Abstract
Rootstocks serve as a strategic tool for grapevine adaptation to specific biotic and abiotic conditions and for managing vine growth, grape yield, and berry composition in commercial vineyards. This study investigates the influences of four different rootstock varieties (101-14 MGt, 3309 C, 110 R, and 140 Ru) on the viticultural performance of ‘Xinomavro’ vines, a prominent Greek red winegrape varietal. By conducting a two-year field experiment using various rootstocks, we assessed parameters related to water status, vegetative growth, yield, and berry composition. Our results revealed that rootstock selection has a significant impact on vine development, especially in terms of berry size and the concentrations of secondary metabolites. Principal component analysis confirmed the complex interaction between rootstock vigor and vine productivity. This study underscores the importance of rootstock variety in manipulating grapevine characteristics, particularly for the ‘Xinomavro’ variety, in response to regional climatic conditions.
1. Introduction
Since the end of the 19th century, phylloxera-resistant rootstocks have played a critical role in European viticulture, with current estimates indicating that 80–85% of global vineyards employ them [1]. Initially adopted to provide resistance against grape phylloxera, these rootstocks have been further selected for traits such as nematode resistance, adaptability to soil pH and salinity, drought resilience, waterlogging tolerance, and nutrient uptake [2]. Additionally, they are vital for controlling vine vigor and yield, which, in turn, affect fruit composition and wine quality [3]. Consequently, rootstocks serve as a strategic tool for grapevine adaptation to specific biotic and abiotic conditions and for managing vine growth and grape yield in commercial vineyards, potentially minimizing the need for more traditional and labor-intensive viticultural practices [2].
Although primarily influenced by the rootstock, the vigor of the scion conferred by the rootstock is not a direct effect of gene expression related to vine growth [4] but rather arises from the differential biomass allocation within the vine, shaped by the complex interplay between the rootstock, scion, and external soil and climatic factors [3]. Further, vigor is influenced by the rootstock’s ability to absorb soil nutrients, predominantly nitrogen, which varies among rootstocks because of differences in gene expression [5] and hormone levels [6] and is contingent upon the nitrogen form and application timing [7]. The rate of nitrogen uptake is primarily rooted in rootstock attributes; however, scion properties also modulate it [8]. Variation in nutrient levels, such as potassium, calcium, magnesium, phosphorus, sodium, iron, and zinc, is also evident across different rootstocks [9,10,11]. Ref. [12] associated rootstock 140 Ruggeri with increased pruning weight and nitrogen in leaf petioles, alongside diminished wine quality in Chardonnay and Pinot noir vines. Conversely, [3] found no significant rootstock effects on vine physiology or productivity when comparing performance among Merlot, Syrah, and Chardonnay varieties, whether grafted onto different rootstocks (5 C, 140 Ru, 1103 P, 3309 C, and 101 CU) or ungrafted, suggesting that prevailing climatic conditions during the vine cycle have a more critical impact. Increased yield in vigorous rootstocks correlates to enhanced light capture by the larger leaf areas of the scion, leading to heightened photosynthesis rates and early-season mobilization of carbohydrates from the root system [13].
According to [10], rootstocks can influence the concentrations of important grape constituents, such as sugars, organic acids, amino acids, anthocyanins, and aromatic compounds. Vigorous rootstocks are often associated with lower-quality wine, particularly in irrigated vineyards [14], because of prolonged vine biological cycles and resultant maturation delays [15]. However, [16] has shown that Cabernet Sauvignon grapevines grafted on 1103 Paulsen had lower sugar and anthocyanin contents in the berries in comparison to grapevines of the same variety grafted on M4 because of differences in the expression of genes related to auxin production. The impact of the rootstock’s vigor on the berries’ composition, though, remains inconsistent across research findings: certain studies report no variation [17,18], while others note significant differences in phenolic compounds without corresponding changes in primary metabolites [19]. For example, increased secondary metabolites have been observed in Pinot noir grapevines grafted to 1103 Paulsen compared to 101-14 MG but without differences in sugar levels [20]. However, [21] determined that ‘Marselan’ grapevines grafted on the less-vigorous rootstock 101-14 MGt had higher sugar and anthocyanin levels and reduced titratable acidity. Rootstocks can also modify the must and wine pH, largely through their role in berries’ potassium accumulation, with effects modulated by seasonal weather variations [11] and notably under arid conditions [22]. Furthermore, the assimilable nitrogen content in the berry juice, which is crucial for alcoholic fermentation, may rise with certain rootstocks, as shown by the authors of [17], who observed higher levels of must assimilable nitrogen in Cabernet Sauvignon grapevines grafted to 110 R compared to the less-vigorous 420 A rootstock.
Considering the intricate interplay among the rootstock, scion variety, and environmental factors, selecting an appropriate rootstock is a multifaceted challenge. Essential rootstock attributes to consider include the compatibility with the scion, effectiveness of grafting techniques, rooting ability in nurseries, and the level of vigor imparted to the scion, which indirectly impact the vine productivity, grape quality, and maturation timing. Furthermore, in the face of climate change, investigating the comprehensive influence of the rootstock on scion traits and their adaptations has become a pivotal aspect of viticultural research [23,24].
Within the modern Greek vineyard landscape, ‘Xinomavro’ is Northern Greece’s distinguished native red winegrape, extensively cultivated in the PDO regions of Naoussa, Amyndeon, Rapsani, and Goumenissa. In addition, amidst climate change prospects, ‘Xinomavro’, a late-maturing variety, is gaining prominence for the Greek wine sector [25]. Despite ‘Xinomavro’ being cultivated across a wide range of environmental conditions, research on how rootstocks affect grapevine performance in terms of vine physiology, yield, and berry quality is notably scarce. Consequently, ‘Xinomavro’ growers are missing critical information for their rootstock selection, tailored to optimize grape production in their unique environmental contexts. Moreover, ‘Xinomavro’ vines are frequently grafted onto rootstocks that impart significant vigor to the scion, even though it is a late-ripening and productive variety. Beyond impacting grape quality, the use of vigorous rootstocks for ‘Xinomavro’ may also complicate compliance with the grape yield limitations officially specified by PDO regulations. Conversely, there is a lack of data regarding the use of less-vigorous rootstocks in ‘Xinomavro’ cultivation.
The objective of this study was to assess the influences of the rootstock variety on the physiological attributes, productivity, and berry composition of ‘Xinomavro’ vines over a two-year field trial. Our aim was to provide ‘Xinomavro’ cultivators with insightful data to guide informed decision-making in rootstock selection according to specific cultivation objectives. These objectives may coincide with enhancing grape quality and adhering to grape yield restrictions for PDO wine production, or they may aim for increased vine productivity in different scenarios. According to these considerations, we selected for this study four rootstocks that differ in the vigor they confer to the scions.
2. Materials and Methods
2.1. Vineyard Site and Experimental Design
This study was conducted for two consecutive years (2016 and 2017) in the experimental vineyard of the Aristotle University of Thessaloniki, located in Northern Greece (40°32′ N–22°59′ E). Own-rooted vines of four rootstocks (101-14 MGt, 3309 C, 110 Richter, and 140 Ru) were planted in 2009 in a layout at 2.2 m × 1.3 between- and within-row distances, respectively. In the spring of the next year, the rootstock vines were field grafted with scions of the ‘Xinomavro’ (Vitis vinifera L.) red winegrape cultivar. Subsequently, the grafted vines were vertically trained to a bilateral Royat cordon system and spur pruned at 6 spurs of 2-count nodes each per vine. The selection of rootstocks for this study was based on the conferred vigor to the scions and their drought tolerance: 110 Richter and 140 Ru are known for high vigor and drought resistance, whereas 101-14 MGt and 3309 C are less vigorous and more drought susceptible. The soil of the experimental vineyard had a sandy loam texture (60% sand, 30% loam, and 10% clay) and was managed in a clean soil surface system. Vines received drip irrigation with consistent water volumes over two years (90 mm in 2016 and 94 mm in 2017), apportioned as half pre-veraison and two subsequent doses post-veraison and pre-harvest. Climate data were recorded on-site by a iMETOS weather station and have been previously published in [26]. Overall, management practices followed the pattern commonly applied in the broad area to which ‘Xinomavro’ is cultivated.
Rootstock treatments consisted of four rootstock varieties: 101-14 MGt, 3309 C, 110 Richter, and 140 Ru arranged in a complete block design with three replications. Each rootstock was planted in a single vine row, which was separated from the rows of the other rootstocks with two buffer rows of 1103 P. In each rootstock treatment row, three plots of ten consecutive vines each were delineated, resulting in a total of 12 plots (4 rootstocks × 1 row/rootstock × 3 plots/row). In this layout, blocks consisted of successive 10-vine segments along the four rows of each rootstock, resulting in non-randomized treatment allocation within blocks, but the soil uniformity for the vineyard parcel was anticipated based on prior knowledge.
2.2. Vine Water Potential and Leaf Gas Exchange
Measurements of predawn (Ψdawn), stem (Ψstem), and leaf (Ψleaf) water potentials were taken from berry set to maturity on selected dates during two growing seasons: 2016 (days of the year—DOYs: 207, 216, 223, 234, 249, 260, and 271) and 2017 (DOYs: 198, 205, 221, 234, 240, 253, and 266). These measurements utilized a pressure chamber, as outlined in [27]. On each measurement date, three mature leaves from the central vines of each plot were sampled. Ψdawn was recorded before sunrise (5:00–6:30 a.m. local time), and Ψstem was taken at solar noon (12:30–14:30 p.m. local time) from leaves positioned between the 7th and 9th nodes of the primary shoots. To facilitate equilibrium between the leaf and stem water statuses for Ψstem measurements, leaves were bagged for 1 h in light-excluding, black plastic bags with an aluminum foil cover for thermal insulation [27]. Similarly, Ψleaf was measured on three sunlit mature leaves located in the same nodal range. The average values of three leaves for each type of water potential were used for statistical analysis.
Concurrently, the net assimilation rate (A, μmol CO2 m−2 S−1), stomatal conductance (gs, mol H2O m−2 S−1), transpiration (E, mmol H2O m−2 S−1), and leaf intrinsic water-use efficiency (WUEi, calculated as A/gs, μmol CO2 mol−1 H2O) were measured using an LCi portable gas exchange system, ADC BioScientific Ltd., Hoddesdon, UK. Data were collected from three fully expanded, recently matured, sunlit leaves in each plot that received a photosynthetic photon flux density exceeding 1200 μmol m−2 s−1, in proximity to the leaves chosen for water potential measurements. Theoretical vine hydraulic conductance (Kplant) values were derived as Kplant = E/ΔΨdawn–leaf, according to [28]. For each gas exchange variable, only the mean values from the sampled leaves were statistically analyzed.
2.3. Leaf Area and Annual Shoot Growth Production
The vine yield and its components were measured at the point of maturity, which aligned with the following commercial harvest dates: 27 September 2016 and 23 September 2017. The harvest process entailed a thorough collection, enumeration, and mass measurement of all the clusters from the quartet of central vines per experimental parcel to calculate the vine yield (kilograms per vine) and average cluster weight (grams). From the aggregate cluster yield of each central vine, a representative sample of 10 clusters was randomly selected and carefully transported to the lab in insulated coolers. These selected clusters were analyzed for weight and linear dimensions—length and width, measured in centimeters (cm). Additionally, the total berry count per cluster was performed, enabling the assessment of the cluster compactness, expressed as the ratio of the berry count to the peduncle length (in cm).
At full ripening, the total leaf area of four vines from each treatment plot was estimated using the non-destructive method outlined in [29]. At dormancy, the total weight of the pruning wood (kg) and the count of canes were recorded for the same four vines previously evaluated for the leaf area. The mean weight per cane was then determined by dividing the total wood weight by the number of canes for each vine.
2.4. Berry Sampling and Must Analysis
To analyze the chemical composition of the berries, six samplings took place from the beginning of veraison to harvest during two consecutive seasons: in 2016 (on days of the year (DOYs) 216, 223, 234, 249, 260, and 271) and in 2017 (on DOYs 218, 226, 235, 244, 253, and 262). At each sampling, 200 berries were randomly selected from the grape clusters on the central four vines of each plot. These berries were promptly placed in portable coolers for transport to the laboratory. Once at the lab, the 200-berry samples were weighed to obtain the average berry weight. Each 200-berry sample was then divided into four 50-berry subsamples for detailed chemical examination. The first subsample was manually pressed to collect the juice, which was tested for the total soluble solids (°Brix) using a digital refractometer (HI96841, HANNA Instruments, Woonsocket, RI, USA), pH with a laboratory pH meter (HI2020-02, HANNA Instruments, Woonsocket, RI, USA), and titratable acidity in grams per liter of tartaric acid equivalent through titration against 0.1 N sodium hydroxide. The remaining 150 berries (3 groups of 50) were preserved at −30 °C for later assessment of phenolic compounds (refer to the following section).
2.5. Phenolic Content and Anthocyanins
The berries’ phenolic content was determined in whole berries, using the analytical protocol in [30]. Briefly, 50 berries from each plot were transferred to a 125 mL plastic beaker and homogenized with a Polytron at 25,000 rpm for 30 s. Then, 1 g of the homogenate (in triplicate) was transferred to 10–15 mL centrifuge tubes, and 10 mL of 50% v/v aqueous ethanol (pH 2) was added to each tube and mixed for 1 h. After centrifugation at 3500 rpm for 10 min, the supernatant was used to measure the absorbance as follows: 0.5 mL of the supernatant was added to 10 mL of 1 M HC1 and mixed thoroughly. After 3 h, absorbances at 520 nm and 280 nm were recorded in a 10 mm cell. Anthocyanins (expressed as milligrams of anthocyanins per berry) were calculated from the absorbance measurement at 520 nm. The total phenolics (expressed as absorbance units per berry) were calculated from the measurement of the absorbance at 280 nm.
2.6. Statistical Analysis
Data were averaged for each plot, and these mean values were utilized for statistical analysis. Results are presented as the means of three replicates (n = 3). An analysis of variance was conducted with the rootstock variety as the main factor, using Duncan’s (multiple range) test for identifying significant differences among the main effect means at p < 0.05. Further, principal component analysis was applied to eleven variables measured at the 2016 and 2017 harvests, and the resulting biplot was generated. All the statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (Armonk, NY, USA, IBM Corp.).
3. Results
3.1. Predawn, Stem, and Leaf Water Potentials
The rootstock significantly affected the Ψdawn, Ψstem, and Ψleaf values of the ‘Xinomavro’ grapevines in both years of the experiment. In 110 R and 140 Ru, Ψdawn mean values generally varied at higher levels—almost 20%—in comparison to −0.52 MPa and −0.51 MPa for 101-14 MGt and 3309 C rootstocks, respectively (Table 1). In addition, significant temporal variation in Ψdawn differences was observed during maturation (Figure 1A), and both 101-14 MGt and 3309 C vines manifested lower Ψdawn values in the first part of the maturation (roughly between DOYs 215 and 240; Figure 1A). At maturity, however, 101-14 MGt had lower Ψdawn values (−0.48 MPa for both years), whereas 3309 C had values similar to those of 110 R (−0.40 MPa and −0.42 MPa for 2016 and 2017, respectively; Figure 1A).

Table 1.
The effects of the rootstock on water potentials Ψdawn, Ψleaf, and Ψstem and their differences (ΔΨdawn–stem and ΔΨstem–leaf) and vine hydraulic conductance (Kplant) as means during the ripening period. Within each column and parameter, means followed by a different letter are significantly different at p < 0.05 based on Duncan’s test; ns: absence of interaction between rootstock and year (R × year).
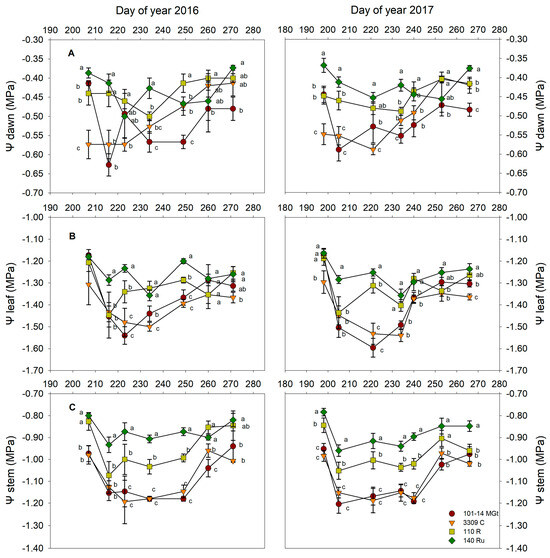
Figure 1.
The effects of the rootstock on water potentials during ripening: (A) Ψdawn, (B) Ψleaf, and (C) Ψstem. Significant differences (p < 0.05) among rootstocks per sampling day are indicated by different letters.
As with Ψdawn, 110 R and 140 Ru vines had higher mean midday leaf and stem water potentials compared to 101-14 MGt and 3309 C (Table 1). Compared to Ψleaf values, Ψstem values differentiated more clearly among the four rootstocks (Figure 1C). In both years, 110 R and 140 Ru had Ψstem values greater than −1.00 MPa, while the Ψstem values of 101-14 MGt and 3309 C approached −1.20 MPa (Figure 1C). Additionally, 140 Ru consistently exhibited higher values at maturity compared to 110 R, which, in 2017, reached a Ψstem value similar to that of 101-14 MGt at maturity (Figure 1C). At maturity, 3309 C vines tended to have lower Ψleaf and Ψstem values (Figure 1B,C). The year had no significant effects on any of the three water potential measures (Table 1).
3.2. Gas Exchange
The single-leaf gas exchange was significantly impacted by the rootstock variety (Table 2). ‘Xinomavro’ vines grafted onto 140 Ru maintained higher rates of stomatal conductance (gs), transpiration (E), and net assimilation (A) in both years (Table 2). Following 140 Ru, vines grafted onto 110 R had the second-highest gs, E, and A rates, while those grafted onto 3309 C and 101-14 MGt had the lowest mean values (Table 2). The significantly higher mean rates of gs, E, and A in vines grafted onto 140 R were expressed consistently at maturity (Figure 2A–C). In terms of single-leaf water-use efficiency mean values, significant differences were observed primarily between the less-vigorous 101-14 MGt and the more-vigorous 140 Ru (Table 2). No significant effects of the year on the variables of the gas exchange were found (Table 2).

Table 2.
The effects of the rootstock on stomatal conductance (gs), leaf transpiration (E), photosynthesis (A), and intrinsic water-use efficiency (A/gs) as means during the ripening period. Within each column and parameter, means followed by a different letter are significantly different at p < 0.05 based on Duncan’s test; ns: absence of interaction between rootstock and year (R × year).
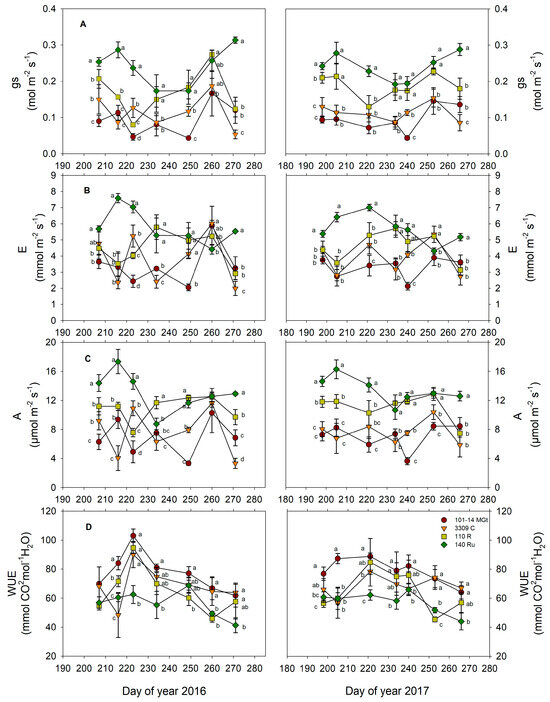
Figure 2.
The effects of the rootstock on gas exchange parameters during ripening: (A) stomatal conductance (gs), (B) leaf transpiration, (C) photosynthesis, and (D) intrinsic water-use efficiency (A/gs). Significant differences (p < 0.05) among rootstocks per sampling day are indicated by different letters.
3.3. Vine Vegetative Growth and Grape Yield Components
Significant differences among the four rootstocks were observed in both the total leaf area and pruning weight (Figure 3A,B). However, the effect of the rootstock was more pronounced and consistent on the total leaf area than on the pruning weight: 140 Ru and 110 R produced larger canopies compared to 101-14 MGt and 3309 C in both years (Figure 3A), but 140 Ru and 110 R had higher pruning weights only in 2016; whereas in 2017, 140 Ru, 110 R, and 3309 C exhibited similar dormant cane productions (Figure 3B). Consequently, only the 101-14 MGt vines consistently had a lower total leaf area and biomass of dormant canes (Figure 3A,B).
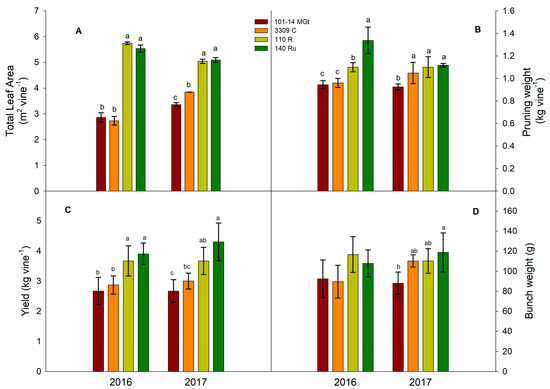
Figure 3.
The effects of the rootstock on the (A) total leaf area, (B) pruning weight, (C) yield, and (D) bunch weight. Significant differences (p < 0.05) among rootstocks are indicated by different letters.
The vines’ grape yield responses (Figure 3C) mirrored those of the total leaf area (Figure 3A), with 140 Ru and 110 R rootstocks being more productive compared to 101-14 MGt and 3309 C (Figure 3C). Regarding the individual components of the vine yield, the mean cluster weight did not consistently respond to the rootstock variety; only 140 Ru produced significantly heavier clusters than 101-14 MGt (Figure 3D). Moreover, 101-14 MGt consistently produced looser clusters in both years, attributable to fewer berries per cluster despite the reduced cluster length (Table S1). In terms of the berry size, both 101-14 MGt and 3309 C vines yielded significantly smaller berries compared to those on 140 Ru and 110 R vines, as indicated by their decreased mean berry weights (Figure 4). The berry weight gradually increased for all the rootstock treatments during maturation in 2016, whereas in 2017, the 140 Ru, 101-14 MGt, and 3309 C treatments exhibited a decline in berry weight at some point of maturation, starting earlier—almost 10 days after veraison—in 101-14 MGt (Figure 4). The rootstock variety had no significant effect on the mean number of clusters per vine.
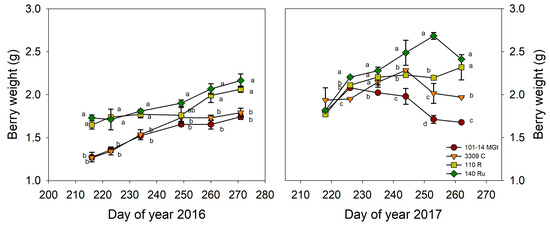
Figure 4.
The effect of the rootstock on the berry weight. Significant differences (p < 0.05) among rootstocks are indicated by different letters.
3.4. Berry Composition
Most berry constituents varied in response to both rootstock variety and year effects (Table 3). However, the juice pH and phenolic content (au berry−1) remained unaffected by the rootstock, while the anthocyanin content (mg berry−1) did not change across the two years (Table 3). Regarding berry anthocyanins and phenolics (Figure 5 and Figure S2), their responses to the rootstock and year differed depending on the expression of the results—on a ‘content’ basis (quantity per berry) or as a ‘concentration’ (quantity per berry mass). On a ‘content’ basis, 101-14 MGt and 3309 C consistently had higher anthocyanin contents compared to 140 Ru and 110 R, but no significant differences were observed among the four rootstocks in terms of the total phenolic content (Table 3, Figure 5A and Figure S2A). On a ‘concentration’ basis, the total phenolic concentrations were consistently higher for 101-14 MGt and 3309 C, with an accompanying higher anthocyanin concentration in the first year (Table 3, Figure 5B and Figure S2B). In the second year, 3309 C had the highest anthocyanin concentration, and 140 Ru had the lowest.

Table 3.
The effects of the rootstock on berry chemical attributes at harvest. Within each column and parameter, means followed by a different letter are significantly different at p < 0.05 based on Duncan’s test. ** and ***: interaction between the rootstock and year (R × year) at p < 0.01 and p < 0.001; ns: absence of interaction between the rootstock and year (R × year).
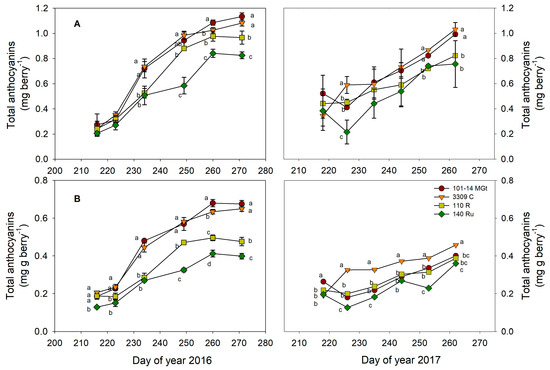
Figure 5.
The effects of the rootstock on the anthocyanin content (A) per berry and (B) per berry weight. Significant differences (p < 0.05) among rootstocks are indicated by different letters.
Significant rootstock × year interactions were noted for all the measured berry constituents except for the total phenolic content (Table 3). Owing to these interactions, the total soluble solid values were consistently higher and titratable acidity values were consistently lower for 101-14 MGt vines (Table 3). The overall means for TSS and TA at maturity were markedly lower in 2017 compared to 2016 (Table 3). In addition, pH was lower in 2017 despite the lower TA—about 35% compared to 2016—in the same year (Table 3).
3.5. Multivariate Analysis
To take an overall view of the responses of ‘Xinomavro’ vines to the rootstock treatments, we conducted an analysis of the principal components on eleven vine parameters related to the water status, vegetative growth, grape yield, and berry composition (Figure 6). The first two principal components with eigenvalues greater than one (Kaizer’s criterion) accounted for 74.83% of the total variance. Nine of the eleven variables were significantly correlated (>0.50 in absolute value) with PC1, while the pruning weight and WUEi correlated significantly with PC2. Within each year, the rootstock types were effectively clustered in pairs: the first consisting of less-vigorous 101-14 MGt and 3309 C and the second consisting of vigorous 110 R and 140 Ru (Figure 6). Across the years, these pairs were scattered in the biplot, reflecting the significant effect of the year on some of the variables, as discussed before. PC1 expresses the negative relationship of the biomass productivity variables (grape yield, leaf area, and berry weight) and higher water availability (Ψstem) with the total phenolics, total anthocyanins, TSS, and pH (Figure 6). In summary, 101-14 MGt and 3309 C were characterized by higher TSS and total anthocyanins and lower biomass productivity in 2016 than in 2017. In the opposite direction, 110 R and 140 Ru promoted biomass production but denoted TSS and total anthocyanins mainly in 2017. We can add that in the more productivity-conducive year of 2017, the differences between the rootstock treatments were less evident.
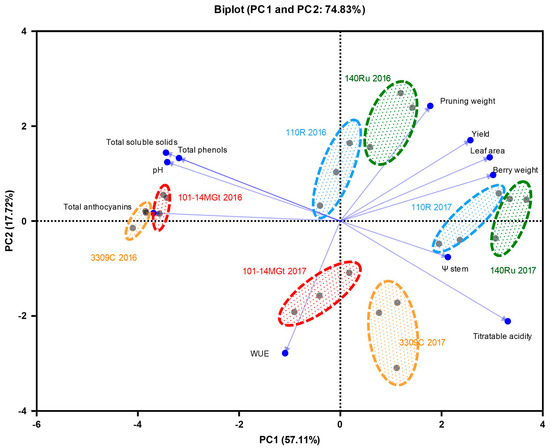
Figure 6.
Biplot of the principal component analysis (PCA) of cv. Xinomavro’s water status, vigor, yield, and berry composition parameters from different rootstocks. Ψstem, midday stem water potential; WUE, water-use efficiency.
4. Discussion
4.1. Vine Water Status and Single-Leaf Gas Exchange
The higher mean predawn water potentials (Ψdawn) of 110 R and 140 Ru vines (Table 1), and their variations within the season (Figure 1A), revealed that ‘Xinomavro’ vines exhibited more favorable water statuses around veraison (28/07 for 2016 and 31/07 for 2017) toward maturity. Despite minor fluctuations during maturation, these patterns were consistent. Conversely, the lower Ψdawn means for 101-14 MGt and 3309 C (Table 1) vines indicate water deficit conditions [31]. Consistently, ref. [32] reported that Cabernet Sauvignon vines grafted onto 101-14 MGt had significantly lower Ψdawn values than those grafted onto 110 R and 140 Ru. These findings suggest a more effective soil exploitation by 110 R and 140 Ru vines, corroborating previous studies that highlighted an expanded root system for these rootstocks and their classification as highly drought-resistant [3,33]. Although this study did not directly investigate root distribution in the soil for the four rootstocks, the Ψdawn variations may reflect differences in soil colonization by the vine roots because Ψdawn serves as an indirect indicator of the soil colonization extent by the roots of rootstocks [27]. Drought-resistant rootstocks, such as 1103 P, demonstrated a surge in new root growth under water-restricted conditions [34]. Upon soil moisture restoration, 110 R vines displayed rapid resumption of new root growth compared to 101-14 MGt [35]. The authors of [36] observed that the fine roots of 101-14 MGt vines under water deficit conditions exhibited expedited suberin deposition to endodermal cell walls, potentially inhibiting new root growth for 101-14 MGt during drought conditions relative to 110 R or 1103 P.
Apart from Ψdawn, the differences in the soil water exploitation by the four rootstocks were also reflected in the Ψleaf and Ψstem values (Table 1). Although Ψleaf does not represent the vine water status consistently and immediately [27], its seasonal trend indicates that 110 R and 140 Ru maintained higher values until maturity (Figure 1B). In the case of Ψstem, according to [37], vines on 110 R and 140 Ru rootstocks experienced from low to moderate water deficits, whereas those on 101-14 MGt and 3309 C experienced a strong water deficit. These results mostly agree with the findings of [38,39] for the ‘Monastrell’ variety under similar conditions in SE Spain.
In grapevines experiencing from mild to moderate water deficits, stomatal conductance (gs) is a sensitive indicator of the intensity of the water stress [40]. According to [41], gs rates between 0.15 and 0.05 mol H2O m−2s−1 are indicative of a moderate level of water deficit. Such responses were observed in the mean gs values for the less-vigorous 101-14 MGt and 3309 C rootstocks (Table 2). However, on some measurement days during maturation, gs in 101-14 MGt vines approached the critical limit of 0.05 mol molm−2s−1, below which severe water stress conditions occur [41]. Although a reduction in gs under water deficit conditions is typically accompanied by an increase in the intrinsic water-use efficiency measured in single grapevine leaves [41], significant and consistent differences in WUEi were only observed between the less-vigorous 101-14 MGt and the more-vigorous 140 Ru rootstocks (Table 2). These findings point to subtle variances among the rootstocks in their responses to fluctuations in water availability and in how they regulate gas exchange at the single-leaf level.
The lower ΔΨdawn–stem difference for the 140 Ru vines (Table 1) suggests a reduced overall resistance to transpiration flow, likely because of this rootstock’s greater water uptake capacity. Conversely, the higher ΔΨdawn–stem differences in 101-14 MGt and 3309 C (Table 1) indicate higher overall resistances or lower plant hydraulic conductances (Kplant; Table 1), alongside a smaller gradient between the water potentials of the stem and leaf blade (ΔΨstem–leaf), which are presumably the result of lower gs rates [27]. As stomatal conductance variation is contingent upon the total resistance to the water flow within the plant [42], the observed differences in gs among rootstocks might be attributed to varying resistances to the internal water’s movement. However, according to [43], the rootstocks primarily confer drought resistance to the scion through enhanced root growth and soil colonization rather than through significant changes in the hydraulic conductivity of woody vessels or leaf stomatal conductance.
According to [44], the difference in Ψ between the stem/petiole and leaf blade (ΔΨstem–leaf) for a given transpiration rate is proportional to the resistances encountered during water movement from the stem to the blade. The higher ΔΨstem–leaf observed in 140 Ru ‘Xinomavro’ vines (Table 1) may be attributable to elevated gs, likely resulting in increased water loss through transpiration.
4.2. Vine Growth and Yield
As discussed previously, rootstock varieties differ in their capacities for water and nutrient uptake from the soil, which in turn influence the vigor they confer to the scions [2]. In this regard, 140 Ru and 110 R are considered highly vigorous and vigorous rootstocks, respectively [45]. Despite both 101-14 MGt and 3309 C being V. riparia × V. rupestris hybrids, evidence suggests that 101-14 MGt confers less vigor to scions compared to 3309 C [46]. The differences observed in the canopy size among the four rootstocks, as indicated by their total leaf areas (Figure 3A), are likely linked to their varying capacities for water and nutrient uptake. Similar responses to different rootstocks were reported previously by the authors of [47], who investigated the effects of several rootstock varieties on the performance of ‘Syrah’ vines drip-irrigated with moderately saline water. In the present study, the rankings of 101-14 MGt and 3309 C in terms of the total leaf area and cane biomass production align with these previous findings but only for the year 2017 (Figure 3A,B), suggesting an interaction between the growth capacity and year. Additionally, rootstocks may influence shoots’ vigor by altering the efficiency with which photosynthetic products are transported. Under the conditions of our study, 140 Ru and 110 R, which exhibited higher net assimilation rates (Table 2), likely allocated more photosynthates to shoot biomass production [38]. However, extrapolating gas exchange measurements from single leaves to the whole canopy requires caution [48].
Effects similar to those observed in the grape yields of ‘Xinomavro’ scions have been reported by other researchers. For instance, the authors of [49] investigated the responses of ‘Syrah’ when grafted onto 12 different rootstocks and found lower yields for 101-14 MGt compared to 3309 C and 110 R. Although not consistent in both years of our study, rootstocks’ similar effects on the mean cluster weight have also been reported in [47]. The larger berries in 140 Ru and 110 R, in comparison to 101-14 MGt and 3309 C, agree with the findings of other studies on 140 Ru and 110 R [50]. As observed in our study, the larger berry size in grapevines with more-vigorous rootstocks has been reported in previous studies and is attributed to the greater capacity of those rootstocks to supply water to the scion [51]. The berry size at maturity is considered as an important quality variable for red wine grapes [52], as smaller berries are generally associated with improved wine quality because of their higher skin-to-berry volumetric ratio [53]. This ratio leads to the increased dissolution of key skin constituents in the must at vinification.
Despite the significant differences in the mean berry weight among the four rootstocks, the observed declines in the mean berry weights for 140 Ru, 101-14 MGt, and 3309 C treatments during the latter part of the maturation in 2017 suggest berry shriveling, a phenomenon that was not evident in the 110 R vines (Figure 4). Consequently, berry maturation in 2017 manifested atypical berry growth (Figure 4).
4.3. Berry Composition
Berry composition is primarily influenced by the scion variety but also by the rootstock genotype through its effects on the vine’s vegetative and reproductive growths and nutrient uptake capacity [3]. This influence happens either indirectly—through effects of the rootstock on the vine yield, for example—or by directly affecting berry development [45]. Overall, these effects are significantly influenced by the variation in climate conditions within and across different years [45], as observed for most of the berry composition variables in our study and further evidenced by the significant interactions between the year and rootstock variety (Table 3). In this context, berries from vines with different rootstocks have been found to differ in their juice’s TSS content, but these responses were not always consistent. For example, mature berries of ‘Marselan’ vines grafted onto 101-14 MGt have been found to be richer in TSS than 110 R and 3309 C [21] but in the case of ‘Pinot Noir’, did not differ from vines grafted onto the vigorous 1103 P rootstock [20]. Such inconsistent responses have also been observed for TA and pH [3].
The differences in the maturation profiles in 2016 and 2017 provide us with some insights into how anthocyanins and the total phenolics may by modulated by the four rootstocks in this study. In the ‘normal’ maturation period in 2016, the berry weight and anthocyanin content both increased gradually from veraison to maturity (Figure 4 and Figure 5), but the magnitude of the increase was much higher for the anthocyanin content. Thus, any ‘dilution’ effects of the increasing berry size were overwhelmed by the higher increase in the anthocyanin content, explaining the simultaneous increase in the anthocyanin concentration in all the rootstock treatments (Figure 5). The expression of berry constituents on a ‘content’ basis represents the balance among various events—biosynthesis, degradation, conversion, and transport into or out of the berry—that finally lead to an increase or decrease in a specific constituent [54]. Therefore, the higher contents and concentrations of anthocyanin in the berries of 101-14 MGt and 3309 C (Table 3) were probably the result of a combination of direct rootstock effects on berry metabolism and functioning. Increased anthocyanin biosynthesis in 101-14 MGt and 3309 C vines, compared to 140 Ru and 110 R, was likely due to the higher water deficit experienced during the crucial berry maturation period. This is supported by the lower stem water potential (Ψstem) values observed in 101-14 MGt and 3309 C vines (approximately −1.2 MPa), which were more aligned with literature values associated with the enhanced activation of the berries’ secondary metabolism [55]. In the case of the total phenolics, however, the magnitude of their increase during maturation in 2016 was comparable to that of the berry weight (Figure 4 and Figure S2), while the rootstocks did not differ in their total phenolic contents at maturity (Table 3). Consequently, the higher phenolic concentrations in the mature berries in the 101-14 MGt and 3309 C (Table 3) treatments were actually the result of lower berry weights for these rootstocks (Table S1).
The atypical berry growth observed during the 2017 maturation period, characterized by berry shriveling (Figure 4), coincided with significantly lower mean values for the measured berry composition variables, with the exception of the anthocyanin content (Table 3). This suggests an irregular year for berry development. However, as for 2016, the anthocyanin content gradually increased during the maturation period, albeit reaching lower levels compared to 2016 (Figure 5). But contrary to 2016, the increases in the anthocyanin contents in 101-14 MGt and 3309 C were not accompanied by simultaneous increases in berry weight (Figure 4 and Figure 5). Given that 3309C and 101 MGt had similar anthocyanin contents at maturity but 101-14 MGt had a much lower berry weight from all the other rootstock treatments, we would expect a higher anthocyanin concentration in 101-14 MGt than in 3309 C. Instead, 3309 C had a higher concentration of anthocyanins (Figure 5, Table 3). This was probably an adverse effect of berry shriveling that affected the efficiency of the berry sampling, being more pronounced in the 101-14 MGt treatment, where berry shriveling started early in the maturation period (Figure 4).
As for TSS, TA, and pH, inconsistent anthocyanin responses to the rootstock genotype have been reported. For example, [21] reported higher anthocyanin concentrations in berries of the ‘Marselan’ variety when grafted onto 101-14 MGt compared to 3309 C and 110 R, which had similar concentrations. On the contrary, the authors of [56] did not observe any rootstock effects on the anthocyanin concentration in ‘Cabernet Sauvignon’ vines grafted onto SO4 and 1103 Paulsen. Overall, the inconsistent responses of berry composition variables reported in the scientific literature may be attributed to the possibly higher impact of the interaction between the scion and rootstock genotype compared to the rootstocks’ main effects [1]. In addition, as our data suggest, the expression of berry constituents solely on a concentration basis may inhibit the interpretation of the influence exerted on berry constituents by different rootstock genotypes. This is because a given concentration of a berry constituent can be the outcome of different combinations between the content of this constituent and the berry’s size [54].
Regardless of the underlying mechanisms of the rootstock effects in our study, the resulting berry compositions of 101-14 MGt and 3309 C vines generally align with desirable quality markers for ‘Xinomavro’ grapes. Although certain aspects, such as berry size, may be modulated by viticultural practices, like irrigation and leaf removal [57], their effectiveness can be constrained by local soil and seasonal weather patterns, especially during the key growth phases commonly observed in the spring within the ‘Xinomavro’ growing region. Ultimately, choosing the appropriate rootstock is a critical, long-term decision for influencing ‘Xinomavro’ vineyard attributes, laid down at the time of the vineyard’s establishment.
5. Conclusions
Our research demonstrates that the rootstock variety significantly affects ‘Xinomavro’ vine performance, with certain rootstocks promoting biomass production, while others enhance grape quality by increasing the total soluble solids and anthocyanin levels. For instance, the less-vigorous 101-14 MGt increased the concentration of secondary metabolites that are crucial for wine quality. Conversely, the more-robust 110 R and 140 Ru rootstocks favored vegetative growth over grape composition. These findings highlight the importance of the careful selection of rootstocks for ‘Xinomavro’ based on desired vine characteristics and regional growing conditions. For instance, employing vigorous rootstocks, such as 110 R and 140 Ru, is advisable where increased yields are sought or at sites with lower potential and water scarcity. In contrast, the less-vigorous rootstocks, 101-14 MGt and 3309 C, are preferable when the goal is to enhance grape quality. Additionally, the observed year-to-year variation suggests that environmental factors play a substantial role, indicating a need for further research on adaptive viticulture practices in light of climate change.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10050490/s1: Table S1. The effects of the rootstock on grape berry and bunch features; Figure S1. The effects of the rootstock on the total soluble solids, titratable acidity, and pH; Figure S2. The effects of the rootstock on the total phenolics per berry and per-berry weight.
Author Contributions
Conceptualization, S.T. and S.K.; data curation, S.T., D.T., T.G., C.K. and A.A.; methodology, S.T. and S.K.; supervision, S.K.; writing—original draft preparation, S.T., A.A. and D.T.; writing—review and editing, S.T., D.T. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tandonnet, J.-P.; Cookson, S.J.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Aust. J. Grape Wine Res. 2010, 16, 290–300. [Google Scholar] [CrossRef]
- Jones, T.H.; Cullis, B.R.; Clingeleffer, P.R.; Rühl, E.H. Effects of novel hybrid and traditional rootstocks on vigour and yield components of Shiraz grapevines. Aust. J. Grape Wine Res. 2009, 15, 284–292. [Google Scholar] [CrossRef]
- Keller, M.; Mills, L.J.; Harbertson, J.F. Rootstock Effects on Deficit-Irrigated Winegrapes in a Dry Climate: Vigor, Yield Formation, and Fruit Ripening. Am. J. Enol. Vitic. 2012, 63, 29–39. [Google Scholar] [CrossRef]
- Cookson, S.J.; Ollat, N. Grafting with rootstocks induces extensive transcriptional re-programming in the shoot apical meristem of grapevine. BMC Plant Biol. 2013, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Cochetel, N.; Escudié, F.; Cookson, S.J.; Dai, Z.; Vivin, P.; Bert, P.-F.; Muñoz, M.S.; Delrot, S.; Klopp, C.; Ollat, N.; et al. Root transcriptomic responses of grafted grapevines to heterogeneous nitrogen availability depend on rootstock genotype. J. Exp. Bot. 2017, 68, 4339–4355. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, N.; Koukourikou, M.; Karagiannidis, N.; Koukourikou, M.A. Effects of various rootstocks on xylem exudates cytokinin content, nutrient uptake and growth patterns of grapevine Vitis vinifera L. cv. Thompson seedless. EDP Sci. 2000, 20, 363. [Google Scholar]
- Lang, C.P.; Merkt, N.; Zörb, C. Different nitrogen (N) forms affect responses to N form and N supply of rootstocks and grafted grapevines. Plant Sci. 2018, 277, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, N.; Monte, R.; Varanini, Z.; Cesco, S.; Pinton, R. Induction of nitrate uptake in Sauvignon Blanc and Chardonnay grapevines depends on the scion and is affected by the rootstock. Aust. J. Grape Wine Res. 2015, 21, 331–338. [Google Scholar] [CrossRef]
- Csikasz-Krizsics, A.; Diofasi, L. Effects of rootstock-scion combinations on Macroelements availability of the vines/Alany-nemesfajta Kombinaciok Hatasa a Szolo Makroelem Felvetelere. J. Cent. Eur. Agric. 2008, 9, 495–505. [Google Scholar]
- Jin, K.; White, P.J.; Whalley, W.R.; Shen, J.; Shi, L. Shaping an Optimal Soil by Root-Soil Interaction; Trends in Plant Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 22. [Google Scholar]
- Kocsis, L.; Lehoczky, É. The Significance of Yield Production and Sugar Content of the Grapejuice with Macronutrients in Grape Rootstock–Scion Combinations on Dry Climatic Condition. Commun. Soil Sci. Plant Anal. 2002, 33, 3159–3166. [Google Scholar] [CrossRef]
- Wooldridge, J.; Louw, P.; Conradie, W.J. Effects of Rootstock on Grapevine Performance, Petiole and Must Composition, and Overall Wine Score of Vitis vinifera cv. Chardonnay and Pinot noir. S. Afr. J. Enol. Vitic. 2016, 31, 45–48. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Franck, N.; Zamorano, D.; Sanhueza, C.; Carvajal, D.E.; Ibacache, A. Rootstock effect on irrigated grapevine yield under arid climate conditions are explained by changes in traits related to light absorption of the scion. Sci. Hortic. 2017, 218, 284–292. [Google Scholar] [CrossRef]
- Ozden, M.; Vardin, H.; Simsek, M.; Karaaslan, M. Effects of rootstocks and irrigation levels on grape quality of Vitis vinifera L. cv. Shiraz. Afr. J. Biotechnol. 2010, 9, 3801–3807. [Google Scholar]
- Gambetta, G.A.; Manuck, C.M.; Drucker, S.T.; Shaghasi, T.; Fort, K.; Matthews, M.A.; Walker, M.A.; McElrone, A.J. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Vannozzi, A.; Ziliotto, F.; Zouine, M.; Maza, E.; Nicolato, T.; Vitulo, N.; Meggio, F.; Valle, G.; Bouzayen, M.; et al. Grapevine Rootstocks Differentially Affect the Rate of Ripening and Modulate Auxin-Related Genes in Cabernet Sauvignon Berries. Front. Plant Sci. 2016, 7, 69. [Google Scholar] [CrossRef]
- Lee, J.; Steenwerth, K.L. Rootstock and vineyard floor management influence on ‘Cabernet Sauvignon’ grape yeast assimilable nitrogen (YAN). Food Chem. 2011, 127, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.R.d.; Da Mota, R.V.; França, D.V.C.; Pimentel, R.M.d.A.; Regina, M.d.A. Cabernet Sauvignon grapevine grafted onto rootstocks during the autumn-winter season in Southeastern Brazilian. Sci. Agric. 2015, 72, 138–146. [Google Scholar] [CrossRef]
- Satisha, J.; Ramteke, S.D.; Karibasappa, G.S. Physiological and Biochemical Characterisation of Grape Rootstocks. S. Afr. J. Enol. Vitic. 2007, 28, 163–168. [Google Scholar] [CrossRef]
- Zombardo, A.; Mica, E.; Puccioni, S.; Perria, R.; Valentini, P.; Mattii, G.B.; Cattivelli, L.; Storchi, P. Berry Quality of Grapevine under Water Stress as Affected by Rootstock–Scion Interactions through Gene Expression Regulation. Agronomy 2020, 10, 680. [Google Scholar] [CrossRef]
- Li, M.; Guo, Z.; Jia, N.; Yuan, J.; Han, B.; Yin, Y.; Sun, Y.; Liu, C.; Zhao, S. Evaluation of eight rootstocks on the growth and berry quality of ‘Marselan’ grapevines. Sci. Hortic. 2019, 248, 58–61. [Google Scholar] [CrossRef]
- Brancadoro, L.; Valenti, L.; Reina, A.; Scienza, A. Potassium content of grapevine during the vegetative period: The role of the rootstock. J. Plant Nutr. 1994, 17, 2165–2175. [Google Scholar] [CrossRef]
- Duchene, E. How can grapevine genetics contribute to the adaptation to climate change? OENO One 2016, 50. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Destrac-Irvine, A. Modified grape composition under climate change conditions requires adaptations in the vineyard. OENO One 2017, 51, 147. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; García de Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the world’s future wine-growing regions. Nat. Clim. Chang. 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Theocharis, S.; Taskos, D.; Gkrimpizis, T.; Nikolaou, K.-E.; Miliordos, D.-E.; Koundouras, S. Optimizing ‘Xinomavro’ (Vitis vinifera L.) Performance by Post-Bloom Basal Leaf Removal Applications. Horticulturae 2024, 10, 340. [Google Scholar] [CrossRef]
- Choné, X. Stem Water Potential is a Sensitive Indicator of Grapevine Water Status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Romero, P.; Fernández-Fernández, J.I.; Martinez-Cutillas, A. Physiological Thresholds for Efficient Regulated Deficit-Irrigation Management in Winegrapes Grown under Semiarid Conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar] [CrossRef]
- Lopes, C.M.; Pinto, P.A. Estimation de la surface foliaire principale et secondaire d’un sarment de vigne. Progrés Agric. Vitic. 2000, 177, 160–166. [Google Scholar]
- Iland, P. Techniques for Chemical Analysis and Quality Monitoring during Winemaking; Patrick Iland Wine Promotions: Campbelltown, Australia, 2000; ISBN 064638435X. [Google Scholar]
- van Leeuwen, C. Soils and Terroir Expression in Wines. In Soil and Culture; Landa, E.R., Feller, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 453–465. ISBN 978-90-481-2960-7. [Google Scholar]
- Nikolaou, N.; Angelopoulos, K.; Karagiannidis, N. Effects of drought stress on mycorrhizal and non-mycorrhizal Cabernet Sauvignon grapevine, grafted onto various rootstocks. Exp. Agric. 2003, 39, 241–252. [Google Scholar] [CrossRef]
- Peiró, R.; Jiménez, C.; Perpiñà, G.; Soler, J.X.; Gisbert, C. Evaluation of the genetic diversity and root architecture under osmotic stress of common grapevine rootstocks and clones. Sci. Hortic. 2020, 266, 109283. [Google Scholar] [CrossRef]
- Bauerle, T.L.; Smart, D.R.; Bauerle, W.L.; Stockert, C.; Eissenstat, D.M. Root foraging in response to heterogeneous soil moisture in two grapevines that differ in potential growth rate. New Phytol. 2008, 179, 857–866. [Google Scholar] [CrossRef]
- Cuneo, I.F.; Barrios-Masias, F.; Knipfer, T.; Uretsky, J.; Reyes, C.; Lenain, P.; Brodersen, C.R.; Walker, M.A.; McElrone, A.J. Differences in grapevine rootstock sensitivity and recovery from drought are linked to fine root cortical lacunae and root tip function. New Phytol. 2021, 229, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Masias, F.H.; Knipfer, T.; McElrone, A.J. Differential responses of grapevine rootstocks to water stress are associated with adjustments in fine root hydraulic physiology and suberization. J. Exp. Bot. 2015, 66, 6069–6078. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.; Trégoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillère, J.-P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? J. Int. Sci. Vigne Vin 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; Navarro, J.M. Selecting rootstocks to improve vine performance and vineyard sustainability in deficit irrigated Monastrell grapevines under semiarid conditions. Agric. Water Manag. 2018, 209, 73–93. [Google Scholar] [CrossRef]
- Bota, B.J.; Flexas, J.; Medrano, H. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Ann. Appl. Biol. 2001, 138, 353–361. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.). Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S. Effects of the experimental blockage of the major veins on hydraulics and gas exchange of Prunus laurocerasus L. leaves. J. Exp. Bot. 2003, 54, 1213–1219. [Google Scholar] [CrossRef]
- Alsina, M.M.; Smart, D.R.; Bauerle, T.; de Herralde, F.; Biel, C.; Stockert, C.; Negron, C.; Save, R. Seasonal changes of whole root system conductance by a drought-tolerant grape root system. J. Exp. Bot. 2011, 62, 99–109. [Google Scholar] [CrossRef]
- Begg, J.E.; Turner, N.C. Water potential gradients in field tobacco. Plant Physiol. 1970, 46, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Neal, S.; Trought, M.; West, B. Rootstock Evaluation for Premium Wine 2010-11; The New Zealand Institute for Plant & Food Research: Auckland, New Zealand, 2011. [Google Scholar]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Holt, H.; Pearson, W.; Francis, I.L. Effect of rootstock on yield, grape composition and wine sensory attributes of Shiraz grown in a moderately saline environment. Aust. J. Grape Wine Res. 2019, 25, 414–429. [Google Scholar] [CrossRef]
- Poni, S.; Bernizzoni, F.; Civardi, S.; Gatti, M.; Porro, D.; Camin, F. Performance and water-use efficiency (single-leaf vs. whole-canopy) of well-watered and half-stressed split-root Lambrusco grapevines grown in Po Valley (Italy). Agric. Ecosyst. Environ. 2009, 129, 97–106. [Google Scholar] [CrossRef]
- Agut, C.; Rodríguez-Lovelle, B.; Fabre, F. Incidence du porte-greffe sur le comportement du cépage Syrah. In Proceedings of the XIVth International GIESCO Viticulture Congress, Geisenheim, Germany, 23–27 August 2005; pp. 148–154. [Google Scholar]
- Satisha, J.; Somkuwar, R.G.; Sharma, J.; Upadhyay, A.K.; Adsule, P.G. Influence of Rootstocks on Growth Yield and Fruit Composition of Thompson Seedless Grapes Grown in the Pune Region of India. S. Afr. J. Enol. Vitic. 2010, 31, 1–8. [Google Scholar] [CrossRef][Green Version]
- Marguerit, E.; Brendel, O.; Lebon, E.; van Leeuwen, C.; Ollat, N. Rootstock control of scion transpiration and its acclimation to water deficit are controlled by different genes. New Phytol. 2012, 194, 416–429. [Google Scholar] [CrossRef]
- Gil, M.; Pascual, O.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I.; Zamora, F.; Canals, J.M. Influence of berry size on red wine colour and composition. Aust. J. Grape Wine Res. 2015, 21, 200–212. [Google Scholar] [CrossRef]
- Roby, G.; Matthews, M.A. Relative proportions of seed, skin and flesh, in ripe berries from Cabernet Sauvignon grapevines grown in a vineyard either well irrigated or under water deficit. Aust. J. Grape Wine Res. 2004, 10, 74–82. [Google Scholar] [CrossRef]
- Iland, P.; Dry, P.; Proffitt, T.; Tyerman, S. The Grapevine: From the Science to the Practice of Growing Vines for Wine; Patrick Iland Wine Promotions: Campbelltown, Australia, 2011; ISBN 978-0-9581605-5-1. [Google Scholar]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Koundouras, S.; Hatzidimitriou, E.; Karamolegkou, M.; Dimopoulou, E.; Kallithraka, S.; Tsialtas, J.T.; Zioziou, E.; Nikolaou, N.; Kotseridis, Y. Irrigation and rootstock effects on the phenolic concentration and aroma potential of Vitis vinifera L. cv. cabernet sauvignon grapes. J. Agric. Food Chem. 2009, 57, 7805–7813. [Google Scholar] [CrossRef] [PubMed]
- Diakou-Verdin, P.; Carde, J.-P.; Gaudillère, J.-P.; Barrieu, F.; Ollat, N.; Moing, A. Grape berry development: A review. J. Int. Des Sci. Vigne Vin 2002, 36, 109–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).