Promising and Failed Breeding Techniques for Overcoming Sterility and Increasing Seed Set in Bananas (Musa spp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Banana Genotypes Used
2.2. Early and Evening Pollination Techniques
2.3. Hormonal Treatment
2.4. Saline Solution Treatment
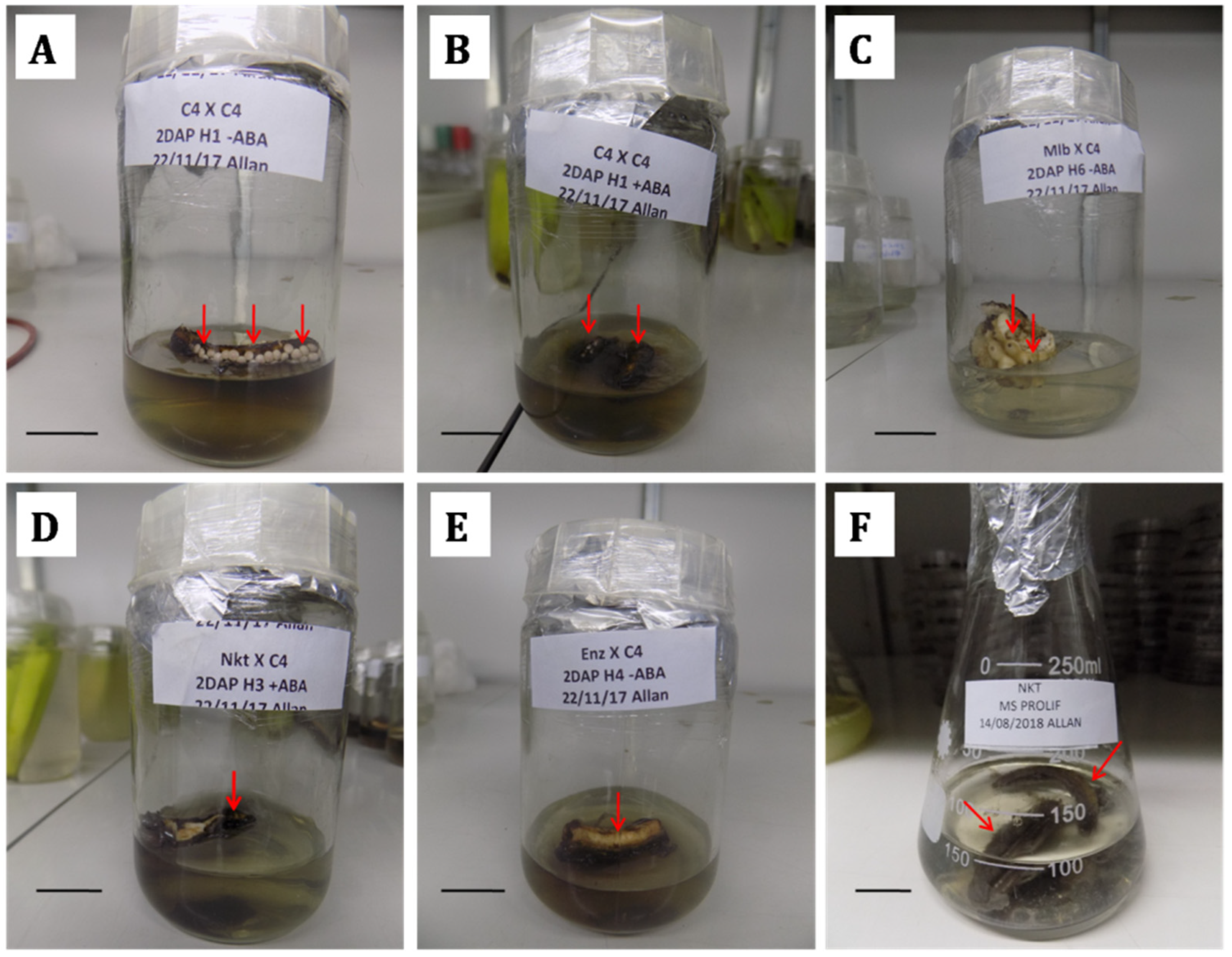
2.5. Ovule Culture
2.6. Seed Extraction
2.7. Data Analysis
3. Results
3.1. Early and Evening Pollinations
3.2. Hormonal Treatment
3.3. Saline Solution Treatment
3.4. Ovule Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. FAO. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 21 November 2023).
- FAO. Banana Market Review; FAO: Rome, Italy, 2023. [Google Scholar]
- Maymon, M.; Sela, N.; Shpatz, U.; Galpaz, N.; Freeman, S. The origin and current situation of Fusarium oxysporum f. sp. cubense tropical race 4 in Israel and the Middle East. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Mostert, D.; Chiconela, T.; Beukes, I.; Fraser, C.; Dwyer, J.; Murray, H.; Amisse, J.; Matabuana, E.L.; Tazan, G.; et al. Occurrence and spread of the banana fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020, 116, 1–11. [Google Scholar] [CrossRef]
- Aguayo, J.; Cerf-Wendling, I.; Folscher, A.B.; Fourrier-Jeandel, C.; Ioos, R.; Mathews, M.C.; Mostert, D.; Renault, C.; Wilson, V.; Viljoen, A. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 (TR4) Causing Banana Wilt in the Island of Mayotte. Plant Dis. 2021, 105, 219. [Google Scholar] [CrossRef]
- Sardos, J.; Rouard, M.; Hueber, Y.; Cenci, A.; Hyma, K.E.; van den Houwe, I.; Hribova, E.; Courtois, B.; Roux, N. A genome-wide association study on the seedless phenotype in banana (Musa spp.) reveals the potential of a selected panel to detect candidate genes in a vegetatively propagated crop. PLoS ONE 2016, 11, e0154448. [Google Scholar] [CrossRef]
- Amah, D.; Turner, D.W.; Gibbs, J.D.; Waniale, A.; Gram, G.; Swennen, R. Overcoming the fertility crisis in bananas (Musa spp). In Achieving Sustainable Cultivation of Bananas Vol 2: Germplasm and Genetic Improvement; Kema, G., Drenth, A., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Fortescue, J.A.; Turner, D.W. Reproductive Biology. In Banana Breeding: Progress and Challenges; Pillay, M., Tenkouano, A., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2011; pp. 145–179. [Google Scholar] [CrossRef]
- Waniale, A.; Swennen, R.; Mukasa, S.B.; Tugume, A.K.; Kubiriba, J.; Tushemereirwe, W.J.; Batte, M.; Brown, A.; Tumuhimbise, R. Seed Set Patterns in East African Highland Cooking Bananas Are Dependent on Weather before, during and after Pollination. Horticulturae 2021, 7, 165. [Google Scholar] [CrossRef]
- Waniale, A.; Swennen, R.; Mukasa, B.; Tugume, A.K.; Kubiriba, J.; Tushemereirwe, W.K.; Amah, D.; Tumuhimbise, R. Application of pollen germination media on stigmas during pollination increases seed set in East African Highland Cooking Bananas (Musa spp.). Agronomy 2021, 11, 1085. [Google Scholar] [CrossRef]
- Waniale, A.; Mukasa, S.B.; Tugume, K.; Kubiriba, J.; Tushemereirwe, W.K.; Tumuhimbise, R. Early Withering of Enlarged Ovules in Pollinated Fruits of Bananas (Musa spp.) Suggest Abortion after Fertilization. Horticulturae 2022, 8, 426. [Google Scholar] [CrossRef]
- Swennen, R.; Vuylsteke, D. Aspects of plantain breeding at IITA. In Proceedings of the INIBAP Workshop on Sigatoka Leaf Spot Diseases (Mycosphaerella spp.), San Jose, Costa Rica, 28 March–1 April 1989; pp. 252–266. [Google Scholar]
- Ssebuliba, R.; Talengera, D.; Makumbi, D.; Namanya, P.; Tenkouano, A.; Tushemereirwe, W.; Pillay, M. Reproductive efficiency and breeding potential of East African highland (Musa AAA-EA) bananas. Field Crops Res. 2006, 95, 250–255. [Google Scholar] [CrossRef]
- Van Creij, M.G.M.; Kerckhoffs, D.M.F.J.; Van Tuyl, J.M. Application of three pollination techniques and of hormone treatments for overcoming interspecific crossing barriers in Tulip. Acta Hortic. 1997, 430, 547–557. [Google Scholar] [CrossRef]
- Sun, C.-Q.; Huang, Z.-Z.; Wang, Y.-L.; Chen, F.-D.; Teng, N.-J.; Fang, W.-M.; Liu, Z.-L. Overcoming pre-fertilization barriers in the wide cross between Chrysanthemum grandiflorum (Ramat.) Kitamura and C. nankingense (Nakai) Tzvel. by using special pollination techniques. Euphytica 2011, 178, 195–202. [Google Scholar] [CrossRef]
- Simmonds, N.W. The development of the banana fruit. J. Exp. Bot. 1953, 4, 87–105. [Google Scholar] [CrossRef]
- Simmonds, N.W. Experiments on banana fruit development. Ann. Bot. 1960, 24, 212–222. [Google Scholar] [CrossRef]
- Serrani, J.C.; Ruiz-Rivero, O.; Fos, M.; Garcia-Martinez, J.L. Auxin-induced fruit set in tomato is mediated in part by gibberellins. Plant J. 2008, 56, 922–934. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhao, X.Y.; Shao, X.X.; Wang, F.; Zhou, C.; Liu, Y.G.; Zhang, Y.; Zhang, X.S. Abscisic Acid Regulates Early Seed Development in Arabidopsis by ABI5-Mediated Transcription of SHORT HYPOCOTYL UNDER BLUE1. Plant Cell 2014, 26, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, A.; Pahuja, S.K.; Dhingra, H.R. Overcoming Interspecific Hybridization Barriers in Cyamopsis Species. Int. J. Biotechnol. Bioeng. Res. 2013, 4, 181–190. [Google Scholar]
- Van Tuyl, J.M.; De Jeu, M.J. Methods for overcoming interspecific crossing barriers. In Pollen Biotechnology for Crop Production and Improvement; Sawhney, V.K., Shivanna, K.R., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 273–293. [Google Scholar]
- Gray, D.J.; Mortensen, J.A.; Benton, C.M. Ovule culture to obtain progeny from hybrid seedless bunch grapes. J. Am. Soc. Hortic. Sci. 1990, 115, 1019–1024. [Google Scholar] [CrossRef]
- Ssebuliba, R.N.; Rubaihayo, P.; Tenkouano, A.; Makumbi, D.; Talengera, D.; Magambo, M. Genetic diversity among East African highland banana for female fertility. Afr. Crop Sci. J. 2005, 13, 13–26. [Google Scholar]
- Perrier, X.; Jenny, C.; Bakry, F.; Karamura, D.; Kitavi, M.; Dubois, C.; Hervouet, C.; Philippson, G.; De Langhe, E. East African diploid and triploid bananas: A genetic complex transported from South-East Asia. Ann. Bot. 2019, 123, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Batte, M.; Swennen, R.; Uwimana, B.; Akech, V.; Brown, A.; Tumuhimbise, R.; Hovmalm, H.P.; Geleta, M.; Ortiz, R. Crossbreeding East African Highland Bananas: Lessons Learnt Relevant to the Botany of the Crop After 21 Years of Genetic Enhancement. Front. Plant Sci. 2019, 10, 421463. [Google Scholar] [CrossRef]
- Vuylsteke, D.; Ortiz, R.; Ferris, R.S.B.; Crouch, J.H. Plantain improvement. Plant Breed. Rev. 1997, 14, 267–320. Available online: https://hdl.handle.net/10568/101043 (accessed on 20 February 2024).
- Mutsaers, M. Natural pollination of plantain and banana at Onne. Musa Africa 1993, 2, 2–3. [Google Scholar]
- Ray, P.K. Breeding Tropical and Subtropical Fruits; Norosa Publishing House: New Delhi, India, 2002. [Google Scholar]
- Waniale, A.; Swennen, R.; Mukasa, S.B.; Tugume, A.K.; Kubiriba, J.; Tushemereirwe, W.K.; Uwimana, B.; Gram, G.; Amah, D.; Tumuhimbise, R. Use of timelapse photography to determine flower opening time and pattern in banana (Musa spp.) for efficient hand pollination. Sci. Rep. 2021, 11, 19480. [Google Scholar] [CrossRef] [PubMed]
- Nyine, M.; Pillay, M. Banana nectar as a medium for testing pollen viability and germination in Musa. Afr. J. Biotechnol. 2007, 6, 1175–1180. [Google Scholar]
- Shepherd, K. Seed Fertility of the Gros Michel Banana in Jamaica. J. Hortic. Sci. 1954, 29, 1–11. [Google Scholar] [CrossRef]
- Simmonds, N.W. Experiments on the pollination of seeded diploid bananas. J. Genet. 1952, 51, 32–40. [Google Scholar] [CrossRef]
- Shepherd, K. Seed fertility of edible bananas. J. Hortic. Sci. 1960, 35, 6–20. [Google Scholar] [CrossRef]
- Montalt, R.; Prósper, L.; Vives, M.C.; Navarro, L.; Ollitrault, P.; Aleza, P. Breakdown of self-incompatibility in Citrus by temperature stress, bud pollination and polyploidization. Agriculture 2022, 12, 273. [Google Scholar] [CrossRef]
- Carafa, A.M.; Carratu, G. Stigma treatment with saline solutions: A new method to overcome self-incompatibility in Brassica oleracea L. J. Hortic. Sci. 1997, 72, 531–535. [Google Scholar] [CrossRef]

| Name of PGR | Rate (ppm) | Method of Application | Period |
|---|---|---|---|
| B-Nine, gibberellic acid (GA) inhibitor | 5000 (recommended rate) | Foliar, freshly pollinated fruits | Marchto September 2018 |
| Abscisic acid (ABA), plant hormone | 500 and 1000 | Foliar, freshly pollinated fruits | March to September 2018 |
| 6-benzylamino purine (6BAP), cytokinin | 500, 1000, and 2000 | Foliar, freshly pollinated fruits | August to December 2018 |
| Thiourea | 1000, 7612, 15,224, and 38,060 | Foliar, freshly pollinated fruits | May to June 2018 |
| Triiodo benzoic acid (TIBA), auxin inhibitor | 500 and 1000 | Foliar, freshly pollinated fruits | May to June 2018 |
| Salicylic acid (SA), plant hormone | 100 and 200 | Injection, foliar, freshly pollinated fruits | December 2017 to January 2018 |
| Salt Concentration (M) | Number of Bunches |
|---|---|
| 0.000 (Control) | 9 |
| 0.031 | 8 |
| 0.063 | 9 |
| 0.125 | 9 |
| 0.250 | 16 |
| 0.500 | 9 |
| 1.000 | 2 |
| Pollination Technique | Bunches Pollinated | Seed/Bunch | t-Probability | Pollination Period |
|---|---|---|---|---|
| Early pollination | 47 | 0.48 | 0.735 | May 2016–April 2017 |
| Control | 37 | 0.66 | ||
| Evening pollination | 34 | 0.27 | 0.884 | June 2016–April 2017 |
| Control | 32 | 0.62 |
| PGR Name | Rate (ppm) | PGR Treated Bunches | Control (+/− 15 Days) | t-prob. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Landrace Name | No. Poll. | No. W/Seed | Max. Seed | Av. Seed | No. Poll | No. W/Seed | Max. Seed | Av. Seed | |||
| B-Nine | 5000 | ‘Enzirabahima’ | 5 | 0 | 0.0 | 0.0 | 6 | 1 | 1.4 | 0.2 | |
| 5000 | ‘Mlelembo’ | 3 | 0 | 0.0 | 0.0 | 19 | 0 | 0.0 | 0.0 | ||
| 5000 | ‘Mshale’ | 1 | 1 | 51.9 | 51.9 | 7 | 5 | 30.6 | 12.0 | ||
| 5000 | ‘Nakitembe’ | 3 | 0 | 0.0 | 0.0 | 27 | 0 | 0.0 | 0.0 | ||
| 5000 | ‘Nshonowa’ | 7 | 4 | 12.5 | 3.6 | 15 | 13 | 89.7 | 18.4 | ||
| Average | 3.8 | 1.0 | 12.9 | 11.1 | 14.8 | 3.8 | 24.3 | 6.1 | 0.308 | ||
| ABA | 500 | ‘Enzirabahima’ | 5 | 1 | 12.5 | 2.5 | 17 | 4 | 6.5 | 2.2 | |
| 500 | ‘Mlelembo’ | 4 | 0 | 0.0 | 0.0 | 18 | 0 | 0.0 | 0.0 | ||
| 500 | ‘Mshale’ | 2 | 1 | 3.7 | 1.9 | 8 | 6 | 30.6 | 11.5 | ||
| 500 | ‘Nakitembe’ | 5 | 0 | 0.0 | 0.0 | 14 | 0 | 0.0 | 0.0 | ||
| 500 | ‘Nshonowa’ | 2 | 0 | 0.0 | 0.0 | 14 | 11 | 26.0 | 9.9 | ||
| Average | 3.6 | 0.4 | 3.24 | 0.88 | 14.2 | 4.2 | 12.62 | 4.72 | 0.907 | ||
| 6BAP | 1000 | ‘Mshale’ | 1 | 0 | 0.0 | 0.0 | 6 | 3 | 26.8 | 20.2 | |
| 2000 | ‘Mshale’ | 1 | 0 | 0.0 | 0.0 | 5 | 3 | 26.8 | 7.8 | ||
| Average | 1.0 | 0.0 | 0.0 | 0.0 | 5.5 | 3.0 | 26.8 | 14.0 | 0.867 | ||
| Thiourea | 1000 | ‘Mlelembo’ | 2 | 0 | 0.0 | 0.0 | 8 | 0 | 0.0 | 0.0 | |
| 7612 | ‘Mbwazirume’ | 3 | 0 | 0.0 | 0.0 | 1 | 0 | 0.0 | 0.0 | ||
| 15224 | ‘Nakitembe’ | 1 | 0 | 0.0 | 0.0 | 11 | 0 | 0.0 | 0.0 | ||
| 38060 | ‘Nakitembe’ | 1 | 0 | 0.0 | 0.0 | 11 | 0 | 0.0 | 0.0 | ||
| Average | 1.8 | 0.0 | 0.0 | 0.0 | 7.8 | 0.0 | 0.0 | 0.0 | - | ||
| TIBA | 500 | ‘Enzirabahima’ | 1 | 1 | 9.3 | 9.3 | 18 | 10 | 15.2 | 3.4 | |
| 500 | ‘Mbwazirume’ | 2 | 0 | 0.0 | 0.0 | 3 | 0 | 0.0 | 0.0 | ||
| 500 | ‘Mshale’ | 1 | 1 | 13.6 | 13.6 | 16 | 12 | 24.6 | 6.4 | ||
| 500 | ‘Nakitembe’ | 2 | 0 | 0.0 | 0.0 | 24 | 0 | 0.0 | 0.0 | ||
| 500 | ‘Nshonowa’ | 1 | 0 | 0.0 | 0.0 | 6 | 3 | 7.1 | 1.5 | ||
| 1000 | ‘Nakitembe’ | 1 | 0 | 0.0 | 0.0 | 17 | 0 | 0.0 | 0.0 | ||
| 1000 | ‘Mlelembo’ | 1 | 0 | 0.0 | 0.0 | 6 | 0 | 0.0 | 0.0 | ||
| Average | 1.3 | 0.3 | 3.3 | 3.3 | 12.9 | 3.6 | 6.7 | 1.6 | 0.123 | ||
| SA spray | 100 | ‘Enzirabahima’ | 2 | 1 | 0.9 | 0.5 | 6 | 3 | 7.6 | 1.8 | |
| SA injection | 200 | ‘Enzirabahima’ | 1 | 1 | 7.1 | 7.1 | 14 | 9 | 10.7 | 2.3 | |
| 200 | ‘Mlelembo’ | 1 | 0 | 0.0 | 0.0 | 6 | 0 | 0.0 | 0.0 | ||
| 100 | ‘Mshale’ | 1 | 1 | 75.0 | 75.0 | 2 | 2 | 38.2 | 36.0 | ||
| 100 | ‘Nshonowa’ | 1 | 1 | 5.7 | 5.7 | 8 | 4 | 29.2 | 5.5 | ||
| Average | 1.2 | 0.8 | 17.7 | 17.7 | 7.2 | 3.6 | 17.1 | 9.1 | 0.164 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waniale, A.; Mukasa, S.B.; Tugume, A.K.; Barekye, A.; Tumuhimbise, R. Promising and Failed Breeding Techniques for Overcoming Sterility and Increasing Seed Set in Bananas (Musa spp.). Horticulturae 2024, 10, 513. https://doi.org/10.3390/horticulturae10050513
Waniale A, Mukasa SB, Tugume AK, Barekye A, Tumuhimbise R. Promising and Failed Breeding Techniques for Overcoming Sterility and Increasing Seed Set in Bananas (Musa spp.). Horticulturae. 2024; 10(5):513. https://doi.org/10.3390/horticulturae10050513
Chicago/Turabian StyleWaniale, Allan, Settumba B. Mukasa, Arthur K. Tugume, Alex Barekye, and Robooni Tumuhimbise. 2024. "Promising and Failed Breeding Techniques for Overcoming Sterility and Increasing Seed Set in Bananas (Musa spp.)" Horticulturae 10, no. 5: 513. https://doi.org/10.3390/horticulturae10050513
APA StyleWaniale, A., Mukasa, S. B., Tugume, A. K., Barekye, A., & Tumuhimbise, R. (2024). Promising and Failed Breeding Techniques for Overcoming Sterility and Increasing Seed Set in Bananas (Musa spp.). Horticulturae, 10(5), 513. https://doi.org/10.3390/horticulturae10050513






