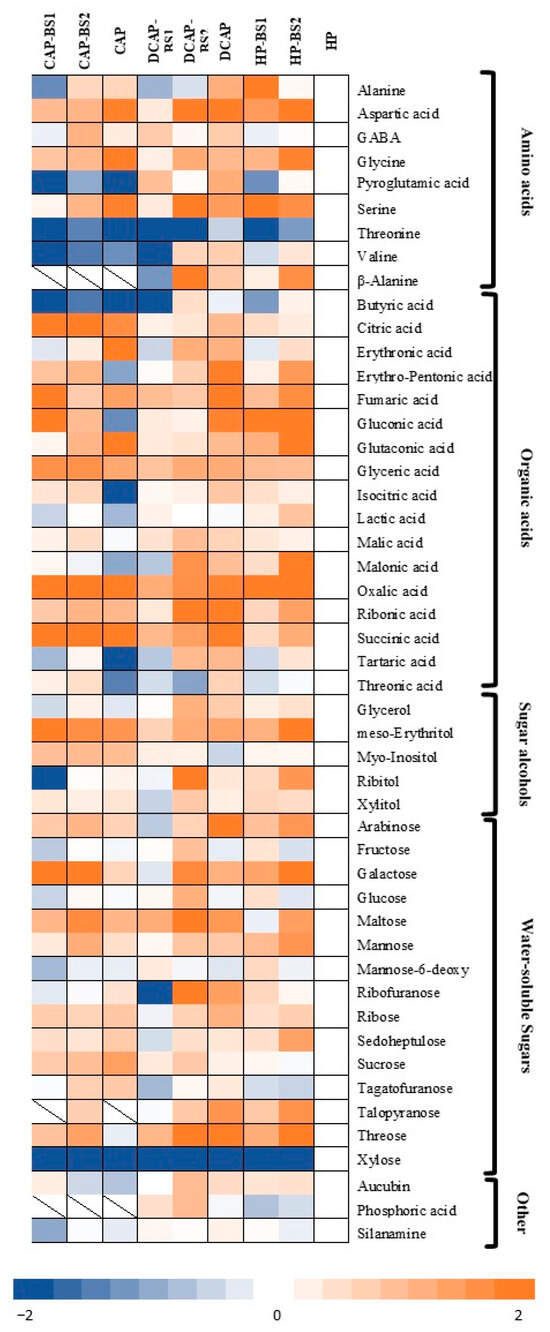
Figure 5.
Heatmap of the quantitative changes in the detected metabolites in the lettuce samples of the treatments compared to the control (HP treatment). The blue color indicates decreased and the orange color increased metabolite levels. The amounts of each treatment are expressed as the relative abundance based on the relative response compared to the internal standard (n = 4).
In the amino acid group, the DCAP treatment had the highest number of detected compounds, which were also found in higher intracellular abundance (except for threonine) compared to the HP treatment. In the DCAP-BS1, CAP, CAP-BS2, and HP-BS1 treatments, amino acids were present in lower abundance, with more than half of them having similar or lower levels than in the HP treatment. In the CAP-BS1 treatment, all the amino acids except aspartic acid and glycine were recorded at the lowest amounts compared to HP. Noteworthy is the high abundance of the amino acid β-alanine in DCAP, DCAP-BS2, and HP-BS2.
Most organic acids showed a higher intracellular abundance in DCAP compared to the control group, as well as the other treatments. In particular, isocitric acid, fumaric acid, erythropentonic acid, ribonic acid, tartaric acid and threonic acid were found in the highest amounts. The DCAP-BS2 and HP-BS2 treatments also resulted in an increase in organic acid abundance compared to the control. On the other hand, in the CAP, CAP-BS1 and DCAP-BS1 treatments, many organic acids showed either no significant differences or a decrease in the intracellular concentration compared to HP.
Regarding sugars, the greatest abundance was found in the DCAP, DCAP-BS2, HP-BS1, and HP-BS2 treatments compared to the HP control. The DCAP treatment was particularly noteworthy as, despite the high availability of sugars, the concentration of fructose, glucose, mannose, and sucrose was limited. All the CAP treatments resulted in minor changes compared to the HP control, regardless of the application of a BS. The only exceptions were the increased maltose content and reduced xylose. Likewise, small differences compared with the control were observed in the sugar alcohols. Nevertheless, meso-erythrol showed a significant increase in all the treatments. The actual amount of each detected metabolite is presented in Table S2.
4. Discussion
This study aimed to examine the feasibility of integrating BSs and aquaponics, both coupled and decoupled, in lettuce cultivation for the first time in the relevant literature. We investigated this combination to assess its potential to overcome the nutrient limitations frequently reported for aquaponics crops. This was performed to propose an alternative management practice that would ensure the sustainability and low-input nature of aquaponics systems.
Lettuce growth was significantly lower in the CAP treatments compared to all the HP and DCAP treatments (Figure 1), while the application of a BS had no effect. The limitations of CAP became evident from the initial harvest on D26, and by the final harvest on D54, there was an 80% reduction in aerial part fresh weight and a 28.7% decrease in leaf number. Previous studies have also reported lower yields in coupled systems compared to hydroponics [8,9,11,23]. Lettuce is a frequently studied species in the aquaponics literature [24]. However, its growth performance varies considerably depending on many factors, such as the experimental conditions, crop cultivar, and paired fish species, stocking, and feeding characteristics. Therefore, drawing comparisons can be challenging. In a similar experimental setup, Yang and Hye-Ji [25] found a comparable yield in their HP treatments with ours, yet a better performance of the CAP system, which reached 68% of their HP. Analogous with above, HP-CAP differences are reported by Nozzi et al. [26], with the CAP lettuce fresh weight reaching 260 g, the 75% of HP production. In this later work, they achieved higher lettuce yields by adding iron, potassium, and phosphorous to the aquaponics water on a daily basis. Additionally, Delaire et al. [27] reported a surprisingly low yield for the HP treatments, which was similar to the CAP and almost 90 g/plant, while the DCAP treatment outperformed both with 136 g/plant. Our results show a discrepancy with the two last-mentioned studies, since DCAP showed similar growth performance to HP lettuce, again irrespective the presence of a BS.
The growth inferiority of the CAP plants in all the above-mentioned works is mainly associated with the lower nutrient concentrations in the leaf tissues, reflecting the sub-optimal levels of the corresponding solution concentrations. Although lettuce is considered a low-demand crop compared to fruit-bearing crops such as tomatoes and cucumbers, it seems that CAP does not meet its nutritional requirements [11,28]. The elemental analysis performed in the present study (Table 2) showed that the cultivation system, but not the BS, shaped the elemental profile of lettuce leaves. All three CAP treatments exhibited a similarly deteriorated nutritional state compared to all the DCAP and HP plants. The total N content did not seem to act as the major limiting nutrient for crop growth, since it did not differ among treatment until D38 and in all cases fell within the range of concentrations usually found in healthy hydroponically grown lettuce (30–60 mg g−1) [29]. Similar values of total N have been reported for hydroponically grown lettuce at a pH between 5.8 and 7 [30]. In a coupled aquaponics system, plants derive their nitrogen for nutrition mainly from fish excretions. Fish excretions are estimated to be 30–65% nitrogen and 40% phosphorus, with the exact proportions varying depending on the composition of the fish feed [31]. The K content of the CAP leaves was significantly lower than all the other treatments in both harvests. K is a functional macronutrient that plays a crucial role in photosynthesis, regulation of stomatal movements, protein synthesis, and osmotic potential [32,33]. In aquaponics systems, the K levels are typically sub-optimal, leading to leaf deficiencies [20,34,35]. Roosta et al. [32] reported that foliar application of K at levels similar to HP and DCAP in the present study significantly enhanced the biomass gain and subsequently leaf K+ content. In a previous study by our team, lettuce was grown using a ‘minimal input’ approach to limit the external nutrient supplementation to a level that would not compromise the sustainability of the system [11]. The results showed that the addition of only K+ and Fe2+ to CAP, at the concentrations used in hydroponics, was sufficient to achieve a yield comparable to conventionally hydroponics-produced lettuce. On the other hand, there are several works that, in an attempt to tackle the nutrient limitations of aquaponic solutions, have included high rates of both micro- and macro-nutrients, reaching the level of hydroponic inputs [27,36]. Although this approach is effective in increasing plant productivity, it raises concerns about the sustainability of aquaponics and its environmental footprint. In the present study, Na+ reached higher levels in the leaves of all the CAP treatments compared to the others, exhibiting values of 8 mg g DW−1 and 12 mg g DW−1 at the intermediate and final harvest, respectively. Similar results are typically reported for coupled aquaponics systems in comparison with HP systems [27,37,38]. Yang and Kim [25] reported the same Na concentration in lettuce grown in a CAP system, showing values of 11.8 mg g DW−1 and 3.6 mg g DW−1 in the HP. They stated that this concentration had no adverse effect on lettuce growth, but it is possible that Na+ played a role in the Ca2+ and Mg2+ deficiencies through mineral interactions. It is well known that high levels of Na+ have a negative effect on the crop yield, but this appears to be concentration-dependent [39,40]. In lettuce irrigated with a relatively low salt concentration, i.e., 5 mM [41], several growth parameters as well as the yield remained unaffected. According to Hniličková et al. [42], it takes a much higher Na content (30 mg g DW−1) in lettuce leaves than ours to lead to a reduction in growth and disturbance of function. Their conclusion is corroborated by Brés et al. [43], who reported similar control growth of lettuce under 10 mmol NaCl, although Na+ content reached 11 mg g DW−1, as well as by Delaire et al. [27], with the extreme 9.3 times increase in the Na in their aquaponics system compared to a hydroponics one. However, a side effect of Na accumulation is the interference with K+ and Ca2+ absorption, inducing deficiencies [44]. In our case, the Na+/Ca2+ balance did not seem to follow a clear pattern between the two measurements (Table 2). The interaction of Na+ with K+ was obvious in both measurements but may have played an important role in the final one. According to the analysis of Hartz et al. [45], the K+ levels of all the plant groups fell within the accepted leaf concentration limits for optimal growth, although in the final harvest they were found to be lower than the minimum accepted value, possibly due to Na+-induced competition. Despite the reported contribution of the BS to the improvement of the nutritional state of plant through various mechanisms [46,47], such an impact was not observed in the present study. Applying different BSs on maize and soybean, de Vasconcelos [48] corroborated our results, since she not find any difference in the leaf nutrient content, suggesting that BSs cannot substitute for fertilization, especially in crops with high nutrient demands.
The time point of D12 appears to be crucial in determining the course of chl biosynthesis and degradation in the present experiment’s no external input CAP system (SPAD index, Figure 2a). A similar response has been reported for lettuce [11], spinach [20] and rocket [49] grown in analogous systems. Low chlorophyll levels can reduce the LUE, i.e., the efficiency of utilizing absorbed photosynthetically active radiation for biomass production, ultimately resulting in inefficient rates of photosynthesis [50]. This was the case in the CAP leaves, regardless of the BS application, where reduced LUE values were implied by the marked decline in the PRI (Figure 2b) already from D12. The PRI correlates strongly with the LUE and is often used as a predictor of it [50]. In addition to consistently lower PRI values in CAP compared to DCAP and HP, negative values were recorded on D19. The low levels of the PRI may be attributed to a combination of low chl and impaired photosynthetic function. The latter is supported by the fluorescence parameters of lettuce described later. Furthermore, there is strong evidence in the literature that the PRI is positively correlated with the relative photosynthetic rate [51] and PSII quantum yield and negatively correlated with non-photochemical quenching [52]. Both the DCAP and HP plants maintained high and similar PRI values up to D27, but then declined, possibly due to developmental reasons.
The chl a in vivo fluorescence assessment indicated that a functional impairment of the photosynthetic machinery began to appear in the CAP plants as early as D12. This was evidenced by limitations in e-transport and the decreased relative pool size of total e-carriers (Sm) compared to the DCAP and HP treatments. Over the course of the experiment, the CAP plants experienced a marked increase in ABS/RC, implying a decrease in the number of active RCs, and subsequently, an enhancement of non-photochemical energy dissipation. Indeed, an upward trend in thermally dissipated energy per active RC (DIo/RC) was observed, except for the last measurements, where all the differences from HP (control) were relaxed. The aforementioned results of the energy fluxes, as well as the increasing limitations of the PSI-related events, collectively contributed to the observed decline in the photosynthetic performance index (PItotal) from D12 onwards. It is well established that nutrient deficiencies, such as those experienced by the CAP plants, typically trigger changes in PSII photochemistry, similar to those observed in lettuce [53,54]. Jardim et al. [55] correlated the lower PSII quantum yield and the enhanced non-photochemical quenching with reduced chl levels due to a sub-optimal leaf K content, which was also the case in our experiment. Furthermore, the inferiority of aquaponics-grown basil compared to hydroponics in the experiment by Roosta [56] was depicted in pronounced decreases in the PSII quantum yield and was attributed to K+, Fe2+ and Mn deficiencies. The time point of D12–14 is crucial for the appearance of the first stress signs in the performance of the photosynthetic apparatus, as has also been documented by other aquaponics works of our group with lettuce, spinach and spiny chicory [11,20,57].
The antioxidant activity, as depicted in the leaf total phenolics content of the CAP plants, irrespective of the BS presence, remained at the same levels as the other treatments at D26 (Figure 4), but it was slightly increased from the middle until the end of the growing period. The phenolic content of plants is directly linked to their nutritional state and serves as an antioxidant mechanism. A deficiency in certain elements can cause the accumulation of reactive oxygen species, as is documented with Fe and K deficiency, and the oxidative damage is aggravated by long-term stress [33]. However, the response of plants to nutrient availabilities is species-specific concerning the antioxidant mechanisms involved, whereas the absence of significant increases in the total phenolic content of the present study may reflect the employment of other antioxidant compounds or processes [58].
The complex interplay between environmental factors, such as those imposed by the cultivation system, and the plant responses is mediated by changes in the plant’s metabolome. The metabolic profile of plants is strongly influenced by nutrient deficiencies, among other stresses or imbalances [59]. The metabolomic analysis of lettuce leaves revealed an overall trend toward greater changes in the amino acid levels, followed by organic acids, with the lowest magnitude of changes being observed in the sugars compared to the control group (HP). A distinct response of the DCAP- and DCAP-BS2-treated plants compared to the others was evident. These two treatments, along with HP-BS2, showed a significant increase in the abundance of more than half of the identified metabolites compared to the control (HP). In contrast, CAP and CAP-BS2 showed a significant reduction in 10 compounds and slight or non-significant increases compared to HP.
The DCAP and DCAP-BS2 treatments resulted in higher intracellular abundance of many amino acids compared to the HP control. This might indicate an increased level of activity in the metabolic pathways of amino acid biosynthesis in these treatments. Of particular interest are both the accumulation of aspartic acid and the decreased abundance of threonine in all the treatments compared to the control. The aspartic acid content exhibited a four-fold increase in the DCAP, DCAP-BS2 and HP-BS2 treatments and was more than doubled in all the CAP treatments, indicating a strong activation of nitrogen assimilation processes [60]. The lower than HP abundance of threonine suggests a decreased intensity of metabolic activation in response to abiotic stress [61], possibly indicating the absence of noticeable stress in these treatments. Finally, the high abundance of the amino acid β-alanine in the DCAP-BS2, and HP-BS2 treatments, which is a precursor of acetyl-CoA, suggests an intensification of metabolism, which conforms with the aforementioned activation of the TCA cycle in these treatments [62].
The increase of organic acids in the DCAP- and DCAP-BS2-treated plants, and especially the accumulation of citric, malic, succinic, and isocitric acids, intermediates of the TCA cycle, indicates an activation of energy production processes [63]. In contrast, in the CAP, CAP-BS1 and DCAP-BS1 treatments, many organic acids either exhibited no noticeable differences or an intracellular decrease in abundance compared to the HP control. Furthermore, the increased abundance of threonic acid and tartaric acid, which are products of ascorbic acid oxidation [64], in all the CAP, DCAP, and HP treatments compared to the control probably indicates the regulation of antioxidant mechanisms. This finding requires further exploration regarding the possible reprogramming of oxidative homeostasis in plants as a response to the cultivation system. As mentioned before, the total phenolics content appeared to be marginally affected by both the cultivation system and BS, but other pathways of antioxidant defense not determined in the present study may be upregulated compared to control.
The BS2 application either in DCAP or in HP, along with the DCAP and HP-BS1 treatments, showed a considerable accumulation of sugars compared to HP. Of particular interest is that in the DCAP treatment, although a high accumulation of sugars was recorded, as at the same time, the concentration of fructose, glucose, mannose and sucrose was limited. This may be due to the increased breakdown of these metabolites to produce energy and other compounds; as mentioned above, TCA cycle organic acids and amino acids were highly accumulated in this treatment. The metabolic profiles of the CAP-BS1, CAP-BS2 and CAP treatments showed similar or reduced fructose and glucose content, with moderate accumulation of sucrose and mannose, compared to HP. It is noteworthy that a decrease in the abundance of the structural sugar xylose was observed in all the treatments in comparison to the control. Since this sugar is associated with the presence of xyloglucans and the composition of the cell wall [65], this fact needs further investigation.
The combination of physiological and metabolomic data suggests that the CAP-related plants down-regulate processes and metabolism to adapt to the low nutrient content in leaves rather than being damaged. The sensitive chlorophyll fluorescence indicators did not indicate any damage to the photosynthetic apparatus of these plants, nor did their metabolic profile show any pronounced stress-related changes in comparison with the HP. In parallel, the growth reduction caused by nutrient limitations decreases the sink strength, thereby triggering a negative feedback mechanism in terms of photochemical performance [66]. Thus, the overall picture suggests an acclimation process of lettuce to the prevailing nutrient conditions, with the down-regulation of metabolism achieving a balance between optimal nutrient use and plant function under nutrient limitations.
5. Conclusions
The present study demonstrated the limitations that the CAP system poses in relation to lettuce growth, function, and metabolism. The application of BSs did not optimize the CAP plants’ performance in any parameter measured. On the contrary, the DCAP cultivation system succeeded in achieving the same levels of yield, as well as a similar physiological state to the HP system. The application of BS2 to the DCAP plants promoted their metabolism, as indicated by the accumulation of sugars, amino acids, and organic acids, which were significantly increased (together with DCAP) compared to the analysis control (HP). However, the improvement in metabolism did not have a clear effect on the growth and physiological response of DCAP-BS2, except for parameters related to the state and efficiency of the photosynthetic apparatus. Despite this, we may consider BS2 as a promising factor that may have the potential to enhance the performance of crops with a longer life cycle. This work was a first step toward integrating BS application with aquaponics systems; thus, not all the mechanisms underlying the observed physiological and metabolomic responses were elucidated. Further research is necessary to gain a comprehensive understanding of all the aspects of this integration. As the DCAP cultivation system has proven successful in achieving yields comparable to HP with lower chemical inputs, future studies should focus on investigating other BSs in this system to further improve its efficiency while maintaining its environmental advantages within a circular economy framework.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10050514/s1, Table S1: The parameters derived from the fast JIP fluorescence induction; Table S2: The metabolic profiles of lettuce samples of the individual treatments.
Author Contributions
Conceptualization, N.K. and E.L.; methodology, E.C., S.F., A.M., M.G.K. and K.K.; formal analysis, A.M., M.G.K. and K.K.; investigation, E.C., S.F. and M.G.K.; writing—original draft preparation, N.K., K.K. and E.L.; writing—review and editing, E.L.; supervision, E.L.; project administration, E.-M.P.; funding acquisition, N.K. and E.-M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out under the PestNu project, which has received funding from the European Union’s Horizon 2020 research and innovation programme under the Green Deal grant agreement No. 101037128—PestNu.
Institutional Review Board Statement
All the experimental procedures were conducted according to the guidelines of EU Directive 2010/63/EU regarding the protection of animals used for scientific purposes. The experimental protocol was approved by the Ethics Committee and conducted at the registered experimental facility (EL-43BIO/exp-02) of the Pilot Aquaponics Greenhouse of the Department of Agriculture Crop Production and Rural Environment, University of Thessaly (n. 35488/2021).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Joyce, A.; Goddek, S.; Kotzen, B.; Wuertz, S. Aquaponics: Closing the Cycle on Limited Water, Land and Nutrient Resources. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–34. ISBN 978-3-030-15943-6. [Google Scholar]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. (Eds.) Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-15942-9. [Google Scholar]
- Junge, R.; König, B.; Villarroel, M.; Komives, T.; Jijakli, M. Strategic Points in Aquaponics. Water 2017, 9, 182. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Comparisons of Nitrogen and Phosphorus Mass Balance for Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Baganz, G.F.M.; Junge, R.; Portella, M.C.; Goddek, S.; Keesman, K.J.; Baganz, D.; Staaks, G.; Shaw, C.; Lohrberg, F.; Kloas, W. The Aquaponic Principle—It Is All about Coupling. Rev. Aquac. 2022, 14, 252–264. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Haïssam Jijakli, M.; Kotzen, B. Towards Commercial Aquaponics: A Review of Systems, Designs, Scales and Nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae 2023, 9, 1077. [Google Scholar] [CrossRef]
- Mourantian, A.; Aslanidou, M.; Mente, E.; Katsoulas, N.; Levizou, E. Basil Functional and Growth Responses When Cultivated via Different Aquaponic and Hydroponics Systems. PeerJ 2023, 11, e15664. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Uzinger, N.; Gyulai, G.; Mathis, A.; Junge, R.; Villarroel, M.; Kotzen, B.; Kőmíves, T. Nutrient Supply of Plants in Aquaponic Systems. Ecocycles 2016, 2, 17–20. [Google Scholar] [CrossRef]
- Tsoumalakou, E.; Mente, E.; Kormas, K.A.; Katsoulas, N.; Vlahos, N.; Kapsis, P.; Levizou, E. Precise Monitoring of Lettuce Functional Responses to Minimal Nutrient Supplementation Identifies Aquaponic System’s Nutrient Limitations and Their Time-Course. Agriculture 2022, 12, 1278. [Google Scholar] [CrossRef]
- Rayhan, M.Z.; Rahman, M.A.; Hossain, M.A.; Akter, T.; Akter, T. Effect of Stocking Density on Growth Performance of Monosex Tilapia (Oreochromis niloticus) with Indian Spinach (Basella alba) in a Recirculating Aquaponic System. Int. J. Environ. Agric. Biotechnol. 2018, 3, 343–349. [Google Scholar] [CrossRef]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, Variety salanova) Production in Decoupled Aquaponic Systems: Same Yield and Similar Quality as in Conventional Hydroponic Systems but Drastically Reduced Greenhouse Gas Emissions by Saving Inorganic Fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef] [PubMed]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants Research in Some Horticultural Plant Species—A Review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Irani, H.; ValizadehKaji, B.; Naeini, M.R. Biostimulant-Induced Drought Tolerance in Grapevine Is Associated with Physiological and Biochemical Changes. Chem. Biol. Technol. Agric. 2021, 8, 5. [Google Scholar] [CrossRef]
- Abdelkader, M.; Voronina, L.; Baratova, L.; Shelepova, O.; Zargar, M.; Puchkov, M.; Loktionova, E.; Amantayev, B.; Kipshakbaeva, A.; Arinov, B. Biostimulants-Based Amino Acids Augment Physio-Biochemical Responses and Promote Salinity Tolerance of Lettuce Plants (Lactuca sativa L.). Horticulturae 2023, 9, 807. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Baudoin, W. Good Agricultural Practices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas; Organisation des Nations Unies pour L’alimentation et L’agriculture, Société Internationale de la Science Horticole, Centre National pour la Recherche Agricole et la Vulgarisation, Eds.; FAO Plant Production and Protection Paper; FAO: Rome, Italy, 2013; ISBN 978-92-5-107649-1. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Tsoumalakou, E.; Mente, E.; Vlahos, N.; Levizou, E. Spinach Responds to Minimal Nutrient Supplementation in Aquaponics by Up-Regulating Light Use Efficiency, Photochemistry, and Carboxylation. Horticulturae 2023, 9, 291. [Google Scholar] [CrossRef]
- Ainalidou, A.; Tanou, G.; Belghazi, M.; Samiotaki, M.; Diamantidis, G.; Molassiotis, A.; Karamanoli, K. Integrated Analysis of Metabolites and Proteins Reveal Aspects of the Tissue-Specific Function of Synthetic Cytokinin in Kiwifruit Development and Ripening. J. Proteom. 2016, 143, 318–333. [Google Scholar] [CrossRef]
- Mellidou, I.; Ainalidou, A.; Papadopoulou, A.; Leontidou, K.; Genitsaris, S.; Karagiannis, E.; Van De Poel, B.; Karamanoli, K. Comparative Transcriptomics and Metabolomics Reveal an Intricate Priming Mechanism Involved in PGPR-Mediated Salt Tolerance in Tomato. Front. Plant Sci. 2021, 12, 713984. [Google Scholar] [CrossRef]
- Roosta, H.R.; Hamidpour, M. Mineral Nutrient Content of Tomato Plants in Aquaponic and Hydroponic Systems: Effect of Foliar Application of Some Macro- and Micro-Nutrients. J. Plant Nutr. 2013, 36, 2070–2083. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Junge, R.; Schmautz, Z.; Sambo, P.; Borin, M. Hydroponic Systems and Water Management in Aquaponics: A Review. Ital. J. Agron. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient Management in Aquaponics: Comparison of Three Approaches for Cultivating Lettuce, Mint and Mushroom Herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M. Lettuce (Lactuca sativa L. Var. Sucrine) Growth Performance in Complemented Aquaponic Solution Outperforms Hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Collado-González, J.; López-Marín, J.; Del Amor, F.M. Effects of Selenium on the Chlorophylls, Gas Exchange, Antioxidant Activity and Amino Acid Composition of Lettuce Grown under an Aquaponics System. Horticulturae 2021, 8, 30. [Google Scholar] [CrossRef]
- Lenz, G.L.; Loss, A.; Lourenzi, C.R.; Luiz De Alcantara Lopes, D.; Siebeneichler, L.D.M.; Brunetto, G. Lettuce Growth in Aquaponic System and in Soil Fertilized with Fish Sludge. Aquac. Res. 2021, 52, 5008–5021. [Google Scholar] [CrossRef]
- Anderson, T.; Martini, M.; De Villiers, D.; Timmons, M. Growth and Tissue Elemental Composition Response of Butterhead Lettuce (Lactuca sativa, Cv. Flandria) to Hydroponic Conditions at Different PH and Alkalinity. Horticulturae 2017, 3, 41. [Google Scholar] [CrossRef]
- Schneider, O.; Sereti, V.; Eding, E.H.; Verreth, J.A.J. Analysis of Nutrient Flows in Integrated Intensive Aquaculture Systems. Aquac. Eng. 2005, 32, 379–401. [Google Scholar] [CrossRef]
- Roosta, H.R. Effects of Foliar Spray of K on Mint, Radish, Parsley and Coriander Plants in Aquaponic System. J. Plant Nutr. 2014, 37, 2236–2254. [Google Scholar] [CrossRef]
- Patel, M.; Fatnani, D.; Parida, A.K. Potassium Deficiency Stress Tolerance in Peanut (Arachis hypogaea) through Ion Homeostasis, Activation of Antioxidant Defense, and Metabolic Dynamics: Alleviatory Role of Silicon Supplementation. Plant Physiol. Biochem. 2022, 182, 55–75. [Google Scholar] [CrossRef]
- Krastanova, M.; Sirakov, I.; Ivanova-Kirilova, S.; Yarkov, D.; Orozova, P. Aquaponic Systems: Biological and Technological Parameters. Biotechnol. Biotechnol. Equip. 2022, 36, 305–316. [Google Scholar] [CrossRef]
- Harika, N.; Verma, A.K.; Krishnani, K.K.; Hittinahalli, C.M.; Reddy, R.; Pai, M. Supplementation of Potassium in Aquaculture Wastewater and Its Effect on Growth Performance of Basil (Ocimum basilicum L.) and Pangasius (Pangasianodon Hypophthalmus) in NFT-Based Aquaponics. Sci. Hortic. 2024, 323, 112521. [Google Scholar] [CrossRef]
- Ru, D.; Liu, J.; Hu, Z.; Zou, Y.; Jiang, L.; Cheng, X.; Lv, Z. Improvement of Aquaponic Performance through Micro- and Macro-Nutrient Addition. Environ. Sci. Pollut. Res. 2017, 24, 16328–16335. [Google Scholar] [CrossRef] [PubMed]
- Kasozi, N.; Kaiser, H.; Wilhelmi, B. Effect of Bacillus spp. on Lettuce Growth and Root Associated Bacterial Community in a Small-Scale Aquaponics System. Agronomy 2021, 11, 947. [Google Scholar] [CrossRef]
- Patloková, K.; Pokluda, R. Optimization of Plant Nutrition in Aquaponics: The Impact of Trichoderma Harzianum and Bacillus Mojavensis on Lettuce and Basil Yield and Mineral Status. Plants 2024, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Avdouli, D.; Max, J.F.J.; Katsoulas, N.; Levizou, E. Basil as Secondary Crop in Cascade Hydroponics: Exploring Salinity Tolerance Limits in Terms of Growth, Amino Acid Profile, and Nutrient Composition. Horticulturae 2021, 7, 203. [Google Scholar] [CrossRef]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Lessons from Crop Plants Struggling with Salinity. Plant Sci. 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C.; Kwon, D.Y. Salt in Irrigation Water Affects the Nutritional and Visual Properties of Romaine Lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of Salt Stress on Growth, Electrolyte Leakage, Na+ and K+ Content in Selected Plant Species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Breś, W.; Kleiber, T.; Markiewicz, B.; Mieloszyk, E.; Mieloch, M. The Effect of NaCl Stress on the Response of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 244. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Mineral Element Acquisition and Growth Response of Plants Grown in Saline Environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Hartz, T.K.; Johnstone, P.R.; Williams, E.; Smith, R.F. Establishing Lettuce Leaf Nutrient Optimum Ranges Through DRIS Analysis. HortScience 2007, 42, 143–146. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.; Kyriacou, M.; et al. Biostimulant Application with a Tropical Plant Extract Enhances Corchorus Olitorius Adaptation to Sub-Optimal Nutrient Regimens by Improving Physiological Parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking Abiotic Stress, Plant Metabolites, Biostimulants and Functional Food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Vasconcelos, A.C.F.D. Effect of Biostimulants on the Nutrition of Maize and Soybean Plants. Int. J. Environ. Agric. Biotechnol. 2019, 4, 240–245. [Google Scholar] [CrossRef]
- Stathopoulou, P.; Tsoumalakou, E.; Levizou, E.; Vanikiotis, T.; Zaoutsos, S.; Berillis, P. Iron and Potassium Fertilization Improve Rocket Growth without Affecting Tilapia Growth and Histomorphology Characteristics in Aquaponics. Appl. Sci. 2021, 11, 5681. [Google Scholar] [CrossRef]
- Vanikiotis, T.; Stagakis, S.; Kyparissis, A. MODIS PRI Performance to Track Light Use Efficiency of a Mediterranean Coniferous Forest: Determinants, Restrictions and the Role of LUE Range. Agric. For. Meteorol. 2021, 307, 108518. [Google Scholar] [CrossRef]
- Filella, I.; Porcar-Castell, A.; Munné-Bosch, S.; Bäck, J.; Garbulsky, M.F.; Peñuelas, J. PRI Assessment of Long-Term Changes in Carotenoids/Chlorophyll Ratio and Short-Term Changes in de-Epoxidation State of the Xanthophyll Cycle. Int. J. Remote Sens. 2009, 30, 4443–4455. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Gromova, E.; Nerush, V.; Vodeneev, V.; Sukhov, V. A Light-Induced Decrease in the Photochemical Reflectance Index (PRI) Can Be Used to Estimate the Energy-Dependent Component of Non-Photochemical Quenching under Heat Stress and Soil Drought in Pea, Wheat, and Pumpkin. Photosynth. Res. 2020, 146, 175–187. [Google Scholar] [CrossRef]
- Larbi, A.; Abadía, A.; Abadía, J.; Morales, F. Down Co-Regulation of Light Absorption, Photochemistry, and Carboxylation in Fe-Deficient Plants Growing in Different Environments. Photosynth. Res. 2006, 89, 113–126. [Google Scholar] [CrossRef]
- Morales, F.; Belkhodja, R.; Abadıa, A.; Abadıa, J. Photosystem II Efficiency and Mechanisms of Energy Dissipation in Iron-Deficient, Field-Grown Pear Trees (Pyrus communis L.). Photosynth. Res. 2000, 63, 9–21. [Google Scholar] [CrossRef]
- da Rosa Ferraz Jardim, A.M.; Santos, H.R.B.; Alves, H.K.M.N.; Ferreira-Silva, S.L.; de Souza, L.S.B.; Júnior, G.D.N.A.; de Sá Souza, M.; de Araújo, G.G.L.; de Souza, C.A.A.; da Silva, T.G.F. Genotypic Differences Relative Photochemical Activity, Inorganic and Organic Solutes and Yield Performance in Clones of the Forage Cactus under Semi-Arid Environment. Plant Physiol. Biochem. 2021, 162, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Roosta, H.R. Comparison of the Vegetative Growth, Eco-Physiological Characteristics and Mineral Nutrient Content of Basil Plants in Different Irrigation Ratios of Hydroponic:Aquaponic Solutions. J. Plant Nutr. 2014, 37, 1782–1803. [Google Scholar] [CrossRef]
- Tsoumalakou, E.; Mente, E.; Vlahos, N.; Levizou, E. Cultivating the Mediterranean Wild Edible Species Cichorium spinosum L. in Aquaponics: Functional and Growth Responses to Minimal Nutrient Supplementation. Sustainability 2023, 15, 5572. [Google Scholar] [CrossRef]
- Jin, C.-W.; Liu, Y.; Mao, Q.-Q.; Wang, Q.; Du, S.-T. Mild Fe-Deficiency Improves Biomass Production and Quality of Hydroponic-Cultivated Spinach Plants (Spinacia oleracea L.). Food Chem. 2013, 138, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Corrado, G.; Zhang, L.; Senizza, B.; Righetti, L.; Bruni, R.; El-Nakhel, C.; Sifola, M.I.; Pannico, A.; Pascale, S.D.; et al. The Metabolic Reprogramming Induced by Sub-Optimal Nutritional and Light Inputs in Soilless Cultivated Green and Red Butterhead Lettuce. Int. J. Mol. Sci. 2020, 21, 6381. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P.; Gantt, J.S. Control of Nitrogen and Carbon Metabolism in Root Nodules. Physiol. Plant. 1992, 85, 266–274. [Google Scholar] [CrossRef]
- Trotta, A.; Suorsa, M.; Rantala, M.; Lundin, B.; Aro, E. Serine and Threonine Residues of Plant STN 7 Kinase Are Differentially Phosphorylated upon Changing Light Conditions and Specifically Influence the Activity and Stability of the Kinase. Plant J. 2016, 87, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The Synthesis and Role of β-Alanine in Plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar] [CrossRef]
- Yang, X.; Feng, L.; Zhao, L.; Liu, X.; Hassani, D.; Huang, D. Effect of Glycine Nitrogen on Lettuce Growth under Soilless Culture: A Metabolomics Approach to Identify the Main Changes Occurred in Plant Primary and Secondary Metabolism. J. Sci. Food Agric. 2018, 98, 467–477. [Google Scholar] [CrossRef]
- Loewus, F. Biosynthesis and Metabolism of Ascorbic Acid in Plants and of Analogs of Ascorbic Acid in Fungi. Phytochemistry 1999, 52, 193–210. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vera, U.M.; De Souza, A.P.; Long, S.P.; Ort, D.R. The Role of Sink Strength and Nitrogen Availability in the Down-Regulation of Photosynthetic Capacity in Field-Grown Nicotiana tabacum L. at Elevated CO2 Concentration. Front. Plant Sci. 2017, 8, 998. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).