Water Use Efficiency in a Deficit-Irrigated Orange Orchard
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Climatic Data
2.2. Experimental Design
2.3. Tree Monitoring
2.4. Mineral Concentration Determination
2.5. Yield and Fruit Quality Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Applied Water for Irrigation Treatments
| Years | T1 | T2 | WS2 | T3 | WS3 | T4 | WS4 |
|---|---|---|---|---|---|---|---|
| 2013–2014 | 259 | 191 | 26.5 | 159 | 38.8 | 109 | 57.9 |
| 2014–2015 | 266 | 195 | 26.8 | 157 | 41.0 | 111 | 58.5 |
| 2015–2016 | 279 | 204 | 26.8 | 174 | 37.6 | 158 | 43.4 |
| 2016–2017 | 279 | 213 | 23.7 | 198 | 29.0 | 155 | 44.5 |
| 2017–2018 | 209 | 168 | 19.5 | 163 | 21.9 | 126 | 39.9 |
| Average | 258 | 194 | 24.6 | 170 | 33.6 | 131 | 48.8 |
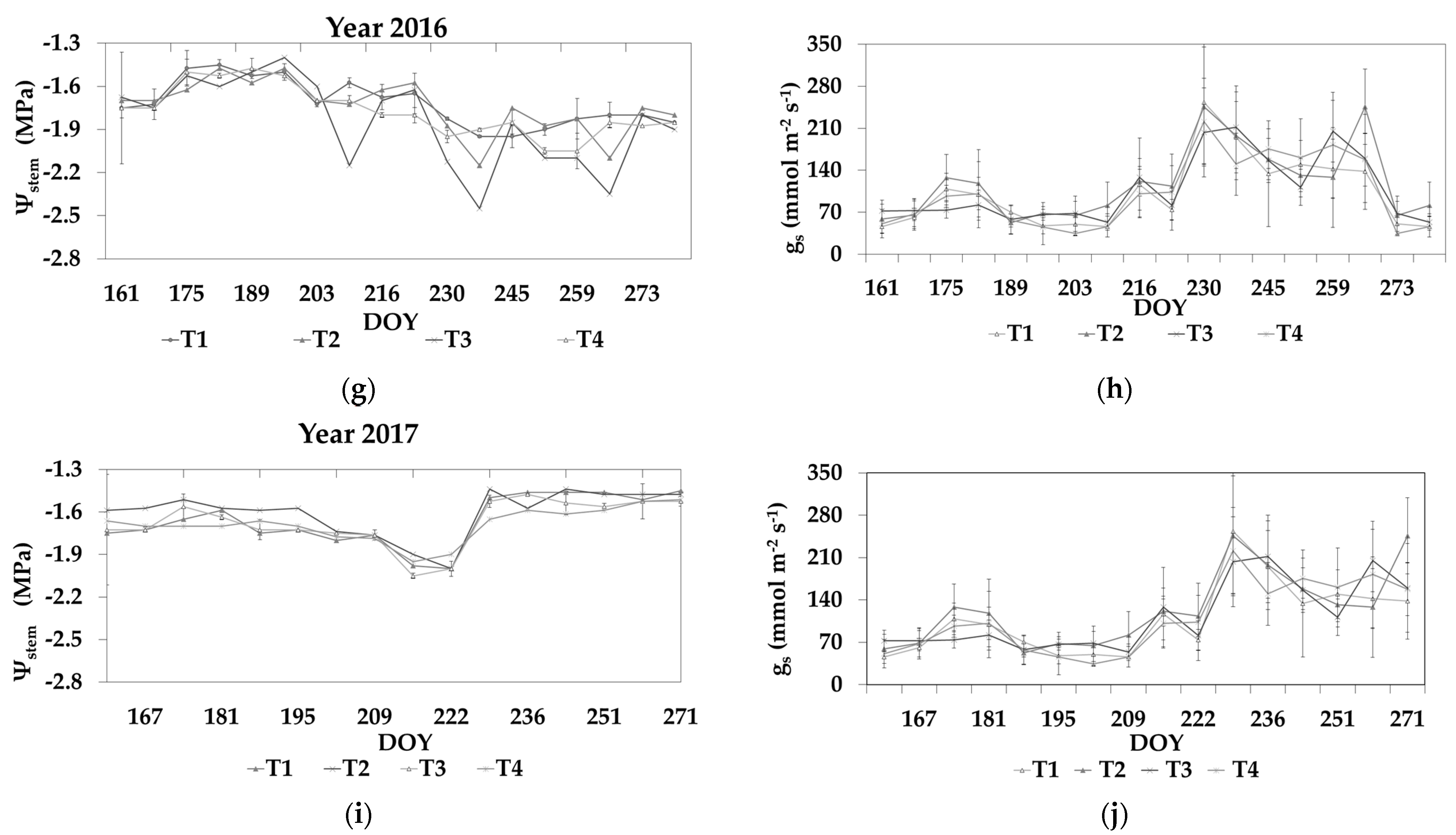
3.2. Stem Water Potential and Stomatal Conductance
3.3. Leaf Area Index
3.4. Mineral Concentration of Leaves
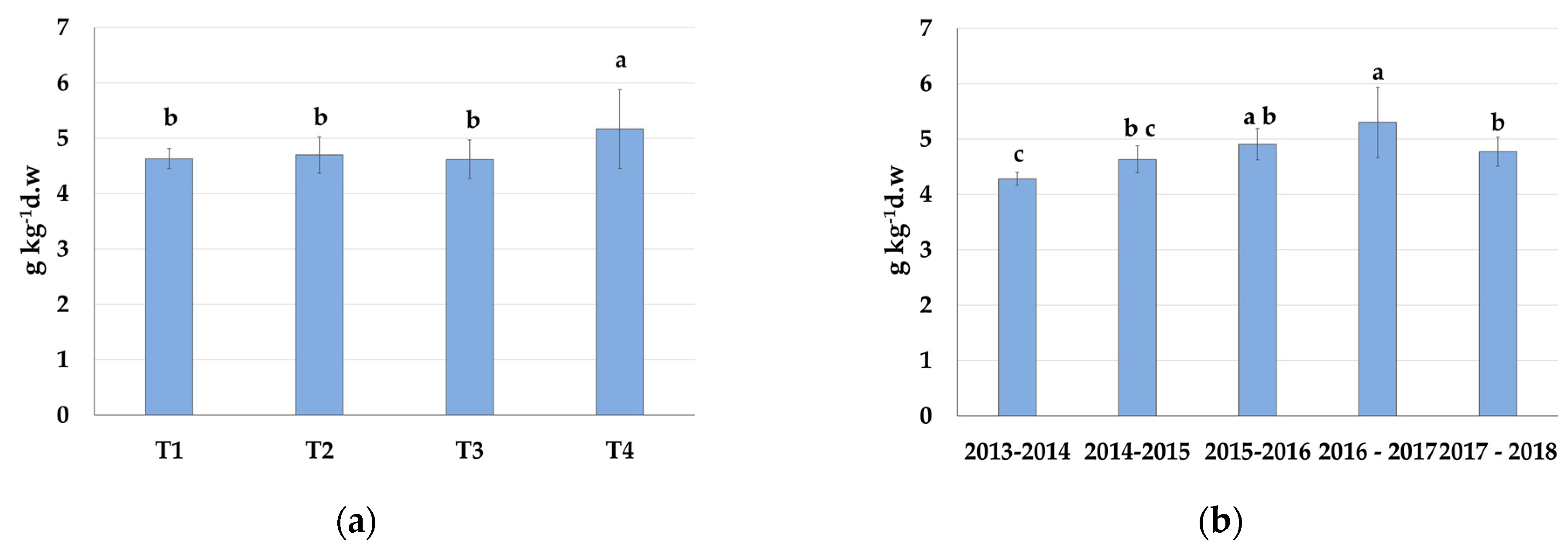
3.4.1. Nitrogen
3.4.2. Phosphorus
3.4.3. Potassium
3.4.4. Calcium and Magnesium
3.4.5. Iron, Zinc, Manganese, and Copper
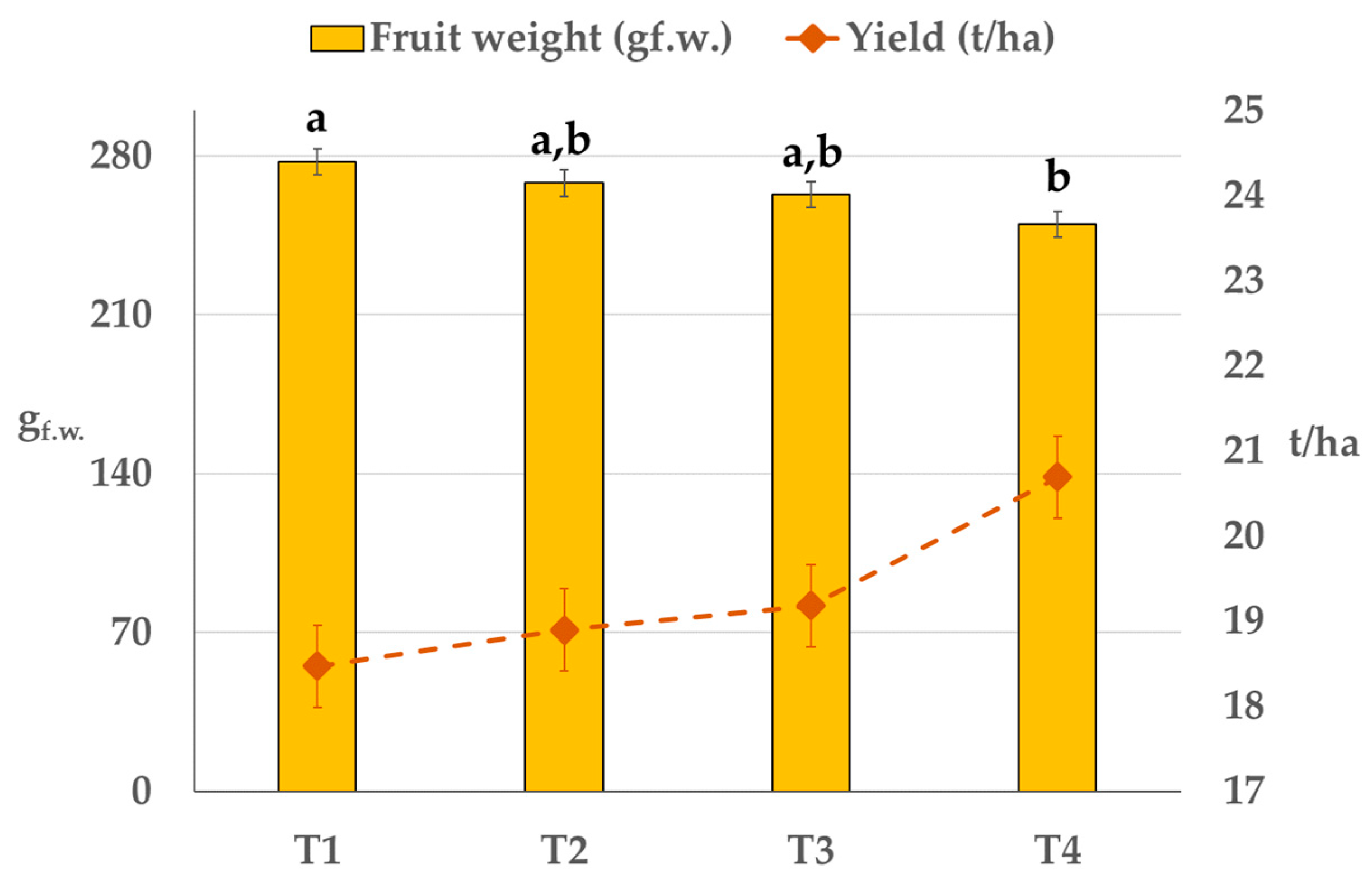
3.5. Yield and Fruit Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Citrus: World Markets and Trade; USDA: Washington, DC, USA, 2023. [Google Scholar]
- Carr, M.K.V. The Water Relations and Irrigation Requirements of Citrus (Citrus Spp.): A Review. Exp. Agric. 2012, 48, 347–377. [Google Scholar] [CrossRef]
- Kourgialas, N.N.; Karatzas, G.P. A Modeling Approach for Agricultural Water Management in Citrus Orchards: Cost-Effective Irrigation Scheduling and Agrochemical Transport Simulation. Environ. Monit. Assess. 2015, 187, 462. [Google Scholar] [CrossRef]
- Ginestar, C.; Castel, J.R. Responses of Young Clementine Citrus Trees to Water Stress during Different Phenological Periods. J. Hortic. Sci. 1996, 71, 551–559. [Google Scholar] [CrossRef]
- Giménez-Sanchis, A.; Zhong, K.; Pintor, A.; Farina, V.; Besada, C. Understanding Blood versus Blond Orange Consumption: A Cross-Cultural Study in Four Countries. Foods 2022, 11, 2686. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Rufty, T.W. Nitrogen and Water Resources Commonly Limit Crop Yield Increases, Not Necessarily Plant Genetics. Glob. Food Sec. 2012, 1, 94–98. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Durand, J.-L.; Gastal, F. Water Deficit and Nitrogen Nutrition of Crops. A Review. Agron. Sustain. Dev. 2010, 30, 529–544. [Google Scholar] [CrossRef]
- Sadras, V.O.; Hayman, P.T.; Rodriguez, D.; Monjardino, M.; Bielich, M.; Unkovich, M.; Mudge, B.; Wang, E. Interactions between Water and Nitrogen in Australian Cropping Systems: Physiological, Agronomic, Economic, Breeding and Modelling Perspectives. Crop Pasture Sci. 2016, 67, 1019–1053. [Google Scholar] [CrossRef]
- Kourgialas, N.N.; Koubouris, G.C.; Dokou, Z. Optimal Irrigation Planning for Addressing Current or Future Water Scarcity in Mediterranean Tree Crops. Sci. Total Environ. 2019, 654, 616–632. [Google Scholar] [CrossRef] [PubMed]
- English, M.; Raja, S.N. Perspectives on Deficit Irrigation. Agric. Water Manag. 1996, 32, 1–14. [Google Scholar] [CrossRef]
- Lez-Altozano, P.G.; Castel, J.R. Regulated Deficit Irrigation in ‘Clementina de Nules’ Citrus Trees. I. Yield and Fruit Quality Effects. J. Hortic. Sci. Biotechnol. 1999, 74, 706–713. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Bonet, L.; Nortes, P.A.; Puerto, H.; Nicolas, E.; Bartual, J. Pomegranate Trees Performance under Sustained and Regulated Deficit Irrigation. Irrig. Sci. 2013, 31, 959–970. [Google Scholar] [CrossRef]
- Boland, A.M.; Jerie, P.H.; Mitchell, P.D.; Goodwin, I.; Connor, D.J. Long-Term Effects of Restricted Root Volume and Regulated Deficit Irrigation on Peach: II. Productivity and Water Use. J. Am. Soc. Hortic. Sci. 2000, 125, 143–148. [Google Scholar] [CrossRef]
- Kang, S.; Liang, Z.; Hu, W.; Zhang, J. Water Use Efficiency of Controlled Alternate Irrigation on Root-Divided Maize Plants. Agric. Water Manag. 1998, 38, 69–76. [Google Scholar] [CrossRef]
- Orgaz, F.; Testi, L.; Villalobos, F.J.; Fereres, E. Water Requirements of Olive Orchards–II: Determination of Crop Coefficients for Irrigation Scheduling. Irrig. Sci. 2006, 24, 77–84. [Google Scholar] [CrossRef]
- Stagno, F.; Intrigliolo, F.; Consoli, S.; Continella, A.; Roccuzzo, G. Response of Orange Trees to Deficit Irrigation Strategies: Effects on Plant Nutrition, Yield, and Fruit Quality. J. Irrig. Drain. Eng. 2015, 141, 04015014. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit Irrigation for Reducing Agricultural Water Use. J. Exp. Bot. 2006, 58, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Kriedemann, P.E.; Goodwin, I. National Program for Sustainable Irrigation (Australia). In Regulated Deficit Irrigation and Partial Rootzone Drying: An Overview of Principles and Applications; Land & Water Australia: Canberra, Australia, 2003; ISBN 0642760896. [Google Scholar]
- Kang, S.; Zhang, J. Controlled Alternate Partial Root-Zone Irrigation: Its Physiological Consequences and Impact on Water Use Efficiency. J. Exp. Bot. 2004, 55, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Martin-Vertedor, A.I.; Dodd, I.C. Root-to-Shoot Signalling When Soil Moisture Is Heterogeneous: Increasing the Proportion of Root Biomass in Drying Soil Inhibits Leaf Growth and Increases Leaf Abscisic Acid Concentration. Plant Cell Environ. 2011, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Dry, P.R.; Loveys, B.R.; Düring, H. Partial Drying of the Rootzone of Grape. II. Changes in the Pattern of Root Development. Vitis 2000, 39, 9–12. [Google Scholar]
- Stoll, M.; Loveys, B.; Dry, P. Hormonal Changes Induced by Partial Rootzone Drying of Irrigated Grapevine. J. Exp. Bot. 2000, 51, 1627–1634. [Google Scholar] [CrossRef]
- Eliades, G.; Georgiu, A.; Papadopoulos, I. Effect of Conventional Irrigation and Alternate Rootzone Drying with Sufficient or Deficient Irrigation Water on the Production of Marsh Seedless Grapefruit. Agric. Mediterr. 2004, 134, 178–184. [Google Scholar]
- Treeby, M.T.; Henriod, R.E.; Bevington, K.B.; Milne, D.J.; Storey, R. Irrigation Management and Rootstock Effects on Navel Orange [Citrus sinensis (L.) Osbeck] Fruit Quality. Agric. Water Manag. 2007, 91, 24–32. [Google Scholar] [CrossRef]
- Consoli, S.; Stagno, F.; Vanella, D.; Boaga, J.; Cassiani, G.; Roccuzzo, G. Partial Root-Zone Drying Irrigation in Orange Orchards: Effects on Water Use and Crop Production Characteristics. Eur. J. Agron. 2017, 82, 190–202. [Google Scholar] [CrossRef]
- Behboudian, M.H.; Mills, T.M. Deficit Irrigation in Deciduous Orchards. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 2010; pp. 105–131. [Google Scholar]
- García-Tejero, I.; Romero-Vicente, R.; Jiménez-Bocanegra, J.A.; Martínez-García, G.; Durán-Zuazo, V.H.; Muriel-Fernández, J.L. Response of Citrus Trees to Deficit Irrigation during Different Phenological Periods in Relation to Yield, Fruit Quality, and Water Productivity. Agric. Water Manag. 2010, 97, 689–699. [Google Scholar] [CrossRef]
- Camp, C.R. Subsurface Drip Irrigation: A Review. Trans. ASAE 1998, 41, 1353–1367. [Google Scholar] [CrossRef]
- Phene, C.J. Subsurface Drip Irrigation Increased Yield, Quality and Water Use Efficiency of Alfalfa. Available online: https://sswm.info/sites/default/files/reference_attachments/PHENE%202004%20Subsurface%20Irrigation.pdf (accessed on 25 May 2023).
- Romero, P.; Botia, P.; Garcia, F. Effects of Regulated Deficit Irrigation under Subsurface Drip Irrigation Conditions on Vegetative Development and Yield of Mature Almond Trees. Plant Soil 2004, 260, 169–181. [Google Scholar] [CrossRef]
- Consoli, S.; Stagno, F.; Roccuzzo, G.; Cirelli, G.L.; Intrigliolo, F. Sustainable Management of Limited Water Resources in a Young Orange Orchard. Agric. Water Manag. 2014, 132, 60–68. [Google Scholar] [CrossRef]
- Bhattacharya, A. Mineral Nutrition of Plants Under Soil Water Deficit Condition: A Review. In Soil Water Deficit and Physiological Issues in Plants; Springer: Singapore, 2021; pp. 287–391. [Google Scholar]
- Chen, Y.; Zhang, J.-H.; Chen, M.-X.; Zhu, F.-Y.; Song, T. Optimizing Water Conservation and Utilization with a Regulated Deficit Irrigation Strategy in Woody Crops: A Review. Agric. Water Manag. 2023, 289, 108523. [Google Scholar] [CrossRef]
- Lynch, J. The Role of Nutrient-Efficient Crops in Modern Agriculture. J. Crop Prod. 1998, 1, 241–264. [Google Scholar] [CrossRef]
- Alva, A.K.; Mattos, D.; Quaggio, J.A. Advances in Nitrogen Fertigation of Citrus. J. Crop Improv. 2008, 22, 121–146. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. FAO Irrigation and Drainage Paper 66, Crop Yield Response to Water; FAO: Rome, Italy, 2012. [Google Scholar]
- Puig-Sirera, A.; Provenzano, G.; González-Altozano, P.; Intrigliolo, D.S.; Rallo, G. Irrigation Water Saving Strategies in Citrus Orchards: Analysis of the Combined Effects of Timing and Severity of Soil Water Deficit. Agric. Water Manag. 2021, 248, 106773. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Andersen, M.N.; Jensen, C.R. Improved Plant Nitrogen Nutrition Contributes to Higher Water Use Efficiency in Tomatoes under Alternate Partial Root-Zone Irrigation. Funct. Plant Biol. 2010, 37, 175–182. [Google Scholar] [CrossRef]
- Fereres, E.; Goldhamer, D.A.; Parsons, L.R. Irrigation Water Management of Horticultural Crops. HortScience 2003, 38, 1036–1042. [Google Scholar] [CrossRef]
- Naor, A. Irrigation Scheduling and Evaluation of Tree Water Status in Deciduous Orchards. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 2006; pp. 111–165. [Google Scholar]
- Stagno, F.; Giuffrida, A.; Intrigliolo, F.; Germanà, C.; Continella, A. Prediction of Water Status by Means of an Empirical Model in Orange Bearing Trees. Acta Hortic. 2011, 889, 339–345. [Google Scholar] [CrossRef]
- Ruiz-Canales, A.; Melián-Navarro, A.; Sacristán-Beltrí, E.; Puerto-Molina, H.; Molina-Martínez, J.M. Irrigation Scheduling Systems Based on Water Content Gauges for Citrus Trees-Some Data of Several Case Studies in the Southeast of Spain. Acta Hortic. 2011, 889, 537–541. [Google Scholar] [CrossRef]
- Ballester, C.; Castel, J.; Intrigliolo, D.S.; Castel, J.R. Response of Navel Lane Late Citrus Trees to Regulated Deficit Irrigation: Yield Components and Fruit Composition. Irrig. Sci. 2013, 31, 333–341. [Google Scholar] [CrossRef]
- Gonzalez-Altozano, P.; Castel, J.L. Effects of Regulated Deficit Irrigation on ‘Clementina de Nules’ Citrus Trees Growth, Yield and Fruit Quality. Acta Hortic. 2000, 537, 749–758. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Carroquino, J.; Dufo-López, R.; Bernal-Agustín, J.L. Sizing of Off-Grid Renewable Energy Systems for Drip Irrigation in Mediterranean Crops. Renew. Energy 2015, 76, 566–574. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Requirements; FAO: Rome, Italy, 1998. [Google Scholar]
- Palazzolo, E.; Laudicina, V.A.; Roccuzzo, G.; Allegra, M.; Torrisi, B.; Micalizzi, A.; Badalucco, L. Bioindicators and Nutrient Availability through Whole Soil Profile under Orange Groves after Long-Term Different Organic Fertilizations. SN Appl. Sci. 2019, 1, 468. [Google Scholar] [CrossRef]
- Soil Survey Staff. Ustepts Great Groups. In Illustrated Guide to Soil Taxonomy, Version 2; U.S. Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2015; pp. 435–442. [Google Scholar]
- Embleton, T.W.; Jones, W.W.; Labanauskas, C.K.; Reuther, W. Leaf Analysis as a Diagnostic Tool and Guide to Fertilization. Citrus Ind. 1973, 3, 183–210. [Google Scholar]
- Intrigliolo, F.; Roccuzzo, G. Evaluation of Different Fertilization Strategies on Orange. In Improved Crop Quality by Nutrient Management; Anac, D., Martin-Prével, P., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 23–26. [Google Scholar]
- Kimball, D.A. Citrus Processing; Springer: Boston, MA, USA, 1999; ISBN 978-0-8342-1258-9. [Google Scholar]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of Analytical Methods for Determining Anthocyanins in Blood Orange Juices. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project (Version 2.2) Jamovi 2022 [Computer Software]. Available online: https://www.jamovi.org (accessed on 24 May 2023).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.1; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- The Jamovi Project GAMLj: General Analyses for the Linear Model in Jamovi. Available online: https://gamlj.github.io/ (accessed on 24 May 2023).
- Castel, J.R.; Buj, A. Response of Salustiana Oranges to High Frequency Deficit Irrigation. Irrig. Sci. 1990, 11, 121–127. [Google Scholar] [CrossRef]
- Ballester, C.; Castel, J.; Intrigliolo, D.S.; Castel, J.R. Response of Clementina de Nules Citrus Trees to Summer Deficit Irrigation. Yield Components and Fruit Composition. Agric. Water Manag. 2011, 98, 1027–1032. [Google Scholar] [CrossRef]
- Adu, M.O.; Yawson, D.O.; Armah, F.A.; Asare, P.A.; Frimpong, K.A. Meta-Analysis of Crop Yields of Full, Deficit, and Partial Root-Zone Drying Irrigation. Agric. Water Manag. 2018, 197, 79–90. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Villalobos, F.J.; Testi, L.; Fereres, E. Effects of Water Deficits on Whole Tree Water Use Efficiency of Orange. Agric. Water Manag. 2014, 140, 61–68. [Google Scholar] [CrossRef]
- Coppin, P.; Jonckheere, I.; Nackaerts, K.; Muys, B.; Lambin, E. Digital Change Detection Methods in Ecosystem Monitoring: A Review. Int. J. Remote Sens. 2004, 25, 1565–1596. [Google Scholar] [CrossRef]
- Delalieux, S.; Somers, B.; Hereijgers, S.; Verstraeten, W.W.; Keulemans, W.; Coppin, P. A Near-Infrared Narrow-Waveband Ratio to Determine Leaf Area Index in Orchards. Remote Sens. Environ. 2008, 112, 3762–3772. [Google Scholar] [CrossRef]
- Benton Jones, J. Plant Nutrition Manual; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Munson, R.D. Principles of Plant Analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 1–25. [Google Scholar]
- Morgan, K.T.; Obreza, T.A. Irrigation Management to Improve Nutrient Uptake. In Nutrition of Florida Citrus Trees (SL253); Obreza, T.A., Morgan, K.T., Eds.; University of Florida, IIFAS Extension: Lake Alfred, FL, USA, 2011; pp. 60–61. [Google Scholar]
- Alva, A.K.; Paramasivam, S.; Graham, W.D.; Wheaton, T.A. Best Nitrogen and Irrigation Management Practices for Citrus Production in Sandy Soils. Water Air Soil Pollut. 2003, 143, 139–154. [Google Scholar] [CrossRef]
- Alva, A.K.; Paramasivam, S.; Obreza, T.A.; Schumann, A.W. Nitrogen Best Management Practice for Citrus Trees. Sci. Hortic. 2006, 107, 233–244. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and Nitrogen Relationships in Leaves of C3 Plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Baral, D.R. Performance of Mandarin Trees (Citrus Reticulata Blanco.) at Different Altitudes and Nutrient Management in Mid-Hills of Nepal. Ph.D. Thesis, Tribhuvan University, Katmandu, Nepal, 2008. [Google Scholar]
- Srivastava, A.K.; Singh, S. Potassium Nutrition in Citrus; National Research Centre for Citrus: Nagpur, India, 1999. [Google Scholar]
- Obreza, T.A.; Zekri, M.; Futch, S.H. General Soil Fertility and Citrus Tree Nutrition. In Nutrition of Florida Citrus Trees, 3rd ed.; Morgan, K.T., Kadyampakeni, D.M., Eds.; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2020; Volume 2020, pp. 13–19. [Google Scholar]
- Srivastava, A.K. Site Specific Potassium Management for Quality Production of Citrus. Karnataka J. Agric. Sci. 2011, 24, 60–66. [Google Scholar]
- Ernani, P.R.; Dias, J.; Flore, J.A. Annual Additions of Potassium to the Soil Increased Apple Yield in Brazil. Commun. Soil Sci. Plant Anal. 2002, 33, 1291–1304. [Google Scholar] [CrossRef]
- Raveh, E. Citrus Leaf Nutrient Status: A Critical Evaluation of Guidelines for Optimal Yield in Israel. J. Plant Nutr. Soil Sci. 2013, 176, 420–428. [Google Scholar] [CrossRef]
- Robles, J.M.; Botía, P.; Pérez-Pérez, J.G. Subsurface Drip Irrigation Affects Trunk Diameter Fluctuations in Lemon Trees, in Comparison with Surface Drip Irrigation. Agric. Water Manag. 2016, 165, 11–21. [Google Scholar] [CrossRef]
- Martínez-Gimeno, M.A.; Bonet, L.; Provenzano, G.; Badal, E.; Intrigliolo, D.S.; Ballester, C. Assessment of Yield and Water Productivity of Clementine Trees under Surface and Subsurface Drip Irrigation. Agric. Water Manag. 2018, 206, 209–216. [Google Scholar] [CrossRef]
- Cebadera-Miranda, L.; Domínguez, L.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Igual, M.; Martínez-Navarrete, N.; Fernández-Ruiz, V.; Morales, P.; Cámara, M. Sanguinello and Tarocco (Citrus sinensis [L.] Osbeck): Bioactive Compounds and Colour Appearance of Blood Oranges. Food Chem. 2019, 270, 395–402. [Google Scholar] [CrossRef]
- Francis, F.J. Quality as Influenced by Color. Food Qual. Prefer. 1995, 6, 149–155. [Google Scholar] [CrossRef]
- Heiman, A.; Karo, T.; Goldschmidt, E.E.; Neale, R.; Bustan, A. Fruit Quality Perception by Growers, Retailers and Consumers: The Case of Oranges. Acta Hortic. 2002, 584, 177–184. [Google Scholar] [CrossRef]
- Navarro, J.M.; Gomez-Gomez, A.G.; Perez-Perez, J.G.; Porras, I.; Botia, P. Effects of Differing Deficit Irrigation Treatments on Fruit Quality of Citrus Reticulata Blanco. In Proceedings of the International Society of Citriculture, Wuhan, China, 26–30 October 2008; pp. 701–706. [Google Scholar]



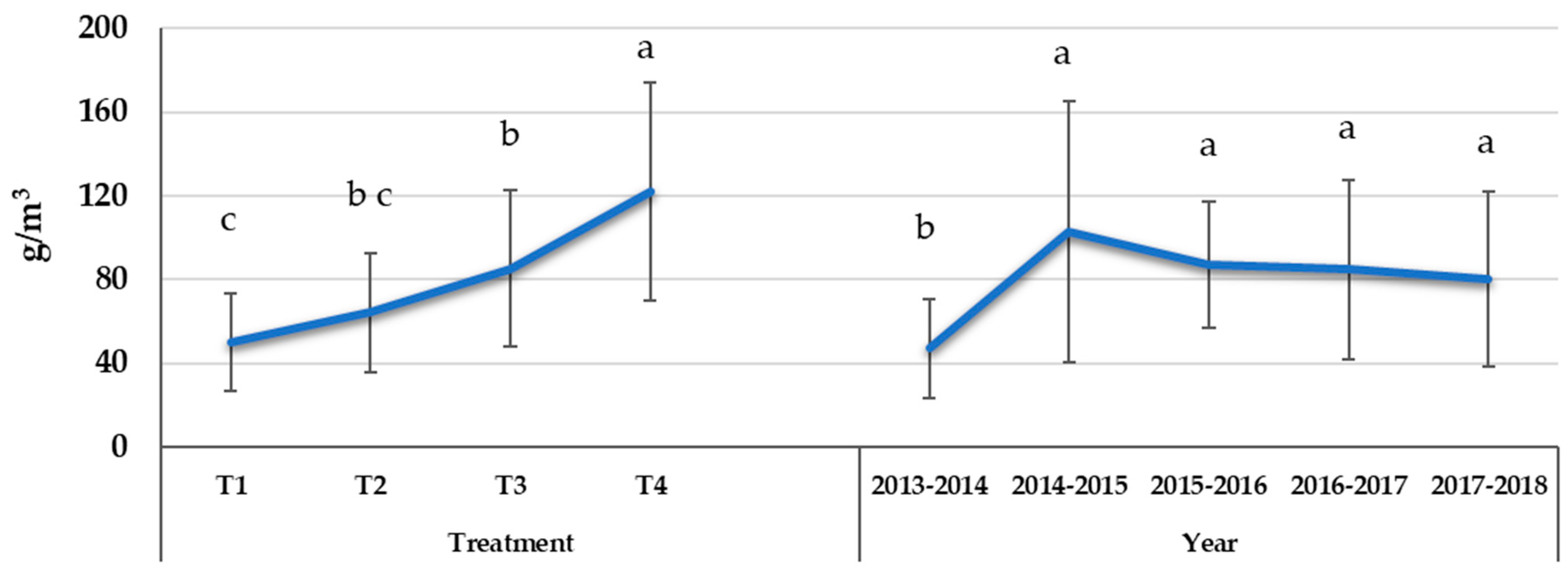
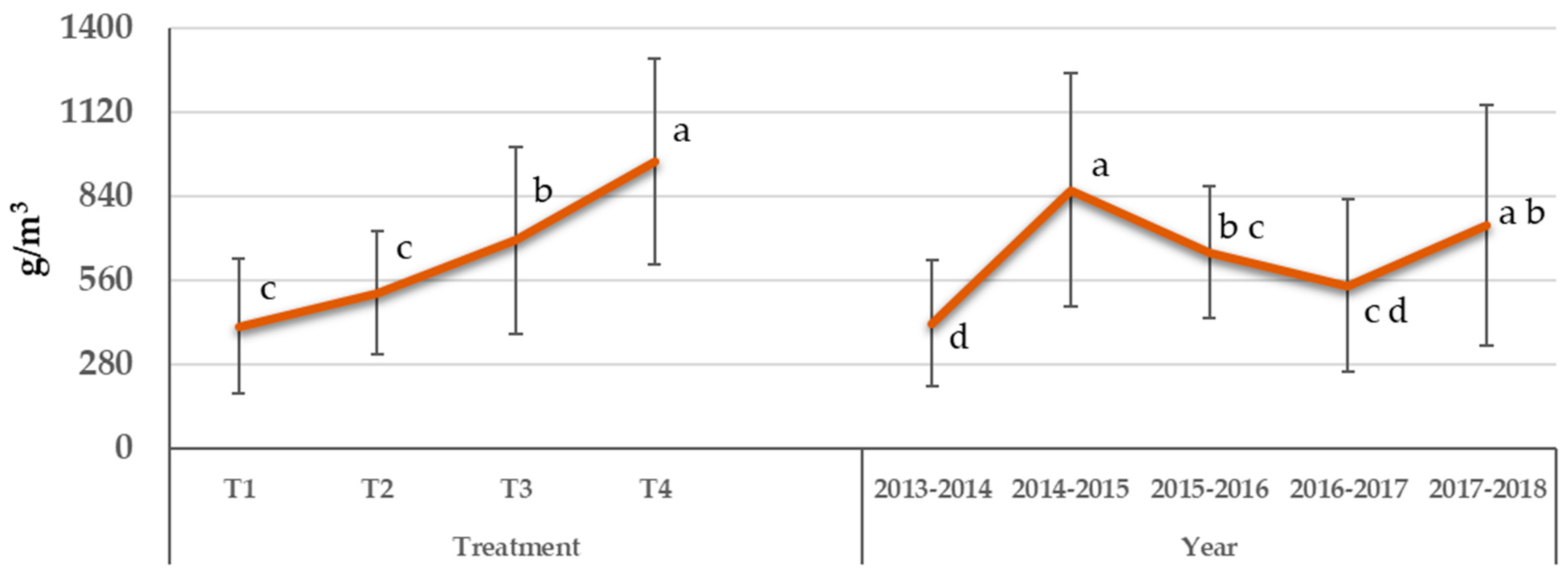
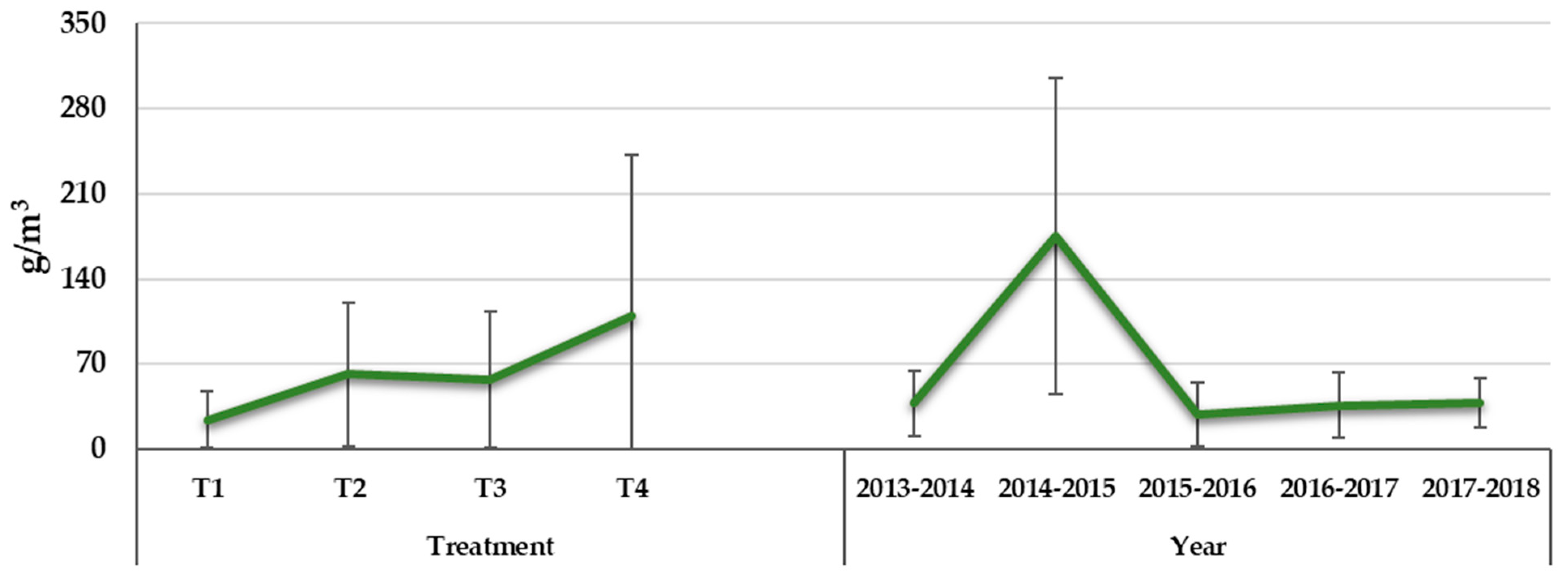
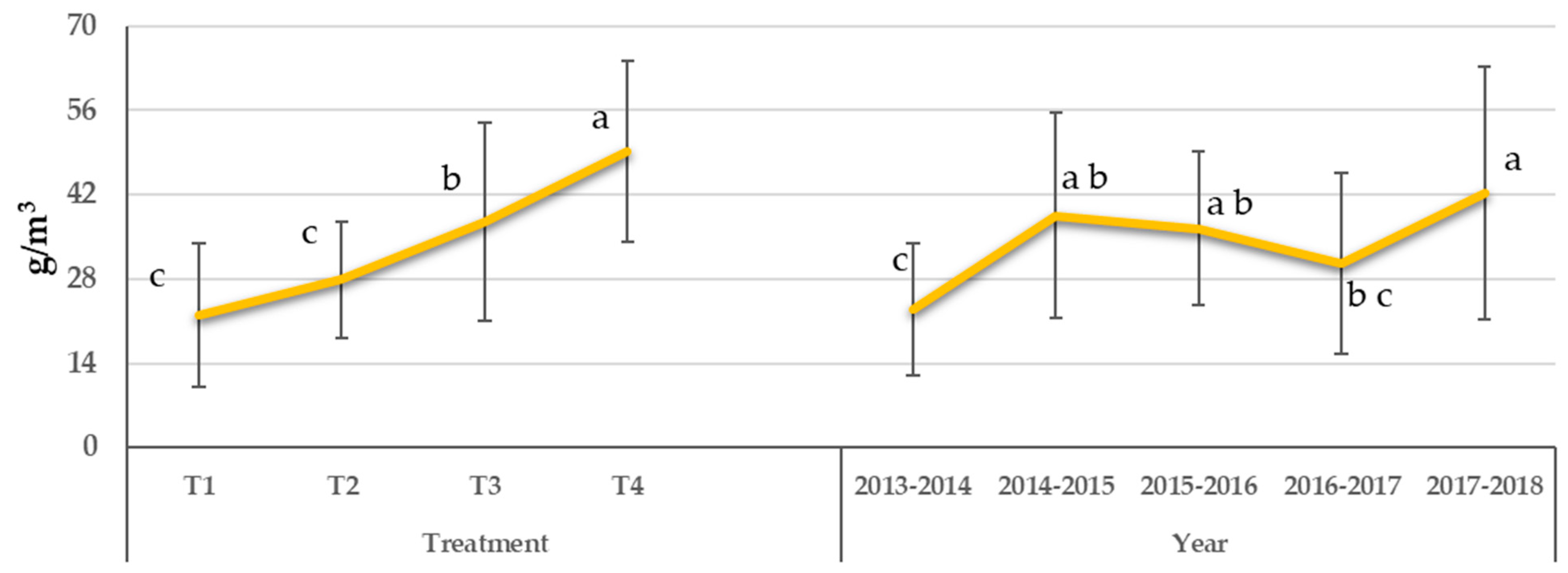
| Water Treatment | WUE (kgfresh weight m−3) | Grouping * |
|---|---|---|
| Control—T1 | 7.31 ± 3.02 | c |
| SSDI—T2 | 9.71 ± 2.00 | b |
| RDI—T3 | 11.32 ± 3.37 | b |
| PRD—T4 | 15.85 ± 3.95 | a |
| Years | Average LAI | Grouping * |
|---|---|---|
| 2013–2014 | 4.52 ± 0.65 | a |
| 2014–2015 | 4.16 ± 0.84 | a |
| 2015–2016 | 3.90 ± 0.94 | a |
| 2016–2017 | 4.40 ± 0.77 | a |
| 2017–2018 | 3.14 ± 0.80 | b |
| Treatment | Average N | Grouping * |
|---|---|---|
| Control—T1 | 26.99 ± 1.39 | ab |
| SSDI—T2 | 27.56 ± 3.37 | a |
| RDI—T3 | 25.88 ± 2.16 | b |
| PRD—T4 | 26.50 ± 1.29 | ab |
| Years | Average N | Grouping * |
| 2013–2014 | 28.20 ± 4.01 | a |
| 2014–2015 | 25.77 ± 0.87 | b |
| 2015–2016 | 26.98 ± 1.96 | ab |
| 2016–2017 | 26.34 ± 1.18 | ab |
| 2017–2018 | 26.38 ± 1.07 | ab |
| Treatment | Average P | Grouping * |
|---|---|---|
| Control—T1 | 1.55 ± 0.16 | n.s. |
| SSDI—T2 | 1.50 ± 0.08 | n.s. |
| RDI—T3 | 1.72 ± 0.12 | n.s. |
| PRD—T4 | 1.62 ± 0.11 | n.s. |
| Years | Average P | Grouping * |
| 2013–2014 | 1.61 ± 0.09 | n.s. |
| 2014–2015 | 1.59 ± 0.10 | n.s. |
| 2015–2016 | 1.62 ± 0.14 | n.s. |
| 2016–2017 | 1.56 ± 0.13 | n.s. |
| 2017–2018 | 1.63 ± 0.17 | n.s. |
| Water Treatment | Average Concentration | Grouping * |
|---|---|---|
| Control—T1 | 45.0 ± 11.0 | b |
| SSDI—T2 | 50.9 ± 14.1 | a |
| RDI—T3 | 51.0 ± 16.2 | a |
| PRD—T4 | 45.2 ± 11.2 | b |
| Water Treatment | Average Concentration | Grouping * |
|---|---|---|
| Control—T1 | 24.1 ± 4.56 | a |
| SSDI—T2 | 14.3 ± 4.58 | b |
| RDI—T3 | 20.9 ± 4.58 | ab |
| PRD—T4 | 26.0 ± 4.56 | a |
| Control T1 | SSDI T2 | RDI T3 | PRD T4 | |
|---|---|---|---|---|
| Total soluble solids—TSS (°Brix) | 10.8 ± 0.64 b | 10.9 ± 0.66 b | 11.6 ± 0.87 a | 11.4 ± 0.74 a |
| Titratable acidity—TA (%) | 1.36 ± 0.18 b | 1.36 ± 0.28 b | 1.44 ± 0.31 a,b | 1.46 ± 0.26 a |
| Maturity Index—MI (dmnl) | 8.07 ± 1.04 | 8.38 ± 1.38 | 8.23 ± 1.57 | 8.01 ± 1.28 |
| Juice yield (%) | 51.3 ± 5.27 a,b | 48.9 ± 8.89 b | 53.5 ± 5.93 a | 52.4 ± 4.56 a,b |
| Anthocyanin (mg L−1) | 6.49 ± 4.30 | 12.3 ± 8.90 | 9.25 ± 6.94 | 11.5 ± 10.2 |
| Vit C (mg 100 mL−1) | 58.5 ± 8.15 b | 60.5 ± 9.38 a,b | 62.7 ± 6.71 a | 60.0 ± 6.23 a,b |
| Pulp colour index—CI | 8.44 ± 2.33 b | 10.01 ± 2.33 a | 9.07 ± 1.83 a,b | 9.90 ± 3.06 a |
| Equatorial Diameter (mm) | 81.1 ± 4.20 a | 79.7 ± 4.78 a,b | 78.6 ± 4.06 b | 78.6 ± 3.64 b |
| Longitudinal Diameter (mm) | 88.5 ± 4.66 | 87.0 ± 6.98 | 86.1 ± 6.77 | 85.5 ± 5.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stagno, F.; Brambilla, M.; Roccuzzo, G.; Assirelli, A. Water Use Efficiency in a Deficit-Irrigated Orange Orchard. Horticulturae 2024, 10, 498. https://doi.org/10.3390/horticulturae10050498
Stagno F, Brambilla M, Roccuzzo G, Assirelli A. Water Use Efficiency in a Deficit-Irrigated Orange Orchard. Horticulturae. 2024; 10(5):498. https://doi.org/10.3390/horticulturae10050498
Chicago/Turabian StyleStagno, Fiorella, Massimo Brambilla, Giancarlo Roccuzzo, and Alberto Assirelli. 2024. "Water Use Efficiency in a Deficit-Irrigated Orange Orchard" Horticulturae 10, no. 5: 498. https://doi.org/10.3390/horticulturae10050498
APA StyleStagno, F., Brambilla, M., Roccuzzo, G., & Assirelli, A. (2024). Water Use Efficiency in a Deficit-Irrigated Orange Orchard. Horticulturae, 10(5), 498. https://doi.org/10.3390/horticulturae10050498








